Abstract
Background
Right ventricular function is a key determinant of exercise capacity and survival in pulmonary arterial hypertension (PAH). We aimed to study the predictors of right ventricular ejection fraction (RVEF) in patients with newly diagnosed PAH.
Methods
We performed a cross-sectional analysis of a retrospective cohort of consecutive patients with idiopathic, familial, or anorexigen-associated PAH who underwent equilibrium radionuclide angiography for measurement of RVEF at baseline.
Results
Of the 84 patients in the cohort, 63 underwent equilibrium radionuclide angiography and right heart catheterization and were included. The mean age was 41 ± 13 years, and 79% of the patients were female. The mean RVEF was 30 ± 8%. RVEF was directly associated with right ventricular stroke volume index and cardiac index, and inversely associated with pulmonary vascular resistance index from right heart catheterization (all p < 0.001). Older age and male sex were associated with lower RVEF (p < 0.05) after adjustment for pulmonary vascular resistance index and left ventricular ejection fraction. Higher plasma von Willebrand factor levels were also independently associated with lower RVEF (p = 0.01) (n = 55). Body size and type of PAH were not associated with RVEF.
Conclusions
Older patients and males with PAH had lower RVEF at baseline than younger patients and females, even after controlling for left ventricular function and hemodynamics. Higher plasma von Willebrand factor levels, a marker of endothelial dysfunction, were also associated with lower RVEF.
Keywords: hypertension, pulmonary, right ventricle, von Willebrand factor
Pulmonary arterial hypertension (PAH) is characterized by exercise limitation and a high risk of death.1 Although there are a variety of etiologies of PAH, the physiologic consequences are similar. Increased right ventricular (RV) afterload leads to RV hypertrophy, decreased RV ejection fraction (RVEF), and eventually, depressed stroke volume, low cardiac output, and progressive RV failure.2,3 The importance of RV structure and function in PAH is supported by data showing that baseline RVEF, right atrial pressure, RV end-diastolic volume, stroke volume index, and cardiac index predict survival in patients with PAH,4–6 whereas pulmonary artery pressure does not, in the current era.7 A 5% lower RVEF at baseline in patients with PAH confers a > 60% increase in the risk of death.7 Lower RVEF is associated with increased RV wall stress,8 and RV function predicts outcome in congestive heart failure. 9,10 The determinants of RVEF in healthy persons and patients with PAH are poorly understood, although investigators have started to focus on these questions,11 and a recent review comprehensively summarized what is currently known about the RV.12,13
A variety of factors may affect cardiac function and morphology in health and disease. Age, sex, and race may have an impact on RV performance. The severity and duration of pulmonary vascular disease in patients with PAH are also probably important. Unfortunately, RV function is hard to assess in healthy volunteers or patients with PAH. The morphology of the RV defies the assumptions necessary for echocardiography to accurately interrogate this chamber. In addition, the development of effective oral therapies, which are easier to prescribe, has made studies of large numbers of patients with newly diagnosed PAH much more difficult to conduct, since patients are more likely to be treated before (or without) referral to academic centers. Such studies in untreated patients are necessary to understand the determinants and mechanisms of RV compensation in the face of increased afterload.
MRI is considered the gold standard for measurement of RVEF.13 Drawbacks include the requirement for breath-holding and laying flat, claustrophobia, availability, and expense. On the other hand, we have validated equilibrium radionuclide angiography against MRI for measurement of RVEF,14 and the former technique does not require breath-holding, is easier to tolerate, and is less expensive. We aimed to study the determinants of RVEF measured by equilibrium radionuclide angiography in a cohort of patients with newly diagnosed PAH. We hypothesized that age, sex, and severity of PAH would be associated with RVEF.
MATERIALS AND METHODS
Study Design
We performed a cross-sectional analysis within a retrospective cohort study of consecutive adult patients with PAH who underwent initial evaluation at our center between January 1994 and June 2002. The study was approved by the Institutional Review Board.
Study Subjects
Details of the cohort have been published previously.7,15,16 The following criteria were required for inclusion in this study: (1) PAH that was idiopathic, familial, or associated with anorexigen use; (2) age > 16 years; (3) initial evaluation was performed at our center during the study period; and (4) availability of data from equilibrium radionuclide angiography and right heart catheterization. The exclusion criteria were (1) previous right heart catheterization with acute vasodilator study and initiation of chronic PAH therapy; and (2) other forms of PAH, such as PAH associated with connective tissue disease, portal hypertension, or congenital heart disease. We excluded other forms of PAH as these associated conditions may directly affect RV function.
Measurements
All patients evaluated for PAH routinely underwent laboratory testing, pulmonary function testing, transthoracic echocardiography, cardiopulmonary exercise testing, equilibrium radionuclide angiography, and right heart catheterization at baseline. Acute vasoreactivity was defined as a mean pulmonary artery pressure < 40 mm Hg (with an absolute decrease > 10 mm Hg) and preserved cardiac output after acute administration of inhaled nitric oxide or IV epoprostenol.
Equilibrium radionuclide angiography was performed at rest in the supine position with use of an in vivo RBC labeling method. After the cells were labeled, images were acquired with dual headed single photon emission CT, as described in detail.14 RVEF and left ventricular ejection fraction (LVEF) were computed with semi-automated software.14
Statistical Analysis
Continuous variables were summarized by mean ± SD or median (interquartile range). Categorical variables were summarized by frequency and percentage. Pearson correlation coefficients and Spearman rank correlation coefficients were used, as appropriate. Simple linear regression was used to assess the association between RVEF (dependent variable) and potential predictors. Multivariate linear regression was performed using purposeful selection of covariates to construct the models that best explained the variability in RVEF. Variables with p < 0.05 were retained in the model. LVEF was forced into the final models. The results are presented as β (standard error) or least squares mean (standard error). All analyses were performed using available data; no imputation was performed. Statistical software (SPSS version 11.0.1; SPSS; Chicago, IL) was used for all analyses.
RESULTS
Patient Characteristics
The cohort included 84 patients evaluated during the study period. Of those, 63 had equilibrium radionuclide angiography and right heart catheterization performed at baseline and constituted the study sample. Of these patients, 86% had radionuclide angiography performed within seven days of the right heart catheterization. Table 1 shows the characteristics of the study sample and the excluded patients. The mean age in the study sample was 41 ± 13 years, 79% of the patients were female, 78% were non-Hispanic white, and 78% had idiopathic PAH. The age and sex distribution of the final cohort was similar to that of excluded patients; however, there were more non-Hispanic white patients in the study sample than in the excluded subset. PAH diagnosis, laboratory values, and echocardiographic values were similar between the groups, but excluded patients may have had more severe PAH by hemodynamic measurements. The median time from onset of symptoms to evaluation of PAH by right heart catheterization in the study sample was 1.6 years (interquartile range 0.7 to 3.8 years; n = 60).
Table 1.
Baseline Characteristics*
| Characteristics | Study Sample (n = 63) |
Excluded Patients (n = 21) |
|---|---|---|
| Age, yr | 41 ± 13 | 45 ± 15 |
| Male sex | 13 (21) | 3 (14) |
| Race/ethnicity | ||
| Non-Hispanic white | 49 (78) | 12 (57) |
| Black | 3 (5) | 3 (14) |
| Hispanic | 7 (11) | 2 (10) |
| Asian | 4 (6) | 4 (19) |
| Body mass index, kg/m2 | 28 ± 6 | 27 ± 8 |
| Diagnosis | ||
| Idiopathic PAH | 49 (78) | 17 (81) |
| Familial PAH | 11 (17) | 3 (14) |
| Anorexigen-induced PAH | 3 (5) | 1 (5) |
| Laboratory values | ||
| BUN, mg/dL | 18 ± 8 | 19 ± 8 |
| Creatinine, mg/dL | 0.9 ± 0.2 | 1.0 ± 0.3 |
| Hemoglobin, g/dL | 15 ± 2 | 15 ± 2 |
| Hematocrit, % | 44 ± 4 | 46 ± 5 |
| Platelet count, 109/L | 234 ± 72 | 203 ± 71 |
| Prothrombin time, s | 14 ± 3 (n = 62) | 17 ± 7 (n = 20) |
| International normalized ratio | 1.2 ± 0.7 (n = 62) | 1.4 ± 0.7 (n = 20) |
| Erythrocyte sedimentation rate, mm/h | 12 ± 17 (n = 57) | 9 ± 8 (n = 17) |
| Fibrinogen, mg/dL | 389 ± 119 (n = 53) | 400 ± 85 (n = 13) |
| von Willebrand factor, % | 149 ± 65 (n = 55) | — |
| Echocardiography | ||
| Right atrial dilation | 60 (95) | 20 (100) [n = 20] |
| RV dilation | 61 (97) | 20 (100) [n = 20] |
| RV hypertrophy | 58 (92) | 20 (100) [n = 20] |
| RV dysfunction | 57 (90) | 18 (90) [n = 20] |
| Paradoxical septal motion | 62 (98) | 19 (95) [n = 20] |
| Tricuspid regurgitation | n = 62 | n = 20 |
| None | 3 (5) | — |
| Mild | 31 (50) | 5 (25) |
| Moderate | 24 (39) | 11 (55) |
| Severe | 4 (6) | 4 (20) |
| Pulmonary function testing | ||
| EV1, L | 2.5 ± 0.8 | 2.3 ± 0.6 |
| FEV1, % predicted | 86 ± 19 | 84 ± 18 |
| FVC, L | 3.2 ± 1.0 | 3.0 ± 0.9 |
| FVC, % predicted | 91 ± 20 | 87 ± 20 |
| FEV1/FVC | 0.78 ± 0.06 | 0.79 ± 0.01 |
| Dlco, mL/min/mm Hg | 16 ± 6 (n = 60) | 15 ± 5 (n = 19) |
| Dlco, % predicted | 66 ± 24 (n = 60) | 65 ± 24 (n = 19) |
| Hemodynamic data | ||
| Right atrial pressure, mm Hg | 9 ± 6 | 12 ± 6 (n = 17) |
| Mean pulmonary artery pressure, mm Hg | 54 ± 12 | 60 ± 12 (n = 17) |
| Pulmonary capillary wedge pressure, mm Hg | 8 ± 3 | 10 ± 5 (n = 17) |
| Pulmonary vascular resistance index , dyne-s ·cm−5·m2 | 2,005 ± 1,033 | 2,851 ± 1,081 (n = 17) |
| Cardiac index, L/min/m2 | 2.1 ± 0.8 | 1.6 ± 0.5 (n = 17) |
| RV stroke volume index, mL/m2 | 27 ± 11 | 19 ± 7 (n = 17) |
| RV stroke work index, mL · mm Hg/m2 | 1,189 ± 520 | 899 ± 371 (n = 17) |
| Acute vasoreactivity | 8 (14) [n = 58] | 1 (6) [n = 16] |
Values are given as the mean ± SD or No. (%). Dlco = diffusing capacity of the lung for carbon monoxide.
Univariate and Bivariate Analyses
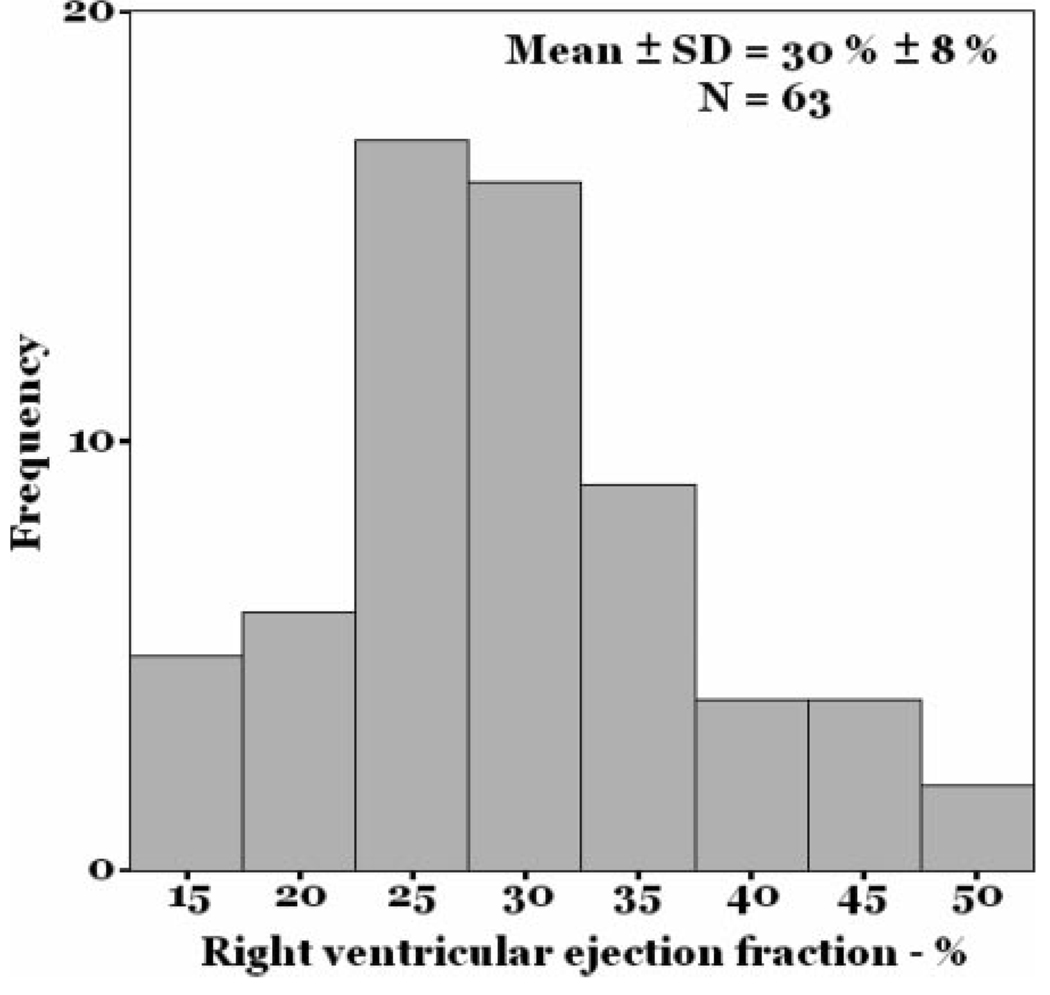
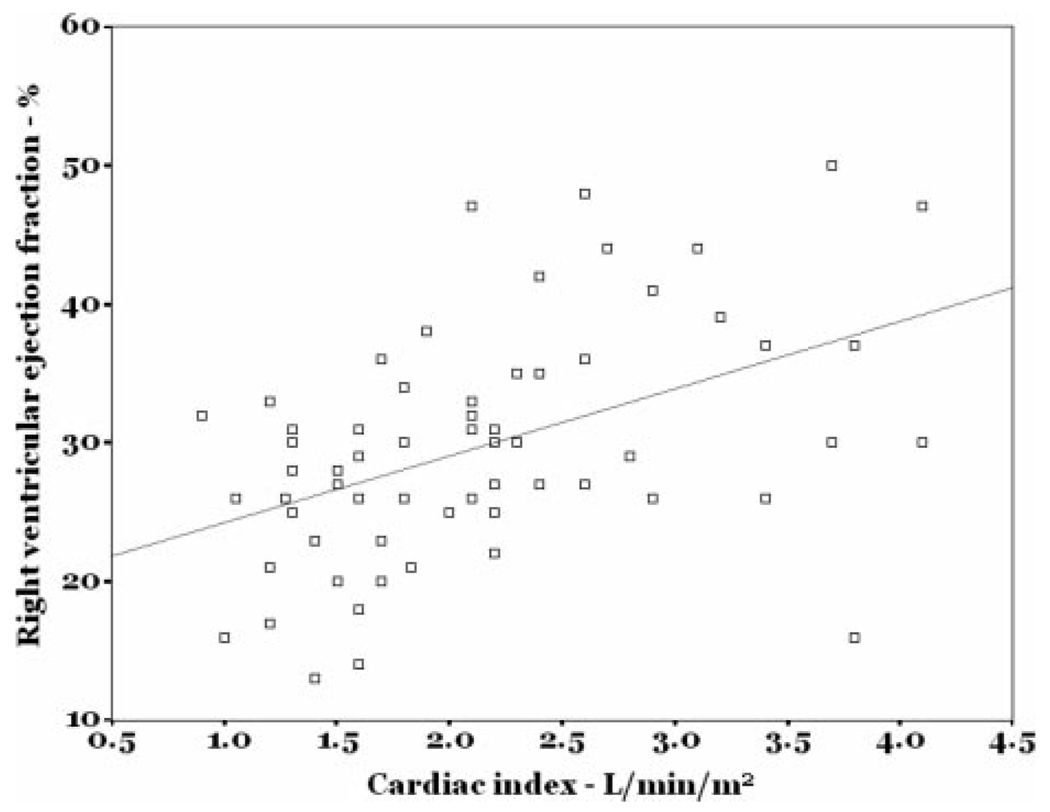
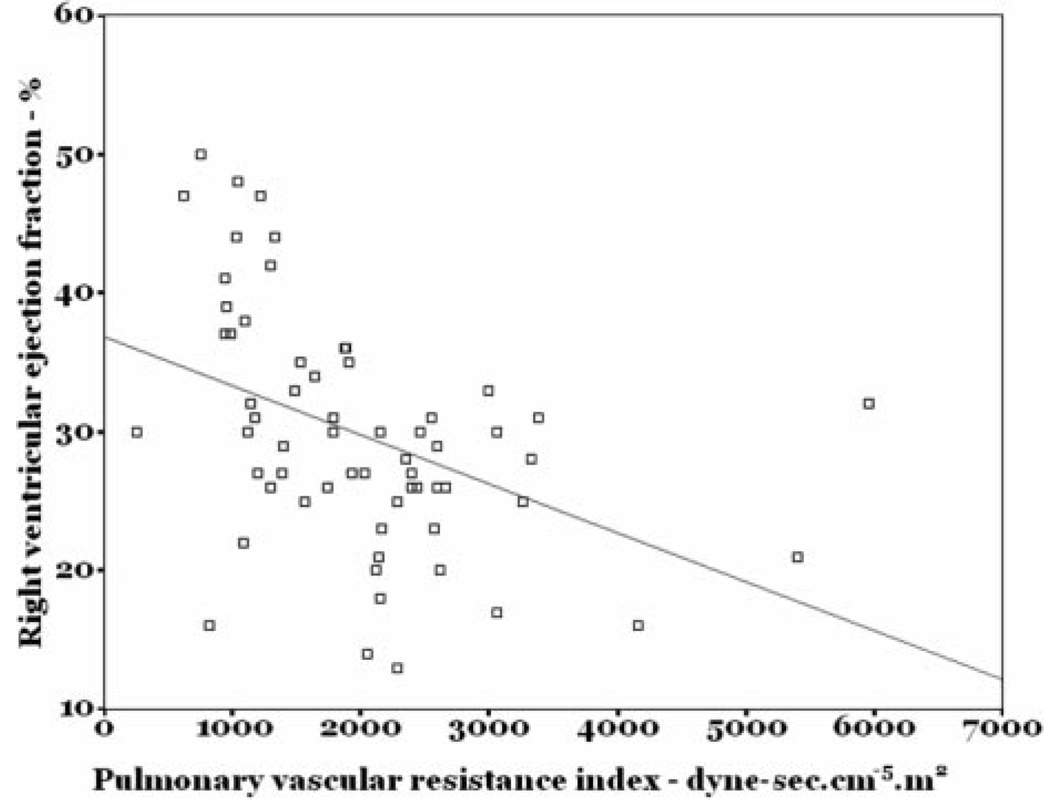
The mean RVEF in the study sample was 30 ± 8% (Fig 1) [lower limit of normal = 40–45%],14 and the mean LVEF was 67 ± 7%. RVEF was directly correlated with cardiac index (r = 0.47; p < 0.001) [Fig 2] and RV stroke volume index (r = 0.46; p < 0.001) and was inversely correlated with pulmonary vascular resistance index (r = −0.51; p < 0.001) [Fig 3]. While statistically significant, these were not particularly tight correlations. There was no association between the number of days from symptom onset to evaluation and RVEF (r = −0.15; p = 0.26).
Figure 1.
Histogram of RVEF.
Figure 2.
Scatterplot of RVEF vs cardiac index.
Figure 3.
Scatterplot of RVEF vs pulmonary vascular resistance index.
Table 2 shows the simple linear regression results. There were no associations between RVEF and age, sex, race/ethnicity, body mass index, or type of PAH in bivariate analyses. However, lower RVEF was associated with higher serum BUN, creatinine, hemoglobin, and international normalized ratio (INR), which were determined at the time of right heart catheterization. There was no association between RVEF and oxygen consumption or systemic blood pressures at peak exercise (data not shown), but lower RVEF was associated with higher ventilatory equivalent1 for carbon dioxide at peak exercise (r = −0.49; p < 0.001; n = 59).
Table 2.
Simple Linear Regression of RVEF on Other Variables*
| Variables | β | SE | p Value |
|---|---|---|---|
| Age (per 5-yr increment) | −0.38 | 0.41 | 0.35 |
| Male sex | − 2.88 | 2.61 | 0.28 |
| Non-white or Hispanic race/ethnicity |
2.54 | 3.38 | 0.46 |
| Body mass index | − 0.02 | 0.18 | 0.89 |
| Diagnosis (vs idiopathic PAH) | |||
| Familial PAH | − 2.41 | 2.81 | 0.40 |
| Anorexigen-induced PAH | − 5.08 | 5.02 | 0.32 |
| BUN (per 10 mg/dL increment) | − 2.94 | 1.35 | 0.03 |
| Creatinine (per 0.5 mg/dL increment) |
− 8.00 | 2.25 | 0.001 |
| Hemoglobin | − 1.70 | 0.63 | 0.009 |
| Hematocrit (per 5% increment) | − 3.42 | 1.24 | 0.008 |
| International normalized ratio (per 0.5 increment) |
− 1.57 | 0.77 | 0.05 |
| Erythrocyte sedimentation rate | − 0.03 | 0.07 | 0.64 |
| Fibrinogen | − 0.007 | 0.01 | 0.51 |
| von Willebrand factor (per 50% increment) |
− 1.63 | 0.88 | 0.07 |
| Right atrial dilation | − 5.87 | 4.96 | 0.24 |
| RV dilation | − 1.30 | 6.09 | 0.83 |
| RV hypertrophy | − 6.91 | 3.76 | 0.07 |
| RV dysfunction | − 6.56 | 3.56 | 0.07 |
| Paradoxical septal motion | 0.00 | 8.40 | 1.00 |
| FEV1, % predicted | 0.02 | 0.06 | 0.71 |
| FVC, % predicted | 0.03 | 0.05 | 0.62 |
| FEV1/FVC | 11.85 | 16.04 | 0.46 |
| Dlco, % predicted | − 0.05 | 0.05 | 0.91 |
| Right atrial pressure (per 5 mm Hg increment) |
− 1.83 | 0.94 | 0.06 |
| Mean pulmonary artery pressure (per 5 mm Hg increment) |
− 0.89 | 0.44 | 0.05 |
| Pulmonary capillary wedge pressure |
0.11 | 0.32 | 0.73 |
| Pulmonary vascular resistance index (per 200 dyne-s · cm−5 · m2 increment |
− 0.71 | 0.19 | < 0.001 |
| Cardiac index (per 0.5 L/min/m2 increment) |
2.42 | 0.58 | < 0.001 |
| RV stroke volume index (per 10 mL/m2 increment) |
3.52 | 0.87 | < 0.001 |
| RV stroke work index (per 10 mL · mm Hg/m2) |
0.05 | 0.33 | 0.008 |
See Table 1 for expansion of abbreviation.
There were no significant associations between RVEF and right atrial or RV dilation assessed by echocardiography (Table 2), but RV hypertrophy and dysfunction may have been associated with lower RVEF. We found that the presence of more severe tricuspid regurgitation was associated with lower RVEF (r = −0.30; p = 0.02; n = 62). The associations between RVEF and right atrial pressure and mean pulmonary artery pressure from right heart catheterization were of borderline statistical significance, whereas measures of RV output were significantly associated with RVEF. Patients with acute vasoreactivity had a higher RVEF (39%[3%]) than those without acute vasoreactivity (28%[1%]; p = 0.001; n = 58).
Multivariate Analysis
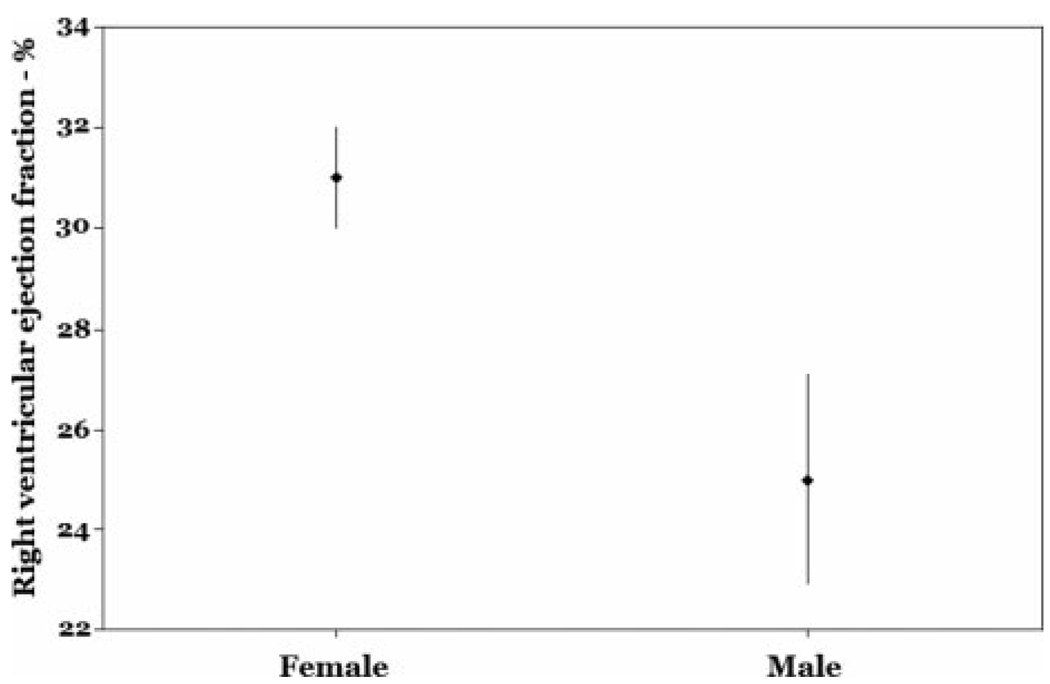
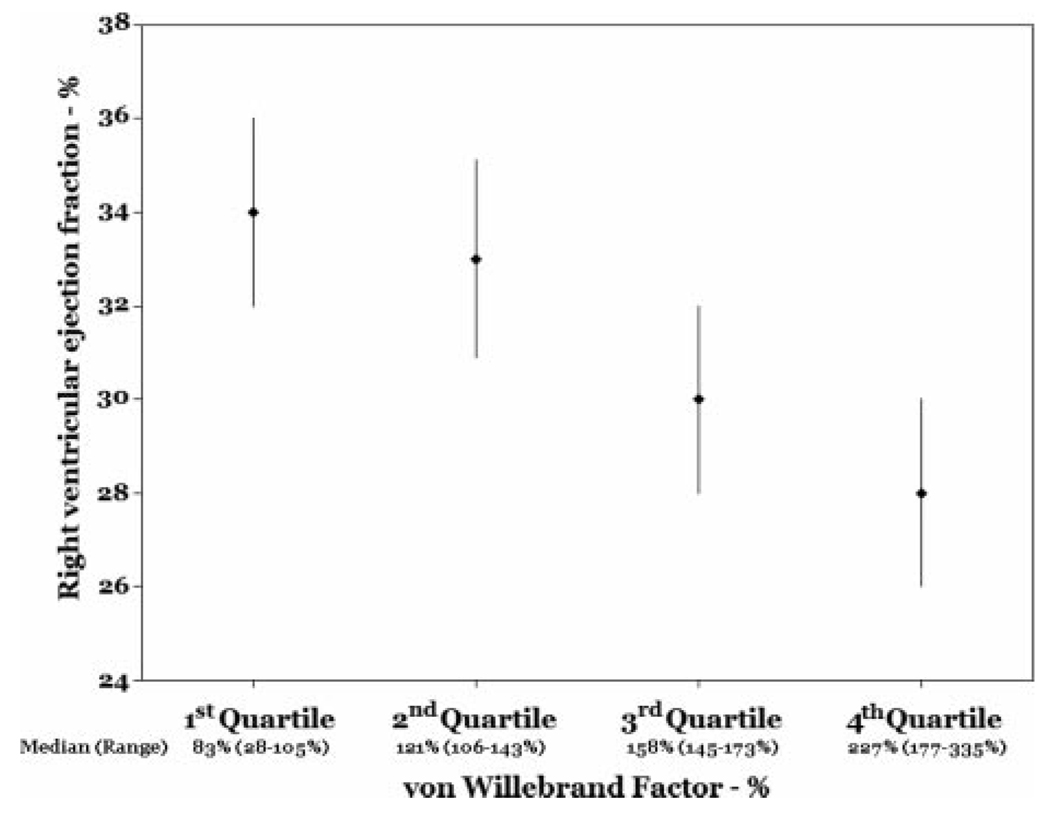
Older age, male sex, and higher pulmonary vascular resistance index were independently associated with lower RVEF after adjustment for LVEF (Table 3, Model 1). The least squares mean RVEF for males vs females from the multivariate model is shown in Figure 4. Additional adjustment for anthropomorphics, mean pulmonary artery pressure, RV stroke volume index, or cardiac index did not significantly change these results (data not shown). Higher levels of von Willebrand factor (vWF) were also significantly associated with a lower RVEF when added to the multivariate model (n = 55) [Table 3, Model 2; Fig 5]. The fit of the multivariate models was adequate, and there were no particularly influential patients.
Table 3.
Multivariate Linear Regression of RVEF on Other Variables
| Variables | β | SE | p Value |
|---|---|---|---|
| Model 1 | |||
| Constant | 51.48 | 9.87 | < 0.001 |
| Age (per 5-yr increment) | − 0.88 | 0.37 | 0.02 |
| Male sex | −5.94 | 2.40 | 0.02 |
| Pulmonary vascular resistance index (per 200 dyne-s · cm−5 · increment) |
−0.85 | 0.18 | <0.001 |
| LV ejection fraction | −0.07 | 0.13 | 0.58 |
| Model 2 | |||
| Constant | 61.66 | 10.39 | <0.001 |
| Age (per 5-yr increment) | −0.98 | 0.39 | 0.01 |
| Male sex | −9.15 | 2.61 | 0.001 |
| Pulmonary vascular resistance index (per 200 dyne-s · cm−5 · m2 increment) |
−0.88 | 0.18 | <0.001 |
| LV ejection fraction | −0.11 | 0.13 | 0.40 |
| von Willebrand factor (per 50% increment) |
−1.96 | 0.75 | 0.01 |
Figure 4.
RVEF for males vs females (adjusted for age, pulmonary vascular resistance index, and LVEF) [p = 0.02].
Figure 5.
RVEF by quartile of vWF level (adjusted for age, sex, pulmonary vascular resistance index, and LVEF) [test for trend, p = 0.04].
DISCUSSION
RV structure and function are important determinants of long-term survival in PAH; however, little is known about the factors present at the time of diagnosis that affect RV performance in this disease. The mean RVEF at baseline in our cohort was 30%, well below the lower limit of normal, and only three patients in the cohort had normal RVEF. Other studies of patients with PAH have shown similar results.5,17 While RVEF was significantly associated with hemodynamics, there was substantial variability, indicating that measures such as RV stroke volume index or cardiac index do not fully characterize RV function in PAH. This could be due to specific structural changes in the RV in PAH. For example, cardiac fibrosis may have a greater effect on systolic performance (reflected by RVEF) than on other parameters, such as RV diastolic function (which contributes more significantly to hemodynamic abnormalities). 18 Older and male patients with PAH had significantly lower RVEF at initial diagnosis than younger and female patients. This finding persisted despite adjustment for numerous covariates, including measures of body size, invasive hemodynamics, and LVEF. In a subset of patients, higher plasma vWF levels were associated with reduced RVEF. Lower RVEF was also associated with worse kidney function and higher INR, however these associations did not remain significant in multivariate analyses.
Age-related changes in RVEF have not been demonstrated previously either in healthy persons or in patients with pulmonary vascular disease.13 It is possible that the RV in older patients is less able to adapt to increased afterload or is more prone to fibrosis, predisposing to worse function. Age-related decrements in RV function could explain some of the differences in survival between certain types of PAH that affect older patients (such as systemic sclerosis-related PAH) and types that affect younger patients (such as idiopathic PAH);19,20 however, there are few comparative studies of RV function in patients with different types of PAH.21 Age-related differences in preload or afterload could explain this finding as well. For example, increased stiffness of the pulmonary vasculature with older age could result in a lower RVEF.
RVEF has been shown to be lower in men than in women in healthy, cardiovascular disease-free volunteers. The largest study of RVEF in healthy volunteers showed a difference of approximately 7% in RVEF between men and women,22 similar in magnitude to the difference found in our study of patients with PAH. The hearts of male patients with dilated cardiomyopathy or left ventricular hypertrophy in response to pressure overload do not adapt as well as those of female patients, contributing to worse contractility.23–26 Males with congestive heart failure have worse outcomes than females, even after consideration of potential confounders.27 Interestingly, animal models of different types of left ventricular failure also demonstrate worse cardiac function in males than in females.23,28 For example, a male animal model of myocardial infarction and systemic hypertension demonstrates eccentric ventricular hypertrophy and cavity dilation, whereas the female model undergoes concentric hypertrophy without dilation.29,30
The mechanism for sex-based differences in right and left ventricular function is unclear. Estrogen may be protective as it induces insulin-like growth factor, which has been associated with improved myocardial contractility.31 Lower estrogen levels may lead to up-regulation of nuclear estrogen receptors (seen in heart failure),32,33 resulting in the increased risk of heart failure seen in men and post-menopausal women.34,35 Other sex-related metabolic changes may also play a role.23
Notably, despite these differences in RVEF at baseline in PAH, there are no distinctions in long-term survival from diagnosis of PAH between the sexes,6,7,36 although male fetal wastage has been postulated to explain the female predominance of familial PAH.37 Several possible scenarios could account for this apparent inconsistency. First, it is possible that, despite having worse RV function at baseline, men have a better response to therapy in terms of either pulmonary vascular remodeling or recovery of RV function. Second, men may have a slower decline in RV function after disease diagnosis. Last, it is possible that RVEF may not reliably predict survival.5,7 These speculations require further studies for confirmation.
We also found that higher levels of vWF were associated with lower RVEF. vWF is a multimeric glycoprotein which is released into the circulation after endothelial disruption. There are several possible explanations for the inverse association between RVEF and plasma vWF level. First, pulmonary vascular capacitance and impedence may be insufficiently gauged by pulmonary vascular resistance and could affect RVEF.38,39 Circulating vWF level could serve as a better indicator of capacitance or impedance at rest or during activity or sleep. Second, plasma vWF may be from a systemic source, reflecting peripheral and coronary vascular endothelial dysfunction and myocardial perfusion. Recent studies have shown important abnormalities in RV perfusion in PAH which could contribute to worse RVEF.40,41 Third, vWF is an acute phase reactant which may be upregulated in systemic inflammation, which could directly affect RVEF.42 However, other markers of inflammation, such as erythrocyte sedimentation rate and fibrinogen, were not associated with RVEF in our cohort. Last, elevated vWF levels could result from impaired RV performance, rather than be a risk factor. Lower RVEF leading to systemic vWF release may be less likely in our cohort, since the association persisted despite adjustment for cardiac index, a measure of systemic perfusion.
There are some potential limitations of this study. We used equilibrium radionuclide angiography rather than MRI to measure RVEF. While the former is a validated technique that is more easily tolerated by patients, the latter is still considered the gold standard measure and should be used in future studies. Twenty-one of the 84 patients in the cohort were excluded due to missing data. These patients were more likely to be nonwhite and may have had more severe PAH than the study sample, suggesting possible selection bias. Even so, we studied RVEF at baseline in a large number of patients with idiopathic, familial, or anorexigen-associated PAH. The availability of oral medications and the decentralization of medical care for PAH will likely make similar studies of large numbers of patients with newly diagnosed PAH more difficult to perform in the future. While we presume that the worse kidney function and higher INR seen in relation to lower RVEF are consequences of right heart failure and multiorgan dysfunction, we cannot rule out a contribution of these factors to lower RV function.43 Finally, although the clinical utility of measuring RVEF is presently unknown, future studies should validate variables such as RVEF and other parameters for the assessment of clinical improvement or worsening of patients with PAH.
In summary, older age, male sex, and higher plasma vWF levels were associated with lower RVEF in PAH, even after controlling for pulmonary vascular resistance index and other measures of heart function. These findings shed light on the differences in cardiac compensation and outcome in patients with PAH. Future studies should focus on these determinants of RVEF in animal models and affected patients and the predictors of change in RVEF after PAH treatment.
Acknowledgments
This study was supported by National Institutes of Health grants HL67771 and HL086719.
Abbreviations
- INR
international normalized ratio
- LVEF
left ventricular ejection fraction
- PAH
pulmonary arterial hypertension
- RV
right ventricular/right ventricle
- RVEF
right ventricular ejection fraction
- vWF
von Willebrand factor
Footnotes
The authors have no conflicts of interest to disclose.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
REFERENCES
- 1.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 2.Chen EP, Craig DM, Bittner HB, et al. Pharmacological strategies for improving diastolic dysfunction in the setting of chronic pulmonary hypertension. Circulation. 1998;97:1606–1612. doi: 10.1161/01.cir.97.16.1606. [DOI] [PubMed] [Google Scholar]
- 3.Szabo G, Soos P, Bahrle S, et al. Adaptation of the right ventricle to an increased afterload in the chronically volume overloaded heart. Ann Thorac Surg. 2006;82:989–995. doi: 10.1016/j.athoracsur.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval J, Bauerle O, Palomar A, et al. Survival in primary pulmonary hypertension: validation of a prognostic equation. Circulation. 1994;89:1733–1744. doi: 10.1161/01.cir.89.4.1733. [DOI] [PubMed] [Google Scholar]
- 5.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 6.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 7.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95:199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Quaife RA, Chen MY, Lynch D, et al. Importance of right ventricular end-systolic regional wall stress in idiopathic pulmonary arterial hypertension: a new method for estimation of right ventricular wall stress. Eur J Med Res. 2006;11:214–220. [PubMed] [Google Scholar]
- 9.de Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Salvo TG, Mathier M, Semigran MJ, et al. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 11.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Doyle R, Murphy DJ, et al. Right ventricular function in cardiovascular disease: Part II. Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 13.Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease: Part I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 14.Nichols K, Saouaf R, Ababneh AA, et al. Validation of SPECT equilibrium radionuclide angiographic right ventricular parameters by cardiac magnetic resonance imaging. J Nucl Cardiol. 2002;9:153–160. doi: 10.1067/mnc.2002.119464. [DOI] [PubMed] [Google Scholar]
- 15.Kawut SM, Horn EM, Berekashvili KK, et al. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther. 2006;19:370–374. doi: 10.1016/j.pupt.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Kawut SM, Horn EM, Berekashvili KK, et al. von Willebrand factor independently predicts long-term survival in patients with pulmonary arterial hypertension. Chest. 2005;128:2355–2362. doi: 10.1378/chest.128.4.2355. [DOI] [PubMed] [Google Scholar]
- 17.Quaife RA, Lynch D, Badesch DB, et al. Right ventricular phenotypic characteristics in subjects with primary pulmonary hypertension or idiopathic dilated cardiomyopathy. J Card Fail. 1999;5:46–54. doi: 10.1016/s1071-9164(99)90024-6. [DOI] [PubMed] [Google Scholar]
- 18.McCann GP, Gan CT, Beek AM, et al. Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. AJR Am J Roentgenol. 2007;188:349–355. doi: 10.2214/AJR.05.1259. [DOI] [PubMed] [Google Scholar]
- 19.Kawut SM, Taichman DB, Archer-Chicko CL, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–350. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 20.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 21.Overbeek MJ, Lankhaar JW, Westerhof N, et al. Different right ventricular contractility in limited cutaneous systemic sclerosis-associated pulmonary arterial hypertension and idiopathic pulmonary arterial hypertension. Eur Respir J. 2008;31:1160–1166. doi: 10.1183/09031936.00135407. [DOI] [PubMed] [Google Scholar]
- 22.Tandri H, Daya SK, Nasir K, et al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007;116:2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 24.De Maria R, Gavazzi A, Recalcati F, et al. Comparison of clinical findings in idiopathic dilated cardiomyopathy in women versus men: the Italian Multicenter Cardiomyopathy Study Group (SPIC) Am J Cardiol. 1993;72:580–585. doi: 10.1016/0002-9149(93)90355-g. [DOI] [PubMed] [Google Scholar]
- 25.Douglas PS, Katz SE, Weinberg EO, et al. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 27.Alla F, Al-Hindi AY, Lee CR, et al. Relation of sex to morbidity and mortality in patients with heart failure and reduced or preserved left ventricular ejection fraction. Am Heart J. 2007;153:1074–1080. doi: 10.1016/j.ahj.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Tamura T, Said S, Gerdes AM. Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension. 1999;33:676–680. doi: 10.1161/01.hyp.33.2.676. [DOI] [PubMed] [Google Scholar]
- 29.Jain M, Liao R, Podesser BK, et al. Influence of gender on the response to hemodynamic overload after myocardial infarction. Am J Physiol Heart Circ Physiol. 2002;283:H2544–H2550. doi: 10.1152/ajpheart.00338.2002. [DOI] [PubMed] [Google Scholar]
- 30.Podesser BK, Jain M, Ngoy S, et al. Unveiling gender differences in demand ischemia: a study in a rat model of genetic hypertension. Eur J Cardiothorac Surg. 2007;31:298–304. doi: 10.1016/j.ejcts.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 31.Vitale G, Galderisi M, Colao A, et al. Circulating insulin-like growth factor-I levels are associated with increased biventricular contractility in top level rowers. Clin Endocrinol (Oxf) 2008;69:231–236. doi: 10.1111/j.1365-2265.2008.03177.x. [DOI] [PubMed] [Google Scholar]
- 32.Grohe CBG, Stimpel M, Karas RH, et al. Functional estrogen receptors in myocardial and myogenic cells [abstract] Circulation. 1994;90:528. [Google Scholar]
- 33.Mahmoodzadeh S, Eder S, Nordmeyer J, et al. Estrogen receptor alpha up-regulation and redistribution in human heart failure. Faseb J. 2006;20:926–934. doi: 10.1096/fj.05-5148com. [DOI] [PubMed] [Google Scholar]
- 34.Mondul AM, Rodriguez C, Jacobs EJ, et al. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–1097. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 35.Schocken DD, Arrieta MI, Leaverton PE, et al. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 36.Thenappan T, Shah SJ, Rich S, et al. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J. 2007;30:1103–1110. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 37.Loyd JE, Butler MG, Foroud TM, et al. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;152:93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lankhaar JW, Westerhof N, Faes TJ, et al. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 39.Lankhaar JW, Westerhof N, Faes TJ, et al. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 40.van Wolferen SA, Marcus JT, Westerhof N, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 41.Gomez A, Bialostozky D, Zajarias A, et al. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/s0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 42.Hennein HA, Ebba H, Rodriguez JL, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–635. [PubMed] [Google Scholar]
- 43.Shah SJ, Thenappan T, Rich S, et al. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation. 2008;117:2475–2483. doi: 10.1161/CIRCULATIONAHA.107.719500. [DOI] [PubMed] [Google Scholar]