Abstract
Ghrelin is a recently discovered hormone which has profound effects on food intake and lipogenesis in mammals. In all mammals studied thus far, plasma ghrelin concentrations are increased before a meal and decrease immediately following a meal; ghrelin levels increase with fasting. The golden-mantled ground squirrel Spermophilus lateralis (also known as Callospermophilus lateralis (see Helgen et al., 2009)) is a diurnal hibernator which has a robust annual cycle of body mass gain and loss that is primarily controlled by food intake. We hypothesized that in spring, summer, and autumn, the endogenous ghrelin concentrations of hibernators would be similar to those of non-hibernators, but that during the winter hibernation season, plasma ghrelin concentrations would be low or undetectable. We found that peripherally injected ghrelin significantly increased food intake in June. Plasma ghrelin concentrations were significantly increased through 5 days of fasting during a short-term fast in summer. Over a 24 hour period, ghrelin concentrations increased at night and decreased during the day with drops corresponding to times when squirrels were eating. In January, ghrelin concentrations are low but measurable even while animals are at low body temperature (Tb). The reason for the persistence of ghrelin in plasma at this time is unclear, but circulating ghrelin in hibernators may be involved with the control of sleep in these animals. This is the first report of ghrelin concentrations in a non-photoperiodic hibernator. We suggest that ghrelin may be important for the regulation of food intake and the body mass cycle in mammals that hibernate.
Keywords: Ghrelin, hibernator, fasting, sleep
1. Introduction
Mammals that hibernate (hibernators) provide unique models for studies on food intake and body condition because of their circannual cycles of obesity and anorexia (Mrosovsky, 1985). Ghrelin is a recently discovered orexigenic hormone which has profound effects on food intake and generally facilitates lipogenesis in mammals (Cummings, 2006; Sangiao-Alvarellos et al., 2009), but there are no published studies on the effects of ghrelin in non-photoperiodic hibernators. Ghrelin is a 28-amino acid protein produced primarily by X/A-like cells in the fundus of the stomach, and in smaller amounts by the intestine, pancreas, and hypothalamus (Dornonville et al., 2001; Hosoda et al., 2000; Kojima et al., 1999; Sakata et al., 2002). The release of ghrelin from the fundus of the stomach occurs in a pulsatory manner, and its circulating levels are dependent on feeding condition (Beck et al., 2003; Toshinai et al., 2001). Ghrelin is known to stimulate growth hormone (GH) secretion and helps regulate energy balance in rodents (Strassburg et al., 2008).
In all mammals studied thus far, fasting results in an elevated concentration of plasma ghrelin (Kinzig et al., 2005; Ortiz et al., 2003; Toshinai et al., 2001). The Siberian hamster (Phodopus sungorus) is a rodent that undergoes daily torpor in a seasonal cycle regulated by photoperiod, and as such is not considered a true hibernator. These animals exhibit a two-fold increase in ghrelin levels during a 48 hour fast, but show no changes in circulating ghrelin levels during a 6 week period of food restriction (Tups et al., 2004). In diurnal mammals, ghrelin levels are highest during nocturnal fasting (Cummings, 2006; Gordon et al., 2005). In rats and humans, plasma ghrelin levels are increased before a meal and decrease immediately following a meal (Beck et al., 2003; Toshinai et al., 2001).
The main physiological effect of ghrelin is an increase in appetite, supported by a food-intake independent increase in adiposity (Depoortere, 2009, Sangiao-Alvarellos et al., 2009). Ghrelin is known to increase food intake when injected interperitoneally (IP) or intracerebroventricularly (ICV) in rodents (Andersson et al., 2004; Gluck et al., 2006; Keen-Rhinehart et al., 2005; Kojima et al., 1999; Mustonen et al., 2002; Tschop et al., 2000). This increase in food intake is seen in tandem with increased fat mass in most animals studied. Peripherally injected ghrelin increases the respiratory quotient in rodents, indicating an increased dependence on carbohydrate utilization and a decreased fat utilization (Nieminen et al., 2004; Tschop et al., 2000). Ghrelin has other physiological effects, including the promotion of slow wave sleep, which may be important for entry into torpor (Heller & Ruby, 2004; Wiekel et al., 2002).
Hibernators undergo multi-day torpor bouts in winter during which food intake ceases and body temperature (Tb) drops to near ambient temperature (Ta). Golden mantled ground squirrels (GMGS) are diurnal mammals that hibernate and have a robust annual cycle of body mass gain and loss controlled primarily by changes in food intake. During the hyperphagic prehibernation period (late August through mid October in a laboratory setting), GMGS double their food intake and increase body mass nearly 50%, mostly in the form of fat (Dark, 2005). During this autumnal period, animals also decrease metabolic rate and activity, which promotes fat deposition (Kenagy et al., 1989). GMGS tend to forage in the morning and evening, but are opportunistic feeders (Kenagy et al., 1989). The orexigenic effects of ghrelin may be important in the control of body mass and food intake during this prehibernation period. During the winter hibernation period (Oct. – Mar.), GMGS cease food intake, and many metabolic processes fall to very low rates. During each torpor bout throughout the winter, animals drop Tb to near Ta for several days at a time, and then rewarm to normal Tb of 36°C for a few hours (become euthermic) before returning to low Tb (usually around 6°C when Ta is held at 5°C in a laboratory setting). During these euthermic inter-bout arousals (IBAs), animals do not eat and spend most of the time undergoing sleep (Heller & Ruby, 2004). GMGS emerge from hibernation between March and May, depending on environmental conditions and sex (males emerge earlier than females), and resume normal food intake.
The control of the food intake pathway in hibernators is not fully understood, but hormones such as leptin, insulin, and adiponectin (Florant et al., 2004) are known to play an important role in the seasonal changes in food intake seen in these animals. We hypothesized that ghrelin, which is a highly evolutionarily conserved and orexigenically potent hormone, is likely to be involved in control of food intake in hibernators. In order to test this hypothesis, we carried out several experiments designed to examine changes in plasma ghrelin concentrations in hibernators in different seasons and under different physiological conditions (including a short term fast in summer, a long term fast in winter, and animals undergoing torpor). As an addition to this experiment, we peripherally injected ghrelin into GMGS in June, to test the hypothesis that this would increase food intake, as it does in most other animals. We hypothesized that a short term fast (1–5 days) in summer would increase plasma ghrelin levels in GMGS. In early autumn (when animals are hyperphagic) it is likely that plasma ghrelin concentrations will be elevated compared to summer levels because increased ghrelin leads to increased food intake. Alternatively, increased food intake during this hyperphagic stage could lead to decreased ghrelin concentrations (due to the effect of a meal decreasing plasma ghrelin). By winter, when animals have ceased feeding and are undergoing torpor bouts, plasma ghrelin concentrations should be low or undetectable. As ghrelin stimulates food intake, an absence of appetite would seem to indicate an absence of ghrelin in the plasma. However, due to the varied physiological effects of ghrelin, it is possible that ghrelin is still circulating in the plasma during hibernation.
2. Methods and Materials
2.1. Animals and treatment
Forty adult GMGS of both sexes were trapped in Larimer County in the springs and early summers of 2007–2009 and kept in an animal facility at Colorado State University under an approved IACUC protocol. Animals were provided with ad libitum food and water and maintained in a warm room (20 ± 2°C) under natural photoperiod until the beginning of November, when the room temperature was reduced to 5°C and animals were kept in constant darkness. The lights in the room in which the animals are kept are controlled by a timer which is set to change at the same rate as the external photoperiod. The constant darkness is designed to simulate the entrance of the animal into an underground hibernaculum, where no light is seen from burrow sequestration until emergence from burrow. Animals were separated into the following four groups for specific ghrelin experiments: (1) Peripheral injection of ghrelin in June (N=8), (2) Short term fast in July (N=18), (3) 24hr ghrelin secretory profile conducted in October (N=5), and (4) Ghrelin levels during the hibernation season (in January) (N=9).
2.2. Peripheral ghrelin injection
To examine the effects of peripheral ghrelin injection on normophagic GMGS, eight animals were randomly assigned to two groups, one group to be injected intraperitoneally (IP) with ghrelin (N=4), and the other with saline (control, N=4). In June, rat ghrelin (Bachem Corporation) was dissolved in 1 ml sterile diH2O and administered to animals at doses of 10 μg/kg and 50 ug/kg, which is within the range used to elicit an orexigenic response in most rodents (Chen et al., 2004; Keen-Rhinehart et al., 2005). Cumulative food intake (in grams) was measured for each animal for the 6 hours after injections (each animal was injected at 1000 and food intake was measured at 1600). All animals had food and water available ad lib.
2.3. Summer short term fasting
In order to determine if a short term fast would alter plasma ghrelin concentrations, eighteen animals were fasted 0, 1, 3, or 5 days in July (control (fed) N=4, 1-day fast N=5, 3-day fast N=5, 5-day fast N=4) and then euthanized at 1200. Body masses were measured prior to starting the fast, and again on the day of sacrifice to determine how much mass was lost. Change in body mass for control animals was calculated by measuring body mass of animals 5 days before sacrifice and comparing this measurement to that taken at time of sacrifice. Animals were anesthetized with an intramuscular injection of ketamine-acepromazine-xylazine (75%-20%-5%). Blood samples were collected by cardiac puncture, were centrifuged, and plasma was removed and stored at −80°C until analysis.
2.4. 24hr ghrelin circulation profile
In mid-October (at the end of the GMGS hyperphagic period), five animals were catheterized in the jugular vein under sterile conditions. An intramuscular injection of ketamine-acepromazine-xylazine was used to initially anesthetize the animals, and then deep anesthesia was maintained using a 2–3% isoflurane gas. The internal jugular vein was isolated through a 1–1.5 cm incision in the ventral neck. A sterile 0.6mm diameter polyethylene tapered catheter was inserted into the vein and extended into the caudal aspect of the vessel (toward the heart) approximately 1.5 cm. The catheter was secured into the vein and surrounding musculature with 4-0 absorbable monofilament suture. The internal jugular vein cranial to the catheter-insertion site was ligated using the same suture material. A 2 mm skin incision was made on the midline of the back between the two scapulae and a subcutaneous tunnel approximately 2 mm in diameter was made between this incision and the ventral neck incision using a surgical trocar. The free end of the catheter was then passed through this tunnel from its position in the neck area to the dorsal incision site. The skin incision on the back was closed and the free end of the catheter (injection port) was secured into place using 3-0 sterile monofilament nylon suture. The neck incision was closed with the same nylon suture. Animals recovered from surgery on heating pads and were administered 0.05 ml buprenorphine twice a day for three days for post-surgical analgesia.
The animals were allowed to recuperate from surgery for three days, and then 0.3 ml of blood was drawn through each animal’s catheter every two hours for 24 hours under a 14 L: 10 D photoperiod with food and water available ad libitum. Blood collection was performed as non-invasively as possible during dark hours. A red safe light (3–5 lux) was used when entering the room, and blood was withdrawn from catheters while squirrels were allowed to remain in their nests. Blood samples were centrifuged and plasma was removed and stored at −80°C. Red blood cells were re-suspended in 0.3 ml sterile heparinized saline and reinjected into each animal through its catheter. Animals were remotely monitored via video camera and the number of times animals fed per hour was recorded. Three weeks later, the animals were again anesthetized and the indwelling catheters were removed.
2.5. Ghrelin levels during hibernation
For the fourth experiment, five animals undergoing torpor in January (at LTT (6–9°C)) were sacrificed by decapitation at 1200 and blood samples were collected. Low Tb was confirmed by thermocouple readings of skin, mouth, blood, and body cavity temperatures. Blood samples were collected by cardiac puncture, were centrifuged, and plasma was removed and stored at −80°C until analyzed. Stomachs were dissected out and examined for traces of food.
To determine euthermic ghrelin levels in January, four animals were aroused from torpor. After animals became completely euthermic (Tb>30°C, moving freely around cage) they were anesthetized for blood sampling. Blood samples were collected by cardiac puncture, were centrifuged, and plasma was removed and stored at −80°C until analysis. Animals were then allowed to return to torpor.
2.6. Validation and use of assay
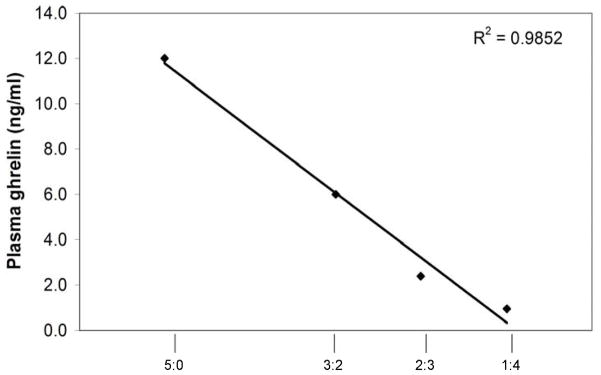
All plasma ghrelin concentrations were determined using an EIA assay (measuring total ghrelin) from Phoenix Pharmaceuticals (EK-031-31). The assay was validated using a GMGS serial plasma dilution curve. Samples were assayed in duplicate and diluted as follows: 50μl sample: 0μl assay buffer (5:0), 30μl sample: 20μl assay buffer (3:2), 20μl sample: 30μl assay buffer (2:3), and 10μl sample: 40μl assay buffer (1:4) (results shown in figure 1). The sensitivity of this assay is 0.08 ng/ml; intra-assay variation is <5% and inter-assay variation is <14%. All statistical analysis was performed using SAS 9.1. A Student t-test was used to evaluate mean differences, which were considered significant if p<0.05. A single-factor ANOVA was used to evaluate differences between hours in the 24 hour circulating ghrelin experiment. When ANOVA indicated significant variations, the Student-Newman-Keuls test was used to compare hourly means. All differences were considered statistically significant at p<0.05
Figure 1.
Validation of total ghrelin EIA assay: dilution curve for GMGS (sample diluted at 5:0, 3:2, 2:3, and 1:4 with assay buffer)
3. Results
3.1. Effect of peripheral ghrelin injection on summer food intake
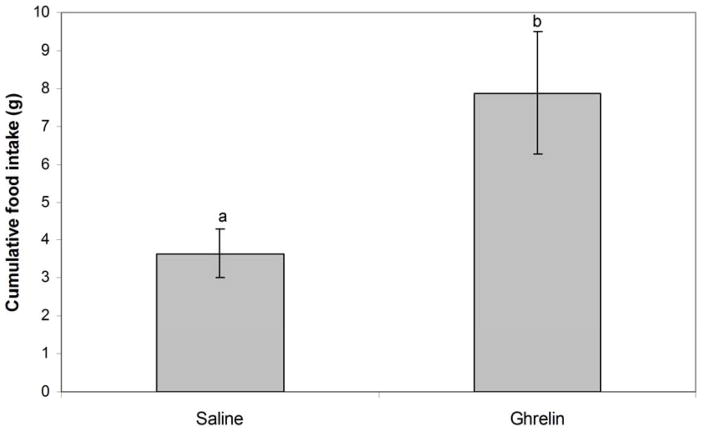
Our dose titration experiment showed that an IP injection of rat ghrelin (at either 10 or 50 μg/kg) stimulated food intake in ad libitum fed GMGS. There was no difference in food intake response between the 10 and the 50 μg/kg groups, so they were analyzed together for statistical purposes. Animals that received IP ghrelin injections significantly increased food intake compared with animals injected IP with saline (p=0.026). Animals injected with ghrelin ate an average of 7.9 grams over a period of six hours after injections whereas saline-injected controls ate an average of 3.6 grams over the same time period (Figure 2).
Figure 2.
Mean cumulative food intake (in grams) for the six hours after peripheral saline and ghrelin injections in June (control N=4, ghrelin N=4)
3.2. Ghrelin levels after a short term fast in summer
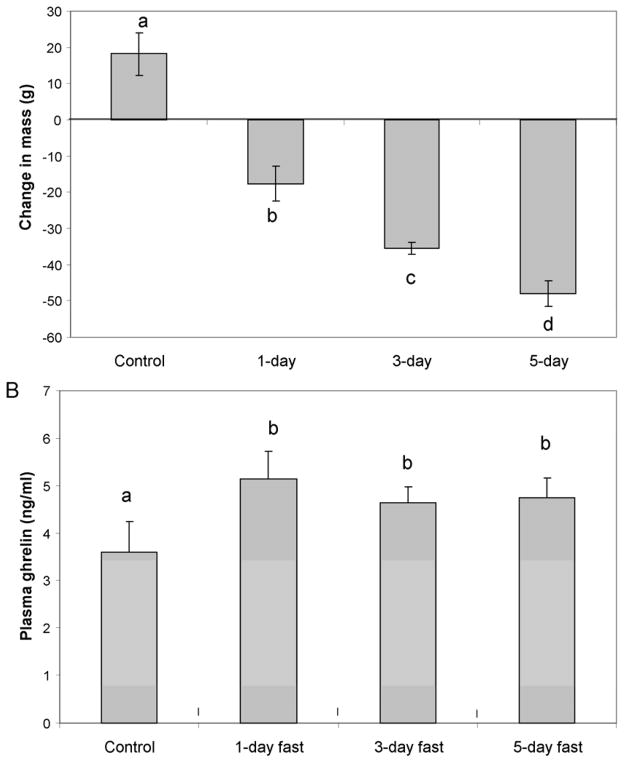
Animals that had been fasted for one day had significantly elevated (p=0.043) plasma ghrelin concentrations compared with controls (5.44 ng/ml vs. 3.60 ng/ml respectively) (Figure 3B). Animals fasted for three and five days also had elevated ghrelin concentrations that were significantly higher than controls (p=0.049), but not significantly different from animals fasted for one day. Figure 3A illustrates the average change in body mass caused by each fasting condition. Control animals gained mass while animals under each of the fasting conditions lost progressively and significantly more mass.
Figure 3.
Figure 3A. Mean change in body mass with fasting ± SEM during short term fast in July (control N=4, 1-day N=5, 3-day N=5, 5-day N=4). Different letters are statistically different (p<0.05); 3B: Mean ghrelin concentrations ± SEM in short-term fast in July. Letters a & b are statistically different (p<0.05)
3.3. 24hr pattern of circulating ghrelin in October
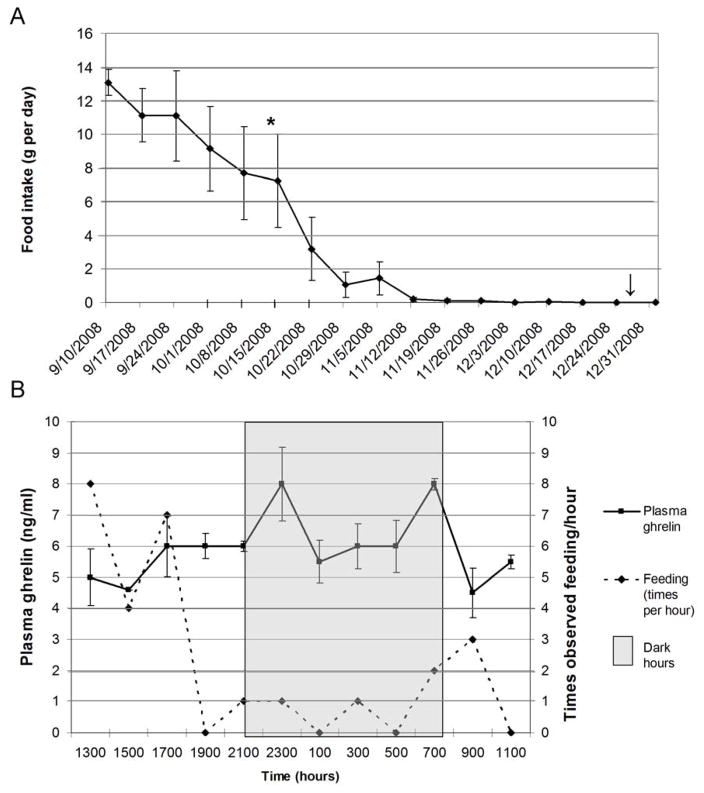
The 24hr plasma ghrelin concentrations are illustrated in Figure 4B and plotted against observed feeding times for catheterized squirrels. Plasma ghrelin increased within the first 2 hours of the dark period (2100–2300), decreased through the night (2100-0700), and then rose again prior to lights on (0700); these two peaks were statistically higher than preceding points at p<0.05. When lights came on (0700), animals were observed eating and ghrelin concentrations were decreased, remaining at a low level from 0900 until 1500 after which ghrelin rose slightly just before the dark period began. The mean plasma ghrelin concentration during the dark period was 6.6 ng/ml which was significantly greater than the mean ghrelin concentration during the light period (5.3 ng/ml) (p=0.019). Figure 4A illustrates the average food intake for the catheterized GMGS during the month of October before, during, and after the catheterization experiment. Animals were eating between 8–12 grams/day at the end of their hyperphagic period (late August to mid October in the lab); by November, food intake was not significantly different from zero.
Figure 4.
Figure 4A. Each point represents mean food intake ± SEM per day from September to January 2008–2009 (N=5), including times of 24-hour ghrelin secretion experiment and January ghrelin experiment; *=animals catheterized for 24 hour ghrelin experiment; ↓=animals sacrificed for January ghrelin experiment; 4B: 24-hour plasma ghrelin secretory pattern and times observed feeding/hour in GMGS—shaded area denotes dark hours (lights out); each point on ghrelin line represents the mean ± SEM for each time period; each point on feeding line represents the total number of times animals were observed feeding for each time period (N=5)
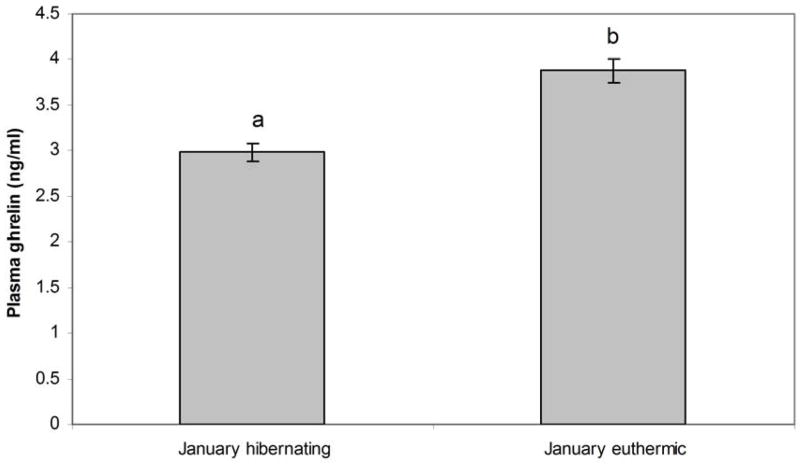
3.4. Ghrelin levels during hibernation
In January, plasma ghrelin levels were significantly higher (p=0.01) in euthermic animals (animals undergoing an interbout arousal (IBA)) than in animals hibernating at low tissue temperature (LTT) in January (Figure 5). Food was present in the animals’ cages throughout the winter, but weekly measurement of food intake indicated that animals were not eating during this time (Figure 4A). The stomachs of animals euthanized at LTT were examined and found empty of food.
Figure 5.
Mean ghrelin concentrations ± SEM in animals at low tissue temperature in January (LTT) (N=5) and in animals euthermic in January (N=4). Letters a & b are statistically different (p<0.05).
4. Discussion
This is the first report of plasma ghrelin concentrations in an obligate hibernator. Circulating ghrelin has been shown to promote an orexigenic response in all mammals studied thus far (Depoortere, 2009). We found that GMGS responded to peripheral ghrelin injections with a significant increase in food intake, in concurrence with studies on non-hibernating rodents (Gluck et al., 2006; Keen-Rhinehart et al., 2005; Nieminen et al., 2004; Tschop et al., 2000). Ghrelin receptors are located in the pituitary and in multiple nuclei of the hypothalamus, and when bound by ghrelin stimulate particular food intake pathways. The activation of the ghrelin receptor leads to the phosphorylation and concomitant activation of adenosine-monophosphate protein kinase (AMPK) (Anderson et al., 2004), which causes an increase in food intake and fatty acid synthesis via the orexigenic peptides neuropeptide Y (NPY) and agouti-related protein (AGRP) (Chen et al., 2004), and down-regulation of the anorexigenic pro-opiomelanocortin (POMC) (Anderson et al., 2004). It has been proposed that peripheral ghrelin and hypothalamic ghrelin take part in two parallel outputs (stimulation of food intake and control of sleep-wake cycle) (Szentirmai et al., 2007a,b), and that a baseline ghrelin concentration must be met for stimulation of hunger (Schuessler et al., 2006).
During short fasts in summer, ghrelin levels increased significantly (p<0.05) between control animals and one, three, or five day fasted animals, consistent with previous studies in rodents (Tups et al., 2004; Toshinai et al., 2001; Ortiz et al., 2003; Mustonen et al., 2002). Ghrelin levels did not change significantly between days of fasting; this result lends support to the hypothesis that ghrelin is a short-acting hormone (Cummings, 2006; Tups et al., 2004). Others, however, have hypothesized that ghrelin can act over the long term (Epelbaum et al., 2009; Strassburg et al., 2008), and some of our results support this hypothesis. Ghrelin levels in mid October (when animals are nearing the end of their hyperphagic period) are significantly higher than those measured at the same time of day in July (5.6 ng/ml vs. 3.6 ng/ml respectively, p<0.05), and both summer and autumn levels are higher than those seen in hibernating GMGS (when animals are not eating). These results indicate that ghrelin secretion may be altered by season, and therefore could play a role in the seasonal changes in food intake seen in hibernators. Scrimgeour et al. (2008) provide evidence that ghrelin may reflect long term energy balance, acting as a feedback signal to the hypothalamus to defend an optimum body mass by controlling food intake. If a hibernator’s energy stores are depleted to a sufficiently low level during torpor, it will arouse and attempt to eat to make up the difference (Mrosovsky et al., 1970). These arousals to euthermia are energetically expensive (incurring up to 86% of the energetic cost of hibernation (Wang, 1978)), and it behooves an animal to arouse as little as possible during the hibernation season (Humphries et al., 2002). By maintaining plasma ghrelin at a low level, the urge to eat is suppressed, removing one possible stimulus to arousal. The ability to maintain low plasma ghrelin levels during the hibernation season may be important in regulating the delicate energy balance maintained by a hibernating mammal.
Ghrelin is known to facilitate torpor bouts in food deprived mice, and this action appears to be mediated by the arcuate nucleus (Gluck et al., 2006). Peripheral ghrelin administration in fasted mice deepened already occurring torpor bouts by several degrees. This may occur through the stimulation of the release of NPY by ghrelin, since ICV injected NPY is known to cause a torpor-like hypothermia in Siberian hamsters (Dark & Pelz, 2008). As torpor in mice is initiated through fasting, ghrelin levels in torpid mice are expected to be high. Conversely, GMGS cease food intake (fast voluntarily) some time before entering hibernation. Since the drive to eat is reduced, we expected that ghrelin levels during hibernation would be very low or undetectable.
Interestingly, although GMGS do not eat during the hibernation season, ghrelin was still present in the plasma of our experimental animals hibernating at low tissue temperature. Since ghrelin is usually strongly orexigenic, its presence in a time of aphagia suggests that it may be fulfilling other physiological processes, perhaps stimulating non-rapid eye movement (NREM) sleep. The long-accepted paradigm is that torpor is entered through sleep—as brain temperature falls, NREM sleep predominates and REM sleep decreases (Walker et al., 1977). Unfortunately, it is difficult to ascertain the differences in hibernating sleep states because electrical processes slow as temperature drops (Heller & Ruby, 2004; Deboer, 1998). Since neurons in certain parts of the brain show specific EEG patterns for each sleep state, there has been some success at differentiating periods homologous to NREM and wakefulness at brain temperatures down to 14°C (Krilowicz et al., 1988). The effects of ghrelin on sleep are controversial and seem to vary by species (for a review see Steiger, 2006). Ghrelin is known to increase during the first hours of sleep and promotes slow wave sleep (SWS) in humans and mice (Obal et al., 2003; Weikel et al., 2003). The low concentrations of plasma ghrelin measured in torpid and euthermic GMGS may be functioning to facilitate NREM sleep during torpor bouts and interbout arousals (IBAs). This could be tested by establishing a ghrelin secretion profile for a torpor bout and associated IBA and recording sleep patterns at the same time, followed by injecting ghrelin to measure the effect on sleep patterns.
In previous studies on diurnal mammals, plasma ghrelin levels stay fairly high throughout the dark period compared with daytime levels, but rise sharply just before waking (Cummings et al., 2001). The 24hr ghrelin secretion profile for our GMGS was determined at the end of the hyperphagic period (mid October), and shows that mean plasma ghrelin levels were significantly higher during the dark period (when GMGS had gone the longest without eating) than during the light period (p<0.05). This is mainly due to two peaks—one occurring shortly after the lights were turned off, and the other occurring immediately prior to lights being turned on. It is possible that the first peak observed in plasma ghrelin was involved in stimulating slow wave sleep, and the second in stimulating feeding. Several recent articles have proposed that plasma ghrelin must reach a threshold level to stimulate food intake, and that smaller increases in plasma ghrelin may be involved in control of sleep (Steiger, 2007; Szentirmai et al., 2007b).
In our experiment, plasma ghrelin concentrations dropped after squirrels ate at the start of light period. In previous studies on rats, which are nocturnal, ghrelin levels were highest during light hours and dropped during dark hours when animals were awake (Bodosi et al., 2004; Murakami et al., 2002; Tolle et al., 2002). The secretion profiles illustrated in these studies show a common pattern; our secretion profile for GMGS shows a similar pattern when adjusted for their diurnal lifestyle (plasma ghrelin levels were highest when animals had gone the longest without eating—early morning for GMGS, late evening for nocturnal animals).
Ghrelin may be important for regulation of the prehibernation food intake cycle in hibernators, and is possibly linked with the cyclic obesity shown in these animals. Ghrelin is thought to be primarily a short-term acting hormone, but recent studies (Strassburg et al., 2008), including ours, suggest that it may have long-term effects on food intake and body mass. Further research is necessary to elucidate the various physiological effects of ghrelin, but it may be an important hormone in regulating the food intake and circannual body mass cycle of hibernators.
Acknowledgments
The authors would like to thank Heather Craven, Ashley Fenn, and Nicole Prentice for help with assays, and Cassandra Gearhart, Melanie Richter, Thomas Barnett, and Julie Dudak for animal care. We thank Denise Pearson of Fox Acres Country Club for allowing us to trap ground squirrels on their property. This work was supported by a Sigma Xi Grant-in-aid of research to JEH and an NIH grant (DK 067017-3) to GLF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Bio Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S, Stricker-Krongrad A. Ghrelin and body weight regulation in the obese Zucker rat in relation to feeding state and dark/light cycle. Exp Biol Med. 2003;228:1124–1131. doi: 10.1177/153537020322801005. [DOI] [PubMed] [Google Scholar]
- Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:550–553. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–497. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R236–245. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J Sleep Res. 1998;7:254–262. doi: 10.1046/j.1365-2869.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Depoortere I. Targeting the ghrelin receptor to regulate food intake. Regulatory Peptides. 2009 doi: 10.1016/j.regpep.2009.04.002. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Håkanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regulatory Peptides. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- Epelbaum J, Bedjaoui N, Dardennes R, Feng DD, Gardette R, Grouselle D, Loudes C, Simon A, Tolle V, Yang SK, Zizzari P. Role of the ghrelin/obestatin balance in the regulation of neuroendocrine circuits controlling body composition and energy homeostasis. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.08.026. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Florant GL, Porst H, Peiffer A, Hudachek SF, Pittman C, Summers SA, Rajala MW, Scherer PE. Fat-cell mass, serum leptin and adiponectin changes during weight gain and loss in yellow-bellied marmots (Marmota flaviventris) J Comp Physiol B. 2004;174:633–9. doi: 10.1007/s00360-004-0454-0. [DOI] [PubMed] [Google Scholar]
- Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol. 2006;291:R1303–9. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- Gordon ME, McKeever KH. Diurnal variation of ghrelin, leptin, and adiponectin in Standardbred mares. J Anim Sci. 2005;83:2365–2371. doi: 10.2527/2005.83102365x. [DOI] [PubMed] [Google Scholar]
- Helgen KM, Cole FR, Helgen LE, Wilson DE. Generic revision in the Holarctic ground squirrel genus Spermophilus. J Mammalogy. 2009;90:270–305. [Google Scholar]
- Heller HC, Ruby NF. Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol. 2004;66:275–289. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Thomas DW, Speakman JR. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature. 2002;418:313–316. doi: 10.1038/nature00828. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness T. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Kenagy GJ, Sharbaugh SM, Nagy KA. Annual cycle of energy and time expenditure in a golden-mantled ground squirrel population. Oecologia. 1989;78:269–282. doi: 10.1007/BF00377166. [DOI] [PubMed] [Google Scholar]
- Kinzig K, Scott K, Hyun J, Bi S, Moran T. Altered hypothalamic signaling and responses to food deprivation in rats fed a low-carbohydrate diet. Obesity Research. 2005;13:1672–1682. doi: 10.1038/oby.2005.205. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Krilowicz BL, Glotzbach SF, Heller HC. Neuronal activity during sleep and complete bouts of hibernation. Am J Physiol Regul Integr Comp Physiol. 1988;255:R1008–R1019. doi: 10.1152/ajpregu.1988.255.6.R1008. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Fisher KC. Sliding set points for body weight in ground squirrels during the hibernation season. Can J Zool. 1970;48:241–247. doi: 10.1139/z70-040. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Cyclical obesity in hibernators: the search for the adjustable regulator. In: Hirsch J, Van Itallie TB, editors. Recent Advances in Obesity Research: IV. Libbey; London: 1985. pp. 45–56. [Google Scholar]
- Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- Mustonen A, Nieminen P, Hyvarinen H. Melatonin and the Wintering Strategy of the Tundra Vole, Microtus oeconomus. Zoological Science. 2002;19:683–687. doi: 10.2108/zsj.19.683. [DOI] [PubMed] [Google Scholar]
- Nieminen P, Mustonen A. Effects of peripheral ghrelin on the carbohydrate and lipid metabolism of the tundra vole (Microtus oeconomus) Gen Comp Endocrinol. 2004;138:182–187. doi: 10.1016/j.ygcen.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Alt J, Taishi P, Gardi J, Krueger JM. Sleep in mice with nonfunctional growth hormone-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol. 2003;284:R131–139. doi: 10.1152/ajpregu.00361.2002. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Noren D, Talamantes F. GH and ghrelin increase with fasting in a naturally adapted species, the northern elephant seal (Mirounga angustirostris) J Endocrinol. 2003;178:533–539. doi: 10.1677/joe.0.1780533. [DOI] [PubMed] [Google Scholar]
- Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T. Ghrelin-producing cells exist as two types of cells, closed and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531–536. doi: 10.1016/s0196-9781(01)00633-7. [DOI] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S, Vázquez MJ, Varela L, Nogueiras R, Saha AK, Cordido F, López M, Diéguez C. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinol. 2009 doi: 10.1210/en.2009–0482. First published ahead of print July 16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler P, Uhr M, Ising M, Schmid D, Weikel J, Steiger A. Nocturnal ghrelin levels—relationship to sleep EEG, the levels of growth hormone, ACTH and cortisol— and sex differences. J Sleep Res. 2005;14:329–336. doi: 10.1111/j.1365-2869.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- Scrimgeour K, Gresham MJ, Giles LR, Thomson PC, Wynn PC, Newman RE. Ghrelin secretion is more closely aligned to energy balance than with feeding behaviour in the grower pig. J Endocrinol. 2008;198:135–145. doi: 10.1677/JOE-07-0627. [DOI] [PubMed] [Google Scholar]
- Steiger A. Ghrelin and sleep-wake regulation. Am J Physiol Integr Comp Physiol. 2006;292:R573–R574. doi: 10.1152/ajpregu.00618.2006. [DOI] [PubMed] [Google Scholar]
- Strassburg S, Anker S, Castaneda T, Burget L, Perez-Tilve D, Pfluger PT, Nogueiras R, Halem H, Dong JZ, Culler M, Datta R, Tschop MH. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. Am J Physiol Endocrinol Metab. 2008;295:E78–E84. doi: 10.1152/ajpendo.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Spontaneous sleep and homeostatic sleep regulation in ghrelin knockout mice. Am J Physiol Integr Comp Physiol. 2007a;293:R510–R517. doi: 10.1152/ajpregu.00155.2007. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Research. 2007b;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, Bluet- Pajot MT. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–1361. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Comm. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tups A, Helwig M, Khorooshi RM, Archer ZA, Klingenspor M, Mercer JG. Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus) J Neuroendocrinol. 2004;16:922–928. doi: 10.1111/j.1365-2826.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol Regul Integr Comp Physiol. 1977;233:R213–R221. doi: 10.1152/ajpregu.1977.233.5.R213. [DOI] [PubMed] [Google Scholar]
- Wang LCH. Energetics and field aspects of mammalian torpor: the Richardson’s ground squirrel. In: Wang LCH, Hudson JW, editors. Strategies in the Cold. Academic Press; New York: 1978. pp. 109–145. [Google Scholar]
- Weikel JC, Wichniak A, Ising M, Brunner H, Friess E, Held K, Mathias S, Schmid DA, Uhr M, Steiger A. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab. 2003;284:E407–E415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]