Abstract
During cancer progression, tumor cells eventually invade the surrounding collagen-rich extracellular matrix. Here we show that squamous cell carcinoma cells strongly adhere to Type I collagen substrates but display limited motility and invasion on collagen barriers. Further analysis revealed that in addition to the α2β1 integrin, a second collagen receptor was identified as Syndecan-1 (Sdc1), a cell surface heparan sulfate proteoglycan. We demonstrate that siRNA-mediated depletion of Sdc1 reduced adhesion efficiency to collagen I, whereas knockdown of Sdc4 was without effect. Importantly, silencing Sdc1 expression caused reduced focal adhesion plaque formation and enhanced cell spreading and motility on collagen I substrates, but did not alter cell motility on other ECM substrates. Sdc1 depletion ablated adhesion-induced RhoA activation. In contrast, Rac1 was strongly activated following Sdc1 knockdown, suggesting that Sdc1 may mediate the link between integrin-induced actin remodeling and motility. Taken together, these data substantiate the existence of a co-adhesion receptor system in tumor cells, whereby Sdc1 functions as a key regulator of cell motility and cell invasion by modulating RhoA and Rac activity. Downregulation of Sdc1 expression during carcinoma progression may represent a mechanism by which tumor cells become more invasive and metastatic.
Introduction
Cell migration is essential for a number of biological and pathological processes, including normal development, angiogenesis, wound repair, and tumor invasion and metastasis. With the ECM scaffolding, cells use their unique complement of adhesion receptors to form stable but dynamic adhesion contacts that are regulated by complex sets of signaling pathways under control by growth factor receptors and other effectors. The process of cell spreading and migration is regulated by the extracellular matrix (ECM) and their receptors that include the integrin family of heterodimer receptors and cell surface heparan sulfate proteoglycans such as syndecans (Sdc) [1].
The Sdc family are composed of four members that structurally consist of an extracellular domain carrying heparan sulfate, a transmembrane domain, and a cytoplasmic domain [2]. Syndecans are an important class of cell surface receptors that have varied roles including their ability to bind to a variety of ECM ligands and also bind and concentrate growth factors [3, 4]. Members of the syndecan family tend to show strict tissue distribution, but Sdc-1 is strongly expressed on epithelial cells, whereas syndecan-4 is widespread and expressed at high levels on fibroblasts [5].
Syndecans bind to a diverse set of ECM ligands including fibronectin, laminins, vitronectin and collagens [6]. For some time it has been suggested that syndecans like Sdc1 can interact and mediate adhesion to collagens via its heparan sulfate chains [7–9]. The role of syndecans in adhesion is complicated by their interactions with other adhesion receptors. Syndecans are signaling co-receptors that are able to regulate cell adhesion to the ECM in collaboration with the associated family of integrin receptors. It is now established that syndecans and integrins participate in the formation and stability of focal adhesions and regulate polymerization of the actin cytoskeleton [4]. Other studies have shown that in MEF cells Sdc4 does not influence cell migratory velocity but does enhance the directional character of motility on fibronectin matrices [10]. Similarly, syndecans along with integrins bind to the ECM and modulate Rho family members that control activation of focal adhesion kinase (FAK) at focal adhesions. Thus, partnering of the two receptor systems has a major function in controlling not only initial adhesions but also dynamic activity such as cell spreading, migration, and invasion.
Syndecans have been implicated as important co-receptors during cancer progression but their role in such processes is complex and context dependent. The expression of the various syndecans during transformation can have either negative or positive influences on aggressive behavior of the cancer cells. In a number of different types of human cancer, Syndecan-1 expression was reported as decreased, including head and neck carcinoma [11–13], lung cancer [14], liver cancer [15], mesothelioma [16] and cancers of the GI track [17–19]. In the case of HNSCC, Scd1 expression was found to be inversely correlated with both the level of epithelial differentiation and the potential for favorable clinical outcome [11]. However, in pancreatic cancer [20], endometrial cancer [21] and ovarian cancer [22], Sdc1 expression is increased in progressed specimens. For breast cancer the results are mixed regarding Scd1 as a marker in outcome studies [23, 24].
In the present study, experiments targeting heparan sulfate residues suggested a role for heparan sulfate proteoglycans in regulating cell adhesion and motility of HNSCC cells on Type I collagen substrates. Although adhesion to collagen I was shown to be primarily mediated by the integrin α2β1, a second receptor was identified as Sdc1. Sdc1 was found to be variably expressed in a panel of human HNSCC cell lines but its expression correlated with invasive capacity. Using siRNA strategies, we found that silencing of Sdc1 influenced α2β1 integrin-dependent focal adhesion formation and RhoA activation leading to enhanced cell adhesion, migration and invasion of collagen I substrates. Thus, these data indicate that Sdc1 and α2β1 integrin are co-receptors for collagen I whose functions are coupled together thereby regulating the cytoskeletal and cell motility systems in HNSCC cells.
Materials and Methods
Cell Culture
HSC-3, LMF-4 and UM-SCC10A cells derived from human HNSCC have been previously described [25, 26]. Cells were routinely maintained in DMEM medium (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C. For most assays, cells were serum-starved overnight. Preparation of laminin 332 (laminin-5) substrates produced by UM-SCC-10A cells has been described [25].
Reagents and Antibody
Heparin and Heparinase III were purchased from Sigma (Saint Louis, MO) and Y27632 was from Calbiochem (San Diego, CA). Bovine Type I collagen was obtained from INAMED (Fremont, CA). Rabbit polyclonal antibody against Sdc1 (H-174) and mouse monoclonal antibody to Sdc4 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Mouse monoclonal antibodies against paxillin, FAK and phospho-FAK (Y397) were purchased from BD Transduction Laboratories (Lexington, KY). Antibodies to pan cofilin (rabbit 1439) and to the phosphorylated form of cofilin (p-cofilin) have been characterized previously [27] and were provided by Dr. James Bamburg (Colorado State University, CO). Rhodamine-phalloidin was purchased from Invitrogen Molecular Probes (Eugene, OR). Mouse monoclonal antibody (VM-1) against human integrin α2 subunit was described previously [28]. Secondary antibodies conjugated with fluorescein isothiocyanate or horseradish peroxidase were purchased from Jackson ImmunoResearch Lab Inc. (West Grove, PA).
Cell Spreading Measurements
Cells were seeded onto substrates previously coated with Type I collagen. After incubation for 1 h at 37°C in serum-free DMEM, the cells were fixed with 1% formaldehyde PBS and then stained with 2% Coomassie Brilliant Blue (Sigma) in 45% methanol/10% acetic acid for 10 min. The cell spreading area was measured using NIH Image software from more than 20 individual cells. In some experiments, cells were processed in parallel for staining with phalloidin-rhodamine by fluorescence microscopy.
Flow Cytometry
Cells were detached from the tissue culture plate by treatment with 5 mM EDTA and resuspended in PBS containing 1% BSA. The cells were then incubated with primary antibody against Sdc1 or Sdc4 for 45 min at 4°C. Next, the cells were processed for staining with fluorescein isothiocyanate-conjugated secondary antibody at 4°C. After 45 min, the cells were washed and resuspended in PBS containing 0.1% BSA, 5% Cell Dissociation Buffer (Invitrogen, Carlsbad, CA) and 0.1% propidium iodide (Sigma, St. Louis, MO). Sdc1 and Sdc4 expression was analyzed by using a FACS (Beckman Coulter, Fullerton, CA). For negative control, rabbit IgG (Jackson ImmunoResearch Labs Inc., West Grove, PA) for Sdc1 and Mouse IgG for Sdc4 were substituted for primary antibody. Mean Fluorescence was measured by the software Cell Quest.
Adhesion Assay
Cell adhesion was measured as described previously [25]. Briefly, 96-well plates were coated with Type I collagen at 37°C for 1 h, followed by blocking with 0.5% bovine serum albumin. Cells were incubated with or without functional blocking antibodies to α2 integrin subunit at 4°C. After 30 min, 2×105 cells were added to each well and allowed to attach at 37°C. Adherent cells were stained with crystal violet and quantified by a microcolorimetric assay.
Time-lapse Migration Assay
Cells were plated onto collagen I or laminin-5 substrates in 12-well tissue culture plates (Falcon, Becton Dickinson Labware). Cells were maintained at 37°C, 5% CO2, and examined with a Zeiss Axiovert 200 inverted microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY). Images were taken at 20 min intervals for 3 h, and then analyzed by AxioVision software (Carl Zeiss, Jena, Germany). The positions of individual nuclei were tracked and at least 60 cells were analyzed to determine the migration rates. In some experiments, prior to the addition of cells, collagen-coated plates were incubated for two sequential periods of 30 min each with serum-free medium containing various concentrations of heparin (Sigma). For treatment with heparinase III, cells were preincubated with the indicated concentration of heparinase in serum-free medium for 6 h. For treatment with Y27632, an inhibitor of Rho-associated coiled-coil kinase (ROCK), cells were incubated in serum-free medium for 30 min prior to assay for migration.
Immunofluorescence Staining
Cells were plated onto coverslips coated with Type I collagen and incubated at 37°C for 1 h. Next, cells were fixed with 1% formaldehyde for 15 min and permeabilized with 0.5% Nonidet P-40 for 5 min. Afterwards cells were incubated with primary antibodies to FAK for 1 hr at room temperature and then incubated with fluorescein isothiocyanate-conjugated secondary antibody. To visualize actin polymerization, cells were incubated with rhodamine-conjugated phalloidin as previously described [28].
Western Blot Analysis
Cell lysates were prepared by extraction with 50 mM Tris, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and complete protease inhibitor mixture (Roche Molecular Biochemicals). The cell lysates were then analyzed in SDS-PAGE after transfer to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). Proteins were probed with primary antibodies overnight at 4°C, and then with horseradish peroxidase-conjugated secondary antibodies against rabbit or mouse IgG (Jackson ImmunoResearch Laboratories) for 1 h. The immune complexes were visualized by chemiluminescence using the ECL system (Amersham Biosciences).
RhoA and Rac1 Activity Assays
RhoA and Rac1 activities were determined using RhoA G-LISA assay and Rac1 G-LISA activation assay kits (Cytoskeleton, Denver, CO), respectively. The cells were plated onto Type I collagen for 30 min and 90 min, and then cells were processed according to the manufacturer’s instructions. In brief, the lysate was incubated in a RhoA-GTP or Rac1- GTP affinity plates to bind the active GTP-bound form. Bound active RhoA and Rac1 protein were then detected with RhoA and Rac1 primary antibody followed by a secondary antibody conjugate to HRP. The signal was then developed, and the chemiluminescence signal was detected by reading on a SpectroMax M5.
siRNA and Transfection
siRNA specific for Sdc1 and Sdc4 as well as control non-targeting siRNA were purchased from Dharmacon (Lafayette, CO). For transfection, cells were seeded in 60 mm culture dishes overnight and then transfected with 100 nM of the siRNA using Lipofectamine 2000 and Opti-MEM I medium (Invitrogen). 24–48 h following transfection, the cells were detached with 5 mM EDTA and used in various assays for adhesion and invasion.
Invasion Assay
Invasion assays were performed using Cell Culture Inserts (8.0 µm pore, Becton Dickinson Labware, Franklin Lakes, NJ). Type I collagen solution was diluted with a 10× fold concentrated stock solution of DMEM to yield a final concentration of 1.2 mg/ml of collagen. After thermal gelation on the membrane of each insert, 2 × 105 cells were seeded onto the collagen gel in serum-free DMEM. Serum-free DMEM was added to the lower chamber and the cultures were incubated for 24 h at 37°C. For analysis, the upper side of the filter and collagen gel was removed along with any noninvasive cells. Invading cells remaining on the bottom surface were fixed and stained with crystal violet. Cells were counted in 10 randomly chosen microscopic fields using a 20× objective and expressed as the mean and S.D.
RNA Isolation and RT-PCR
RNA was prepared by lysing cells with TRIZOL (Invitrogen), according to the manufacturer's recommendations. RNA was reverse transcribed by SuperScript III Reverse Transcriptase at 42°C for 60 min using 50 ng of random primers (Invitrogen). Subsequently, 0.5 µl aliquots of the products were optimized and subjected to limited cycle PCR amplification. PCR was carried out using Taq PCR Master Mix Kit (QIAGEN, Miami, FL). To amplify Sdc1 (271 bp), the sequences used were 5’-GCC GCA AAT TGT GGC TAC TAA-3’as an upstream primer and 5’-CTC CAC TTC TGG CAG GAC TAC A-3’ as a downstream primer; to amplify Sdc2 (274 bp) the sequences used were 5’-CCT GCT CAG ACA AAG TCA CCT G-3’ as an upstream primer and 5’-GTT TGC GTT CTC CAA GGT CAT A-3’ as a downstream primer; to amplify Sdc3 (330 bp) the sequences used were 5’-GAC AAA ACC ATA CGC AAT CCT C -3’ as an upstream and 5’-CCT CAG AAC ACT CCA TCA CAG G -3’ as a downstream primer; to amplify Sdc4 (255 bp) the sequences used were 5’-GTC TGG CTC TGG AGA TCT GGA T-3’ as an upstream primer and 5’-TCC GTT CTC TCA AAG ATG TTG C-3’ as a downstream primer; to amplify GAPDH, the sequences used were 5’-TGA AGG TCG GAG TCA ACG GAT-3’ as an upstream primer and 5’-CGC CCC ACT TGA TTT TGG A-3’ as a downstream primer. PCR products were separated on 1.2% agarose gel and visualized after staining with ethidium bromide.
Results
Migratory and invasive capacity of HNSCC cells
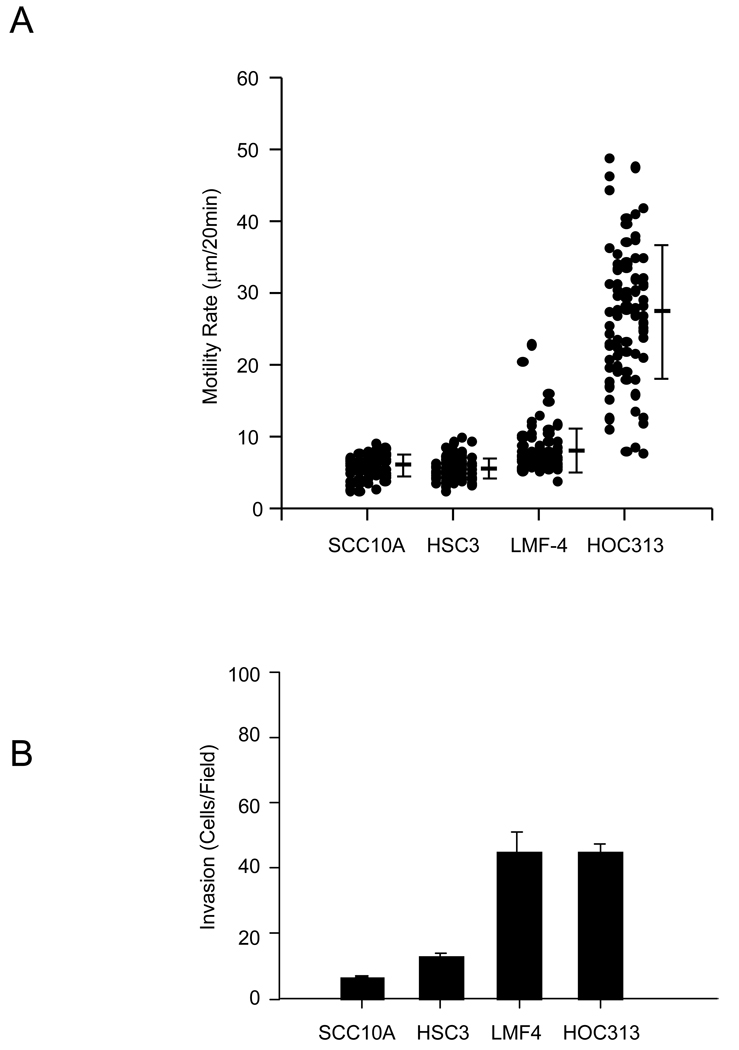
As a first step in investigating the behavior of epithelial cells on collagen substrates, we assessed the ability of Type I collagen to promote cell migration by a panel of SCC cells using time-lapse video microscopy. Analysis of cell tracts revealed that although all cell lines adhered efficiently to type I collagen, HSC-3 and UM-SCC-10A cells migrated poorly on this substrate (Fig. 1A). In contrast, LMF-4 and HOC-313 cells showed higher levels of motility. The LMF-4 cells were originally derived from the HSC-3 parental cells by selection for increased lymph node metastasis. The inefficient motility of HSC-3 on type I collagen is in contrast to their rapid locomotion on laminin-5 [28]. Next, we compared the invasive potential of the cell lines through a three-dimensional type I collagen matrix barrier (Fig.1B). In general, tumor cell invasion paralleled their locomotion on collagen substrates. LMF-4 and HOC-313 cells, compared to HSC-3 and SCC-10A cells, displayed a much higher invasive capacity through the collagen barrier, nearly four times greater than that of the HSC-3 or SCC-10A cells. These results suggest that for this set of SCC cell lines there is heterogeneity in their capacity to migrate on or invade collagen type I barriers. Previous studies have identified the α2β1 integrin as the primary receptor for mediating adhesion of these cells to collagen substrates [25].
Figure 1.
Relative motility and invasion of oral SCC cells. (A) For cell motility assays, head and neck SCC cell lines were plated on 1 µg/ml collagen I substrates. Time-lapse images were taken at 20 min intervals for 3 hr. Cell motility rates were analyzed from at least 60 cells. The data represents the mean and S.D of cell motility rate for each cell type (B) Head and neck SCC cell lines were assessed for the invasion of collagen I gels as described under the “Material and Methods”. The data represent the mean and S.D. of cells that invaded through the collagen gel barrier.
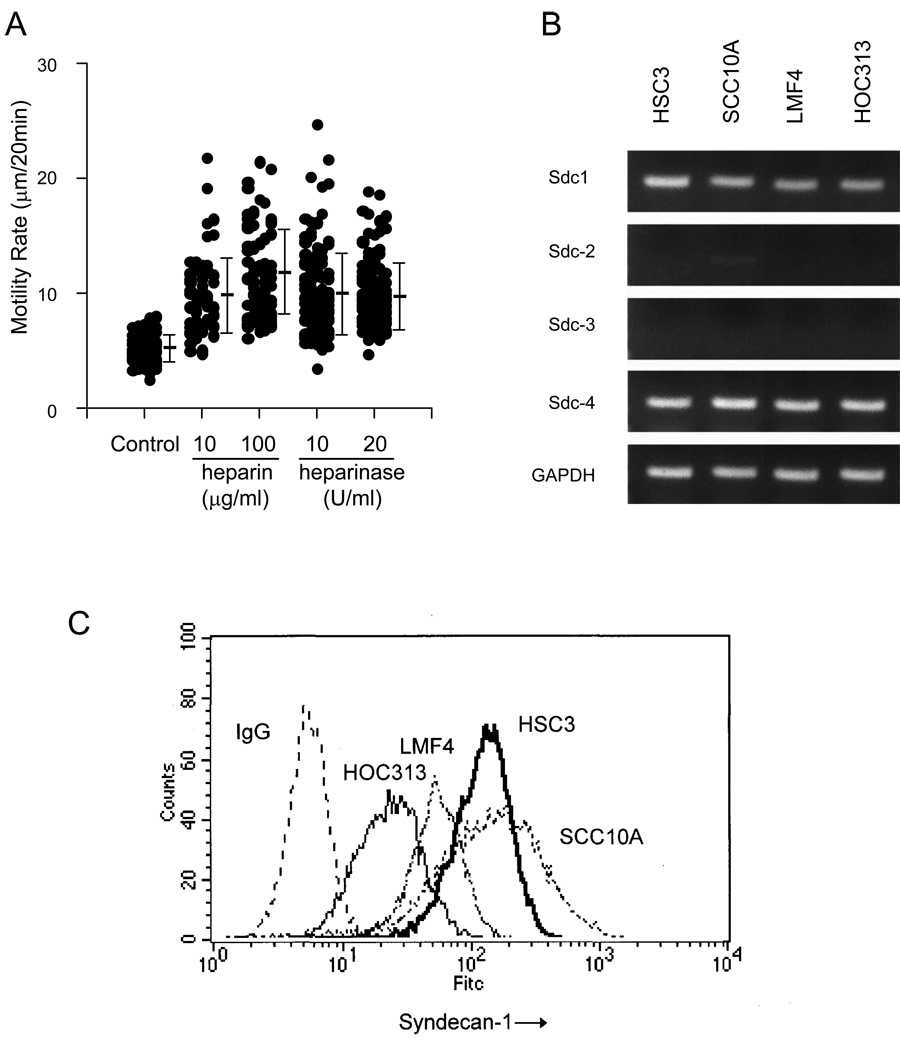
Since it has long been recognized that certain co-receptors may interact with collagen, we investigated whether a heparin-binding receptor may play a role in hindering motility on type I collagen. It is well established that other cell surface receptors, such as heparan sulfate proteoglycans, may participate in adhesive events in collaboration with integrin receptors. In order to evaluate the potential interaction between potential cell surface heparan sulfate proteoglycans and collagen I, we first subjected HSC-3 cells to treatment with either heparin or heparinase, and then tested for the effect of such treatment on their migratory rate on collagen substrates (Fig. 2A). Heparin treatment produced over a two-fold enhancement of cell motility on type I collagen in a dose-dependent manner. Similarly, cells preincubated with heparinase, an enzyme that specifically removes cell surface heparan sulfate residues, produced a 1.5-fold increase in motility compared to control untreated cells. These results suggest that cell surface heparan sulfate-containing molecules may interact with type I collagen and attenuate cell motility.
Figure 2.
Cell motility on collagen I involves Sdc1. (A) Effect of heparin and heparinase treatment on cell migration. Cells were treated with heparinase or alternatively, the Type I collagen substrates were treated with heparin as detailed in Materials and Methods. Cells were then plated on the substrates and assessed for cell migration using time-lapse microscopy. Data represents the mean and S.D. of the rate of at least 60 cells per assay condition. (B) Expression of Sdc-1, -2, -3 and -4 in SCC cell lines. Semiquantitative RT-PCR showing the relative mRNA expression of syndecans 1, 2, 3 and 4. Primers specific for Sdc 1, 2, 3, 4 and GAPDH cDNAs generated fragments of 271 bp, 274 bp, 330bp, 295 bp and 251 bp, respectively. (C) FACS analysis showing Sdc1 expression. Cell surface expression of Sdc1 was assayed by flow cytometry using pAb H-174. Mean fluorescence intensity was measured by Cell Quest software. Experiments were repeated three times with similar results.
Expression and function of Sdc1 in HNSCC cells
To further explore the nature of potential heparan sulfate containing receptors, we first evaluated the expression of syndecans in the panel of HNSCC cell lines (SCC-10A, HSC-3, LMF-4, and HOC-313) using semiquantitative RT-PCR (Fig. 2B). All cell lines expressed Sdc1 and Sdc4 mRNA, but did not express detectable levels of mRNA for Sdc2 or Sdc3. Next, the surface expression of Sdc1 in the cell lines was confirmed at the protein level by flow cytometric analysis using specific antibodies (Fig. 2C). Both HSC-3 and UM-SCC-10A had high expression of Sdc1. HOC-313 cells expressed nearly 10-fold less Sdc1 compared to HSC-3 and UM-SCC-10A. LMF-4 cells expressed an intermediate level compared to HSC-3 cells. In contrast, there was no significant difference in Sdc4 expression between the four cell lines by flow cytometry (data not shown).
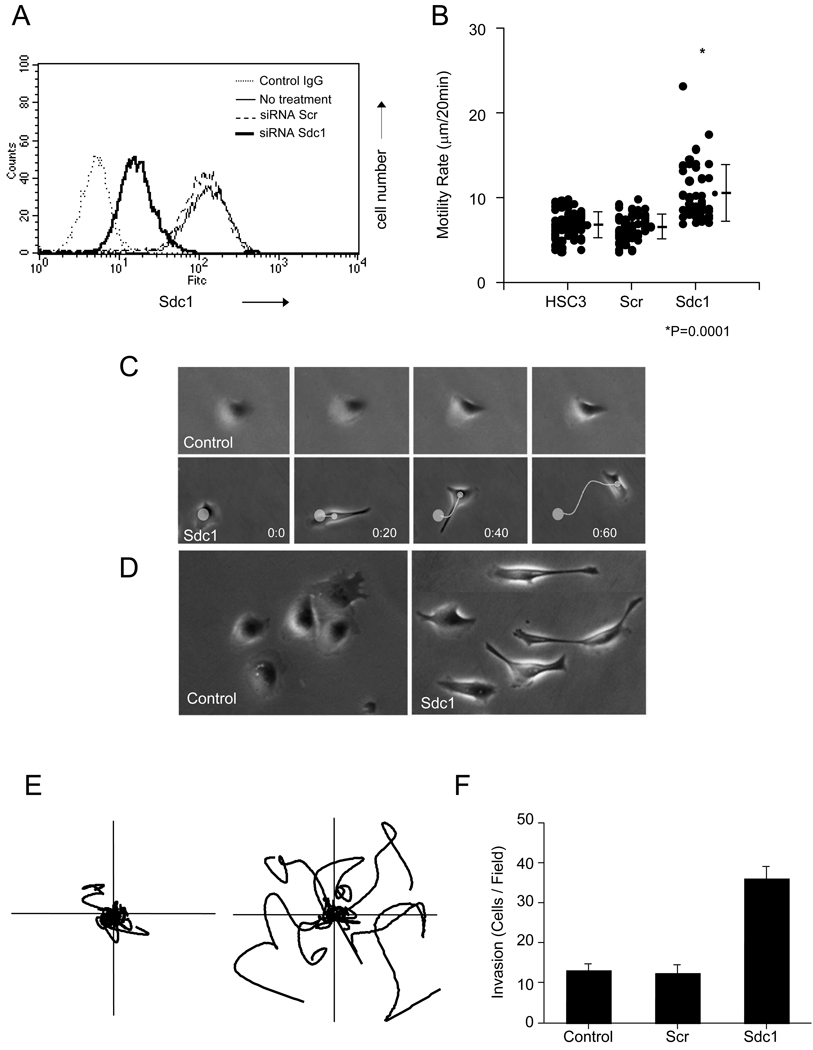
To determine the potential function of Sdc1 in cell motility, we used an RNA interference strategy to alter its expression. Following treatment with specific or scrambled control siRNA, the cell surface expression of Sdc1 of HSC-3 cells was assessed by flow cytometry (Fig. 3A). The Sdc1-specific siRNA suppressed the expression of the receptor by nearly 10-fold compared with untreated HSC-3 cells or cells treated with scrambled siRNA. Next, we tested the potential role of Sdc1 in regulating cell motility by performing time-lapse video microscopy on collagen-coated substrates after targeting the receptor with specific siRNA (Fig. 3B). Analysis of cell tracts revealed that control HSC-3 cells or cells treated with scrambled siRNA migrated poorly on this substrate. In contrast, siRNA Sdc1-transfected cells had a significantly higher average cell velocity being approximately two-fold greater than Scr siRNA Scr-transfected cells. Observation by time-lapse microscopy showed that control cells seeded on collagen type I initially showed spread morphology with lamellopodia around their edges (Fig. 3C, D; and see below). Usually, HSC3 cells on collagen display little movement and tend to form small radial membrane protrusions consisting of slowly forming and retracting lamellopodia and filopodia [1, 28]. However, Sdc1 siRNA-treated cells moved at a higher average velocity with a fairly random course of migration but rapidly emitted a complex set of protrusions consisting of multiple frontal filopodia followed closely behind by lamellopodia as they shuffled forward. Compared to the controls that usually showed a cobblestone epitheloid phenotype (Fig. 3D, left), the majority of siRNA Sdc1-transfected cells showed a polarized morphology with a distended fibroblastic shape (Fig. 3D, right).
Figure 3.
Silencing of Sdc1 expression by siRNA targeting. (A) HSC3 cells were transfected with Sdc1 siRNA or with non-targeting siRNA. After 24 h, Sdc1 expression was analyzed using FACS as described in “Material and Methods”. (B) HSC-3 cells were transfected with non-targeting siRNA and Sdc1 siRNA. HSC-3 and transfected cells were plated on Type I collagen-coated substrates (1 µg/ml) and assessed for cell motility using time-lapse microscopy for 3 h. Motility rates were analyzed as described under Material and Methods. Data represents the motility rate of individual cells along with the mean and S.D. (C) Cell locomotion and morphology on Type I collagen substrate was followed by time-lapse phase-contrast microscopy (20 min intervals). Control HSC3 cells (top panel) display little migration over time, whereas cells transfected with Sdc1 siRNA (lower panel) rapidly migrated with an elongated fibroblastic morphology. (D) Morphology of control HSC3 cells or Sdc1 siRNA-transfected cells on 1 µg/ml collagen following incubation on the substrate for 2 h. (E) HSC3 cells (left) and cells transfected with Sdc1 siRNA (right) were assessed for cell motility using time-lapse microscope on 1 µg/ml collagen I for 3 h. Individual representative cell tracks are shown. (F) Effect of suppression of Sdc1 expression on cell invasion. Control and siRNA-transfected HSC-3 cells were seeded onto collagen I gels. Invasion was assessed as detailed in Materials and Methods; data represents the mean and S.D. Experiments were repeated three times with similar results.
Analysis of cell traces show that scrambled siRNA migrated much less than Sdc1 siRNA and that knockdown of Sdc1 permitted a more persistent course of locomotion (Fig. 3E). In assays for cell invasion, analysis revealed that in HSC-3 cells transfected with Sdc1 siRNA, invasion through the collagen barrier was substantially increased, showing a three-fold enhancement (Fig. 3D). We also tested the effect of downregulating levels of Sdc4. Flow cytometry confirmed strong inhibition of Sdc4 expression after transfection with targeting siRNA and in contrast to the results with Sdc1, suppression of Sdc4 did not produce an effect on cell motility or invasion (data not shown). Thus, cell motility and invasion on collagen matrices does not appear to be modulated by Sdc4 in contrast to the negative regulation by Sdc1.
Sdc1 is a co-receptor for type I collagen
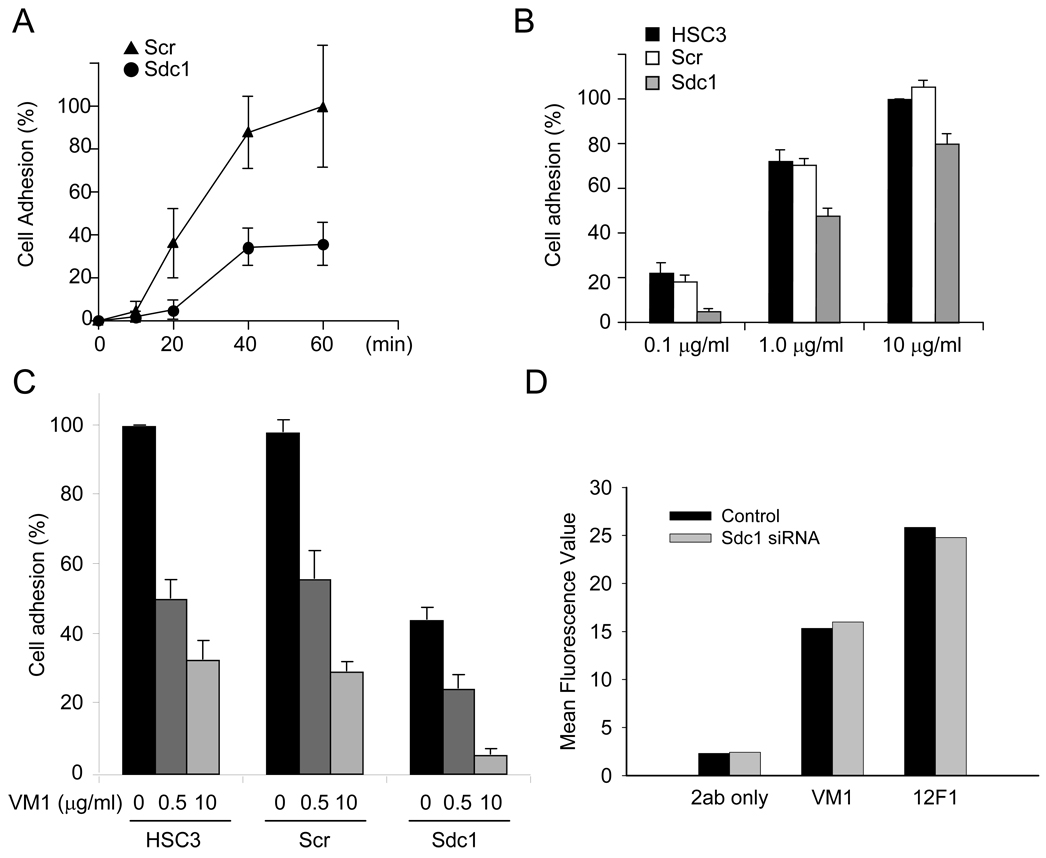
In order to further define the possible interaction between Sdc1 and type I collagen, the process of early cell adhesion was examined with HSC-3 cells transfected with control siRNA or Sdc1 siRNA. A time-course study of control and Sdc1 siRNA-transfected cells showed that the attachment to the collagen substrate was similarly progressed, but attachment of Sdc1 siRNA-transfected cells was lower than control cells (Fig. 4A). Further studies examined the adhesion efficiency to substrates coated with increasing concentrations of type I collagen. At 0.1 µg/ml, cell adhesion of Sdc1 siRNA-treated cells was nearly 90% less compared to the control cells. On substrates coated with 1 and 10 µg/ml of collagen, siRNA Sdc1 transfected cells attached less efficiently by 36.8% and 26.8%, respectively (Fig. 4B). These results indicate that Sdc1 can participate as a co-receptor and contribute to HSC-3 cell adhesion to type I collagen, especially at low ligand-coating concentrations.
Figure 4.
Silencing Sdc1 modulates cell adhesion on collagen I. (A) Sdc1 contributes to adhesion to type I collagen. HSC3 cells were transfected with non-targeting siRNA and siRNA to Sdc1. After transfection (24 h), cells were seeded onto substrates coated with 0.1 µg/ml collagen I. Cell adhesion was determined at indicated times using a colorimetric assay as described under “Material and Methods.” The average number of attached cells was determined at 1 h. Data is presented as a percentage and S.D. of total added cells. (B) HSC3 cells, non-targeting siRNA- and Sdc1 siRNA-transfected cells were plated on substrates coated with indicated concentration of collagen I. Cell adhesion was determined after 20 min as above. The maximum adhesion in the control wells cells on 10 µg/ml collagen I was taken as 100%. Data represents the mean of relative adhesion ratio and S.D. (C) Adhesion of HSC3 cells to collagen I is dependent on Sdc1 and integrin α2 receptors. Adhesion assay was performed in the presence of anti-integrin α2 antibody (VM1) on collagen I as described under Material and Methods. HSC-3 cells, non-targeting siRNA- and Sdc1 siRNA-transfected cells were preincubated with the indicated concentration of antibody and processed for cell adhesion on substrates coated with 1 µg/ml collagen I. The average of the cell adhesion for control HSC-3 was set as 100%. Data represents the mean of the relative adhesion ratio and S.E. (D) Relative levels of total integrin α2 receptor and α2 receptor with active conformation epitope. Cells transfected with non-targeting siRNA or Sdc1 siRNA were processed for flow cytometry to assess levels of total integrin receptor (VM1 mAb) or active form (12F1 mAb) of the α2 integrin receptor as well as the level of background staining by secondary antibody only. Mean fluorescence values are shown. The experiments were repeated three times with similar results.
Sdc1 cooperates with integrin α2β1
Like other HNSCC cells, HSC-3 cells are known to express a variety of integrin receptors and but the α2β1 integrin is the primary receptor that mediates attachment to collagen I [28]. We performed adhesion assays to evaluate the relationship between Sdc1 and integrin receptors on collagen I. Control cells or Sdc1 siRNA cells were untreated or incubated with anti-α2 integrin antibody and then plated on substrates coated with 1 µg/ml collagen I (Fig. 4C). In the absence of antibody, the adhesion of Sdc1 siRNA-transfected cells compared to controls was suppressed by nearly 50%. Treatment of control cells with anti-α2 integrin antibody at 0.5 and 10 µg/ml blocked cell adhesion to collagen I by approximately 50% and 30%, respectively. However, for Sdc1 siRNA-transfected HSC-3 cells adhesion was sensitive to anti-α2 mAb and reduced by 65.9% and 97.9% at 0.5 and 10 µg/ml, respectively. These results suggest that Sdc1 is a co-receptor for collagen I and acts in concert with integrin α2 to interact with the ligand.
In order to further define the potential role of Sdc1 in modulating levels of α2β1 integrin expression and function, we performed flow cytometry analysis (Fig. 4D). The relative level of cell surface α2 integrin receptor in Sdc1 siRNA transfected cells was comparable to that of control cells transfected with non-targeting SiRNA. In addition, we also evaluated the level of activated α2β1 integrin by flow cytometry using the 12F1 mAb that targets the conformation-specific epitope of functionally activated α2 integrin heterodimer [9, 29]. No difference was detected between the control cells and the Sdc1 siRNA-transfected cells suggesting that Sdc1 does not promote a shift in the proportion of integrin receptors in the conformationally active state. These results are in line with those of Ivaska and collaborators.[9] using the 12F1 mAb with breast carcinoma cells.
Role of Sdc1 in cell spreading
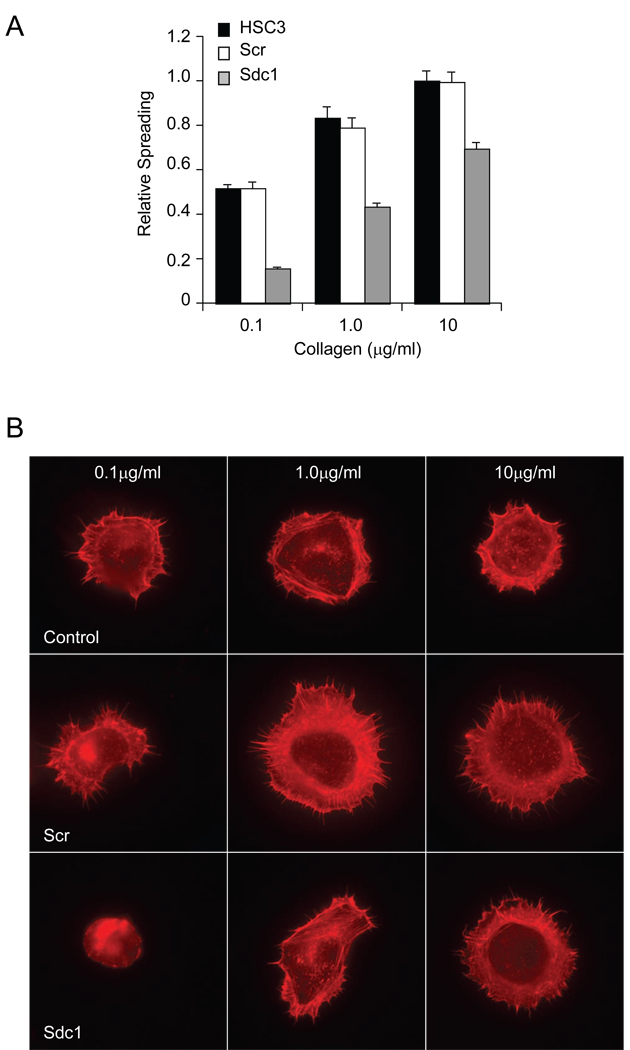
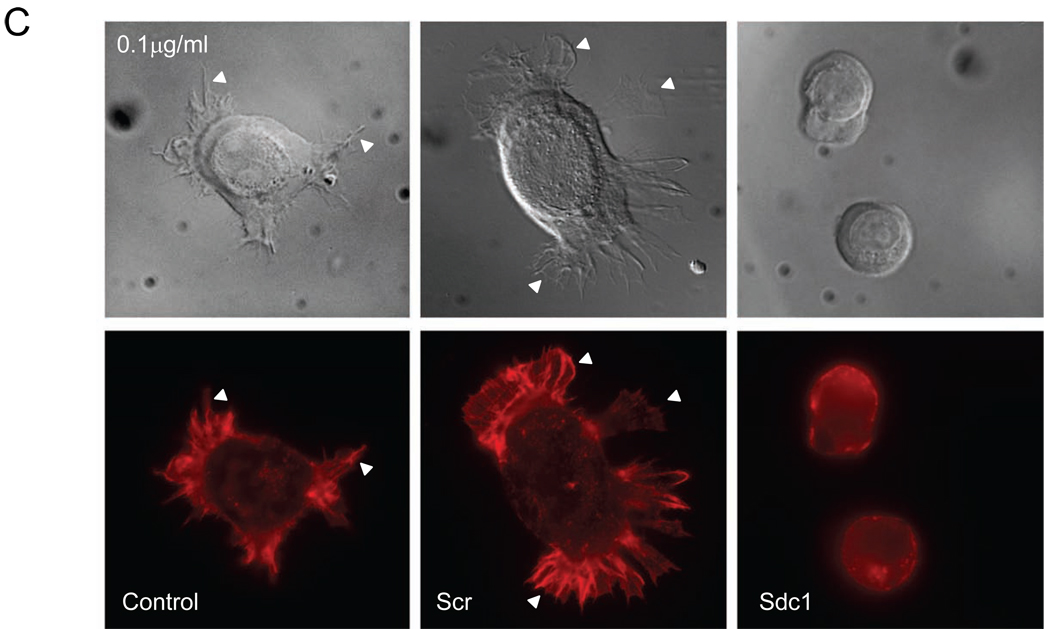
The process of cell spreading is driven by actin polymerization that precedes locomotion and includes the early phase of cell contact formation with the ECM. We evaluated the role of Sdc1 in mediating cell spreading on collagen substrates. HSC-3 control cells, Scr siRNA-transfected HSC3, and Sdc1 siRNA-transfected HSC-3 cells were plated on 0.1, 1.0 and 10 µg/ml collagen I, followed by fixation, staining with rhodamine-phalloidin, and evaluated for cell spreading (Fig. 5). The control cells and Scr siRNA-transfected cells adhered and spread efficently on the various concentrations of collagen I and the cell spreading surface area increased in a dose-dependent manner (Fig. 5A). These cells rapidily emitted lamellopodia around their peripheral edges and polymerized actin visible as a cortical ring surrounding the cell center (Fig. 5B, C). In contrast, Sdc1 siRNA-transfected HSC-3 cells spread poorly on the substrate particularly at the lower coating concentration (0.1 µg/ml) and displayed a mostly non-polarized, spherical shape (Fig. 5B). On the 1 µg/ml collagen I-coated substrates, Sdc1 siRNA-transfected HSC-3 cells began to show enhanced spreading with increased polymerized actin localized as extensive stress fibers at the cell’s leading edge, and when seeded onto substrates coated with 10 µg/ml of collagen I, the Sdc1 siRNA-transfected cells appeared morphologically similar to control cells with highly polarized actin at the peripheral of cells. In this case, the higher density of collagen was able to overcome the lack of Sdc1 in the transfected cells.
Figure 5.
Silencing Sdc1 modulates cell spreading on collagen I. (A) Measurement of cell spreading. Control, non-targeting siRNA-, and siRNA Sdc1-transfected HSC3 cells were seeded on substrates coated with collagen I at indicated concentration and the extent of cell spreading was determined after 1 h as described under Materials and Methods. Data represents the mean of the relative spreading and S.E. (B) HSC3 control cells, cells transfected with non-targeting siRNA, or cells transfected with Sdc1 siRNA were evaluated for morphology after adhering for 1h to collagen substrates coated at the indicated concentration and stained with staining with rhodamine-phalloidin. (C) As in (B) but examined by Differential Interference Contrast microscopy (DIC) microscopy (top) and immunofluorescence microscopy after staining with rhodamine-phalloidin (bottom). Silencing Sdc1 expression resulted in limited spreading on the collagen I substrate. Note extensive emission of actin-rich lamellopodia (arrowheads) in control cells but not in siRNA Sdc1-transfected cells.
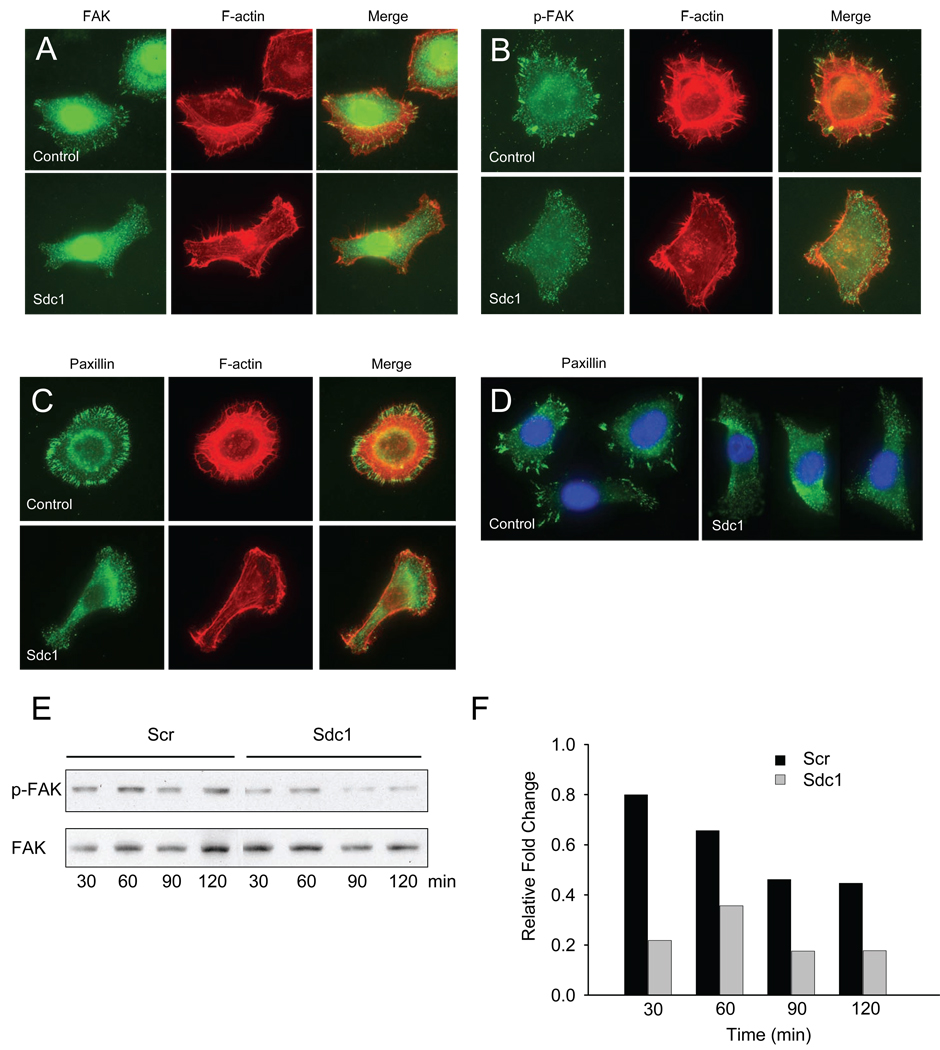
Sdc1 modulates formation of focal adhesions
To further detail the role of Sdc1 in cell adhesion and migration, we evaluated the distribution and quality of the focal adhesion-associated proteins, FAK and paxillin. Sdc1 siRNA-transfected and control non-targeting siRNA-transfected cells plated on collagen I were stained with specific antibodies to focal adhesion and co-stained with rhodamine-phalloidin to localize polymerized actin (Fig. 6A, B, C, D). In control cells, a majority of cells (~80%) displayed a high density of punctate mature focal adhesions associated with condensation of FAK or p-FAK staining along the cells’ perimeter edge with a high density of F-actin as a continuous belt at the peripheral membrane. However, a high percent of Sdc1 siRNA-transfected HSC-3 cells (~65%) showed dispersed small granular condensations of FAK and p-FAK expression throughout the cell with limited well defined focal adhesions along with variable and thin arrays of polymerized F-actin staining along the peripheral cell edge. We also stained for paxillin and as for FAK/p-FAK staining found a similar pattern where control cells displayed mature focal adhesions along with minor actin bundle arrays distributed at the cell edge (Fig. 6C, D). These results indicate that HSC-3 cells form mature focal adhesions on collagen I substrates that involve collaboration between Sdc1 and integrin receptors. Thus, Sdc1 appears to regulate the activation of focal adhesion proteins and induce actin polymerization during the adhesion to collagen.
Figure 6.
Sdc1 modulates the distribution of focal adhesions. Control non-targeting siRNA-, and Sdc1 siRNA-transfected cells were seeded on collagen I (1 µg/ml) coated substrates with for 1h, and then fixed and stained with fluorescein-conjugated mAb to (A) FAK, (B) phospho-FAK, or (C) paxillin, and counterstained with rhodamine-conjugated phalloidin as described under Material and Methods. (D) Clusters of control and siRNA Sdc1-transfected cells were stained with mAb to paxillin as above and counterstained with DAPI. (E) Sdc1 regulates phosphorylation of FAK. Cells were transfected with non-targeting siRNA or Sdc1 siRNA and seeded onto collagen I-coated substrates (1 µg/ml) for indicated times. FAK phosphorylation was detected by immunoblotting with antibody to pY397 or with antibody to total FAK protein as described in Materials and Methods. FAK was used as an internal loading control. The experiments were repeated three times with similar results. (F) Relative fold change of p-FAK as measured by NIH Image.
We demonstrated that suppression of Sdc1 attenuates focal adhesion formation and actin polymerization. Next, we explored the possibility that FAK phosphorylation may be regulated by Sdc1. We seeded control cells and Sdc1 siRNA-transfected HSC-3 cells onto collagen substrates and examined the time-course of the FAK Y397 residue phosphorylation (Fig. 6E, F). The elevation of p-FAK corresponded to the formation of focal adhesions. In control cells, the level of FAK phosphorylation after 30 min gradually decreased. The level of phosphorylation of FAK in Sdc1 siRNA-transfected cells was substantially less than that of control cells and overtime did not increase but tended to remain at basal levels. These results indicate that the formation of focal adhesions correlates with the parallel increase of FAK phosphorylation on collagen and that this phosphorylation may be at least partially regulated by Sdc1.
Sdc1 modulates Rho GTPases on collagen I
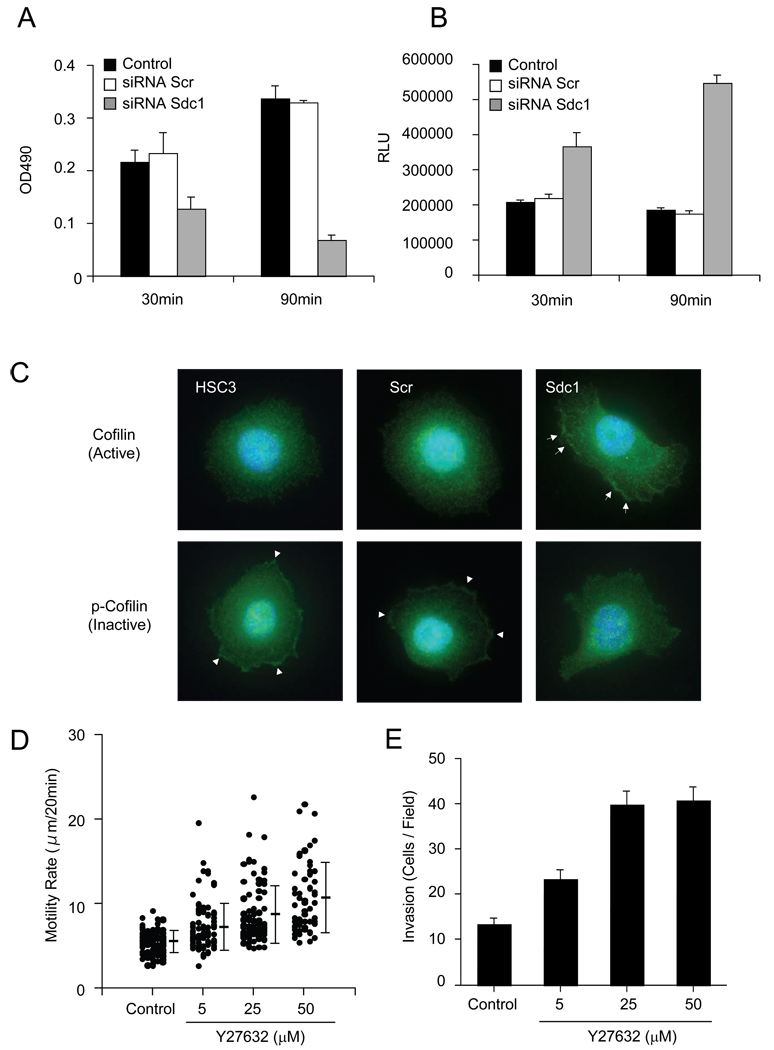
The Rho GTPases mediate the process of cell adhesion and migration. It has been previously reported that RhoA is a key molecule for cell adhesion and migration on collagen I in head and neck squamous carcinoma cells [28], where RhoA was activated during cell adhesion on collagen I, and the suppression of RhoA activity reduced focal adhesion formation and promoted cell migration. To determine the potential role of Sdc1 in regulating these events, the RhoA activity in control and Sdc1 siRNA-transfected cells seeded on collagen I substrates was measured. At 30 min post-seeding, control and Scr siRNA-transfected cells showed equivalent levels of RhoA activity (Fig. 7A). Continued adhesion to collagen I stimulated RhoA activation in control cells and in cells transfected with scrambled siRNA. In contrast, RhoA activation in Sdc1 siRNA-transfected cells was significantly inhibited compared to controls at 30 min and even more so at 90 min post-seeding. These results showing low RhoA activity on collagen I. paralleled the decrease in focal adhesions and significant suppression of FAK phosphorylation (Fig. 6A–D).
Figure 7.
Sdc1 regulates RhoA and Rac1 activity. (A) Sdc1 induces elevated RhoA activity. Cells transfected with non-targeting siRNA or Sdc1 siRNA were seeded on collagen I-coated substrates (1 µg/ml) for the indicated time period and prepared for RhoA activity (see Materials and Methods). Data represents the mean and S.E. of colorimetric values at OD490. (B) Sdc1 inhibits Rac1 activation. For the assay of Rac1 activity, cells were processed as above. Rac1 activity was measured by Rac1 G-LISA activation assay. Data represents the mean of RLU and S.E. (C) Sdc1 promotes cofilin phosphorylation. Cells transfected with non-targeting siRNA and Sdc1 siRNA were plated onto collagen I (1 µg/ml) and after 1 h, cells were stained with antibody to pan-cofilin and p-cofilin. Condensation of p-cofilin and cofilin are marked with arrows or arrowheads, respectively. (D) Inhibition of ROCK accelerates cell motility on collagen I. HSC3 cells were pretreated with or without Y27632 ROCK inhibitor before plating on collagen substrates. Cell motility was analyzed using a time-lapse microscope. Data represents the mean cell velocity and S.D. (E) ROCK inhibitor promotes invasion in collagen I barrier. HSC3 cells were treated with the indicated concentration of ROCK inhibitor and subjected to the invasion assay for 24 h (Materials and Methods). Cells that invaded through the collagen gel were scored. Data represents the mean and S.D. of invading cells.
To gain additional insight on how the interaction between Sdc1 and collagen I may regulate cell motility, we investigated the potential role of Rac, another important Rho GTPase. The activation of Rac1 was similarly followed in cells seeded on collagen I (Fig. 7B). At 30 min post-seeding, Sdc1 siRNA-transfected cells displayed significant elevation of Rac1 activation, compared to control cells and Scr siRNA-transfected cells that both generated low levels of active Rac 1. Rac1 activity was further elevated in Sdc1 siRNA-transfected cells at 90 min whereas control cells continued to show a relatively low level of basal Rac activity. This data indicates that the binding of Sdc1 to collagen I induces enhanced focal adhesion formation with reduced cell motility as a consequence increased a RhoA activity and concomitant suppression of of Rac signaling.
Sdc1 suppresses RhoA downstream signaling
To further evaluate the importance of Sdc1 in cell spreading and motility on collagen I, we tested the effect of silencing Sdc1 on cofilin phosphorylation. Cofilin belongs to the actin depolarizing factor (ADF) family and induces disassembly and rapid turnover of actin filaments. Cofilin activity is inhibited by LIMK, a downstream target of the RhoA/ROCK pathway, and activated by specific phosphatases that can promote the regeneration of actin filaments [30]. The activity is inhibited. To examine the status of cofilin, cells were plated on collagen I and then evaluated for distribution of total cofilin and p-cofilin expression using immunofluorescent staining (Fig. 7C). At 30 min, control and Scr siRNA-transfected cells expressed diffuse and weak cytoplasmic staining active cofilin. However, inactive p-cofilin accumulated at the peripheral membrane edges of these cells. In contrast, for Sdc1 siRNA-transfected cells, active cofilin was localized in the cell membranes but p-cofilin was diffusely stained. This data suggests that Sdc1 by binding to collagen induces RhoA/ROCK signaling, cofilin phosphorylation, inhibition of actin-depolymerization activity, and attenuation of both lamellipodium formation and cell migration. In contrast, when Sdc1 is downregulated, Rac activity is upregulated, RhoA/ROCK signaling is suppressed, resulting in cofilin dephosphorylation and activation, thereby promoting the generation of actin-positive lamelliopodial structures and directed migration.
To evaluate whether loss of RhoA activity is associated with high motility on collagen I, we studied the effect of the ROCK inhibitor, Y27632, on cell motility ROCK is the downstream target of RhoA and plays an important role as an effector protein in the RhoA mediated signaling pathway. Time-lapse motility assays revealed that treatment of HSC-3 cells with Y27632 promoted a dose-dependent stimulation of cell motility on collagen I substrates (Fig. 7D). Cells pretreated with Y27632 moved at a rate several-fold greater than control cells. We next performed a collagen barrier invasion assay to investigate the involvement of the Rho/Rock pathway during cell invasion. Cells were seeded onto collagen gels in the absence or presence of Y27632 inhibitor and then assessed for penetration of the collagen barrier. Analysis revealed that in cells incubated with Y27632, invasion was substantially increased (Fig.7E). These results suggest that inhibition of the RhoA/ROCK signaling pathway may contribute to enhanced cell motility in Sdc1-depleted cells.
Discussion
Previous studies have shown that Sdc1 is expressed in various types of human carcinomas including HNSCC [12, 18, 19, 31, 32], but the functional consequences of regulated syndecan expression are not clear. Several histopathological studies have reported that high Sdc1 expression is correlated with the inhibition of invasiveness [33, 34], and downregulation of Sdc1 resulted in increased invasion [12, 31]. In this study, we examined the functional coupling between integrins and Sdc1 in HNSCC cells that regulates not only adhesion but also spreading and cell motility on type collagen I substrates. In a panel of HNSCC cells, we found that LMF-4 and HOC313 cells were highly motile and extensively invade collagen substrates but HSC3 and SCC 10A cells display limited motility or invasion on collagen I. Our results indicated that in addition to integrins, other receptors may be involved in mediating cell interaction with collagen matrices. Heparan sulfate proteoglycans are known to bind to the ECM and promote cell adhesion. For squamous cell carcinomas, the major class of heparan sulfate proteoglycan are the syndecans [3]. We found that in the presence of exogenous heparin or cleavage of heparan sulfate chains resulted in enhanced cell migration on collagen. In the panel of HNSCC cell lines tested here, the dominant syndecans expressed were Sdc1 and Sdc4. Importantly, there was an inverse correlation between the expression of Sdc1 and motility on Type I collagen.
We used a siRNA knockdown strategy to further evaluate the potential role of Sdc1 in regulating HNSCC cell behavior on collagen I matrices. The loss-of-function studies in the SCC cells showed that cell motility was significantly enhanced following Sdc1 suppression. Similarly, such treatment also stimulated invasion through three-dimensional collagen barriers, suggesting that loss of Sdc1 may provide an advantage during tumor invasion and metastasis in SCC cells. Furthermore, during cell attachment and spreading on collagen, we found that loss of Sdc1 diminished adhesion potential to collagen substrates. This effect was pronounced at low ligand-coating concentrations and was attenuated at high ligand levels. Similar results were found in cell spreading assays, in that cells with poor expression of Sdc1 failed to spread on collagen I with altered actin distribution. In contrast, cells expressing high levels of Sdc1 efficiently spread on wide range of collagen I-coating concentrations. These results are consistent with the recent and elegant study by Vuoriluoto et al. [9] that found a similar dependency between expression of Sdc1 and adhesive function to type I collagen substrates. These studies used both variant CHO and breast carcinoma cell line.
These results suggest that Sdc1 is a regulator of cell adhesion and spreading in HNSCC and modulates cell migration and invasion. Since inhibiting expression of Sdc1 partially blocked cell adhesion and spreading on collagen, we examined whether other potential receptors may work in concert with Sdc1 to regulate cell adhesive activities. For many cells types, the α2β1 integrin is commonly the dominant integrin receptor that promotes cell adhesion to collagens. Furthermore, integrin α2β1 and induced adhesive signaling by collagen have been shown to reduce cell-ECM locomotion and lead to immobilization of HNSCC cells [28]. In the current study, blocking antibody to α2 integrin was able to partially inhibit adhesion. Following siRNA-knockdown of Sdc1, the anti-α2 mAb completely blocked attachment to collagen. This indicates that in HNSCC cells the α2β1 integrin and Sdc1 are co-receptors for collagen I. Similar results were recently reported by Ivaska and collaborators.[9] using a breast carcinoma cell line. Based on ligand density studies in the current studies, it appears that α2β1 integrin interaction with collagen has relatively low affinity. Consequently, cell adhesion would be sensitive to low ligand densities. In contrast, when ligand density is high, the integrin is able to effectively compensate for lack of Sdc1 co-receptors. This is consistent with the understanding that, in general, integrins tend to form relatively low but variable affinity interactions with ECM ligands [35]. Whether this functional coupling of the two receptors is via signaling pathway or through a direct interaction between the two receptor classes is not yet known and further work is needed to determine this possibility.
However, expression of integrin α2β1 was not altered following knockdown of Sdc1 nor was the relative level of activated integrin modified by loss of the HSPG. The present results regarding the function of Sdc1 in adhesion are consistent to the recent report of Vuoriluoto et al. [9] in which Sdc1 was found to partially mediate adhesion to collagen type I in CHO cells and in human breast carcinoma cells. Furthermore, the same group demonstrated the dependency of Sdc1 for the full function of the α2β1 integrin. Cells with reduced HSPG displayed poor spreading and adhesion on collagen substrates with disrupted polymerized actin distribution. In other studies by Rapraeger and colleagues [36, 37], it was revealed that there is a direct physical interaction between the αvβ1/αvβ5 integrins and Sdc1 on vitronectin substrates in mammary carcinoma cells that is required for activation of the integrin. Recently, Chen et al. [38] reported that in lung epithelial cells Sdc1 may have a role in regulating the affinity of α2β1 integrin based on the use of an activation-specific beta-1 integrin antibody (12G10). This suggests that Sdc1 modulation of α2β1 integrin may be cell type specific.
The process of cell migration involves multiple cyclic processes including disruption of focal adhesions, a decrease of cell substrate adhesion, formation of nascent adhesion with new protrusions and, finally, polymerization and depolymerization of actin cytoskeleton [39]. The cooperative interaction between adhesion receptors including the syndecans and integrins provide a dynamic linkage between the ECM scaffolding and the cytoskeleton [40]. In the current studies, we find that components of the focal adhesion plaque (FAK and paxillin) are strongly engaged in the non-migratory cells on type I collagen substrates. In these immobilized cells, peripheral focal contacts were particularly predominant.
However, cells processed for Sdc1 knockdown took on a more migratory phenotype, with a concurrent shift in the FAK and paxillin distribution and stability characterized by minimal mature focal adhesions and a diffuse array of small transient and dynamic focal adhesions. It is believed that integrin clustering at nascent focal adhesions near the newly protruded leading edge of motile cells initiates a cascade where FAK and integrins are activated and assembled into focal adhesions. However, these nascent contacts are transient and shifts to the cell’s rear where FAK and integrins undergo rapid inactivation, focal adhesion disassembly and turnover, thereby permitting enhanced forward motility [41, 42].
Alternatively, persistent, localized activation of FAK at mature focal adhesions leads to stable and mature contact formation inducing the formation of an extensive network of cortical stress fiber bundles, high contractility, and prolonged cell immobilization [40]. Analysis of phosphorylation of FAK at Tyr397 in cells adhering to collagen substrates revealed a robust but persistent activation of FAK. In contrast, when Sdc1 expression was suppressed, the α2β1 integrin-dependent activation of phospho-FAK was effectively reduced, attenuating cell adhesion/spreading on collagen. These results suggest that the α2β1 integrin signaling pathway is regulated by crosstalk with Sdc1 receptors thus coordinating a decrease in its overall functionality and reducing downstream activity of effectors such as FAK.
ECM proteins direct cell motility through integrin-dependent regulation of Rho family members [43, 44]. As an important manager of cytoskeletal organization, the Rho family GTPases that include Rho, Rac, and Cdc42, modulate many aspects of cytoskeletal dynamics that underlie changes during migration. Previously, it was shown that for SCC cells, laminin 322 (laminin-5) promotes rapid cell scattering, whereas fibronectin and collagen I do not [1, 28]. GTPases are important in such integrin-mediated responses to specific ECM ligands during tumor invasion. On laminin-5 substrates, α3β1 integrin preferentially inactivated RhoA and induced activation of Cdc42 and PAK1, promoting migration of oral SCC cells [28, 45]. In contrast, on collagen, RhoA was strongly activated, leading to enhanced focal contact formation, and hindering cell migration. The current results extend these studies and show that forced downregulation of Sdc1 reduced levels of active RhoA but enhanced Rac1 and triggered filopodia and lamellopodia formation at the cell’s leading edge along with stimulation of directed cell movement. This supports the possibility that Sdc1 is involved in regulating Rho signaling thereby controlling the cell’s migratory and invasive phenotype.
Studies indicate that Rac1 directly influences Rho A activity, and that the reciprocal balance between Rac and Rho activity can determine either epithelial or mesenchymal cell morphology and migratory behavior of cancer cells [46]. Rho regulates myosin-mediated contractility through downstream effectors, such as ROCK/Rho kinase [47, 48] and RhoA activation has been shown to limit membrane protrusions in a number of cell types including leukocytes [49], apparently by regulating myosin-dependent contractile force. Enhanced cell spreading and cell motility has also been observed by inhibiting RhoA in fibroblasts [50, 51] or by induction of truncated RhoA in various cancer cells [52–54]. Suppression of ROCK activity by its inhibitor (Y27632) was found to facilitate HSC-3 cell motility and invasion through collagen barriers (Fig. 7 D, E). Previous work has established that inhibition of ROCK can suppress migration and invasion [54–56], yet in other studies downregulation of ROCK activity actually enhanced motility/invasion [28, 54, 57]. It is now appreciated that the process of cell motility and invasion are highly complex and there are two basic forms of invasion: protease-independent amoeboid invasion and mesenchymal invasion [56, 58] which may explain the apparent paradox. ROCK can trigger integrin clustering and generate mature focal adhesions in certain cells (e.g., fibroblastic) and in this situation inhibition of ROCK may lead to enhanced migration and invasion. But in other cells (e.g., lymphoid) that lack well-formed focal adhesions, ROCK has been found to increase formation of mature focal adhesions that are need for initial adhesion and motility. Consequently, the role of Rho/ROCK signaling in modulating invasion is cell type-context dependent.
ROCK phosphorylates LIMK1 and LIMK2 and leads to an increase in LIMK activity that phosphorylates and inactivates cofilin thereby inhibiting actin-depolymerization activity [59, 60]. We found that in immobilized control cells on collagen, p-cofilin was strongly stained and accumulated at the peripheral cell membrane edge. In contrast, for cells in which Sdc1 was silenced, active and dephosphorylated cofilin localized at the migratory cell’s periphery. These results suggest that in HNSCC cells, the α2β1 integrin/Sdc1 co-receptor system leads to activation of RhoA thereby stabilizing focal adhesions and the actin cytoskeleton thus restricting cell locomotion. When Sdc1 is downregulated, there is reciprocal induction of Rac1, loss of active RhoA, induction of cofilin activity, resulting in facilitated directional locomotion.
In summary, this study reports on the mechanism by which Sdc1 and α2β1 integrin act as co-receptors for collagen I and regulate cell motility. Evidence is provided that indicates the activity of α2β1 integrin signaling pathway is positively regulated by Sdc1. The functional coupling between Sdc1 and α2β1 integrin modulates adhesive, migratory and invasive behavior. Similar to the previous report by Ivaska and collaborators [9], we found that modulation of Sdc1 levels did not have any dramatic effect on overall integrin α2β1 levels or on the relative level of activated integrin epitope for α2β1 using activation-specific epitope antibody. Our findings are interesting in the context of tumor progression, where evidence shows a correlation between loss of Sdc1 expression and enhanced invasion and metastasis of HNSCC and other tumor types [33, 34]. The finding that suppression of Sdc1 leads to reduced FAK function with related alterations in the dynamics of focal adhesions and cytoskeletal organization points toward the existence of unique signaling pathways controlling the invasive process. Further work is needed to define how Sdc1 controls these processes at the molecular level.
Acknowledgements
We thank L. Lee and B. Situ for their assistance in preparing the manuscript. This work was supported by NIH grant R01 DE11436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer metastasis reviews. 2005;24:35–45. doi: 10.1007/s10555-005-5046-2. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett AH, Hayashida K, Park PW. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol Cells. 2007;24:153–166. [PubMed] [Google Scholar]
- 3.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Mythreye K, Blobe GC. Proteoglycan signaling co-receptors: Roles in cell adhesion, migration and invasion. Cellular signalling. 2009 doi: 10.1016/j.cellsig.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annual review of biochemistry. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 6.Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell and tissue research. 2009 doi: 10.1007/s00441-009-0829-3. [DOI] [PubMed] [Google Scholar]
- 7.Koda JE, Rapraeger A, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. The Journal of biological chemistry. 1985;260:8157–8162. [PubMed] [Google Scholar]
- 8.Sanderson RD, Sneed TB, Young LA, Sullivan GL, Lander AD. Adhesion of B lymphoid (MPC-11) cells to type I collagen is mediated by integral membrane proteoglycan, syndecan. J Immunol. 1992;148:3902–3911. [PubMed] [Google Scholar]
- 9.Vuoriluoto K, Jokinen J, Kallio K, Salmivirta M, Heino J, Ivaska J. Syndecan-1 supports integrin alpha2beta1-mediated adhesion to collagen. Experimental cell research. 2008;314:3369–3381. doi: 10.1016/j.yexcr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. The Journal of cell biology. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inki P, Jalkanen M. The role of syndecan-1 in malignancies. Ann Med. 1996;28:63–67. doi: 10.3109/07853899608999076. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa H, Zhang M, Matsumoto S, Yamashita Y, Tanaka T, Takamori K, Igawa K, Yoshida M, Fukuyama H, Takahashi T, Sakoda S. Reduced syndecan-1 expression is correlated with the histological grade of malignancy at the deep invasive front in oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:301–306. doi: 10.1111/j.1600-0714.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 13.Pulkkinen JO, Penttinen M, Jalkanen M, Klemi P, Grenman R. Syndecan-1: a new prognostic marker in laryngeal cancer. Acta Otolaryngol. 1997;117:312–315. doi: 10.3109/00016489709117794. [DOI] [PubMed] [Google Scholar]
- 14.Nackaerts K, Verbeken E, Deneffe G, Vanderschueren B, Demedts M, David G. Heparan sulfate proteoglycan expression in human lung-cancer cells. Int J Cancer. 1997;74:335–345. doi: 10.1002/(sici)1097-0215(19970620)74:3<335::aid-ijc18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto A, Ono M, Fujimoto Y, Gallo RL, Bernfield M, Kohgo Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int J Cancer. 1997;74:482–491. doi: 10.1002/(sici)1097-0215(19971021)74:5<482::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Kumar-Singh S, Jacobs W, Dhaene K, Weyn B, Bogers J, Weyler J, Van Marck E. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. J Pathol. 1998;186:300–305. doi: 10.1002/(SICI)1096-9896(1998110)186:3<300::AID-PATH180>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Day RM, Hao X, Ilyas M, Daszak P, Talbot IC, Forbes A. Changes in the expression of syndecan-1 in the colorectal adenoma-carcinoma sequence. Virchows Arch. 1999;434:121–125. doi: 10.1007/s004280050315. [DOI] [PubMed] [Google Scholar]
- 18.Peretti T, Waisberg J, Mader AM, de Matos LL, da Costa RB, Conceicao GM, Lopes AC, Nader HB, Pinhal MA. Heparanase-2, syndecan-1, and extracellular matrix remodeling in colorectal carcinoma. Eur J Gastroenterol Hepatol. 2008;20:756–765. doi: 10.1097/MEG.0b013e3282fc2649. [DOI] [PubMed] [Google Scholar]
- 19.Wiksten JP, Lundin J, Nordling S, Kokkola A, Haglund C. Comparison of the prognostic value of a panel of tissue tumor markers and established clinicopathological factors in patients with gastric cancer. Anticancer Res. 2008;28:2279–2287. [PubMed] [Google Scholar]
- 20.Conejo JR, Kleeff J, Koliopanos A, Matsuda K, Zhu ZW, Goecke H, Bicheng N, Zimmermann A, Korc M, Friess H, Büchler MW. Syndecan-1 expression is up-regulated in pancreatic but not in other gastrointestinal cancers. International Journal of Cancer. 2000;88:12–20. doi: 10.1002/1097-0215(20001001)88:1<12::aid-ijc3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Oh J-H, Kim J-H, Ahn H-J, Yoon J-H, Yoo S-C, Choi D-S, Lee I-S, Ryu H-S, Min CK. Syndecan-1 enhances the endometrial cancer invasion by modulating matrix metalloproteinase-9 expression through nuclear factor [kappa]B. Gynecologic Oncology. 2009;114:509–515. doi: 10.1016/j.ygyno.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, Slade RJ, Martin-Hirsch P, Gallagher JT, Jayson GC. Distribution and Clinical Significance of Heparan Sulfate Proteoglycans in Ovarian Cancer. Clin Cancer Res. 2004;10:5178–5186. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- 23.Loussouarn D, Campion L, Sagan C, Frenel JS, Dravet F, Classe JM, Pioud- Martigny R, Berton-Rigaud D, Bourbouloux E, Mosnier JF, Bataille FR, Campone M. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br J Cancer. 2008;98:1993–1998. doi: 10.1038/sj.bjc.6604400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, Veronese S, Caffo O, Lucenti A, Palma PD, Galligioni E, Doglioni C. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98:474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 25.Kawano K, Kantak SS, Murai M, Yao CC, Kramer RH. Integrin alpha3beta1 engagement disrupts intercellular adhesion. Exp Cell Res. 2001;262:180–196. doi: 10.1006/excr.2000.5083. [DOI] [PubMed] [Google Scholar]
- 26.Momose F, Araida T, Negishi A, Ichijo H, Shioda S, Sasaki S. Variant sublines with different metastatic potentials selected in nude mice from human oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:391–395. doi: 10.1111/j.1600-0714.1989.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 27.Dang D, Bamburg JR, Ramos DM. Alphavbeta3 integrin and cofilin modulate K1735 melanoma cell invasion. Exp Cell Res. 2006;312:468–477. doi: 10.1016/j.yexcr.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Zhou H, Kramer RH. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. The Journal of biological chemistry. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]
- 29.Connors WL, Jokinen J, White DJ, Puranen JS, Kankaanpaa P, Upla P, Tulla M, Johnson MS, Heino J. Two synergistic activation mechanisms of alpha2beta1 integrin-mediated collagen binding. The Journal of biological chemistry. 2007;282:14675–14683. doi: 10.1074/jbc.M700759200. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn TB, Bamburg JR. Tropomyosin and ADF/cofilin as collaborators and competitors. Adv Exp Med Biol. 2008;644:232–249. doi: 10.1007/978-0-387-85766-4_18. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Skacel M, Adams JC. Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: an immunohistochemical study of clinically annotated tumors. BMC Cancer. 2008;8:185. doi: 10.1186/1471-2407-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gokden N, Greene GF, Bayer-Garner IB, Spencer HJ, Sanderson RD, Gokden M. Expression of CD138 (Syndecan-1) in renal cell carcinoma is reduced with increasing nuclear grade. Appl Immunohistochem Mol Morphol. 2006;14:173–177. doi: 10.1097/01.pai.0000168592.58721.7d. [DOI] [PubMed] [Google Scholar]
- 33.Thanakit V, Ruangvejvorachai P, Sampatanukul P. Expression of E-cadherin and syndecan-1 in axillary lymph node metastases of breast cancer with and without extracapsular extension. J Med Assoc Thai. 2008;91:1087–1092. [PubMed] [Google Scholar]
- 34.Roh YH, Kim YH, Choi HJ, Lee KE, Roh MS. Fascin overexpression correlates with positive thrombospondin-1 and syndecan-1 expressions and a more aggressive clinical course in patients with gallbladder cancer. J Hepatobiliary Pancreat Surg. 2009 doi: 10.1007/s00534-009-0046-1. [DOI] [PubMed] [Google Scholar]
- 35.Luo B-H, Carman CV, Springer TA. Structural Basis of Integrin Regulation and Signaling. Annual Review of Immunology. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beauvais DM, Rapraeger AC. Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Exp Cell Res. 2003;286:219–232. doi: 10.1016/s0014-4827(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 38.Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting alpha(2)beta(1) integrin activation. PLoS One. 2009;4:e6565. doi: 10.1371/journal.pone.0006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–2111. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 40.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 42.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Current opinion in cell biology. 2009 doi: 10.1016/j.ceb.2009.05.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 44.Olson MF. Contraction reaction: mechanical regulation of Rho GTPase. Trends Cell Biol. 2004;14:111–114. doi: 10.1016/j.tcb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Zidober B, Humtsoe JO, Kramer RH. Cell Adhesion Molecules in Carcinoma Invasion and Metastasis. Signaling Pathways in Squamous Cancer. 2010 (in press) [Google Scholar]
- 46.Yamazaki D, Kurisu S, Takenawa T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene. 2009 doi: 10.1038/onc.2009.2. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Kuzelova K, Hrkal Z. Rho-signaling pathways in chronic myelogenous leukemia. Cardiovasc Hematol Disord Drug Targets. 2008;8:261–267. doi: 10.2174/187152908786786241. [DOI] [PubMed] [Google Scholar]
- 50.Kole TP, Tseng Y, Huang L, Katz JL, Wirtz D. Rho kinase regulates the intracellular micromechanical response of adherent cells to rho activation. Mol Biol Cell. 2004;15:3475–3484. doi: 10.1091/mbc.E04-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol Biol Cell. 2006;17:4827–4836. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Yang L, Luo Y, Zheng Y. A novel strategy for specifically down-regulating individual Rho GTPase activity in tumor cells. The Journal of biological chemistry. 2003;278:44617–44625. doi: 10.1074/jbc.M308929200. [DOI] [PubMed] [Google Scholar]
- 53.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 54.Rosel D, Brabek J, Tolde O, Mierke CT, Zitterbart DP, Raupach C, Bicanova K, Kollmannsberger P, Pankova D, Vesely P, Folk P, Fabry B. Up-regulation of Rho/ROCK signaling in sarcoma cells drives invasion and increased generation of protrusive forces. Mol Cancer Res. 2008;6:1410–1420. doi: 10.1158/1541-7786.MCR-07-2174. [DOI] [PubMed] [Google Scholar]
- 55.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742–8751. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- 56.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer metastasis reviews. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 57.Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, Rutka JT. Inhibition of Rho-Kinase Affects Astrocytoma Morphology, Motility, and Invasion through Activation of Rac1. Cancer Res. 2005;65:8792–8800. doi: 10.1158/0008-5472.CAN-05-0160. [DOI] [PubMed] [Google Scholar]
- 58.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med. 2007;85:555–568. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]