Abstract
Background
Autism spectrum disorders (ASD) are associated with severe impairments in social functioning. Because faces provide nonverbal cues that support social interactions, many studies of ASD have examined neural structures that process faces, including the amygdala, ventromedial prefrontal cortex and superior and middle temporal gyri. However, increases or decreases in activation are often contingent on the cognitive task. Specifically, the cognitive domain of attention influences group differences in brain activation. We investigated brain function abnormalities in participants with ASD using a task that monitored attention bias to emotional faces.
Methods
Twenty-four participants (12 with ASD, 12 controls) completed a functional magnetic resonance imaging study while performing an attention cuing task with emotional (happy, sad, angry) and neutral faces.
Results
In response to emotional faces, those in the ASD group showed greater right amygdala activation than those in the control group. A preliminary psychophysiological connectivity analysis showed that ASD participants had stronger positive right amygdala and ventromedial prefrontal cortex coupling and weaker positive right amygdala and temporal lobe coupling than controls. There were no group differences in the behavioural measure of attention bias to the emotional faces.
Limitations
The small sample size may have affected our ability to detect additional group differences.
Conclusion
When attention bias to emotional faces was equivalent between ASD and control groups, ASD was associated with greater amygdala activation. Preliminary analyses showed that ASD participants had stronger connectivity between the amygdala ventromedial prefrontal cortex (a network implicated in emotional modulation) and weaker connectivity between the amygdala and temporal lobe (a pathway involved in the identification of facial expressions, although areas of group differences were generally in a more anterior region of the temporal lobe than what is typically reported for emotional face processing). These alterations in connectivity are consistent with emotion and face processing disturbances in ASD.
Introduction
Deficits in social function represent a core feature of autism spectrum disorders (ASD).1 Because emotional facial expressions convey nonverbal information that help to scaffold social interactions, many studies of ASD have examined the network of neural structures involved in face processing, including the amygdala, fusiform gyrus, ventromedial pre-frontal cortex and areas around the superior temporal sulcus.2
Influential models posit that the amygdala is centrally involved in ASD.3,4 However, it is uncertain whether ASD is associated with increased or decreased amygdala activation. Most studies found that participants with ASD show decreased amygdala activation relative to controls in response to neutral and emotional facial displays.5–11 However, one study reported that both groups showed comparable amygdala activation to faces,12 and another study documented that participants with ASD had greater amygdala activation relative to controls.13
Differences in activation between ASD and control groups may depend in part on the cognitive demands specific to each face viewing task during brain imaging. Indeed, varying the cognitive task can determine whether group differences are found in amygdala activation.7,11,13 In particular, attention influences amygdala activation.13 When gaze fixation, an index of attention, was monitored with eye tracking, Dalton and colleagues13 found that participants with ASD showed greater amygdala activation relative to controls when viewing faces. Moreover, within the ASD group, the duration of gaze directed to the eyes of the face stimuli was positively associated with amygdala activation. Thus, coupled with behavioural work indicating that individuals with ASD show abnormal attention to social stimuli,13–16 these findings highlight the importance of considering group differences in attention when examining brain activation.
The ventromedial prefrontal cortex, which includes the ventral anterior cingulate and orbitofrontal cortex, is also highly involved in processing social stimuli.2 Findings from nonhuman primates indicate that there are dense, reciprocal connections between regions of the ventromedial prefrontal cortex and the amygdala.17 Moreover, using functional connectivity, an analytical method that evaluates correlations in neuroimaging activation, recent investigations suggest that these structures interact and that the interactions may be disturbed in various forms of psychopathology.18,19 Consistent with the amygdala findings, most studies found that participants with ASD showed less activation in various areas of the ventral prefrontal cortex relative to controls in response to facial displays.5,7,11,20,21 However, the study that found amygdala hyperactivation in ASD also found increased activation in the ventromedial prefrontal cortex.13 To our knowledge, no study has examined task-related functional connectivity between the amygdala and pre-frontal cortex in participants with ASD.
Whereas the fusiform gyrus is involved in processing invariant aspects of faces (e.g., identity), structures around the superior temporal sulcus, including the superior and middle temporal gyri, are responsible for processing changeable features of faces, such as emotional facial expressions.22 Recently, work involving monkeys showed that electrical microstimulation of a face-processing region of the temporal lobe activated the amygdala.23 In autism, the integrity of the white matter tract between the temporal lobe and amygdala is compromised.24 Although no published study has reported functional connectivity between the amygdala and temporal lobe in ASD, functional magnetic resonance imaging (fMRI) findings indicate that participants with ASD show altered activation in the temporal lobe relative to controls, but the direction of the effects has been inconsistent.5,6,9,11,25
We had 3 goals in the present study. The first was to examine amygdala activation in response to face stimuli in participants with ASD and controls while measuring attention bias. Whereas Dalton and colleagues13 used eye monitoring to measure attention to faces, we implemented an attention cuing paradigm called the probe detection task.26 This paradigm provides a measure of participant attention bias during fMRI data acquisition.19,27 Specifically, participants viewed emotional (happy, sad, angry) and neutral face pairs. After the presentation of each face pair, an asterisk appeared in place of the location of the emotional (congruent) or neutral (incongruent) face (Fig. 1). Participants pressed a button to the location of the asterisk. If attention is drawn to one facial expression over another in the pair (e.g., happy faces relative to neutral faces), the reaction times to the asterisks that follow the location of the happy faces will be faster relative to asterisks that follow the location of the neutral faces. Thus, attention bias scores reflect the difference between mean reaction times for incongruent and congruent trials.26 If attention bias is equivalent between groups, differences in activation are less likely to be driven by attention. Measures of eye gaze allow for the monitoring of overt attention and are sensitive to the precise location of gaze direction. In contrast, although the probe detection task is not sensitive to the precise location of gaze on the face (e.g., the eyes), it does index both overt and covert attention bias between the faces.28 Therefore, the probe detection task may be considered a complement to measures of eye gaze.
Fig. 1.
The visual task presented to the autism spectrum disorder (ASD) and control participants. The columns on the far left and right show the screens that participants saw for the 2 trial types with happy faces. In the left column, the happy face and probe are presented on different sides of the screen (incongruent). In the right column, the happy face and probe are presented on the same side of the screen (congruent). Participants also viewed sad–neutral, angry–neutral and neutral–neutral trials.
Our second goal was to examine functional connectivity between the amygdala and the ventromedial prefrontal cortex. Because these structures are thought to influence one another in social tasks, we sought to determine how these interactions might be altered in ASD. The third goal was to evaluate functional connectivity in areas involved in processing facial expressions, in particular between the amygdala and structures around the superior temporal sulcus (superior and medial temporal gyri).
Following previous work,13 our first hypothesis was that relative to controls, participants with ASD would show greater amygdala activation to emotional faces when attention bias was equivalent between the groups. We also formulated 2 additional hypotheses that are considered prelim inary. Specifically, based on the finding that ASD participants showed greater amygdala and ventromedial prefrontal cortex activation when attention to emotional faces was monitored,13 we predicted that, relative to controls, participants with ASD would show increased functional connectivity between the amygdala and ventromedial prefrontal cortex. In addition, because the integrity of white matter tracts are diminished in ASD between the amygdala and temporal lobe,24 we predicted that participants with ASD would show reduced functional connectivity between these structures.
Methods
Participants
We included 12 with ASD and 12 control adults. These participants were the same as those who were in a study examining resting connectivity.29 All procedures were approved by the University of Michigan Institutional Review Board. All participants signed consent and were between 18 and 40 years of age.
We recruited participants with ASD through the University of Michigan Autism and Communication Disorders Center. We recruited controls through posted flyers distributed in the community. An ASD diagnosis was determined based on the Autism Diagnostic Observation Schedule,30 the Autism Diagnostic Interview–Revised31 and clinical consensus.32 We interviewed parents with the Autism Diagnostic Interview–Revised. Asperger syndrome was diagnosed through clinical consensus using criteria from the DSM-IV.1 To measure cognitive functioning, we gave participants the Peabody Picture Vocabulary Test33 and the Ravens Progressive Matrices.34 We did not include participants with ASD if their nonverbal cognitive functioning was below 85, if they had another neurologic disorder or if they had braces. Because most of the ASD patients were taking medication, we used an approach similar that used in prior work on bipolar disorder35 to perform a post-hoc analyses to examine the influence of the class of medications on group differences.
We excluded controls if their nonverbal cognitive functioning was below 85, if they had a neurologic disorder, a mental disorder or braces or if they were taking a psychotropic medication.
Task
During image acquisition, participants performed the probe detection task. We selected emotional and neutral faces from NimStim.36 Building on prior work,19,27 picture pairs (happy–neutral, sad–neutral, angry–neutral and neutral–neutral expressions) were presented to the participants. There were 72 trials of each emotion pair (36 trials in which the specific emotional face and the probe were on the same side and 36 on the opposite side), 36 trials with neutral–neutral pairs and 36 trials for the implicit baseline (blank screen). In an event-related design, we presented the trials in a different, randomized order for each participant. In addition, we also included trials in which faces were presented for 17 ms and masked. No group differences in amygdala activation for the 17-ms face presentation were found. Results from the 17-ms conditions are not considered further here.
Trials began with a fixation cross in the middle of the screen for 500 ms, followed by a face pair for 500 ms (Fig. 1). The faces were replaced by an asterisk probe in one of the hemifields for 1100 ms. The emotional faces appeared in the right and the left positions with equal probability, with the matched neutral faces of each pair appearing in the other position. The probe was presented in both positions with equal probability. The intertrial interval varied between 250 and 1165 ms, during which time a black screen was displayed. We used E-Prime (Psychological Software Tools) to control the stimulus presentation and record the responses.
We asked the participants to respond quickly when they detected an asterisk by pressing the left button if the asterisk appeared on the left and the right button if the asterisk appeared on the right. Participants first completed a practice session with a different set of faces26 and were allowed to repeat the practice session until they were comfortable with the task.
Data acquisition
We used a 3-T GE Signa scanner at the University of Michigan to collect the MRI images. We acquired T2*-weighted blood oxygen level–dependent images using a reverse spiral sequence37 of 40 contiguous axial with 3.44 × 3.44 × 3 mm voxels (repetition time 2000 ms, echo time 30 ms, flip angle 90°, field of view 22 cm). Slices were prescribed parallel to the anterior–posterior commissural line and acquired contiguously to optimize the effectiveness of movement postprocessing algorithms. Over 4 runs, we acquired 720 volumes. We collected the images and reconstructed them into a 64 × 64 matrix. A T1-weighted gradient echo axial overlay was collected for anatomic localization (repetition time 8.9 ms, echo time 1.8 ms, flip angle 15°, field of view 26 cm, slice thickness 1.4 mm, 124 slices, matrix = 256 × 160). To facilitate normalization, we collected an inversion-prepped T1-weighted anatomic image using spoiled gradient-recalled acquisition in steady state (SPGR) imaging (flip angle 15°, field of view 26 cm, 110 sagittal slices, 1.4-mm slice thickness).
Data analysis of fMRI scans
We performed the following preprocessing steps. We skull-stripped the anatomic data using the Brain Extraction Tool from FSL (www.fmrib.ox.ac.uk/fsl/bet2/index.html). K-space outliers that were greater than 2 standard deviations (SDs) from the mean were replaced with the average of their temporal neighbours. Next, to remove distortions from magnetic field in homogeneity, we reconstructed images using field map correction. In addition, slice timing differences were corrected using local sinc interpolation.38 Finally, MCFLIRT (www.fmrib.ox.ac.uk/fsl/mcflirt/index.html)39 was used to perform motion correction (using the 10th image volume as the reference). Subsequent processing was done using SPM2 (www.fil.ion.ucl.ac.uk/spm).
We performed the following steps to map the fMRI results into a standardized anatomic atlas. First, we registered the functional images to the SPGR images. Second, the transformation to align the SPGR to the SPM2 T1 Montreal Neurological Institute (MNI) template was determined. Third, we applied the transformation to the functional data. Data were smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
We performed analyses of the fMRI scans using the general linear model. For each participant, a design matrix was specified. We derived regressors for the individual participant fMRI analysis from SPM2’s canonical hemodynamic response function. We then performed the group analysis in 2 steps. First, using an in-house Matlab script, mean activation from the amygdala (bilateral) was extracted from the primary contrasts (happy–neutral v. neutral–neutral; sad–neutral v. neutral–neutral; angry–neutral v. neutral–neutral). The structural amygdala region of interest (ROI) was derived from PickAtlas, an atlas that provides ROIs in MNI space based on the Talairach Daemon.40 We then performed a multiple regression analysis with face expression and group using SPSS 17 to determine whether the groups differed in amygdala response to each of the facial expressions.
Second, to better characterize the interaction, we used random effects analyses in SPM2 for the interpretation of data across participants. This approach requires the derivation of a single test-measure from each participant, a contrast value at each voxel within Talairach space. We then subjected these contrast maps from all participants to the general linear model using t tests to test population-level hypotheses. Because our primary hypothesis concerned the amygdala, we followed previous work and used an ROI analytic approach separately for the right and left amygdala.41 Small volume corrections controlled for multiple comparisons within the whole amygdala42 with the corrected family-wise error p value set at 0.05 (2-tailed). We derived the structural amygdala ROI from PickAtlas.40 We included only trials with correct responses in the analysis.
Functional connectivity analysis
Using psychophysiological interaction connectivity analysis, we examined patterns of interrelations between the right amygdala and ventromedial prefrontal cortex and between the right amygdala and the temporal lobe during viewing of emotional faces relative to neutral faces. The premise underlying functional connectivity is that 2 brain regions that are mutually participating at some stage in a cognitive process should have coherent activity across trials or exhibit correlated β values (separate estimates used to model activity during different stages of a task). These methods require a measure of activation at each voxel on each trial and then a search for other voxels that show correlated activation across trials. For the psychophysiological interaction analysis, we modified the procedures from SPM243 such that seed remained in the same location for each participant.
Whereas some functional connectivity procedures examine the correlation between structures across the entire task, psychophysiological interaction connectivity analysis allows for consideration of selected conditions of the task in the analysis. For instance, group differences in functional connectivity to happy relative to neutral faces can be examined. We used the psychophysiological interaction analytic approach in the present study because we were particularly interested in how ASD participants, relative to control participants, would differ in functional connectivity when viewing specific facial expressions. Following previous work on functional connectivity analyses using a similar paradigm, we set the threshold at p < 0.005.19
Behavioural data analysis
For the behavioural measure of attention bias from the probe detection task, there were 2 categories for each emotion: congruent trials (an emotional/neutral face-pair, followed by a probe replacing the emotional face) and incongruent trials (an emotional/neutral face-pair followed by a probe replacing the neutral face). Differences between each participant’s mean reaction time for incongruent and congruent conditions form the attention bias scores, such that positive values indicate bias toward the particular emotion and negative values indicate a bias away from the emotion.26
Group analysis followed 2 steps for attention bias and reaction time. First, we implemented a repeated-measures analysis of variance in SPSS 17 with face expression and group. Second, we performed t tests in SPSS 17 to directly compare attention biases to specific emotions and reaction times to specific emotions between the 2 groups.
Results
Participants
Of the 12 included ASD participants, 7 were diagnosed with autism, 2 with Asperger syndrome and 3 with pervasive developmental disorder not otherwise specified. There were no significant group differences in age, verbal measure of cognitive function, nonverbal measure of cognitive function, sex and handedness (Table 1). One participant with ASD had movement that was greater than 1 voxel during the scan. This participant returned for another scan about 1 month later; the second scan was successful. Eleven ASD participants were taking a psychotropic medication (5 were taking selective serotonin reuptake inhibitors, 4 were taking stimulants, 2 were taking neuroleptics, 1 was taking a tricyclic and 1 was taking benzodiazepine).
Table 1.
Characteristics of the participants
| Group; mean (SD)* |
||
|---|---|---|
| Characteristic | ASD, n = 12 | Control, n = 12 |
| Age, yr | 26 (6) | 27 (6) |
| Sex, male:female | 11:1 | 10:2 |
| Cognitive function | ||
| Verbal | 117 (14) | 110 (18) |
| Nonverbal | 119 (14) | 118 (13) |
| Handedness, left:right | 0:12 | 1:11 |
ASD = autism spectrum disorder; SD = standard deviation.
Unless otherwise indicated.
Behavioural results
Overall, there were no interactions or group differences in attention bias. A repeated-measures analysis showed that there was no significant interaction between group and attention bias to the emotions (F2,21 = 0.430, p = 0.66). To be consistent with previous work of the probe detection task,19,27 we also examined possible group differences in attention bias to each emotion separately using t tests. To happy faces, the ASD group manifested an attention bias of 10.9 (SD 22.4) ms and the control group showed an attention bias of 8.8 (SD 16.9) ms. There was no group difference for attention bias to happy faces (t22 = 0.26, p = 0.80). To sad faces, the ASD group had an attention bias of 7.2 (SD 22.9) ms, and the control group showed an attention bias of 7.9 (SD 30.2) ms. No group difference was found for attention bias to sad faces (t22 = 0.07, p = 0.95). For angry faces, the ASD group showed an attention bias of 18.3 (SD 25.7) ms, and the control group had an attention bias of 8.4 (SD 24.0) ms. There was no group difference for attention bias to angry faces (t22 = 0.97, p = 0.34).
Both groups combined showed an attention bias to happy faces (t22 = 2.48, p = 0.021) and angry faces (t22 = 2.63, p = 0.015), but there was no a significant effect for sad faces (t22 = 1.41, p = 0.17).
A repeated-measures analysis did not reveal a significant interaction between group and overall reaction times to trials to each emotion (F3,20 = 0.460, p = 0.71). Nevertheless, t tests revealed that the ASD participants had shorter response latencies relative to controls in each condition. For trials containing happy–neutral face pairs, mean reaction times were 500.25 (SD 59.90) ms for ASD participants and 547.71 (SD 50.60) ms for controls (t22 = 2.10, p = 0.048). For trials with sad–neutral face pairs, mean reaction times were 497.59 (SD 60.54) ms for ASD participants and 550.96 (SD 48.15) ms for controls (t22 = 2.39, p = 0.026). For the angry–neutral face pair trials, mean reaction times were 498.34 (SD 56.44) ms for ASD participants and 551.69 (SD 53.31) ms for controls (t22 = 2.38, p = 0.026). For trials containing neutral–neutral face pairs, mean reaction times were 494.46 (SD 67.35) ms for ASD participants and 541.74 (SD 47.23) ms for controls. Because the reaction time data for the control group were not normally distributed, we used a Mann–Whitney U test, which showed a significant difference between groups (Mann–Whitney U = 32.00, p = 0.021).
The finding that the ASD group had shorter responses latencies relative to controls was unexpected. However, because the group differences in reaction time were also evident in the comparison condition (neutral faces) of the fMRI activation, any effects were likely cancelled out in the fMRI contrast analyses.
The ASD group had an error rate of 6% (SD 0.13%), and the controls had an error rate of 2% (SD 0.02%). No differences were found in overall error rates between groups in the task (t22 = 1.1, p = 0.29). Incorrect trials were removed from the reaction time and fMRI analyses.
Activation results on fMRI
A repeated-measures analysis of variance was used to examine the effect of group and bilateral amygdala activation (mean activation from the left and right sides combined) to the emotional relative to neutral faces (happy–neutral v. neutral–neutral; sad–neutral v. neutral–neutral; angry–neutral v. neutral–neutral). This analysis showed a significant interaction between group and amygdala activation to the different facial expressions (F2,22 = 7.67, p = 0.003).
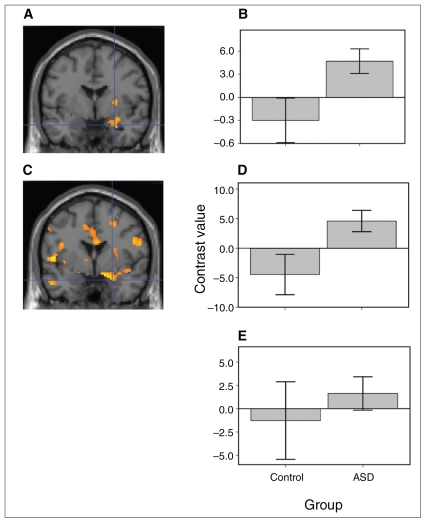
To better characterize this interaction, t tests were conducted in SPM2. Specifically, we separately examined group differences in activation to the emotional (happy–, sad– and angry) and neutral face pair trials versus the neutral–neutral face pair trials. For happy–neutral face pair trials versus neutral–neutral face pair trials, the ASD group showed greater right amygdala activation relative to the control group (MNI coordinates 26 −2 −22, t22 = 3.14, p = 0.046, volume corrected42 for the right amygdala) (Fig. 2). To further evaluate group differences in amygdala function, mean contrast values for the whole right amygdala ROI from Pick -Atlas44 were extracted from each participant for the contrast of happy–neutral face pair trials versus neutral–neutral face pair trials. Relative to controls, the ASD group showed greater activation in the whole right amygdala (t22 = 2.32, p = 0.030) (Fig. 2). For the sad–neutral face pair trials versus neutral–neutral face pair trials, the ASD group showed greater right amygdala activation relative to the control group (MNI coordinates 24 0 −22, t22 = 3.09, p = 0.046, small-volume corrected). Group differences in amygdala function were evaluated by extracting the mean contrast values for the whole right amygdala for the contrast of sad–neutral face pair trials versus neutral–neutral face pair trials. Compared with controls, the ASD group showed greater activation in the whole right amygdala (t22 = 2.35, p = 0.028). There were no group differences in amygdala activation to angry–neutral face pair trials relative to neutral–neutral face pair trials (MNI coordinates 28 −2 −24, t22 = 1.19, p = 0.46 corrected, p = 0.12 uncorrected) (Fig. 2). There were no group differences in activation for the angry–neutral face pair trials versus the neutral–neutral face pair trials (t22 = 0.64, p = 0.53) using the mean of the whole right amygdala.
Fig. 2.
Right amygdala activation in the autism spectrum disorder (ASD) and control groups. (A) In the comparison of happy–neutral face pair trials versus neutral–neutral face pair trials, the ASD group showed greater right amygdala activation relative to the control group. The threshold for the illustration was set at p = 0.02 with a minimum cluster size of 90 voxels. To further evaluate group differences in amygdala function, mean contrast values for the whole right amygdala were extracted for the contrast of happy–neutral face pair trials versus neutral–neutral face pair trials. Contrast values represent the difference in mean activation in the whole right amygdala for the given contrast for all participants averaged together in each group. (B) Relative to controls, those in the ASD group showed greater activation in the whole right amygdala. Error bars are standard errors of the mean. (C) For the sad–neutral face pair trials vs. neutral–neutral face pair trials, the ASD group showed greater right amygdala activation relative to the control group. Group differences in amygdala function were evaluated by extracting the mean contrast values for the whole right amygdala for the contrast of sad–neutral face pair trials vs. neutral–neutral face pair trials. (D) Compared to controls, those in the ASD group also showed greater activation in the whole right amygdala. (E) There were no group differences in activation for the angry–neutral face pair trials vs. the neutral–neutral face pair trials.
Activation in the controls alone did not differ between happy–neutral and neutral–neutral (t11 = −1.04, p = 0.32). Activation in the ASD group was greater for happy–neutral pairs relative to neutral–neutral pairs (t11 = 2.92, p = 0.014). Activation in the controls alone did not differ between sad–neutral and neutral–neutral pairs (t11 = −1.31 p = 0.22). Activation in the ASD group was greater for sad–neutral relative to neutral–neutral (t11 = 2.54, p = 0.028). Activation in the controls alone did not differ between angry–neutral and neutral–neutral (t11 = −0.31 p = 0.76). Activation in the ASD group did not differ between angry–neutral relative to neutral–neutral (t11 = 0.91, p = 0.38).
In the left amygdala, there were no group differences in activation to any emotion that surpassed the small volume corrected threshold. For happy–neutral face pair trials versus neutral–neutral face pair trials, the ASD group did not show significantly greater left amygdala activation relative to the control group (t22 = 1.63, p = 0.35 corrected, p = 0.06 uncorrected). For sad–neutral face pair trials versus neutral–neutral face pair trials, the ASD group did not show significantly greater left amygdala activation relative to the control group (t22 = 1.82, p = 0.25 corrected, p = 0.040 uncorrected). For angry–neutral face pair trials versus neutral–neutral face pair trials, the ASD group did not show significantly greater left amygdala activation relative to the control group (t22 = 1.93, p = 0.20 corrected, p = 0.033 uncorrected) (Fig. 2).
To better understand amygdala activation in the ASD group, we followed prior work45 and entered the Autism Diagnostic Interview–Revised algorithm for reciprocal social interaction, which provides a measure of lifetime social impairment. The goal of this analysis was to determine plausible associations between amygdala activation and symptoms of autism. Because this analysis only involved the 12 participants with ASD, a liberal threshold of p < 0.05 (uncorrected) was used. We found a positive association with right amygdala activation to the happy–neutral face pairs versus neutral–neutral face pairs, sad–neutral face pairs versus neutral–neutral face pairs, and angry–neutral face pairs versus neutral–neutral face pairs. Specifically, right amygdala activation to angry–neutral face pair trials versus neutral–neutral face pair trials was positively correlated with the Autism Diagnostic Interview–Revised Total of Reciprocal Social Interaction (MNI coordinates 22 −8 −10, t10 = 2.63, p = 0.013 uncorrected, cluster size = 3; cluster size here and below is reported at a threshold of p = 0.05 uncorrected). This activation was in a distinct location from the activation in the group contrasts described above but was in the amygdala based on PickAtlas.44 Positive correlations were also found for happy–neutral face pair trials versus neutral–neutral face pair trials (MNI coordinates 22 −8 −10, t10 = 2.01, p = 0.036 uncorrected, cluster size = 2) and sad–neutral face pair trials versus neutral–neutral face pair trials (MNI coordinates 24 −10 −10, t10 = 2.05, p = 0.033 uncorrected, cluster size = 2). Activation did not relate to attention bias to any emotion.
Functional connectivity results
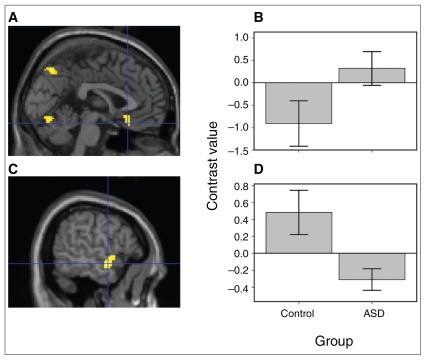
For our exploratory hypothesis concerning group differences in connectivity between the activation in the right amygdala and the ventromedial prefrontal cortex during the viewing of emotional faces, we used a psychophysiological interaction analysis. We used the peak activation from each contrast for the seeds (26 −2 −22 for happy–neutral pairs v. neutral–neutral pairs; 24 0 −22 for sad–neutral pairs v. neutral–neutral pairs; 28 −2 −24 for angry–neutral pairs v. neutral–neutral pairs) with an 8-mm sphere. In selecting the location of the seed based on group differences in activation, we were following our prior work.19 For brevity, we report clusters with 10 or more voxels. Consistent with our hypothesis, participants with ASD showed greater positive connectivity relative to the control group in the contrast of happy–neutral pairs versus neutral–neutral pairs (MNI coordinates 0 24 −18 [ventral anterior cingulate cortex], t22 = 3.14 p = 0.002 uncorrected, cluster size = 161) (Fig. 3). These results indicate that, compared with the control group, the ASD group exhibited a stronger correlation of activation to the happy–neutral pairs relative to the neutral–neutral pairs. For the contrasts of sad–neutral pairs versus neutral–neutral pairs and angry–neutral pairs versus neutral–neutral pairs, we did not find greater coupling of the amygdala–ventral prefrontal cortex in the ASD group relative to the control group.
Fig. 3.
Differences between the autism spectrum disorder (ASD) and control groups in the psychophysiological interaction connectivity analysis. (A) In the analysis of happy–neutral face pair trials versus neutral–neutral face pair trials, the ASD group showed greater positive connectivity between the right amygdala and ventromedial prefrontal cortex than did the control group. The threshold for the illustration was set at p = 0.02 with a minimum of 60 voxels. Contrast values represent the difference in mean activation in the whole right amygdala for the given contrast for all participants averaged together in each group. (B) The bar graph illustrates average contrast values within an 8-mm sphere around the peak ventromedial pre-frontal cortex activation. Error bars are standard errors of the mean. (C) For the same analysis of happy–neutral face pair trials versus neutral–neutral face pair trials, the control relative to the ASD group showed greater positive connectivity between the right amygdala and left middle temporal gyrus. (D) The bar graph illustrates average contrast values within an 8-mm sphere around the peak left middle temporal lobe activation.
To evaluate our exploratory hypothesis regarding amygdala-temporal lobe functional connectivity, the psychophysiological interaction analysis showed that the ASD group relative to the control group had weaker positive connectivity in the contrast of happy–neutral pairs versus neutral–neutral pairs between the right amygdala and left middle temporal gyrus (MNI coordinates −56 −2 −12, t22 = 3.52, p = 0.001 uncorrected, cluster size = 21; Fig 3). For the analysis of sad–neutral pairs versus neutral–neutral pairs, the ASD group relative to the control group showed weaker positive connectivity (MNI coordinates −66 −4 −10 [left middle temporal gyrus], t22 = 3.60, p = 0.001, cluster size = 422; MNI coordinates −64 −14 8 [left superior temporal gyrus], t22 = 3.03, p = 0.003, cluster size = 889; and MNI coordinates 46 −66 16 [right middle temporal gyrus], t22 = 4.09, p < 0.001, cluster size = 498). For the analysis of angry–neutral pairs versus neutral–neutral pairs, the ASD group relative to the control group showed weaker positive connectivity (MNI coordinates 56 −32 −8 [right middle temporal gyrus], t22 = 3.57, p = 0.001, cluster size = 112; MNI coordinates 66 −20 −14 [right middle temporal gyrus], t22 = 3.19, p = 0.002, cluster size = 18). With the exception of one cluster for the sad condition (MNI coordinates 46 −66 16), group differences in connectivity in the temporal lobe were more anterior than what is commonly reported for face processing.46
We found that the control group, relative to the ASD group, showed greater amygdala connectivity within 2 areas of the inferior frontal gyrus for sad–neutral pairs versus neutral–neutral pairs: MNI coordinates −28 24 −2, t22 = 4.25, p < 0.001 uncorrected, cluster size = 829; and MNI coordinates 32 24 −4, t22 = 3.06, p = 0.003 uncorrected, cluster size = 1109. Control participants showed pronounced positive connectivity in these locations, while ASD participants did not show connectivity in these areas.
Effects of medication
To evaluate whether specific medications influenced the findings, we removed from the analysis ASD participants who received each class of medication, and we compared the data from the remaining ASD participants with that from the control participants to determine whether the effects of increased right amygdala activation remained. The results remained significant (p < 0.05 uncorrected) when each medication group was removed from the analysis. In addition, we performed a comparable analysis using the behavioural data and found that none of the classes of medication altered the results. When each medication class was removed, the group differences in attention bias still did not approach significance.
Discussion
In response to emotional faces, ASD participants showed abnormalities in brain function even when attention bias was equivalent to that in the control group. Consistent with our primary hypothesis, the ASD group showed greater right amygdala activation to happy and sad faces than did the control group. In a preliminary analysis, we found that the ASD group, relative to the control group, showed greater positive functional connectivity between the right amygdala and ventromedial prefrontal cortex to happy faces. Another preliminary analysis showed that the ASD group, relative to the control group, showed less positive functional connectivity between the right amygdala superior/medial temporal gyri (primarily anterior regions) to happy, sad and angry faces. Finally, relative to the control group, the ASD group unexpectedly had less positive functional connectivity to sad faces between the right amygdala and inferior frontal gyrus, an area outside the ventromedial prefrontal cortex region of interest.
Most studies have reported decreased amygdala activation in ASD.5–11 However, Dalton and colleagues13 found that ASD participants showed greater amygdala activation to face stimuli and that amygdala activation within the ASD group positively correlated with the duration of gaze directed to the eyes of the face stimuli. The present findings coupled with those of Dalton and colleagues13 suggest that ASD participants may show increased activation to facial displays when potential group differences in attention are considered. In addition to attention, several studies of ASD have reported that group differences in amygdala activation are dependent on the cognitive task being performed during image acquisition.6,7,11 Moreover, in another study, participants with ASD and controls both showed amygdala activation in response to faces, but the ASD participants showed less habituation in the amygdala.47 To build a coherent model of ASD and brain function, it is important that aspects of the task, such as the cognitive elements and habituation rates, be considered.
Our study was designed specifically to examine brain response to emotional faces in ASD. Therefore, we used neutral faces for the comparison condition. However, as is shown in Figure 2, group differences are the result of a combination of differential activation to specific emotional and neutral faces within each group. Thus, it is important that the interpretation of these results be constrained to documenting how individuals with ASD process emotional (happy and sad) faces relative to nonemotional faces differently from controls.
Effective social functioning involves a broad network of structures.2 The amygdala is responsive to stimuli that signal socio-emotional information.13 The ventromedial prefrontal cortex, partly by modulating amygdala activation, is involved in cognitive flexibility, including navigating complex social interactions.3 The superior and medial temporal gyri are involved in identifying facial expressions.22 (However, evidence indicates that face processing areas are generally more posterior46 than what was primarily found in our study.) Although the result was selective to happy faces, the finding that ASD participants had greater positive connectivity between the amygdala and ventromedial prefrontal cortex suggests that this circuit may be more engaged in amplifying responses to emotional stimuli in ASD. The finding that the amygdala–temporal lobe had less positive connectivity in ASD for happy, sad and angry faces indicates that the transmission of information about facial expression identification may be altered and possibly compromised.
These connectivity results may explain an apparent paradox in the present findings. Specifically, participants with ASD had greater right amygdala activation than controls, but there were no group differences in attention bias. Although speculative, our connectivity results suggest that attention bias may not be enhanced, because of abnormalities in connections with other regions involved in emotional face processing. Further work is necessary to directly examine this.
Some have suggested that ASD is a disorder of underconnectivity.48 Indeed, multiple fMRI studies have documented such a relation.25,49,50 However, other studies have found that participants with ASD show areas of stronger connectivity.29,51 Our findings are more consistent with the latter work. In particular, in response to emotional faces, ASD participants show underconnectivity between the amygdala and posterior and anterior portions of the temporal lobe, as well as overconnectivity between the amygdala and ventromedial prefrontal cortex. Such overconnectivity may underlie greater emotional responses. The inclusion of measures of emotional face recognition and subjective emotional responses could be combined with the present procedures to more precisely identify the functional significance of these disturbed connections.
Limitations
This study has several limitations. First, our study had a small sample size. Nevertheless, because small samples reduce the power and our hypotheses were supported, this limitation is less problematic. Of greater concern because of the small sample size are the behavioural results. Reduced power from small samples may underlie the lack of group differences in the behavioural data. This concern is mitigated by the behavioural results, which showed that both groups had highly comparable attention bias scores to the emotional faces (happy and sad) where group differences in fMRI activation were found. In addition, the ASD group showed greater activation in the left amygdala than the control group, but the activation was not significant at the corrected threshold. With a larger sample, this pattern may have become significant. Given the small sample, it is not possible to draw conclusions about group differences in laterality of amygdala activation.
Second, 11 of the ASD participants were taking medication. However, follow-up analyses indicated that none of the medication classes caused the group differences in amygdala activation.
Third, although there were no group differences in attention bias to the emotional faces, the ASD group showed a shorter reaction time than did the control group for the button responses to the emotional face conditions (mean difference 53.39 ms). However, the group differences in reaction time were also evident in the comparison condition (neutral faces). Thus, any effects were likely cancelled out in the contrast analyses.
Fourth, the cognitive functioning of the ASD individuals was high. The advantage of this was that the control sample was matched on cognitive ability. However, the ASD sample was not representative of the majority of people with this disorder.
Fifth, although the probe detection task is useful by providing a measure of attention bias, the task is fairly complex. Of note, when an emotional face is presented, it is paired with a neutral face. Recent evidence indicates that a lack of congruence in emotional stimuli affects neural function.52 Therefore, group differences in the present study may be influenced by the lack of congruence in stimuli when emotional faces were presented.
Future work may wish to examine multiple age ranges to clarify the role of development in our findings. Previous work involving healthy individuals have shown developmental changes in amygdala activation in response to emotional faces.53 Moreover, ASD and control participants show different developmental trajectories in amygdala volume.54 Understanding how amygdala response to social stimuli differentially changes in ASD and controls in specific age ranges would help to identify the influence of development in these findings. In addition, the task used in the present study did not require participants to explicitly identify facial expressions. Given that amygdala activation in individuals with ASD varies depending on whether they explicitly or implicitly process the faces,6 it would be important to include an explicit task of facial expressions to understand how such task conditions affect group differences in amygdala activation. Finally, statistical techniques, such as self-organizing map algorithms, may be used to examine brain function without relying on predetermined locations.55
Conclusion
During a task that yielded no group differences in attention bias, ASD participants showed greater amygdala activation to emotional faces than did control participants. Furthermore, a preliminary analysis showed that, relative to the control group, the ASD group had greater positive connectivity between the amygdala and ventromedial prefrontal cortex and weaker connectivity between the amygdala and primarily anterior regions of the temporal lobe. Thus, even when attention bias is equivalent between groups, individuals with ASD show broad-based alterations in brain function when viewing socially relevant, emotional faces.
Acknowledgements
This research was supported in part by the National Institutes Health (K22 MH068017 to C.S.M, U19 HD35482 to C.L. and MH066496 to C.L.). We thank Dr. D. Noll for methodological advice, and Dr. S.J. Fromm and K. Newnham for technical support. Data from this study were presented at the International Meeting for Autism Research, London, UK, in May 2008.
Footnotes
Competing interests: None declared for Drs. Monk and Weng and Mr. Kurapati, Ms. Wiggins, Ms. Carrasco and Ms. Maslowsky. Drs. Lord and Risi receive royalties from a publisher of diagnostic instruments described in this paper. They donate all profits generated by the University of Michigan Autism and Communication Disorders Center (UMACC) in regards to this paper and all other UMACC projects to a charity.
Contributors: Dr. Monk designed the study. Drs. Monk, Weng, Risi and Lord and Ms. Wiggins, Carrasco and Maslowsky acquired the data. Dr. Monk wrote the article. All authors analyzed the data, reviewed the article and approved its publication.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): the Association; 1994. [Google Scholar]
- 2.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–9. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 3.Baron-Cohen S, Ring HA, Bullmore ET, et al. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 4.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Ashwin C, Baron-Cohen S, Wheelwright S, et al. Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Critchley HD, Daly EM, Bullmore ET, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–12. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- 7.Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grelotti DJ, Klin AJ, Gauthier I, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–85. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Hadjikhani N, Joseph RM, Snyder J, et al. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28:441–9. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelphrey KA, Morris JP, McCarthy G, et al. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2:140–9. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinkham AE, Hopfinger JB, Pelphrey KA, et al. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–75. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce K, Haist F, Sedaghat F, et al. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–16. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 13.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross TF. The perception of four basic emotions in human and nonhuman faces by children with autism and other developmental disabilities. J Abnorm Child Psychol. 2004;32:469–80. doi: 10.1023/b:jacp.0000037777.17698.01. [DOI] [PubMed] [Google Scholar]
- 15.Joseph RM, Tager-Flusberg H. An investigation of attention and affect in children with autism and Down syndrome. J Autism Dev Disord. 1997;27:385–96. doi: 10.1023/a:1025853321118. [DOI] [PubMed] [Google Scholar]
- 16.Klin A, Jones W, Schultz R, et al. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–16. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 17.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh AA, Finger EC, Mitchell DG, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 19.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35:1219–30. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadjikhani N, Joseph RM, Snyder J, et al. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–82. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- 22.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 23.Moeller S, Freiwald WA, Tsao DY. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–9. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnea-Goraly N, Kwon H, Menon V, et al. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Koshino H, Kana RK, Keller TA, et al. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley BP, Mogg K, White J, et al. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol. 1999;38:267–78. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- 27.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 28.Posner MI, Walker JA, Friedrich FJ, et al. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–74. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monk CS, Peltier SJ, Wiggins JL, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–72. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- 31.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 32.Lord C, Risi S, DiLavore PS, et al. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 33.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd ed. Circle Pines (MN): American Guidance Services; 1997. [Google Scholar]
- 34.Raven JC. Guide to using the Standard Progressive Matrices. London (UK): Lewis; 1960. [Google Scholar]
- 35.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–5. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tottenham N, Tanaka JW, Leon AC, et al. NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim A, Schafer R, Buck J. Discrete-time signal processing. 2nd ed. Upper Saddle River (NJ): Prentice Hall; 1999. [Google Scholar]
- 39.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 40.Maldjian JA, Laurienti P, Burdette J, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 41.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–8. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 42.Worsley KJ, Marrett S, Neelin P, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Gitelman DR, Penny WD, Ashburner J, et al. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 44.Maldjian J, Laurienti P, Burdette J, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Nacewicz BM, Dalton KM, Johnstone T, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–28. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hein G, Knight RT. Superior temporal sulcus — It’s my area: Or is it? J Cogn Neurosci. 2008;20:2125–36. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- 47.Kleinhans NM, Johnson LC, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–75. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 48.Just MA, Cherkassky VL, Keller TA, et al. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 49.Kleinhans NM, Richards T, Sterling L, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 50.Welchew DE, Ashwin C, Berkouk K, et al. Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biol Psychiatry. 2005;57:991–8. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 51.Mizuno A, Villalobos ME, Davies MM, et al. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–74. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 52.Amting JM, Miller JE, Chow M, et al. Getting mixed messages: the impact of conflicting social signals on the brain’s target emotional response. Neuroimage. 2009;47:1950–9. doi: 10.1016/j.neuroimage.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 54.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peltier SJ, Polk TA, Noll DC. Detecting low-frequency functional connectivity in fMRI using a self-organizing map (SOM) algorithm. Hum Brain Mapp. 2003;20:220–6. doi: 10.1002/hbm.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]