Abstract
Parallel solution-phase methods for the synthesis of a 72-membered benzo[b]thiophene library are reported. Medicinally-interesting, drug-like, methyl sulfone-substituted benzo[b]thiophenes have been prepared by the palladium-catalyzed substitution of 3-iodobenzo[b]thiophenes by Suzuki-Miyaura, Sonogashira, Heck, carboalkoxylation, and aminocarbonylation chemistry. The key intermediates for library generation, methyl sulfone-containing 3-iodobenzo[b]thiophenes, are readily prepared by iodocyclization and oxidation methodologies from readily available alkynes.
Introduction
Benzo[b]thiophenes are of interest because of their frequent occurrence in nature and their wide range of biological and physiological effects.1-3 Benzo[b]thiophene derivatives currently in pharmaceutical use or development include selective estrogen receptor modulators (SERMs),4-8 tubulin-binding agents,7,9-10 modulators of multidrug resistance,11 and anti-inflammatory12 agents to name but a few.
Traditional non-steroidal anti-inflammatory drugs (NSAIDs) represent one of the most prescribed medications, although the chronic use of such pharmacological agents is commonly associated with numerous side effects. The use of cyclooxygenase-2 (COX-2) selective or preferential inhibitors has opened up new horizons in the search for safer drugs for the management of inflammation.13 Cyclooxygenases exist as two isoforms called COX-1 and COX-2.14-15 In many systems, COX-1 is a constitutively expressed isoform and is responsible for the maintenance of physiological homeostasis, such as renal function and gastrointestinal integrity,16 whereas COX-2 is induced in response to inflammatory stimuli and is responsible for the progression of inflammation.17 In addition to its role in inflammatory disorders, COX-2 is also implicated in a variety of other pathologies, such as cancer, cardiac and cerebral ischemia, and Parkinson's18-22 and Alzheimer's22-24 diseases. Thus, the search for specific inhibitors of COX-2 by modifications of well known non-selective agents has been actively pursued and increasing attention has been devoted to the synthesis of diaryl heterocyclic compounds.25-26
Biological evaluation of known compounds has shown that insertion of the coxib-like scaffold gives rise to very potent and selective COX-2 inhibitors. All structures possess 1,2-diaryl substitution on a central hetero- or carbocyclic ring system with a characteristic sulfonyl group on one of the aryl rings that plays a crucial role in COX-2 selectivity. To date highly selective COX-2 inhibitors have been reported to contain the following functional groups: (i) methyl sulfonyl- or sulfonamide-substituted tricyclic compounds, such as DuP-697,27-30 rofecoxib,27,31-32 etoricoxib,27,33 SC-57666,34 valdecoxib,27,35 celecoxib,27,36 SC-558,36-37 and SC-5812527; (ii) acidic methanesulfoanilides, such as nimesulide,27,38-39 NS-398,27,29-30,40-41; and (iii) modifications of classical NSAIDs, such as substituted 2-phenylsulfonyl-3-phenylindole analogues I,42-44 and substituted 2-phenylsulfonyl-3-phenylbenzofuran analogues II.45 Interestingly, the drug CH3SO2-desmethylarzoxifene (LY2066948,46 CH3SO2-DMA, III) was found to bind with high affinity to estrogen receptors α (ERα) and β (ERβ) by a different mode from the other SERMs and is a potent uterine antagonist with minimal effects on the ovaries as determined by serum biomarkers and histological evaluation (Figure 1).8,46-48 Most importantly, substitution at the para position of one of the aromatic systems with a methanesulfonyl or a sulfonamide group is essential for COX inhibition.
Figure 1.
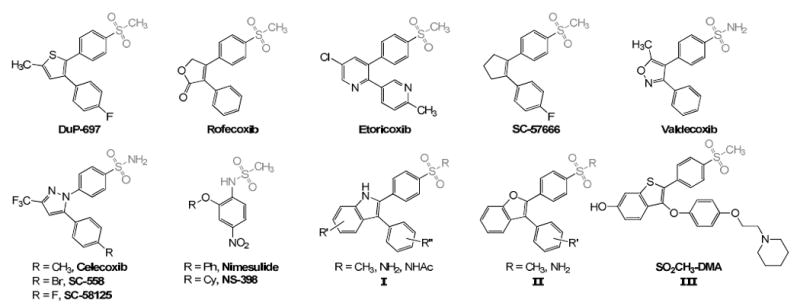
Chemical structures of known selective COX-2 inhibitors and synthetic benzothiophene SERMs, such as CH3SO2-DMA (III)
The pharmacophore of these diarylheterocyclic inhibitors is characterized by a central carbocyclic or heterocyclic ring system bearing two vicinal aryl moieties with one benzene ring being substituted with a methanesulfonyl or aminosulfonyl group at the para position (Figure 1; see I, II, III). For these reasons, 2,3-diarylsubstituted benzo[b]thiophenes with a methanesulfonyl group at the meta and/or para position have been the subject of extensive experimental studies.
In a continuation of our library efforts to adapt heterocyclization chemistry to a high-throughput synthesis format,49-54 we here report the preparation of a methyl sulfone-containing library of multi-substituted benzo[b]thiophenes 6 by electrophilic cyclization chemistry. We demonstrate the significance of this methodology by elaborating the resulting sulfur-containing 3-iodobenzothiophenes 4/5 via various palladium-catalyzed couplings, such as Suzuki-Miyaura, Sonogashira, Heck, carboalkoxylation and aminocarbonylation chemistry, to methyl sulfone-containing multi-substituted benzo[b]thiophenes 6.
Results and Discussion
We have previously developed a general synthesis of 2,3-disubstituted benzo[b]thiophenes by the palladium/copper-catalyzed cross-coupling of various o-iodothioanisoles and terminal alkynes, followed by electrophilic cyclization under mild conditions.55 Very recently, a simple and efficient method for the parallel synthesis of multi-substituted benzo[b]thiophenes has also been described via known palladium-catalyzed couplings for generation of a diverse set of building blocks starting from 3-iodobenzo[b]thiophenes (Scheme 1).54
Scheme 1.
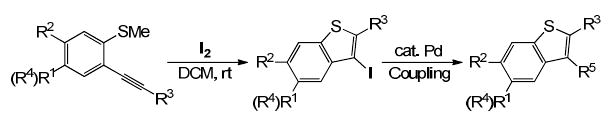
Parallel Synthesis of Multi-substituted Benzo[b]thiophenes
The strategy for library production is outlined in Scheme 2. For generation of the first methyl sulfone-substituted benzothiophene library, we decided to explore strategies using iodocyclization and oxidation methodologies that would allow us considerable flexibility to introduce a diversity of functionalities for medicinally-interesting, drug-like methyl sulfone-substituted benzo[b]thiophenes 6 in a high-throughput manner.
Scheme 2.
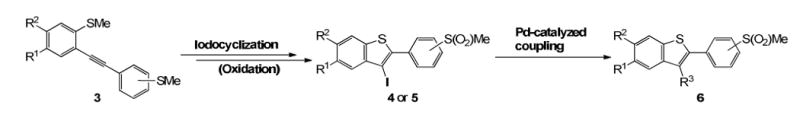
Library Design for a Diverse Methyl Sulfone-substituted Benzo[b]thiophene Library (6)
A sequence of reactions involving Sonogashira coupling of the bromo-iodoarenes 1, and subsequent lithiation of compounds 2{1-7}, followed by methylthiolation with dimethyl disulfide afforded the corresponding disulfide products 3. Initially, the electron-rich substituted dihaloarenes 1 (see Table 1) were chosen as the starting materials, because it was envisioned that the oxygen-containing groups would provide desirable polarity in the resulting library members. The starting materials, dihalobenzenes 1, are easily prepared through regioselective bromination and iodination.54 In general, the requisite precursors bearing a dibromo-substituted alkyne moiety (2) can be easily prepared from bromophenylacetylenes (para- and meta- positions) by palladium/copper-catalyzed Sonogashira coupling of the corresponding dihaloarenes in good yields (Table 1).54 Ortho-Bromophenylacetylene was not chosen as a suitable to avoid competing reactions during iodocyclization. The dibromo-substituted alkynes 2{1-6} provided the corresponding products 3{1-6} in modest yields (Table 1, entries 1-6). In a similar manner, the bromo-containing alkyne 2{7} also afforded the corresponding product 3{7} in modest yields (Table 1, entry 7).
Table 1.
Library Data for Compounds 2{1-7} and 3{1-7}
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | X | 2 | yield (%)a | 3b | yield (%)a | ||
| R1 | R2 | Y | ||||||
| 1 | MeO | H | Br | 4-Br | 2{1} | 87 | 3{1} | 78 |
| 2 | MeO | H | Br | 3-Br | 2{2} | 77 | 3{2} | 70 |
| 3 | H | MeO | Br | 4-Br | 2{3} | 72 | 3{3} | 68 |
| 4 | MeO | MeO | Br | 4-Br | 2{4} | 86 | 3{4} | 73 |
| 5 | MeO | MeO | Br | 3-Br | 2{5} | 72 | 3{5} | 71 |
| 6 |  |
Br | 4-Br | 2{6} | 83 | 3{6} | 58 | |
| 7 | H | H | SMe | 4-Br | 2{7} | 87 | 3{7} | 62 |
Isolated yields after column chromatography. All compounds 2 have been characterized by 1H and 13C NMR spectroscopy.
For 3{1-6}, n-BuLi (3.0 equiv) and Me2S2 (2.4 equiv) were used. For 3{7}, n-BuLi (1.5 equiv) and Me2S2 (1.2 equiv) were used.
The key cyclization step efficiently generates the methyl sulfide-substituted 3-iodo[b]benzothiophenes 4 in 10 min at room temperature by electrophilic cyclization of the corresponding disulfide-containing alkynes 3{1-6} and sulfide alkyne 3{7} using I2 in CH2Cl2 at ambient temperature (Table 2). In all cases, good to excellent yields of methyl sulfide-substituted 3-iodo[b]benzothiophenes 4 have been obtained, except for 4{2}. The methyl sulfides 4 were immediately converted to the corresponding sulfones 5 by oxidation in good to excellent yields by KMnO4 supported on MnO2 under heterogeneous conditions at room temperature.56 Attempts to oxidize species 4 using standard organic solvents, e.g. methylene chloride or acetonitrile, individually under the same reaction conditions proved unsuccessful. Both methylene chloride and acetonitrile must be used as co-solvents for this reaction to afford decent yields. All of the reactions were monitored by thin layer chromatography and the products purified by column chromatography (see the Supporting Information for the experimental details).
Table 2.
3-Iodobenzo[b]thiophene Scaffords 4{1-7} and 5{1-6}
 | |||||
|---|---|---|---|---|---|
| entry | alkyne 3 | 3-iodobenzo[b]thiophenea 4 | yield (%)c | 3-iodobenzo[b]thiopheneb 5 | yield (%)c |
| 1 | 3{1} |  |
92 |  |
90 |
| 2 | 3{2} |  |
nrd | - | - |
| 3 | 3{3} |  |
92 |  |
91 |
| 4 | 3{4} |  |
91 |  |
88 |
| 5 | 3{5} |  |
58 |  |
82 |
| 6 | 3{6} |  |
93 |  |
86 |
| 7 | 3{7} |  |
91 |  |
87 |
All reactions were carried out using alkynes 3 and 1.5 equiv of I2 in CH2Cl2 at room temperature within 0.5 h, unless otherwise indicated.
Reactions of methyl sulfides 4 were carried out at room temperature in CH3CN/DCM (1:1) using KMnO4/MnO2.
Isolated yields after column chromatography. All compounds 4 and 5 have been characterized by 1H and 13C NMR spectroscopy.
I2 (1.5 equiv, followed by an additional 1.0 equiv after 12 h) was used for 24 h. The starting material 3{2} was recovered.
Subsequently, we proceeded to prepare a diverse library of methyl sulfone-substituted benzothiophenes 6 by a variety of palladium-catalyzed processes, such as Suzuki-Miyaura coupling, Sonogashira coupling, Heck coupling, carboalkoxylation, and aminocarbonylation. The Suzuki-Miyaura coupling of the 3-iodobenzo[b]thiophenes 4 or 5 with appropriate boronic acids 7 afforded the desired products 6{1-21} (Table 3). Sonogashira coupling of the 3-iodobenzo[b]thiophenes 4 or 5 with appropriate terminal alkynes 8 provides the corresponding alkynes 6{22-38} under microwave irradiation (Table 4). Moreover, methyl sulfone-substituted, olefin-containing benzothiophenes 6{39-45} have been prepared by Heck coupling of the 3-iodobenzo[b]thiophenes 4 or 5 with a small styrene sublibrary 9 (Table 5). Methyl sulfone-substituted, ester-containing benzothiophenes 6{46-56} have been prepared by carboalkoxylation of the 3-iodobenzo[b]thiophenes 4 or 5 using one atmosphere of carbon monoxide and primary alcohols 10 (Table 6). In addition, aminocarbonylation of the 3-iodobenzo[b]thiophenes 4 or 5 using one atmosphere of carbon monoxide in the presence of a palladium catalyst with appropriate primary and secondary amines 11 afforded the desired amide-containing products 6{57-72} (Table 7). The sublibraries, e.g. boronic acids 7, terminal alkynes 8, styrenes 9, alcohols 10, and amines 11, were chosen for their ability to provide the requisite diversity and drug-like properties to the scaffold (Figure 2).
Table 3.
Library Data for Compounds 6{1-21}a
 | |||||||
|---|---|---|---|---|---|---|---|
| product 6 | 7 | R3 | yield(%)c | purity(%)e | ion HRMS | calcd for HRMS | found HRMS |
| 6{1} | 7{4} | 2,3-dihydrobenzo[1,4]dioxin-6-yl | 69 | 98 | M+ | 420.0854 | NDf |
| 6{2} | 7{5} | 4-hydroxyphenylb | 65d | >99 | M+ | 378.0748 | 378.0753 |
| 6{3} | 7{8} | 2-bromo-5-pyridinyl | 58 | 58 | M+H+ | 441.9935 | 441.9931 |
| 6{4} | 7{9} | 4-carbamoylphenyl | 61 | 99 | M+H+ | 376.0830 | 376.0830 |
| 6{5} | 7{1} | 4-methoxyphenyl | 72d | >99 | M+NH4+ | 442.1147 | 442.1169 |
| 6{6} | 7{2} | 4-(methoxymethyl)phenyl | 72d | >99 | M+NH4+ | 456.1303 | 456.1303 |
| 6{7} | 7{4} | 2,3-dihydrobenzo[1,4]dioxin-6-yl | 77 | 98 | M+MeOH+ | 484.1014 | 485.1102 |
| 6{8} | 7{9} | 4-carbamoylphenyl | 57 | 95 | M+H+ | 438.0834 | 438.0842 |
| 6{9} | 7{11} | 4-(methylthio)phenyl | 71 | >99 | M+MeOH+ | 472.0837 | 473.0939 |
| 6{10} | 7{12} | 2-morpholinopyrimidin-5-yl | 65 | >99 | M+H+ | 482.1208 | 482.1201 |
| 6{11} | 7{13} | 4-sulfamoylphenyl | 47 | >99 | M+MeOH+ | 505.0687 | 506.0761 |
| 6{12} | 7{6} | 4-acetylphenyl | 57 | 99 | M+H+ | 437.0881 | 437.0874 |
| 6{13} | 7{11} | 4-(methylthio)phenyl | 73d | >99 | M+MeOH+ | 472.0837 | 473.0920 |
| 6{14} | 7{2} | 2-morpholinopyrimidin-5-yl | 57d | >99 | M+H+ | 482.1208 | 482.1192 |
| 6{15} | 7{13} | 4-sulfamoylphenyl | 38 | 99 | M+MeOH+ | 505.0687 | 506.0775 |
| 6{16} | 7{3} | benzo[1,3]dioxol-5-yl | 78d | >99 | M+MeOH+ | 500.0963 | 501.1049 |
| 6{17} | 7{13} | 4-sulfamoylphenyl | 18 | 96 | M+MeOH+ | 535.0793 | 536.0887 |
| 6{18} | 7{11} | 4-(methylthio)phenyl | 69 | 98 | M+MeOH+ | 502.0942 | 503.1037 |
| 6{19} | 7{13} | 4-sulfamoylphenyl | 34 | 95 | M+MeOH+ | 535.0793 | 536.0949 |
| 6{20} | 7{7} | 3-fluoro-4-methoxyphenyl | 67d | >99 | M+MeOH+ | 488.0764 | 489.0853 |
| 6{21} | 7{11} | 4-(methylthio)phenyl | 53 | 98 | 2M+Na+ | 931.0632 | NDf |
Suzuki-Miyaura reaction: 5 mol % Pd(PPh3)4, K2CO3 (2.5 equiv), R3B(OH)2 7 (1.5 equiv), toluene/EtOH/H2O (20/5/1), reflux. See the Supporting Information for the experimental details.
The THP ether-protected boronic acid was deprotected in situ using aq. HCl in THF at room temperature.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography. Isolated desired products 6 were characterized by 1H and 13C NMR spectroscopy.
UV purity determined at 214 nm after preparative HPLC.
Not determined by HRMS. Identified by NMR spectroscopy.
Table 4.
Library Data for Compounds 6{22-38}a
 | |||||||
|---|---|---|---|---|---|---|---|
| product 6 | 8 | R3 | yield (%)b | purity (%)d | ion HRMS | calcd for HRMS | found HRMS |
| 6{22} | 8{1} | hydroxymethyl | 57c | 98 | M+ | 378.0748 | NDe |
| 6{23} | 8{3} | (1-hydroxy-1-methyl)ethyl | 77 | >99 | 2M+NH4+ | 754.2153 | 754.2177 |
| 6{24} | 8{6} | 2-pyridinyl | 19 | >99 | M+H+ | 388.0830 | 388.0836 |
| 6{25} | 8{1} | hydroxymethyl | 37c | >99 | M+NH4+ | 390.0834 | 390.0843 |
| 6{26} | 8{3} | (1-hydroxy-1-methyl)ethyl | 58 | 99 | M+NH4+ | 418.1147 | 418.1146 |
| 6{27} | 8{4} | 1-hydroxy-1-cyclohexyl | 48c | 98 | 2M+NH4+ | 898.2576 | 898.2602 |
| 6{28} | 8{5} | 1-methyl-1H-imidazol-5-yl | 45 | >99 | M+H+ | 423.0837 | 423.0826 |
| 6{29} | 8{2} | 2-hydroxyethyl | 57 | >99 | M+H+ | 387.0725 | 387.0701 |
| 6{30} | 8{5} | 1-methyl-1H-imidazol-5-yl | 43 | >99 | M+NH4+ | 440.1103 | 440.1188 |
| 6{31} | 8{7} | 3-thiophenyl | 55 | 97 | M+NH4+ | 442.0605 | 442.0596 |
| 6{32} | 8{1} | hydroxymethyl | 66 | >99 | M+NH4+ | 420.0939 | 420.0949 |
| 6{33} | 8{4} | 1-hydroxy-1-cyclohexyl | 47c | >99 | M+NH4+ | 488.1565 | 488.1577 |
| 6{34} | 8{4} | 1-hydroxy-1-cyclohexyl | 38 | >99 | M+NH4+ | 472.1252 | 472.1266 |
| 6{35} | 8{3} | (1-hydroxy-1-methyl)ethyl | 56c | >99 | M+NH4+ | 432.0939 | 432.0947 |
| 6{36} | 8{2} | 2-hydroxyethyl | 65 | 99 | M+H+ | 357.0619 | 357.0609 |
| 6{37} | 8{4} | 1-hydroxy-1-cyclohexyl | 53 | >99 | M+NH4+ | 428.1354 | 428.1372 |
| 6{38} | 8{5} | 1-methyl-1H-imidazol-5-yl | 37c | >99 | M+H+ | 393.0731 | 393.0716 |
Sonogashira reaction: 3 mol % PdCl2(PPh3)2, 3 mol % CuI, Et2NH, R3C≡CH 8 (1.2 equiv), DMF, 80 °C, 20 min, using microwave irradiation. See the Supporting Information for the experimental details.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography. Isolated desired products 6 were characterized by 1H and 13C NMR spectroscopy.
UV purity determined at 214 nm after preparative HPLC.
Not determined by HRMS. Identified by NMR spectroscopy.
Table 5.
Library Data for Compounds 6{39-45}a
 | |||||||
|---|---|---|---|---|---|---|---|
| product 6 | 9 | R3 | yield (%)b | purity (%)d | ion HRMS | calcd for HRMS | found HRMS |
| 6{39} | 9{2} | 3,4-dimethoxyphenyl | 64c | 95 | M+ | 448.1167 | 448.1181 |
| 6{40} | 9{1} | 4-methoxyphenyl | 77c | 84 | M+H+ | 450.0960 | 451.1040 |
| 6{41} | 9{2} | 3,4-dimethoxyphenyl | 68 | >99 | M+H+ | 481.1143 | NDe |
| 6{42} | 9{1} | 4-methoxyphenyl | 57 | 93 | M+H+ | 451.1038 | 451.1074 |
| 6{43} | 9{2} | 3,4-dimethoxyphenyl | 62 | 97 | M+H+ | 495.0936 | 495.0936 |
| 6{44} | 9{3} | 4-pyridinyl | 61 | 97 | M+H+ | 436.0677 | 436.0691 |
| 6{45} | 9{3} | 4-pyridinyl | 66 | 96 | M+H+ | 392.0779 | 392.0794 |
Heck reaction: 5 mol % Pd(OAc)2, n-Bu4NI (1.0 equiv), Na2CO3 (2.5 equiv), R3CH=CH2 9 (1.2 equiv), DMF, 80 °C. See the Supporting Information for the experimental details.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography. Isolated desired products 6 were characterized by 1H and 13C NMR spectroscopy.
UV purity determined at 214 nm after preparative HPLC.
Not determined by HRMS. Identified by NMR spectroscopy.
Table 6.
Library Data for Compounds 6{46-56}a
 | |||||||
|---|---|---|---|---|---|---|---|
| product 6 | 10 | R3 | yield (%)b | purity (%)d | ion HRMS | calcd for HRMS | found HRMS |
| 6{46} | 10{1} | methyl | 71c | >99 | 2M+Na+ | 711.0979 | 711.0962 |
| 6{47} | 10{3} | 3,4-dimethoxybenzyl | 55 | 98 | M+NH4+ | 498.1409 | 498.1409 |
| 6{48} | 10{1} | methyl | 83 | >99 | 2M+NH4+ | 646.1214 | 646.1214 |
| 6{49} | 10{1} | methyl | 81c | >99 | M+H+ | 377.0517 | 377.0511 |
| 6{50} | 10{1} | methyl | 74c | >99 | M+H+ | 377.0517 | 377.0491 |
| 6{51} | 10{2} | propyl | 47 | >99 | M+NH4+ | 422.1096 | 422.1108 |
| 6{52} | 10{1} | methyl | 41c | 98 | M+H+ | 407.0623 | 407.0617 |
| 6{53} | 10{1} | methyl | 62c | >99 | M+NH4+ | 424.0889 | 424.0886 |
| 6{54} | 10{1} | methyl | 57 | >99 | M+H+ | 391.0310 | 391.0312 |
| 6{55} | 10{1} | methyl | 45 | 99 | M+H+ | 347.0412 | 347.0421 |
| 6{56} | 10{3} | 3,4-dimethoxybenzyl | 49 | >99 | M+H+ | 483.0936 | 483.0944 |
Carboalkoxylation: CO (1 atm), 3 mol % Pd(OAc)2, 5 mol % dppf, TEA (2.0 equiv), R3OH 10 (1.5-5.0 equiv), DMF, 70 °C. See the Supporting Information for the experimental details.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography. Isolated desired products 6 were characterized by 1H and 13C NMR spectroscopy.
UV purity determined at 214 nm after preparative HPLC.
Table 7.
Library Data for Compounds 6{57-72}a
 | |||||||
|---|---|---|---|---|---|---|---|
| product 6 | 11 | N(H)R3 | yield (%)b | purity (%)d | ion HRMS | calcd for HRMS | found HRMS |
| 6{57} | 11{1} | 2-pyridylethylamino | 56 | 62 | M+H+ | 473.1569 | 473.1548 |
| 6{58} | 11{8} | 4-hydroxybutylamino | 27 | 98 | M+H+ | 434.1096 | 434.1100 |
| 6{59} | 11{10} | 1-amino-4-methylpiperazino | 67c | 87 | M+H+ | 460.1365 | 460.1360 |
| 6{60} | 11{12} | 3-(2-oxo-1-pyrrolidinyl)propylamino | 48 | 99 | M+H+ | 487.1361 | 487.1365 |
| 6{61} | 11{5} | 3,4-(methylenedioxy)benzylamino | 65c | >99 | M+H+ | 496.0889 | 496.0889 |
| 6{62} | 11{7} | 1-methyltryptamino | 71 | >99 | M+H+ | 505.1256 | 505.1248 |
| 6{63} | 11{8} | 4-hydroxybutylamino | 72c | >99 | M+H+ | 434.1096 | 434.1098 |
| 6{64} | 11{3} | 1-methyl-2-pyrrolidineethanamino | 48 | 84 | M+H+ | 503.1674 | 503.1679 |
| 6{65} | 11{4} | 5-methyl-2-furanmethanamino | 61 | >99 | M+H+ | 486.1045 | 486.1057 |
| 6{66} | 11{8} | 4-hydroxybutylamino | 58c | >99 | M+H+ | 464.1202 | 464.1190 |
| 6{67} | 11{2} | 1H-imidazole-1-ethanamino | 17 | >99 | M+H+ | 500.1314 | 500.1323 |
| 6{68} | 11{8} | 4-hydroxybutylamino | 51 | >99 | M+H+ | 464.1202 | NDe |
| 6{69} | 11{6} | 4-morpholinepropanamino | 84c | >99 | M+H+ | 503.1311 | 503.1308 |
| 6{70} | 11{9} | 4-morpholinamino | 62c | >99 | M+H+ | 461.0841 | 461.0833 |
| 6{71} | 11{6} | 4-morpholinepropanamino | 68 | >99 | M+H+ | 459.1412 | 459.1398 |
| 6{72} | 11{11} | 1-(4-fluorophenyl)piperazino | 57 | 99 | M+H+ | 495.1212 | 495.1205 |
Aminocarbonylation: CO (1 atm), 10 mol % Pd(OAc)2, 20 mol % PPh3, TEA, R3NH2 or (R3)2NH 11 (1.5 equiv), DMF, 80 °C. See the Supporting Information for the experimental details.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography. Isolated desired products 6 were characterized by 1H and 13C NMR spectroscopy.
UV purity determined at 214 nm after preparative HPLC.
Not determined by HRMS. Identified by NMR spectroscopy.
Figure 2.
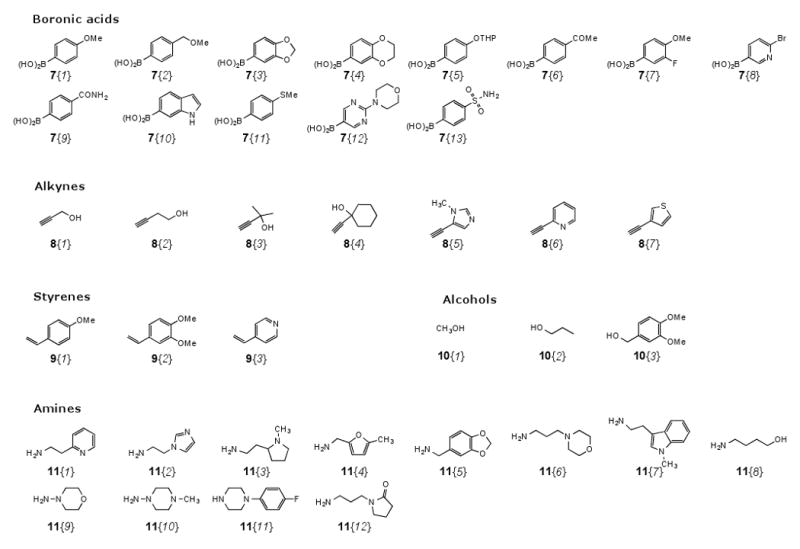
Sublibraries of boronic acids 7, terminal alkynes 8, styrenes 9, alcohols 10, and amines 11
Our goal in synthesizing these relatively low molecular weight heterocycles was to examine their biological properties by high-throughput screening. The overall synthetic process as a whole is quite functional group tolerant and efficient. These reactions have been performed in parallel on approximately a 35-50 mg scale, starting from the 3-iodobenzo[b]thiophenes 4 or 5. All of the crude compounds 6{1-72} were isolated by either column chromatography or preparative HPLC. The purity of the reaction mixtures has been analyzed by TLC, LC-MS, and HPLC.
The selected 72 sulfone-substituted benzo[b]thiophene template 6 has been evaluated computationally for its drug-like properties on the basis of Lipinski's rule of five.57 Lipinski calculations have been performed based on the commercial available of boronic acids 7, terminal alkynes 8, styrenes 9, alcohols 10, and amines 11. The molecular weight (less than 500), clogP (less than 5), number of hydrogen bond donors (less than 5 H) and acceptors (less than 10 H), and the number of rotatable bonds (less than 10) were calculated for each of the library members using the SYBYL58 program. Most of the desired sulfone-substituted benzo[b]thiophene library members were highly Lipinski compliant. A small subset of this virtual library, namely 72 compounds, follows Lipinski's rules with no violations.
Conclusions
We have successfully produced a 72-member library of sulfone-substituted benzo[b]thiophenes 6 via palladium-catalyzed couplings, such as Suzuki-Miyaura, Sonogashira, Heck, carboalkoxylation, and aminocarbonylation chemistry, starting from key intermediate sulfur-containing 3-iodobenzo[b]thiophenes 5. The 72 member sulfone-substituted benzo[b]thiophene library 6 has been added to the Kansas University NIH Center for Chemical Methodologies and Library Development (KU CMLD) collection and will be submitted to the National Institutes of Health Molecular Library Screening Center Network (MLSCN) for evaluation by a broad range of assays. We expect this basic methodlogy to find extensive application in the fields of combinatorial chemistry, diversity-oriented synthesis and drug discovery.
Supplementary Material
Acknowledgments
We would like to thank Dr. Gerald H. Lushington of Kansas University for all calculations for the compounds. We thank the National Institute of General Medical Sciences (GM070620 and GM079593) and the National Institutes of Health Kansas University Chemical Methodologies and Library Development Center of Excellence (GM069663) for support of this research; Johnson Matthey, Inc. and Kawaken Fine Chemicals Co., Ltd. for donations of palladium catalysts; and Frontier Scientific and Synthonix for donations of boronic acids.
Footnotes
Supporting Information Available: Synthetic methods, spectral assignments and copies of 1H and 13C NMR spectra for all previously unreported starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Zhang TY, O'Toole J, Proctor CS. Sulfur Rep. 1999;22:1–47. [Google Scholar]
- 2.Russell RK, Press JB. Compr Heterocycl Chem II. 1996;2:679–729. [Google Scholar]
- 3.Pelkey ET. Prog Heterocycl Chem. 2003;15:116–139. [Google Scholar]
- 4.Jones CD, Jevnikar MG, Pike AJ, Peters MK, Black LJ, Thompson AR, Falcone JF, Clemens JA. J Med Chem. 1984;27:1057–1066. doi: 10.1021/jm00374a021. [DOI] [PubMed] [Google Scholar]
- 5.Palkowitz AD, Glasebrook AL, Thrasher KJ, Hauser KL, Short LL, Phillips DL, Muehl BS, Sato M, Shetler PK, Cullinan GJ, Pell TR, Bryant HU. J Med Chem. 1997;40:1407–1416. doi: 10.1021/jm970167b. [DOI] [PubMed] [Google Scholar]
- 6.Schopfer U, Schoeffter P, Bischoff SF, Nozulak J, Feuerbach D, Floersheim P. J Med Chem. 2002;45:1399–1401. doi: 10.1021/jm015577l. [DOI] [PubMed] [Google Scholar]
- 7.Romagnoli R, Baraldi PG, Carrion MD, Lopez Cara C, Preti D, Fruttarolo F, Pavani MG, Tabrizi MA, Tolomeo M, Grimaudo S, Di Antonella C, Balzarini J, Hadfield JA, Brancale A, Hamel E. J Med Chem. 2007;50:2273–2277. doi: 10.1021/jm070050f. [DOI] [PubMed] [Google Scholar]
- 8.Overk CR, Peng KW, Asghodom RT, Kastrati I, Lantvit DD, Qin Z, Frasor J, Bolton JL, Thatcher GRJ. ChemMedChem. 2007;2:1520–1526. doi: 10.1002/cmdc.200700104. [DOI] [PubMed] [Google Scholar]
- 9.Pinney KG, Bounds AD, Dingeman KM, Mocharla VP, Pettit GR, Bai R, Hamel E. Bioorg Med Chem Lett. 1999;9:1081–1086. doi: 10.1016/s0960-894x(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang SX, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Mauger A, Narayanan VL, Lee KH. J Med Chem. 1999;42:4081–4087. doi: 10.1021/jm990208z. [DOI] [PubMed] [Google Scholar]
- 11.Norman BH, Dantzig AH, Kroin JS, Law KL, Tabas LB, Shepard RL, Palkowitz AD, Hauser KL, Winter MA, Sluka JP, Starling JJ. Bioorg Med Chem Lett. 1999;9:3381–3386. doi: 10.1016/s0960-894x(99)00610-1. [DOI] [PubMed] [Google Scholar]
- 12.Bleavins MR, de la Igelsia FA, McCay JA, White L, Kimber L, Jr, Munson AE. Toxicology. 1995;98:111–123. doi: 10.1016/0300-483x(94)02985-4. [DOI] [PubMed] [Google Scholar]
- 13.Vane JR. Nature. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 14.Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. N Engl J Med. 1992;327:749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- 15.O'Banion MK, Sadowski HB, Winn V, Young DA. J Biol Chem. 1991;266:23261–23267. [PubMed] [Google Scholar]
- 16.Perkins DJ, Kniss DA. Biochem J. 1997;321:677–681. doi: 10.1042/bj3210677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann C. Curr Med Chem. 2000;7:1113–1120. doi: 10.2174/0929867003374282. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang Y, Wei Z, Li H, Zhou H, Zhang Z, Zhang Z. J Neurol Sci. 2009;285:172–177. doi: 10.1016/j.jns.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Etminan M, Carleton BC, Samii A. J Clin Neurosci. 2008;15:576–577. doi: 10.1016/j.jocn.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 20.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Proc Natl Acad Sci. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries EF. J Curr Pharm Design. 2006;12:3847–3856. doi: 10.2174/138161206778559650. [DOI] [PubMed] [Google Scholar]
- 22.Katori M, M M Inflamm Res. 2000;49:367–392. doi: 10.1007/s000110050605. [DOI] [PubMed] [Google Scholar]
- 23.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KI, Farlow MR, Jin S, Thomas RG, Thal LJ. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 24.Liang X, Wu L, Wang Q, Hand T, Bilak M, McCullough L, Andreasson K. J Mol Neurosci. 2007;33:94–99. doi: 10.1007/s12031-007-0058-8. [DOI] [PubMed] [Google Scholar]
- 25.Dannhardt G, Kiefer W. Eur J Med Chem. 2001;36:109–126. doi: 10.1016/s0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 26.Sondhi SM, Singhal N, Johar M, Narayan Reddy BS, Lown W. Curr Med Chem. 2002;9:1045–1074. doi: 10.2174/0929867024606678. [DOI] [PubMed] [Google Scholar]
- 27.Zambre AP, Ganure AL, Shinde DB, Kulkarni V. J Chem Inf Model. 2007;47:635–643. doi: 10.1021/ci6004367. [DOI] [PubMed] [Google Scholar]
- 28.Gans KR, Galbraith W, Roman RJ, Haber SB, Kerr JS, Schmidt WK, Smith C, Hewes WE, Ackerman NR. J Pharmacol Exp Ther. 1990;254:180–187. [PubMed] [Google Scholar]
- 29.Habeeb G, Praveen PN, Knaus EE. J Med Chem. 2001;44:2921–2927. doi: 10.1021/jm0101287. [DOI] [PubMed] [Google Scholar]
- 30.Dannhardt G, Laufer S. Curr Med Chem. 2000;7:1101–1112. doi: 10.2174/0929867003374237. [DOI] [PubMed] [Google Scholar]
- 31.Prasit P, Wang Z, Brideau C, Chan CC, Charleson S, Cromlish W, Ethier D, Evans JF, Ford-Hutchinson AW, Gauthier JY. Bioorg Med Chem Lett. 1999;9:1773–1778. doi: 10.1016/s0960-894x(99)00288-7. [DOI] [PubMed] [Google Scholar]
- 32.Nicoll-Griffith DA, Yergey JA, Trimble LA, Silva JM, Li C, Chauret N, Gauthier JY, Grimm E, Leger S, Roy P, Therien M, Wang Z, Prasit P, Zamboni R, Young RN, Brideau C, Chan CC, Mancini J, Riendeau D. Bioorg Med Chem Lett. 2000;10:2683–2686. doi: 10.1016/s0960-894x(00)00538-2. [DOI] [PubMed] [Google Scholar]
- 33.Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret JP. J Pharmacol Exp Ther. 2001;296:558–566. [PubMed] [Google Scholar]
- 34.Reitz DB, Li JJ, Norton MB, Reinhart EJ, Collins JT, Anderson GD, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Isakson PC. J Med Chem. 1994;37:3878–3881. doi: 10.1021/jm00049a005. [DOI] [PubMed] [Google Scholar]
- 35.Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. J Med Chem. 2000;43:775–777. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 36.Penning T, Talley J, Bertenshaw S, Carter J, Collins P, Docter S, Graneto M, Lee L, Malecha J, Miyashiro J, Rogers R, Rogier D, Yu S, Anderson G, Burton E, Cogburn J, Gregory S, Koboldt C, Perkins W, Seibert K, Veenhuizen A, Zhang Y, Isakson P. J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 37.Sai Ram KVVM, Rambabu G, Sarma JARP, Desiraju GR. J Chem Inf Model. 2006;46:1784–1794. doi: 10.1021/ci050142i. [DOI] [PubMed] [Google Scholar]
- 38.Tavares IA, Bishai PM, Bennett A. Arzneim-Forsch. 1995;45:1093–1095. [PubMed] [Google Scholar]
- 39.Taniguchi Y, Yokoyama K, Inui K, Deguchi Y, Furukawa K, Noda K. Euro J Pharm. 1997;330:221–229. doi: 10.1016/s0014-2999(97)00183-0. [DOI] [PubMed] [Google Scholar]
- 40.Nogawa S, Zhang F, Ross ME, Iadecola C. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Futaki N, Yashikawa K, Hamasaka Y, Arai I, Higuchi S, Iizuka H, Otomo S. Gen Pharmacol. 1993;24:105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- 42.Gupta AK, Gupta RA, Soni LK, Kaskhedikar SG. Euro J Med Chem. 2008;43:1297–1303. doi: 10.1016/j.ejmech.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Hu W, Guo Z, Chu F, Bai A, Yi X, Cheng G, Li J. Bioorg Med Chem. 2003;11:1153–1160. doi: 10.1016/s0968-0896(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 44.Khoshneviszadeh M, Edraki N, Miri R, Hemmateenejad B. Chem Biol Drug Des. 2008;72:564–574. doi: 10.1111/j.1747-0285.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- 45.Huang HC, Chamberlain TS, Selbert K, Koboldt CM, Isakson PC, Reitz DB. Bioorg Med Chem Lett. 1995;5:2377–2380. [Google Scholar]
- 46.Hummel CW, Geiser AG, Bryant HU, Cohen IR, Dally RD, Fong KC, Frank SA, Hinklin R, Jones SA, Lewis G, McCann DJ, Rudmann DG, Shepherd TA, Tian H, Wallace OB, Wang M, Wang Y, Dodge JA. J Med Chem. 2005;48:6772–6775. doi: 10.1021/jm050723z. [DOI] [PubMed] [Google Scholar]
- 47.Qin Z, Kastrati I, Chandrasena REP, Liu H, Yao P, Petukhov PA, Bolton JL, Thatcher GRJ. J Med Chem. 2007;50:2682–2692. doi: 10.1021/jm070079j. [DOI] [PubMed] [Google Scholar]
- 48.Qin Z, Kastrati I, Ashgodom RT, Lantvit DD, Overk CR, Choi Y, van Breemen RB, Bolton JL, Thatcher GRJ. Drug Metab Dispos. 2009;37:161–169. doi: 10.1124/dmd.108.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldo JP, Mehta S, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2008;10:658–663. doi: 10.1021/cc800055x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho CH, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2008;10:941–947. doi: 10.1021/cc800120y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Worlikar SA, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2009;11:875–879. doi: 10.1021/cc900057n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho CH, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2009;11:900–906. doi: 10.1021/cc9000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy S, Roy S, Neuenswander B, Hill D, Larock RC. J Comb Chem. 2009;11:1061–1065. doi: 10.1021/cc9000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy S, Roy S, Neuenswander B, Hill D, Larock RC. J Comb Chem. 2009;11:1128–1135. doi: 10.1021/cc9001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue D, Larock RC. J Org Chem. 2002;67:1905–1909. doi: 10.1021/jo011016q. [DOI] [PubMed] [Google Scholar]
- 56.Shaabani A, Mirzaei P, Naderi S, Lee DG. Tetrahedron. 2004;60:11415–11420. [Google Scholar]
- 57.Lipinski CA, Lombardo F, Dominay BW, Feeney PJ. Adv Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 58.SYBYL, version 8.0. The Tripos Associate; St. Louis, MO: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


