Abstract
Background
Although studies have indicated that the frontal lobe plays an important role in performance on the Wisconsin Card Sorting Test (WCST) and that basal ganglia play a specific role in frontal lobe function, the role of striatal dopamine (DA) activity in performance on the WCST remains unclear.
Methods
We assessed the relation between the availability of striatal dopamine transporters (DATs) and performance on the WCST as a measure of executive function in healthy individuals. We approximated the availability of DATs in 53 healthy volunteers aged 19–61 years by use of single photon emission computed tomography with technetium-99m (99mTc)-TRODAT-1 as the ligand. The WCST was administered to all participants.
Results
The availability of DAT was significantly negatively correlated with perseverative errors on the WCST, both before and after adjustment for body mass index (rbefore = −0.39, p = 0.004; rafter = −0.39, p = 0.005).
Limitations
This was an association study; thus, a causal relation between DAT availability and performance cannot be confirmed.
Conclusion
Our results suggest that striatal DAT availability may play a role in executive function as measured by the WCST.
Introduction
The Wisconsin Card Sorting Test (WCST) is an important neuropsychological test that is generally regarded as a prototype abstract reasoning task. The test is mainly reliant on frontal lobe function and provides a measure of executive function, in that it requires strategic planning, organized searching and the ability to use environmental feedback to adjust cognitive sets, goal-oriented behaviour, impulsive responses and working memory.1
Dopamine (DA) function is associated with performance on the WCST. At the genetic level, genes that modulate dopaminergic activity, such as the catechol-O-methyltransferase gene, dopamine transporter (DAT) gene and the DA receptor gene, have been noted to affect frontal executive function measured by the WCST.2–5 At the neuropsychological level, scores on the WCST appear to be associated not only with function of the frontal lobe but also with function of the complicated corticobasal ganglia circuits.6,7 Basal ganglia are involved in frontal subcortical circuits.8 Because basal ganglia are closely linked to the cortical areas, alterations in the basal ganglia may ultimately result in cognitive deficits.9,10 Brain DA, which is abundant in the basal ganglia area, is thought to be one of the important neurotransmitters that regulate several domains of cognitive function.9 In previous studies, regional DA release was thought to be associated with cognition.11–13 The relation between striatal DA function, measured by different ligands, and performance on the WCST has been studied in schizophrenia patients,14 alcoholic patients15 and healthy controls.16
The DA receptor and transporter are both primary indicators of dopaminergic tone.17 The dopamine transporter can be considered to be a specific marker of DA neurons in the central nervous system.18,19 Hersch and colleagues20 observed that, in striatal terminals, DATs are not concentrated in the active synaptic zones but are widely distributed in the axons and axon membranes. This suggests that DATs probably play a role in the regulation of extracellular DA levels throughout the striatum, rather than just in the DA synapse.21 However, the exact role of the DA receptor or DAT in performance on the WCST remains to be elucidated. Volkow and colleagues16 have demonstrated an association between WCST performance and D2 receptors in the caudate region.
Previous studies have revealed correlations between striatal DAT levels and various cognitive functions,16,22 but no studies have focused on the relation between DAT availability and performance on the WCST. Our hypothesis was that the executive function measured by the WCST may be an indicator of striatal DAT availability in addition to striatal DA receptor availability. The aim of this study was to explore the relation between striatal DAT availability using technetium-99m (99mTc)-TRODAT-1 (a radio-labelled form of tropan derivative for selectively labelling DAT) and the performance on WCST of healthy volunteers to support a relation between striatal DA function and executive function.
Methods
Participants
We included 53 healthy, right-handed volunteers (24 men and 29 women) who were enrolled in various studies as healthy controls recruited through research advertisements.23–25 The characteristics of 50 of these participants have been described previously.26
We excluded participants with any past or present psychiatric or neurologic disorder, alcohol or substance abuse (except for caffeine and nicotine), serious medical or surgical conditions that could alter cognitive function, or a history of head trauma with loss of consciousness. We also excluded those with a family history of psychosis in a first-degree relative. The participants underwent comprehensive medical and neurologic examinations to ensure the absence of diseases. A senior psychiatrist interviewed each participant with the Mini International Neuropsychiatric Interview to exclude individuals with mental disorders.27 None of the participants were taking any medication at the time of the study.
The Ethical Committee for Human Research at the National Cheng Kung University Hospital approved the study protocol. Each participant gave informed consent before any procedure was performed.
Imaging procedures
Before examination by single photon emission computed tomography (SPECT), the patients’ thyroid glands were protected with 9 mL of Lugol’s solution. For brain imaging, each participant was intravenously administered 740 MBq (20 mCi) (99mTc)-TRODAT-1 in a quiet environment about 10 minutes after insertion of the intravenous line. We used a triple-headed rotating gamma camera (Multispect 3; Siemens) with ultra high-resolution fan beam collimators. This camera yields an image resolution of about 8.5 mm full-width at half-maximum. We obtained SPECT data using an energy window of 15% centred on 140 keV for 99mTc. Imaging of (99mTc)-TRODAT-1 was initiated about 240 minutes after administration of (99mTc)-TRODAT-1. The images were reconstructed using Butterworth and ramp filters (cut-off frequency = 0.3 Nyquist, power factor = 7) with attenuations by Chang’s method.28 The reconstructed transverse images were realigned parallel to the canthomeatal line. The slice thickness of each transverse image was 2.89 mm.
To measure the striatal availability of DAT, we combined 6 consecutive transverse slices on which the striatum was best visualized to obtain a 17.34-mm thick slice. By use of magnetic resonance imaging (Signa CV-I, 1.5 T; GE Medical Systems) as a guide, an experienced nuclear medicine doctor who was unaware of the participants’ clinical data drew the regions of interest (ROIs) manually on the SPECT images (the images were not co-registered to make fusion images). The ROIs were drawn around the striatum and occipital cortex. The striatal availability of DAT was calculated as striatal availability of DAT = (St − Oc)/Oc, where St was the average count in the striatal ROI and Oc was the average count in the occipital cortex.29
Wisconsin Card Sorting Test
An experienced clinical neuropsychologist administered the WCST. Using a computerized version of the WCST, the patients were required to match response cards to 4 stimulus cards along 1 of 3 dimensions (colour, form or number) on the basis of verbal feedback (correct or incorrect). The participants were not given any information about the dimensions. After sorting a series of 10 cards in 1 category, participants were asked to sort the cards in a different category. All definitions of the indices were as described in the WCST manual.30 We examined only the index of perseverative errors, which is the most commonly used measure of WCST performance.16,31
Statistical analysis
We used independent t tests to examine the differences in continuous variables. We performed stepwise multiple linear regression analyses to determine the explanatory variable most highly correlated with DAT availability. Only variables that were significantly correlated with DAT were included in the regression analyses, including age, body mass index (BMI) and daily life events.25,26
We used Pearson correlation (r) to explore the relation between 2 continuous variables. According to the results of our previous studies25,26 and the result of multiple linear regression, BMI, rather than age or daily life events, should be considered as a covariate when measuring striatal DAT availability. Therefore, partial correlation was applied to explore the relation between striatal DAT availability and perseverative errors in the WCST. We set the level of significance at p < 0.05 (2-tailed).
Results
The mean age of the participants was 29.40 (standard deviation [SD] 9.69, range 19–55) years. There were no age differences between men and woman (t = 0.07, p = 0.94). Their height ranged from 141.00 cm to 184.00 cm, with a mean height of 165.15 cm. Their BMI ranged from 14.81 to 30.58, with a mean of 22.46. Their mean education level was 14.25 (SD 2.94) years.
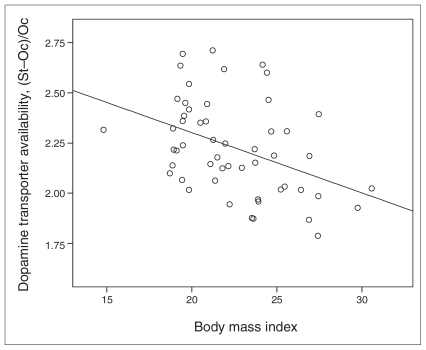
The mean score of perseverative errors on the WCST was 6.91 (SD 3.25). The mean ratio of striatal DAT availability was 1.49 (SD 0.29) for both hemispheres, 1.47 (SD 0.29) for the right side and 1.50 (SD 0.29) for the left side. BMI was also significantly correlated with age (r = 0.60, p < 0.001). The participants’ DAT availability was significantly negatively correlated with age (r = −0.44, p = 0.001), daily life events (r = −0.27, p = 0.04) and BMI (r = −0.44, p = 0.001, Fig. 1). There were no differences between men and women for performance on the WCST or DAT availability.
Fig. 1.
Scatter plot of the relation between body mass index and dopamine transporter availability. St = average count in the striatal region of interest; Oc = average count in the occipital cortex.
Stepwise multiple linear regression analysis with total striatal DAT availability as the dependent variable and BMI, age and daily life events as predictors showed that BMI was the only significant predictor of striatal DAT availability (β = −0.41, t = −3.23, p = 0.002); this result supports our choice of using BMI as a covariate when measuring striatal DAT availability.
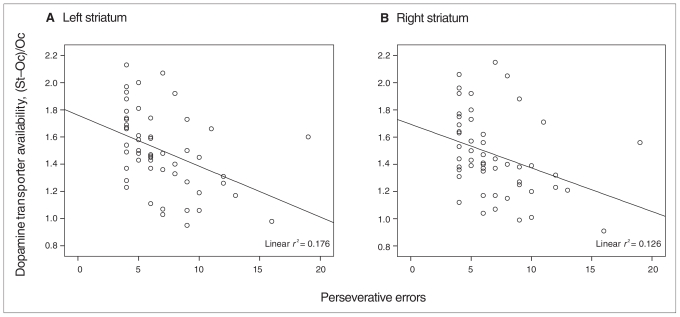
Perseverative error was correlated significantly with striatal DAT availability (r = −0.44, p = 0.001), both on the right and left side (Table 1, Fig. 2); this was significant even after the Bonferroni correction was applied (p < 0.017). After adjustment for the effect of BMI, perseverative error remained significantly negatively correlated with DAT availability (r = −0.39, p = 0.005) for both the right and left sides (Table 1).
Table 1.
Correlation between perseverative errors on the Wisconsin Card Sorting Test and striatal dopamine transporter availability, age and body mass index
| Striatal DAT availability | Age |
BMI |
Perseverative error |
Perseverative error* |
||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Left | −0.42 | 0.002 | −0.44 | 0.001 | −0.42 | 0.002 | −0.42 | 0.002 |
| Right | −0.45 | 0.001 | −0.42 | 0.002 | −0.36 | 0.009 | −0.34 | 0.010 |
| Total | −0.44 | 0.001 | −0.44 | 0.001 | −0.39 | 0.004 | −0.39 | 0.005 |
BMI = body mass index; DAT = dopamine transporter.
After adjustment for the effects of BMI.
Fig. 2.
Scatter plot of the relation between perseverative errors on the Wisconsin Case Sorting Test (WCST) and dopamine transporter availability on the left (A) and right (B) side. St = the average count in the striatal region of interest; Oc = average count in the occipital cortex.
Discussion
In this study, we found a negative association between striatal DAT availability and performance on the WCST. In previous studies, DAT knockout mice have been shown to have hyperactivity and impaired spatial cognitive function.32 It is known that the basal ganglia are closely linked to the cortical areas and that alterations in the basal ganglia may ultimately result in cognitive deficits.9,10 Because dopaminergic influences are mainly exerted over the frontal lobe and basal ganglia, DA activity may play a role in executive function, which is measured by the WCST. In keeping with our results, striatal DA activity has been reported to be associated with various cognitive processes controlled by executive functions.16,22,24 In addition, striatal DA D2 receptor availability has been correlated with tasks involving frontal brain regions, including measures of abstraction and mental flexibility (measured by the WCST), attention, response inhibition and verbal intelligence.16,22,25,33 The correlation between DAT availability and perseverative errors in this study may also imply a putative role of striatal dopaminergic activity in repetitive behaviours. In one study, DAT knockout mice displayed a behavioural profile characterized by perseverative and stereotyped locomotor patterns.34 The nature of repetitive behaviours mandates future investigation.
It is known that DAT distribution in the central nervous system coincides with dopaminergic innervations. Ligands of DATs have been developed for use in neuroimaging as in vivo markers of dopaminergic systems.21 In this study, the negative correlation between the perseverative errors on the WCST and striatal DAT availability remained significant after adjustment for BMI. Thus, higher striatal DAT availability may result in better WCST performance. Taken together with Volkow and colleagues’ finding16 that there is a relation between the D2/D3 receptors and the WCST index, the negative correlations between perseverative errors and both DAT availability and D2 receptor availability suggest that DA may play an important role in the striatum during performance on the WCST.
The negative association between DAT availability and both age and BMI found in this study are consistent with the findings of 2 previous studies.26,35 BMI was highly correlated with age in our study. Our previous study suggested that, when measuring striatal DAT, BMI, instead of age, should be considered to be a covariate.26 After adjustment for the effect of age, there was still a significant negative association between striatal DAT availability and perseverative errors.
Although we did not find asymmetries in this study, several studies have revealed hemispheric asymmetry differences in right-handed healthy participants in the recall of novel and practiced unstructured word lists and visuospatial abstraction.36,37 However, another study did not find hemispheric asymmetries.22 The issue of asymmetry in the association between striatal dopaminergic tone and the indices of cognitive function and/or regional metabolic activity should be confirmed in future studies.
Limitations
The findings of the present study should be interpreted with caution because of the following limitations. First, the volunteers were generally younger than 40 years, especially in the male group, and a urine toxicological screen was not performed for all participants. In the female group, we did not control for the menstrual cycle. Second, we performed an association study that did not depend on experimental manipulations of dopaminergic tone to produce changes in WCST performance. Thus, a causal relation between the 2 cannot be confirmed. Third, there is a chance that the localization of the ROI may not have been accurate because MRI coregistration was not performed in this study. Fourth, the high education level (14.25 yr, SD 2.94) of our participants lead to selection bias and reduces the possibility of generalizing the results. Finally, we only used the WCST as a cognitive test for executive function. More cognitive functions should be evaluated to test our hypothesis.
Conclusion
We have documented an association between perseverative errors on the WCST and striatal DAT availability. These findings may suggest that striatal DAT availability plays a role in executive function as measured by the WCST.
Acknowledgements
The authors thank the Atomic Energy Council of Taiwan (92-Nu-7-006-004) and the National Science Council of Taiwan (NSC-92-2314-B-006-042, NSC-95-2314-B-006-115-MY2, NSC-97-2314-B-006-006-MY3) for financial support. The authors thank Ms. Ching Lin Chu, Ms. Tsai-Hua Chang, Ms. Yun-Hsuan Chang and Ms. Chien Ting Lin for administrative support.
Footnotes
Competing interests: None declared.
Contributors: Drs. Hsieh and Yang designed the study. Drs. Yeh, Lee, Huang, Chiu, Lu and Liao acquired the data, which Dr. Chen analyzed. Dr. Hsieh wrote the article; all other authors reviewed it. All authors provided final approval for publication.
References
- 1.Barcelo F, Sanz M, Molina V, et al. The Wisconsin Card Sorting Test and the assessment of frontal function: a validation study with event-related potentials. Neuropsychologia. 1997;35:399–408. doi: 10.1016/s0028-3932(96)00096-6. [DOI] [PubMed] [Google Scholar]
- 2.Caldu X, Vendrell P, Bartres-Faz D, et al. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37:1437–44. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Lane HY, Liu YC, Huang CL, et al. Prefrontal executive function and D1, D3, 5-HT2A and 5-HT6 receptor gene variations in healthy adults. J Psychiatry Neurosci. 2008;33:47–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Rybakowski JK, Borkowska A, Czerski PM, et al. Performance on the Wisconsin Card Sorting Test in schizophrenia and genes of dopaminergic inactivation (COMT, DAT, NET) Psychiatry Res. 2006;143:13–9. doi: 10.1016/j.psychres.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Rybakowski JK, Borkowska A, Czerski PM, et al. An association study of dopamine receptors polymorphisms and the Wisconsin Card Sorting Test in schizophrenia. J Neural Transm. 2005;112:1575–82. doi: 10.1007/s00702-005-0292-6. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Hernandez JA, Pita-Alcorta C, Cedeno I, et al. Wisconsin Card Sorting Test synchronizes the prefrontal, temporal and posterior association cortex in different frequency ranges and extensions. Hum Brain Mapp. 2002;17:37–47. doi: 10.1002/hbm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YK, Chen CC, Lee IH, et al. Association between regional cerebral blood flow and eye-tracking performance and the Wisconsin Card Sorting Test in schizophrenics: a single photon emission computed tomography study. Psychiatry Res. 2003;123:37–48. doi: 10.1016/s0925-4927(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Mega MS, Cummings JL, Salloway S, et al. The limbic system: an anatomic, phylogenetic, and clinical perspective. J Neuropsychiatry Clin Neurosci. 1997;9:315–30. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- 9.Previc FH. Dopamine and the origins of human intelligence. Brain Cogn. 1999;41:299–350. doi: 10.1006/brcg.1999.1129. [DOI] [PubMed] [Google Scholar]
- 10.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 11.Christian BT, Lehrer DS, Shi B, et al. Measuring dopamine neuro-modulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31:139–52. doi: 10.1016/j.neuroimage.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 12.Riccardi P, Li R, Ansari MS, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31:1016–26. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- 13.Riccardi P, Zald D, Li R, et al. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163:1639–41. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced pre-frontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–71. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 15.Tiihonen J, Vilkman H, Rasanen P, et al. Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry. 1998;3:156–61. doi: 10.1038/sj.mp.4000365. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 17.Jaber M, Jones S, Giros B, et al. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord. 1997;12:629–33. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- 18.Ciliax BJ, Heilman C, Demchyshyn LL, et al. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–23. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freed C, Revay R, Vaughan RA, et al. Dopamine transporter immunoreactivity in rat brain. J Comp Neurol. 1995;359:340–9. doi: 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- 20.Hersch SM, Yi H, Heilman CJ, et al. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–27. [PubMed] [Google Scholar]
- 21.Piccini PP. Dopamine transporter: basic aspects and neuroimaging. Mov Disord. 2003;18(Suppl 7):S3–8. doi: 10.1002/mds.10571. [DOI] [PubMed] [Google Scholar]
- 22.Mozley LH, Gur RC, Mozley PD, et al. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–9. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 23.Yang YK, Yao WJ, Yeh TL, et al. Decreased dopamine transporter availability in male smokers — a dual isotope SPECT study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:274–9. doi: 10.1016/j.pnpbp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Yang YK, Yeh TL, Yao WJ, et al. Greater availability of dopamine transporters in patients with major depression — a dual-isotope SPECT study. Psychiatry Res. 2008;162:230–5. doi: 10.1016/j.pscychresns.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Yeh TL, Lee IH, Chen KC, et al. The relationships between daily life events and the availabilities of serotonin transporters and dopamine transporters in healthy volunteers — a dual-isotope SPECT study. Neuroimage. 2009;45:275–9. doi: 10.1016/j.neuroimage.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen PS, Yang YK, Yeh TL, et al. Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers — a SPECT study. Neuroimage. 2008;40:275–9. doi: 10.1016/j.neuroimage.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 28.Chang L. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;25:638–43. [Google Scholar]
- 29.Hwang WJ, Yao WJ, Wey SP, et al. Reproducibility of 99mTc-TRO-DAT-1 SPECT measurement of dopamine transporters in Parkinson’s disease. J Nucl Med. 2004;45:207–13. [PubMed] [Google Scholar]
- 30.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test manual Revised and expanded. Odessa (FL): Psychological Assessment Resources; 1993. [Google Scholar]
- 31.Stratta P, Mancini F, Mattei P, et al. Association between striatal reduction and poor Wisconsin card sorting test performance in patients with schizophrenia. Biol Psychiatry. 1997;42:816–20. doi: 10.1016/s0006-3223(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 32.Spielewoy C, Biala G, Roubert C, et al. Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl) 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo JF, Yang YK, Chiu NT, et al. The correlation between striatal dopamine D2/D3 receptor availability and verbal intelligence quotient in healthy volunteers. Psychol Med. 2006;36:547–54. doi: 10.1017/S0033291705006732. [DOI] [PubMed] [Google Scholar]
- 34.Ralph RJ, Paulus MP, Fumagalli F, et al. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–13. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozley PD, Acton PD, Barraclough ED, et al. Effects of age on dopamine transporters in healthy humans. J Nucl Med. 1999;40:1812–7. [PubMed] [Google Scholar]
- 36.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Recalling word lists reveals “cognitive dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry. 1999;156:386–92. doi: 10.1176/ajp.156.3.386. [DOI] [PubMed] [Google Scholar]
- 37.Wharton CM, Grafman J, Flitman SS, et al. Toward neuroanatomical models of analogy: a positron emission tomography study of analogical mapping. Cognit Psychol. 2000;40:173–97. doi: 10.1006/cogp.1999.0726. [DOI] [PubMed] [Google Scholar]