Abstract
Background
The molecular mechanisms leading to ascending thoracic aortic aneurysms (ATAAs) remain unknown. We hypothesized that alterations in expression levels of specific fibrillar collagens occur during the aneurysmal process.
Methods
Surgical samples from ascending aortas from patients with degenerative ATAAs were subdivided by aneurysm diameter: small, 5 to 6 cm; medium, 6 to 7 cm; and large, greater than 7 cm; and compared with nonaneurysmal aortas (mean diameter, 2.3 cm).
Results
Histology, immunofluorescence, and electron microscopy demonstrated greater disorganization of extracellular matrix constituents in ATAAs as compared with control with an increase in collagen α1(XI) within regions of cystic medial degenerative lesions. Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) showed collagens type V and α1(XI) were significantly and linearly increased in ATAAs as compared with control (p < 0.001). There was no change in the messenger ribonucleic acid (mRNA) expression levels of collagens type I and III. Western blot analysis showed collagens type I and III were significantly decreased and collagens α1(XI) and V were significantly increased and were linearly correlated with the size of the aneurysm (p < 0.001 for both).
Conclusions
These results demonstrate that increased collagen α1(XI) and collagen V mRNA and protein levels are linearly correlated with the size of the aneurysm and provide a potential mechanism for the generation and progression of aneurysmal enlargement.
Ascending thoracic aortic aneurysms (ATAAs) predominately affect the elderly population with an incidence of 5.9 new cases per 100,000 persons/year [1]. It is expected that the incidence of ATAAs will continue to increase with the potential of severe clinical consequences, including rupture, dissection, and the possibility to cause aortic valve insufficiency [2].
There are two broad categories of ATAAs; namely those associated with genetic syndromes such as Marfan, Ehlers-Danlos, and Loeys-Dietz, and those without genetic involvement, which predominantly occur in the aging population [3]. The pathogenesis of ATAAs, not associated with known genetic syndromes, remains poorly understood. However, previous research has suggested that alterations in the extracellular matrix (ECM), the major constituent of the aortic wall, may play an important role in the formation and expansion of ATAAs [3–5]. The aortic wall is primarily composed of collagen types I and III, the major fibrillar collagens, responsible for the tensile strength of the aortic wall [6, 7]. Under normal conditions collagen types I and III form heterotypic fibrils with collagen type V [6]. Collagen type V is a minor fibrillar collagen, but plays a critical role in the regulation of the size/diameter of the heterotypic fibrils [5]. Greater proportions of collagen type V have been shown to significantly decrease the diameter of the heterotypic fibrils, and in turn decrease tensile strength; however, other minor fibrillar collagens may also be involved. One of these is collagen α1(XI).
Collagen α1(XI) is structurally and biologically related to collagen type V and has the same large globular amino-terminal domain [5]. Experimental data have shown that collagen α1(XI) is expressed in the mouse embryonic aortic tunica media and that the messenger ribonucleic acid (mRNA) of collagen type α1(XI) may be present in human abdominal aortic aneurysms [8, 9]. However, there have been no data to show the involvement of collagen α1(XI) in the normal thoracic aorta or in thoracic aortic aneurysms in humans. The purpose of the present study was to determine the mRNA and protein expression levels of fibrillar collagens I, III, V, and α1(XI) in the aortic wall of ATAAs and to compare these results with control nonaneurysmal aortas during the progression of the aneurysmal enlargement.
Material and Methods
Clinical Data and Aortic Specimens
Research protocols were approved by the Institutional Review Boards in all participating hospitals and informed consents were obtained. Clinical data and aortic specimens were collected during a 2-year period (April 2006 to April 2008) from patients with nonaneurysmal ascending thoracic aortas undergoing heart transplantation (n = 7) and patients undergoing replacement of ATAAs (n = 25). All patients had tricuspid aortic valves and patients with ATAAs were subdivided into three groups according to the maximal diameter of the aneurysm: small ATAAs (n = 9) with diameters between 5 and 6 cm; medium ATAAs (n = 8) between 6 and 7 cm; and large ATAAs with diameters greater than 7 cm. Full-thickness biopsies containing all three layers of the aorta were collected from the right-lateral aspect of the ascending aorta (the greater curvature, roughly in line with the commissure between the right and noncoronary sinuses) in the operating room and fresh frozen in liquid nitrogen and stored at −80°C until analysis.
All patients with ATAAs underwent elective surgery and required graft replacement of the ascending aorta. Aortic samples from relatively young patients (<50 years of age) or from patients with aneurysms secondary to genetic syndromes such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndrome or from patients with bicuspid aortic valves were excluded from this study.
Histology, Immunofluorescence, and Transmission Electron Microscopy
Tissue samples from the site of the maximal diameter of the ascending aorta were used for histological, immunofluorescence, and transmission electron microscopy analysis. For histology, frozen tissue was sectioned (1.5 μm thickness). Thirty sections from each specimen were mounted on glass slides and divided sequentially for Masson’s trichrome staining and Movat’s pentachrome staining using standard techniques and reagents [10]. Some sections were stained with collagen type I (ab292, 1:200 dilution; Abcam Inc, Cambridge MA). Primary antibodies were detected with species-appropriate Alexa 568 conjugated secondary antibodies (Molecular Probes, Invitrogen; Carlsbad, CA) prior to mounting and visualization on a multipoint spinning disk confocal system (Atto; BD Biosciences, Rockville, MD) attached to a Zeiss Axiovert 200M microscope (Zeiss, Thornwood, NY) [10].
For immunohistochemistry, tissue samples were fixed in 5% zinc buffered formalin, embedded in paraffin, cut to 1 μm thickness, and heat fixed onto glass slides. Sections were deparaffinized in xylene, rehydrated through graded ethanol, and incubated for 30 minutes in 0.01 mg/mL hyaluronidase (Sigma-Aldrich Corporation, St. Louis, MO) [11]. Primary antibody to collagen α1(XI) was diluted 1:400 and applied to tissue section for 1 hour at 25°C [12]. An antirabbit secondary antibody conjugated to horseradish peroxidase was applied and incubated for 30 minutes, followed by development with substrate chromogen (DakoCytomation, Dako, North America). Slides were subsequently counterstained with hematoxylin. For transmission electron microscopy tissue samples were fixed with 1.25% formaldehyde, 2.5% glutaraldehyde, and 0.03% picric acid in 100 mM cacodylate buffer, and embedded in resin [10]. Thin sections (1 μm) were stained with 1% uranyl acetate and examined with transmission electron microscopy by an independent pathologist.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Frozen tissue was ground in liquid nitrogen and total RNA extracted by homogenization in Trizol reagent (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s protocol. Real-time qRT-PCR was performed using a Chromo 4 continuous fluorescence detector and Opticon monitor 3 software (MJ Research, Waltham, MA) using an iScript one-step RT-PCR kit with SYBR green solution (Bio-Rad, Hercules, CA) according to manufacturer’s instructions. In brief, 100 ng of total RNA and 600 nM of both forward and reverse primer were added to each reaction. Primers are shown in Table 1. Control samples and ATAAs samples were run for each primer set in duplicate. Control reactions without reverse transcriptase were performed for each reaction. Reaction kinetics were optimized for each primer set: reverse transcription 30 minutes at 60°C, denaturation 2 minutes at 94°C, followed by 60 cycles of denaturation 15 seconds at 94°C; annealing for 30 seconds at 58°C α1(I), 60°C α1(III), 61°C α2(V), 65°C α1(XI), and 60°C β-actin; extension for 2 minutes at 68°C. The RT-PCR products were stored at 4°C until further analysis. Melting curves were constructed for each reaction at the conclusion of the cycling parameters from 60°C to 95°C. Fold changes in gene expression were calculated using the Pfaffl method [13]. Real-time RT-PCR products were validated by agarose gel electrophoresis and sequencing.
Table 1.
Primers Used for Real Time RT-PCR Reactions
| mRNA Target | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | 5′-AGCATTGCTTTCGTGTAAATTATG-3′ | 5′-GTGTGCACTTTTATTCAACTGGTC-3′ |
| Collagen α1 (I) | 5′-CTCTGACTGGAAGAGTGGAGAGTA-3′ | 5′-TTGGTGGTTTTGTATTCAATCACT-3′ |
| Collagen α1 (III) | 5′-AGTGACCGACAAAATTCCAGTTAT-3′ | 5′-CTTTTACTGGTGAGCACAGTCATT-3′ |
| Collagen α2 (V) | 5′-TGAGTTGTGGAGCTGACTCTAATC-3′ | 5′-TAACAGAAGCATAGCACCTTTCAG-3′ |
| Collagen α1 (XI) | 5′-GAAATTGTACCTTGGTGCCACCAAC-3′ | 5′-GGATGGATGAGAATGAGCACCATAT-3′ |
mNRA = messenger ribonucleic acid; RT-PCR = reverse transcription-polymerase chain reaction.
Western Blotting
Full-thickness aortic tissue from the site of the maximal diameter containing all three layers (tunica adventitia, tunica media, and tunica intima) was pulverized under liquid nitrogen and protein extraction was performed using the T-PER reagent (Pierce, Rockford, IL). Protein concentration estimation was performed by using the BCA protein assay (Pierce). Total protein (40 μg) was used for standard denaturing 10% sodium-dodecyl-sulphate polyacrylamide gel electrophoresis under non-reducing (collagen types I and V) or reducing conditions (collagen type III, collagen α1(XI), and β-actin). Protein transfer and blocking was performed as previously described [10]. Immunoblotting was performed using the following antibodies: collagen type I (ab292, 1:2000 dilution; Abcam Inc, Cambridge, MA); collagen type III (ab6310, 1:1000 dilution; Abcam Inc); collagen type V (ab19812, 1:200 dilution; Abcam Inc); collagen α1(XI) (kind gift of Dr J. T. Oxford, Boise State University, Boise, ID) [14]; and beta-actin (ab8227, 1:2000 dilution; Abcam Inc). Antibodies were diluted in tris-buffered saline-Tween and incubated with the membranes at 4°C overnight. Membranes were washed and appropriate secondary antibodies (dilution 1:5,000) (Santa Cruz Biotechnology Inc, Santa Cruz, CA) were used [10]. Blots were detected using ECL Plus (Amersham Pharmacia Biotech, Piscataway, NJ) with species-appropriate secondary antibodies. Densitometric analysis was performed using the ImageJ analysis software (Rasband WS, ImageJ; http://rsb.info.nih.gov/ij).
Statistical Analysis
All continuous variables were expressed as mean ± SD. Discrete variables were summarized by percentages. Independent sample t tests and one way analysis of variance were used for mean comparisons between two or multiple groups, respectively. The Spearman rank correlations, χ2 test, and Fisher exact test were also performed to describe association between different outcome variables. The Bonferroni correction was used to adjust for both multiple comparisons and correlated outcome variables. Two-tailed probability values of p less than 0.01 were considered statistically significant for each test to ensure an overall study significance level of p less than 0.05. All statistical analyses were performed in SPSS 15.0 (SPSS, Inc, Chicago, IL).
Results
Aortic Specimens and Patient Characteristics
Thirty-two surgical ascending aortic samples were analyzed. Seven specimens from patients who underwent heart transplantation procedures with normal ascending aortic diameters (mean diameter, 2.3 cm) were used as controls. Twenty-five patients underwent ascending aortic replacement for ATAAs (mean diameter, 6.8 cm, p < 0.001 vs controls). This subgroup of patients was further divided into small (n = 9, mean diameter 5.6 cm), medium (n = 8, mean diameter 6.5 cm), and large aneurysms (n = 8, mean diameter 8.4 cm) (all p < 0.001 vs controls). Patients with ATAAs had no differences in age, smoking, hypertension, or gender distribution. Patients with control aortas were younger, with decreased percentages of hypertension. All ATAAs specimens analyzed in this study were from patients with tricuspid aortic valves having degenerative ATAAs. None of the specimens analyzed was atherosclerotic and none were lined with thrombus. Control specimens were taken from donors in order to ensure that these aortas would have minimal alterations in matrix composition.
Histology, Immunofluorescence, and Transmission Electron Microscopy
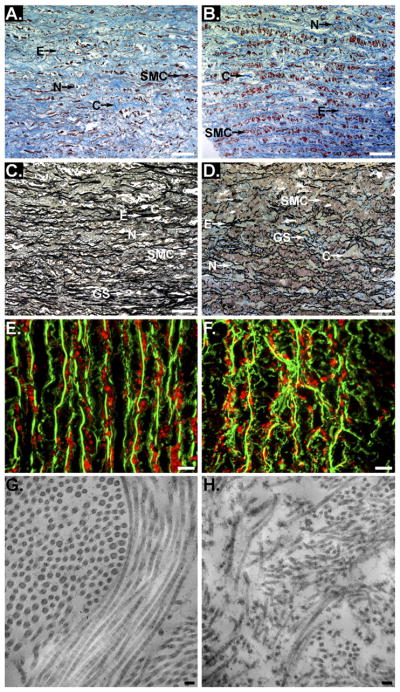
Histological analysis using both Masson’s trichrome and Movat’s pentachrome staining, and transmission electron microscopy demonstrated that both elastin and collagen showed greater disorganization and fragmentation in ATAA samples as compared with controls (Figs 1A–1H). These results were consistent in all samples.
Fig 1.
Histological analysis using Masson’s trichrome (A and B) and Movat’s pentachrome (C and D). Representative serial sections of the tunica media of the aortic wall from control (A and C) and ascending thoracic aortic aneurysms (ATAA) (B and D) are shown. Collagen (C), smooth muscle cells (SMC), elastin (E), nuclei (N), and ground substance (GS) are shown. Scale bars represent 50 μm. Representative serial sections of the tunica media of the aortic wall from control (E) and ATAA (F) are shown. In E and F elastin auto-fluorescence is shown in green and collagen type I is shown in red. Scale bars represent 10 μm. Transmission electron microscopy collagen fibrils are shown in longitudinal and cross sections. Representative serial sections of the tunica media of the aortic wall from control (G) and ATAA (H) are shown. Collagen fibrils in ATAA are fragmented and disorientated as compared with control aorta. Scale bars represent 100 nm.
Immunohistochemical staining demonstrated that collagen α1(XI) was detectable in both control and ATAA samples (Figs 2A, B, D and E). Collagen α1(XI) was more uniformly distributed through the matrix in control tissues while the staining pattern in the ATAA samples appeared more punctuate. Collagen α1(XI) staining intensity was increased in areas of cystic medial degenerative lesions (Figs 2C and 2F).
Fig 2.
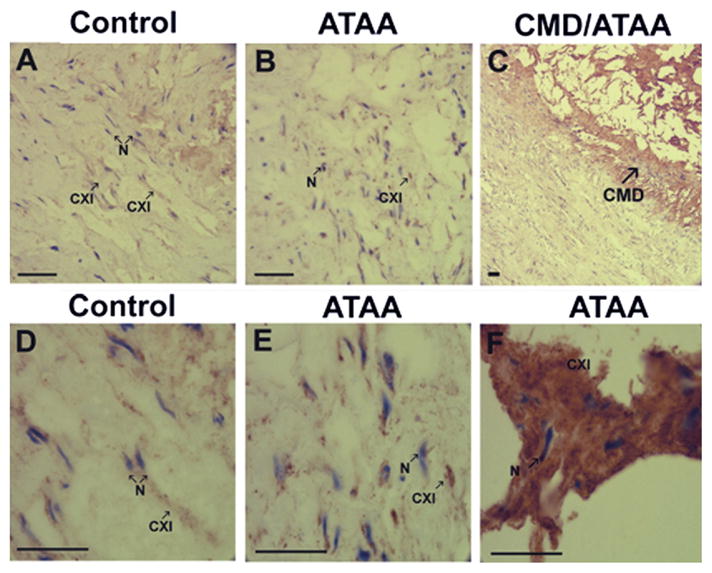
Immunohistological analysis of collagen α1(XI). (A) Control tissue and (B) ascending thoracic aortic aneurysms (ATAA) tissue, at ×200 original magnification. (C) Cystic medial degenerative (CMD) lesion at ×50 original magnification. (D) Control tissue, (E) ATAA tissue, and (F) CMD lesion from ATAA at ×630 original magnification. Tissue sections stained with antibody directed to collagen α1(XI) demonstrated staining within the tunica media in both control tissues and ATAA. Presence of collagen α1(XI) is indicated by brown staining (indicated by arrow CXI). Tissues were counterstained briefly with hematoxylin to stain nuclei (blue, indicated by arrow N). An increase in staining for collagen α1(XI) is shown within regions of CMD lesions. Scale bars in A to F are 100 μm.
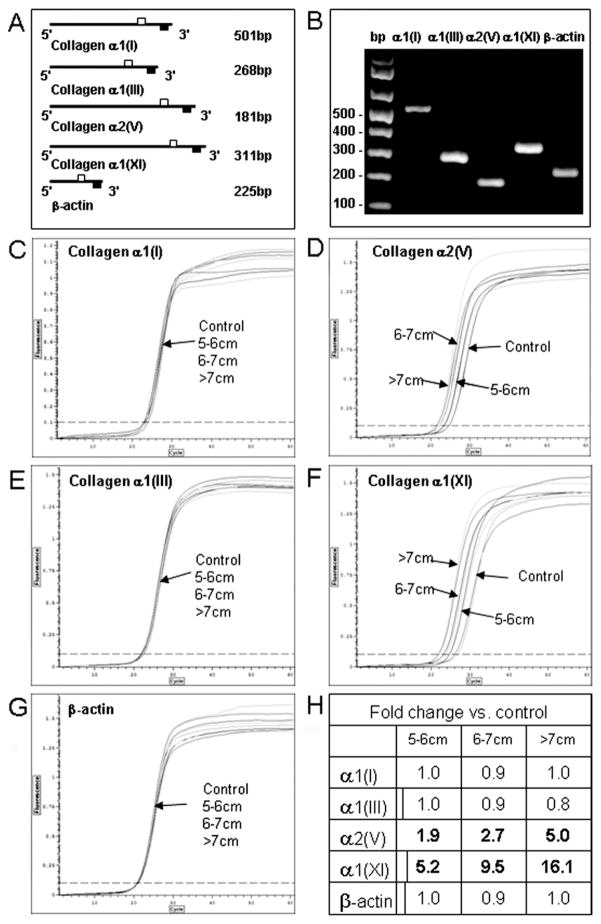
Real-Time qRT-PCR
The qRT-PCR showed only one product per primer set was produced at the predicted molecular size. Sequence analysis confirmed that the products corresponded to the genes analyzed (Fig 3 and results not shown). There was a significant increase in collagens α2(V) and α1(XI) in ATAAs as compared with controls (p < 0.001). Collagen α2(V) expression levels were increased 1.9-, 2.7-, and 5.0-fold and collagen α1(XI) was increased 5.2-, 9.5-, and 16.1-fold in small, medium, and large aneurysms, respectively, as compared with control aortas. There was no significant difference in the expression levels of collagens α1(I) and α1(III) or that of β-actin within or between groups (Fig 3).
Fig 3.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Panel A shows primer sets used for qRT-PCR and expected product size (open squares = forward primer; closed squares = reverse primer). Panel B shows a representative 1.5% agarose gel of amplified products from each primer set. Panels C to G show real-time quantitative amplification curves for collagen α1(I), α1(III), α2(V), α1(XI), and β-actin. For C–G, x-axis = cycle; y-axis = fluorescence. In C, measurements are 0–60 fluorescence units at increments of 10 along x-axis and 0–1.2 fluorescence units at increments of 0.1 along y-axis; in D–G, measurements are 0–60 fluorescence units at increments of 10 along x-axis and 0–1.5 fluorescence units at increments of 0.25 along y-axis. Ascending thoracic aortic aneurysm size is indicated. Panel H shows the fold changes versus controls. Significant differences versus control at p less than 0.001 are shown in bold type.
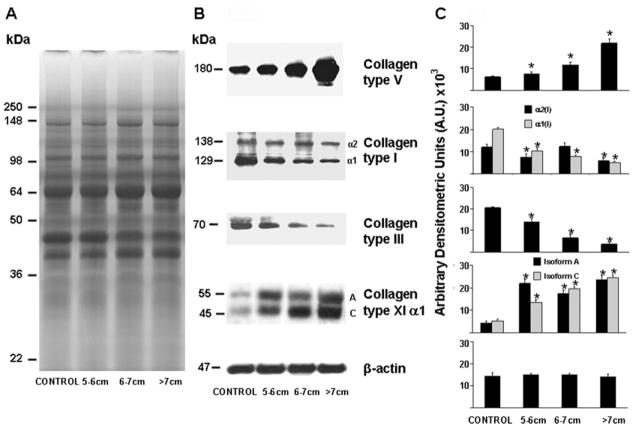
Western Blotting
Western blots (Fig 4) of collagen type I showed two bands at 138 kDa and 129 kDa corresponding to the two α1 and α2 chains, respectively, of collagen type I. Collagen α1(I) protein levels were significantly decreased in all ATAAs categories as compared with control aortas (p < 0.001) while collagen α2(I) protein levels were significantly decreased in small and large aneurysms as compared with controls (p < 0.001). Collagen type III protein levels were significantly decreased in all ATAAs groups as compared with control aortas (p < 0.001). Collagen type V protein levels were significantly increased in all ATAAs groups as compared with control aortas (p < 0.001). Western blots of α1(XI) protein showed two bands, at 55kDa and 45kDa, corresponding to the two different isoforms. Both isoforms were significantly increased in ATAAs as compared with control aortas (p < 0.001).
Fig 4.
Western blot analysis. A representative 10% sodium-dodecyl-sulfate polyacrylamide gel stained with Coomassie brilliant blue for each aneurysm size is shown in panel A. Representative Western immunoblots are shown in panel B. Densitometric analysis of Western blots is shown in panel C. Ascending thoracic aortic aneurysm size is indicated. Significant differences at p less than 0.001 versus control are shown as *.
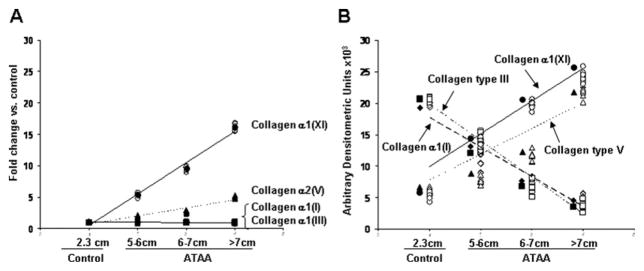
Spearman Rank Correlation Analysis
The Spearman rank correlation analysis was performed for mRNA and for protein levels in comparison to aneurysmal size (Fig 5). Increased mRNA expression levels for collagen α1(XI) and collagen α2(V) showed linear correlation with the size of the aneurysm (p < 0.001 for both). There was no linear correlation between the expression of collagen α1(I) (r = −0.020) or collagen α1(III) (r = −0.063) with the size of the aneurysm (Fig 5A).
Fig 5.
Spearman rank correlations of the messenger ribonucleic acid (A) and protein (B) expression levels of collagens type I, III, V, and α1(XI) with aneurysm size are shown. Control mean diameter was 2.3 cm. (ATAA = ascending thoracic aortic aneurysm.)
Decreased expression levels of collagen α1(I) and collagen type III protein showed linear correlation with the size of the aneurysm (Fig 5B; p < 0.001 for both). Increased expression levels of collagen α1(XI) and collagen type V protein also showed linear correlation with the size of the aneurysm (Fig 5B; p < 0.001 for both).
Comment
Collagen type composition is crucial for the maintenance of vessel wall integrity and tensile strength [5, 7, 15, 16]. In the present study both transmission electron and light microscopy demonstrated that collagen fibrils in ATAAs were fragmented and disorientated, with a less ordered appearance, as compared with control nonaneurysmal samples.
Our results demonstrate that collagen α1(XI) and collagen type V mRNA and protein expression levels are significantly increased within regions of cystic medial degenerative lesions in human ATAAs from patients with tricuspid aortic valves as compared with control aortas, and that there is a linear correlation with the size-diameter of the aneurysm. Our data also show that although collagen types I and III mRNA expression levels are similar in ATAAs as compared with controls there is a significant decrease in their corresponding protein levels, which are also linearly correlated with the size of the aneurysm.
Increased expression of collagen α1(XI) and type V protein may putatively represent a regulatory mechanism for the lateral growth and diameter of fibrils containing collagen types I and III. Previous studies have shown that collagen α1(XI) and type V protein have a large globular amino-terminal domain with similar structure and size [5]. This large globular domain has been suggested to reduce tensile strength through steric hindrance between the large globular amino-terminal domain and the major fibrillar collagens, preventing their assembly into the heterotypic fibrils [7].
The overexpression of collagen α1(XI) and type V would also allow for the formation of heterotrimeric collagen XI/V [17]. These heterotrimeric collagens would inhibit the deposition of collagen types I and III into the fibrils due to the aforementioned steric hindrance. This possible mechanism is supported by basic research studies showing that there is a dose-dependent competition for collagen fibril formation between collagen type I and V as well as collagens type I and α1(XI) [7, 18]. Overexpression of collagen α1(XI) and type V would therefore lead to thinner collagen fibers and decreased tensile strength in the aortic wall increasing the susceptibility to dilatation.
Our data demonstrate that the increase in collagen α1(XI) and type V protein content during ATAA expansion is associated with increased mRNA levels while there is no change in the mRNA levels associated with the decrease in collagen types I and III. This differential regulation has been previously demonstrated in smooth muscle cells in the tunica media of ATAAs [19, 20] and in aortic smooth muscle cells in culture [17], and has been suggested as a possible mechanism modulating ECM remodeling. Recent studies have shown that the amino-terminal propeptide of collagen α1(XI) contains a well-characterized heparin binding domain [21, 22]. This heparin binding domain has been shown to interact with specific integrin receptors that promote the regulation of expression and activity of matrix metalloproteinases (MMPs) [23]. Increased collagens α1(XI) and V mRNA levels could therefore act indirectly to either induce or to further increase MMP synthesis and increase ECM degradation. This cascading effect would further reduce major collagens I and III, which would be unable to be replaced due to static transcription, and increase collagen V and α1(XI) content due to increased mRNA expression levels. These events would ultimately lead to a weakening of the aortic wall and increased susceptibility to dilatation and rupture.
Support for this mechanism comes from Ikonomidis and colleagues [24] who have shown that MMP levels and activity are differentially increased in ATAAs leading to ECM and collagen degradation. The authors showed that in patients having tricuspid aortic valves there was an increase in MMP-13 in ATAAs 4.0 to 5.9 cm and an increase in MMP-7 in larger ATAAs. The MMP-13 and MMP-7 are both expressed by aortic wall cells and have been shown to significantly contribute to ECM remodeling through increased collagen and ECM degradation [25]. Whether MMP degradation proceeds and upregulates collagen V and α1(XI) mRNA and protein expression or is the result of upregulated collagen V and α1(XI) mRNA and protein expression, or acts in a coordinate and additive manner, is beyond the scope of this paper and remains to be determined.
The findings of the present study are in agreement with two recently published studies. Tang and colleagues [16] have also shown that mRNA expression levels of collagen types I and III in ATAAs are unchanged but that there is a 45% decrease in total collagen content as compared with control. Our results also agree with Della Conte and colleagues [25] who have shown a significant reduction in major fibrillar collagens I and III in ATAAs as compared with controls. Our data regarding minor collagens α1(XI) and V mRNA and protein expression levels are unique as none of these studies provided experimental data on minor fibrillar collagen expression and protein synthesis.
In conclusion, we present a molecular mechanism for the formation of ATAAs in patients with tricuspid aortic valves based on the overexpression of minor fibrillar collagen α1(XI) and V, which may regulate the assembly of the major fibrillar collagens I and III. We also report the involvement of collagen α1(XI) in the normal adult human thoracic aorta and its increased expression in ATAAs. Our results demonstrate that increased collagen α1(XI) and collagen V mRNA and protein levels are linearly correlated with the size of the aneurysm and we provide a potential mechanism for the generation and progression of the aneurysmal enlargement.
Acknowledgments
This study was supported by National Heart, Lung and Blood Institute Grant HL-29077, National Center for Research Resources Grant P20RR-016454, the Idaho IDeA Network for Biomedical Research Excellence, and the American Association for Thoracic Surgery (Evarts A. Graham Fellow 2007–2008).
References
- 1.Miniño AM, Heron MP, Murphy SL, Kochanek KD. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 2.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–80. doi: 10.1016/s0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]
- 3.Ince H, Nienaber CA. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nat Clin Pract Cardiovasc Med. 2007;4:418–27. doi: 10.1038/ncpcardio0937. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch EW, Radu NC, Gervais M, Allaire E, Loisance DY. Heterogeneity in the remodeling of aneurysms of the ascending aorta with tricuspid aortic valves. J Thorac Cardiovasc Surg. 2006;132:1010–6. doi: 10.1016/j.jtcvs.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Gelse K, Pöschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000;258:1–14. doi: 10.1002/(SICI)1097-0185(20000101)258:1<1::AID-AR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Hansen U, Bruckner P. Macromolecular specificity of collagen fibrillogenesis: fibrils of collagens I and XI contain a heterotypic alloyed core and a collagen I sheath. J Biol Chem. 2003;278:37352–9. doi: 10.1074/jbc.M304325200. [DOI] [PubMed] [Google Scholar]
- 8.Iyama K, Sumiyoshi H, Khaleduzzaman M, Matsuo N, Ninomiya Y, Yoshioka H. Differential expression of two exons of the alpha1(XI) collagen gene (Col11a1) in the mouse embryo. Matrix Biol. 2001;20:53–61. doi: 10.1016/s0945-053x(00)00130-x. [DOI] [PubMed] [Google Scholar]
- 9.Bhatti AF, Ewing DR, Jordan TP, McCaffrey TA, Gefen JY, Tilson MD. Role of collagen XI in the pathogenesis of the abdominal aortic aneurysm. J Am Coll Surg. 2002;195:S101. [Google Scholar]
- 10.Tansey EE, Kwaku KF, Hammer PE, et al. Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann Thorac Surg. 2006;82:1472–9. doi: 10.1016/j.athoracsur.2006.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hølund B, Clemmensen I. The value of hyaluronidase treatment of different tissues before demonstration of fibronectin by the indirect immunoperoxidase technique. Histochemistry. 1982;76:517–25. doi: 10.1007/BF00489906. [DOI] [PubMed] [Google Scholar]
- 12.Bowen KB, Reimers AP, Luman S, Kronz JD, Fyffe WE, Oxford JT. Immunohistochemical localization of collagen type XI alpha1 and alpha2 chains in human colon tissue. J Histochem Cytochem. 2008;56:275–83. doi: 10.1369/jhc.7A7310.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris NP, Oxford JT, Davies GB, Smoody BF, Keene DR. Developmentally regulated alternative splicing of the alpha1(XI) collagen chain: spatial and temporal segregation of isoforms in the cartilage of fetal rat long bones. J Histochem Cytochem. 2000;48:725–41. doi: 10.1177/002215540004800601. [DOI] [PubMed] [Google Scholar]
- 15.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–37. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 16.Tang PC, Coady MA, Lovoulos C, et al. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation. 2005;112:1098–105. doi: 10.1161/CIRCULATIONAHA.104.511717. [DOI] [PubMed] [Google Scholar]
- 17.Kypreos KE, Birk D, Trinkaus-Randall V, Hartmann DJ, Sonenshein GE. Type V collagen regulates the assembly of collagen fibrils in cultures of bovine vascular smooth muscle cells. J Cell Biochem. 2000;80:146–55. doi: 10.1002/1097-4644(20010101)80:1<146::aid-jcb140>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Adachi E, Hayashi T. In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res. 1986;14:257–66. doi: 10.3109/03008208609017469. [DOI] [PubMed] [Google Scholar]
- 19.Lesauskaite V, Tanganelli P, Sassi C, et al. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol. 2001;32:1003–11. doi: 10.1053/hupa.2001.27107. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar R, Miller DV, Ballman KV, et al. Elevated expressions of osteopontin and tenascin C in ascending aortic aneurysms are associated with trileaflet aortic valves as compared with bicuspid aortic valves. Cardiovasc Pathol. 2007;16:144–50. doi: 10.1016/j.carpath.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Fallahi A, Kroll B, Warner LR, et al. Structural model of the amino propeptide of collagen XI alpha1 chain with similarity to the LNS domains. Protein Sci. 2005;14:1526–37. doi: 10.1110/ps.051363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner LR, Blasick CM, Brown RJ, Oxford JT. Expression, purification, and refolding of recombinant collagen alpha1(XI) amino terminal domain splice variants. Protein Expr Purif. 2007;52:403–9. doi: 10.1016/j.pep.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–36. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 25.Della Conte A, Quarto C, Bancone C. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell-matrix signaling. J Thorac Cardiovasc Surg. 2008;135:8–18. doi: 10.1016/j.jtcvs.2007.09.009. [DOI] [PubMed] [Google Scholar]