Abstract
Objective
Investigate the effect of epigallocatechin gallate (EGCG), on rat leiomyoma (ELT3) cells in vitro and in nude mice model.
Study Design
ELT3 cells were treated with various concentrations of EGCG. Cell proliferation, PCNA and Cdk4 protein levels were evaluated. ELT3 cells were inoculated subcutaneously in female athymic nude mice. Animals were fed 1.25mg EGCG (in drinking water)/mouse/day. Tumors were collected and evaluated at 4 and 8 weeks post-treatment.
Results
Inhibitory effect of EGCG (200 μM) on ELT3 cells was observed after 24 h treatment (p<0.05). At ≥50μM, EGCG significantly decreased PCNA and Cdk4 protein levels (p<0.05). In vivo, EGCG treatment dramatically reduced the volume and weight of tumors at 4 and 8 weeks post-treatment (p<0.05). The PCNA and Cdk4 protein levels were significantly reduced in EGCG treated group (p<0.05).
Conclusion
EGCG effectively inhibits the proliferation and induce apoptosis in rat ELT3 uterine leiomyoma cells in vitro and in vivo.
Keywords: Apoptosis, Epigallocatechin gallate (EGCG), Green Tea Extract, Inhibitory Effect, Uterine Leiomyoma
INTRODUCTION
Tea is one of the most popular beverages consumed worldwide. Based on the manufacturing process, green, black and oolong tea are the three major commercial types of tea. Green tea, without fermentation, is processed to prevent the oxidation of green leaf polyphenols, while majority of polyphenols are oxidized in black tea or oolong tea during fermentation production.1 This fermentation converts catechin to theaflavins and thearubigins, consequently decreasing the catechin content. The polypehnols present in green tea are flavonols, commonly known as catechins, which contains 5 major subtypes: catechin, epicatechin, epicatechin gallate, epigallocatechin and epigallocatechin gallate (EGCG) 1. These natural compounds show diverse chemical and biological activities and are nontoxic under daily dose.2 In recent years, the evidences from epidemiological and animal studies have emerged, showing chemopreventive and anticancer potential of dietary polyphenols.3 Several studies have suggested positive correlations between human consumption of green tea and a lower incidence of gastric, esophageal, ovarian, pancreatic, and colorectal cancers.4-7 EGCG, the major polyphenol in green tea, was found in animal studies to effectively and broadly inhibit carcinogenesis in various organs such as the esophagus, stomach, duodenum.8
Uterine leiomyoma (fibroids) are the most common tumors of the reproductive tract in women of reproductive age.9 It clinically affects 25%-30% of American women; however, incidence of upward of 77% has been reported.10 Although, uterine fibroids are benign tumors and often asymptomatic, they may cause debilitating symptoms: such as abnormal uterine bleeding, abdominal pain and in some cases infertility. Pregnancy complications attributed to uterine fibroids include miscarriage, preterm labor and postpartum hemorrhage. Approaches available for the treatment of uterine fibroids include pharmacologic options, surgical approaches, and uterine artery embolization.11 We have recently reported on the utility of gene therapy approach as a potential alternative treatment for uterine leiomyoma.12,13 However, approaches that are minimally invasive, easy to perform, preserve fertility would be preferable and cost effective. A dietary agent, such as green tea, if proven effective against uterine fibroids, would be a welcome addition since it is safe, inexpensive, well tolerated and readily available.14,15
Cancer chemoprevention is defined as the use of natural, synthetic, or biological chemical agents to reverse, suppress, or prevent either the initial phase of carcinogenesis or the progression of premalignant cells to cancer.16 The basic difference between cancer chemoprevention and cancer treatment lies in that the goal of the former approach is to lower the rate of cancer incidence by delaying or suppressing the process of cancer development. Leiomyoma could be an ideal candidate disease for chemoprevention because of its high prevalence and slow progression. The effects of EGCG on leiomyoma cells in vivo (in an animal model), however, remains unclear. This study was designed to investigate the effects of EGCG in vivo on leiomyoma lesions developing in a nude mice-based model.
MATERIALS AND METHODS
Materials
Smooth muscle cell basal medium (SmBM) were purchased from Lonza (Walkersville, MD, USA). (–)-Epigallocatechin gallate (EGCG) was purchased from Sigma (Sigma, St. Louis, MO). EGCG was dissolved in distilled water and filtered through 0.22um filter to have 10mM stock solution. All other chemicals and biochemicals were of the highest quality available from commercial resources. The 60-day released 17ß-estrodials were purchased from Innovative Research of America (Sarasota, FL.)
Cell Culture of Uterine Leiomyoma Cells
The Eker rat tumor-derived ELT3 uterine leiomyoma cell line was kindly provided by Dr. Cheryl Walker (MD Anderson Cancer Center, Houston TX).17 The cells were cultured in SmBM medium supplemented with 5%FBS, 0.1% insulin, 0.2% hFGF-B, 0.1% GA-1000 and 0.1% hEGF (Lonza, Walkersville, MD, USA). The cells were maintained at 37°C and 5% CO2-95% humidified air.
Morphological Observation
For assessing morphological changes, the cells treated with desired concentrations of EGCG for various durations were observed using a phase-contrast microscope and photographs were taken using Nikon Eclipse TE2000-S microscope.
Leiomyoma Cell Proliferation Assay
The proliferative response of ELT3 cells to EGCG was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay, Sigma) as we have described previously.12 Briefly, ELT3 cells were plated at density of 2×103cells/well in 96-well plates. After 24 hours, the cells were treated with various concentrations of EGCG (0, 1.0, 50, 100 and 200μm) for up to 7 days. Media were changed every other day. At designed time points, the cells treated with various concentrations of EGCG were incubated with 50μl/well of 0.5% MTT solution for 4 hours at 37°C. The MTT solution was removed and the dye was solubilized with 150μl/well of DMSO for 5 minutes. The optical density (OD) of each well was measured with spectrophotometer at 570nm. For each concentration of EGCG, three wells were assayed. Mean values of OD for each concentration were calculated. The MTT data for EGCG treatment were collected at the following time points; day 1, 3, 5 and 7 post-treatment.
Western Blotting for PCNA and Cdk4 in Uterine Leiomyoma Cells and Tumor Tissue
For western blotting, ELT3 rat leiomyoma cells treated with indicated concentrations of EGCG for 48 h were lysed and proteins were harvested. Proteins from tumor tissues developed in nude mice were isolated using standard protocol at 4 and 8 weeks after the inoculation of ELT3 cells. Equivalent amounts of protein extracts, from ELT3 cells or tumor tissue, were separated by NuPAGE Novex 10% Bis-Tris Gel (Invitrogen Life Technologies, Carlsbad, CA) under a reducing condition using 200V for 50 min, as we have described earlier.18 The proteins were then electrophoretically transferred onto PVDF membranes (Millipore Corp., Billerica, MA) using the XCell II Blot Module (Invitrogen). After blocking nonspecific binding sites by incubation for 1 h with PBS containing 5% fat-free milk and 0.1% Tween 20, the membranes were incubated with the corresponding primary antibodies overnight at 4°C. Immunological detection was performed using primary antibodies against human proliferation cell nuclear antigen (PCNA) (1:500 dilution) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and Cdk4 (1:1000 dilution, Sigma, St. Louis, MO). The membranes were then incubated for 1 h with horseradish peroxidase conjugated secondary antibodies diluted 1:5000 with blocking buffer. The antigen-antibody complexes were detected with the ECL chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ, USA). The membranes were reprobed with a monoclonal antibody raised against β-actin (diluted1:5000, Sigma) as an internal control for protein loading and normalization between samples. Films exposed to blots were scanned and optical densities of the positive signals were quantified.
TUNEL Staining for EGCG Treated Leiomyoma Cells
ELT3 leiomyoma cells were seeded onto BioCoat CultureSlide (BD Bioscience, San Jose, CA) and treated with various concentration of EGCG (0, 1.0, 50, 100 and 200μm) for 48 h. Apoptosis in ELT3 cells was determined by the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) technique using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) as we have described earlier.18,19 Briefly, the slides were fixed in 4% paraformaldehyde solution, and then placed in equilibration buffer. The slides were incubated with rTdT incubation buffer consisted of equilibration buffer, nucleotide mix and rTdT enzyme at 37°C for 1 h. Positive control was performed using 10 units/ml DNase I treated slides (Sigma, St. Louis, MO) and negative control omitted rTdT enzyme. The reaction was terminated with 2x SSC buffer. The nuclei were stained by 1 μg/ml of propidium iodide solution (Sigma, St. Louis, MO). Slides were examined under an Eclipse TE2000-S, fluorescence microscope (Nikon, Melville, NY). Cells with green fluorescence were scored as apoptotic.
Animal Experiments
Female athymic nude mice (Hsd:Athymic Nude-Foxn1nu/Foxn1+), 5 to 6 weeks old were purchased from Harlan Sprague Dawley (Indianapolis, Indiana). The mice were maintained in a specific pathogen germ-free environment. The animal experiments were conducted according to the guideline of experimental animals by the IACUC at Meharry Medical College. After quarantine, one 60-day estrogen pellet (17ß-estradiol 1.7mg) (Innovative Research of America, Sarasota, FL) was surgically implanted under the neck skin of each mouse under aseptic conditions. Three days later, all mice were anesthetized and inoculated subcutaneously with 1×107 ELT3 uterine leiomyoma cells in 300μl serum-free media, in the right flank region. All surgical procedures were performed under aseptic conditions. The animals were then randomized into two groups, the control group (n = 10) were fed with autoclaved distilled water, while experimental group (n = 10) were fed with 1.25mg EGCG /mouse/day, dissolved in autoclaved distilled water and dispensed in the drinking water bottles, starting from the day of the inoculation. The animals were fed with standard laboratory chow during the experiment. Animals were examined weekly for body weight and the size of tumors was measured with calipers in three dimensions. The tumor volume was calculated from the formula: (length×width×height×0.5326). Five animals from each group were scarified at 4th week post injection. The tumors were excised, weighed, and stored in 10% Formalin solution or at −80°C until further biochemical analysis. The remaining five animals in each group were allowed to remain in the study until 8 weeks. The tumors and organs were collected at this point for histological and biochemical analysis. The experiment was terminated at 8 weeks when control mice showed severe morbidity with tumor volume exceeding 30% of body weight as per approved animal protocol. Animal experiments were repeated twice.
Histological and Immunohistochemical Analysis
Tumor samples for histology and immunohistochemistry were fixed in 10% neutral formalin, dehydrated, and embedding in paraffin. The blocks were sectioned at a thickness of 5 μm for hemotoxylin and eosin staining. For PCNA immunohistochemical staining, the primary PCNA antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was diluted 1:500 in DPBS, incubated for 1 h at room temperature. After washing with DPBS, a biotinylated secondary antibody (diluted 1:200) was used for 10 min, following streptavidin-conjugated peroxidase for 15 min. The sections were stained with diaminobenzidine (DAB) substrate and counterstained using hematoxylin. The PCNA positive cells were counted in 4 randomly selected high power fields as a percentage of total cells as we have described earlier.18
Statistical analysis
The data were expressed as the mean ± SD of the values. Statistic significance was determined using ANOVA. A difference with p< 0.05 was considered statistically significant.
RESULTS
Effects of EGCG on Cell Morphology in Uterine Leiomyoma Cells
ELT3 rat uterine leiomyoma cells displayed oval or short-spindle morphology and grew fast in complete medium (Figure 1 A). The cells treated with 1μM of EGCG displayed similar morphology compared to that of untreated control. Change in cell morphology was observed after 72 h in cells treated with ≥50μM EGCG. These cells grew slowly and less crowded than the cells treated with lower concentrations of EGCG (Figure 1, B, C). The cell morphology changed markedly as blebbing formation and apoptotic vesicles were observed in cells treated with 100μM EGCG for 72 h (Figure1, D). After treatment with 200 μM EGCG for 48 h, the ELT3 cells were no longer robust and cellular crowding. The cells were shrunken and fewer in number, along with irregular shaped nuclei.
Figure 1.
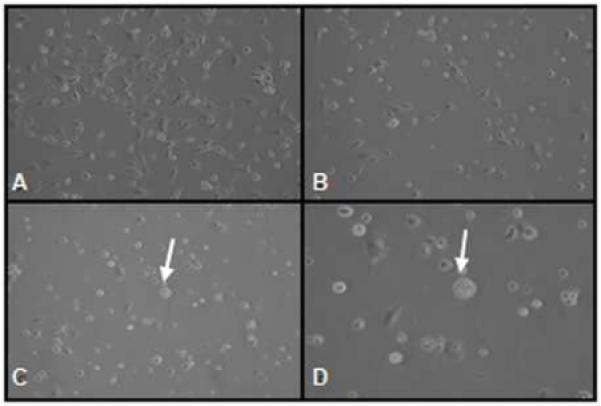
Effect of EGCG on morphology of ELT3 cells at 72 h treatment. The cells were treated with ascending concentration of EGCG for 0(a), 50(b), and 100 μM (c, d). Arrow shows apoptotic vesicles. Pictures were taken using a phase-contrast microscope at 100× (A-C) or 200 × (D) magnifications.
EGCG Inhibits Proliferation of ELT3 Uterine Leiomyoma Cells
To evaluate the effects of EGCG on the proliferation of ELT3 rat uterine leiomyoma cells, the cells were treated with designed concentrations of EGCG (0, 1, 50, 100, and 200μM) for 7 days. There was a gradual decrease in the growth capability through the 7-days culture period compared to vehicle treated ELT3 cells. The inhibitory effect of 200 μM EGCG was observed after 24 h treatment (p<0.05), with 40% reduction in cell proliferation observed in cells after exposure to 200μM EGCG for 72 h. The significant inhibitory effect of ≥50μM EGCG on ELT3 cells was observed by day 3 and continued till day 7 (p<0.05, Figure2).
Figure 2.
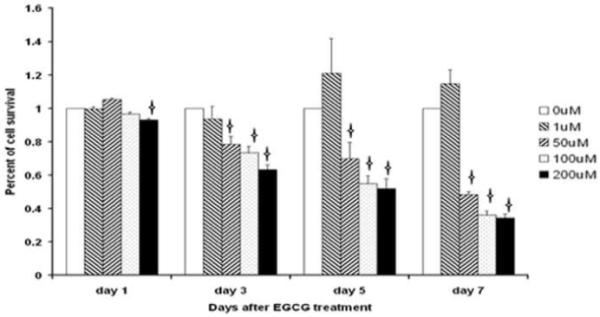
Effects of EGCG on ELT3 cell growth measured by MTT assay. Concentration of EGCG ≥ 50 mM significantly inhibited ELT3 cell growth. Mean ± SD shown. Asterisks indicate significant difference from the vehicle-treated control (p<0.05).
EGCG Decreased PCNA and Cdk4 Protein Expression in Uterine Leiomyoma Cells
Effects of EGCG treatment on PCNA and Cdk4 protein levels in ELT3 rat uterine leiomyoma cells were assessed by western blot analysis. Proteins extracted from ELT3 cells cultured in the presence of indicated concentrations of EGCG for 48 h revealed that ≥50uM EGCG significantly decreased PCNA and Cdk4 protein expression, compared to vehicle control (Figure 3) (p<0.05).
Figure 3.
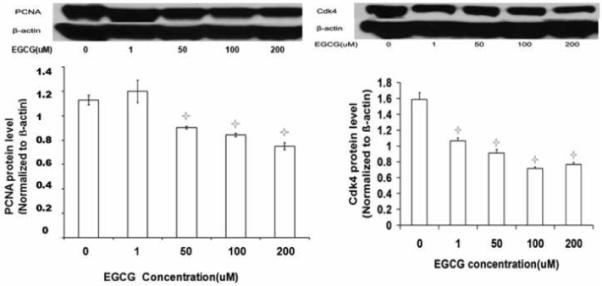
Western blot analyses of the effect of EGCG on PCNA and Cdk4 protein expression in ELT3 cells, in vitro. Asterisks indicate significant difference from the vehicle-treated control (p<0.05).
EGCG Induced Apoptosis in ELT3 Uterine Leiomyoma Cells
The inhibitory effect of EGCG on ELT3 rat uterine leiomyoma cells via apoptosis induction was confirmed by TUNEL staining. ELT3 cells were treated with 0, 1, 50, 100 and 200 μM of EGCG for 48 h. Propidium iodide stains all nuclei red. Fluorescein 12-dUTP incorporation results in localized green fluorescence within the nucleus of apoptotic cells. Enhanced apoptosis was observed in ELT3 cells treated with ≥ 50 μM EGCG (Figure 4 C, D) when compared to vehicle treated cells (Figure 4 A, B).
Figure 4.
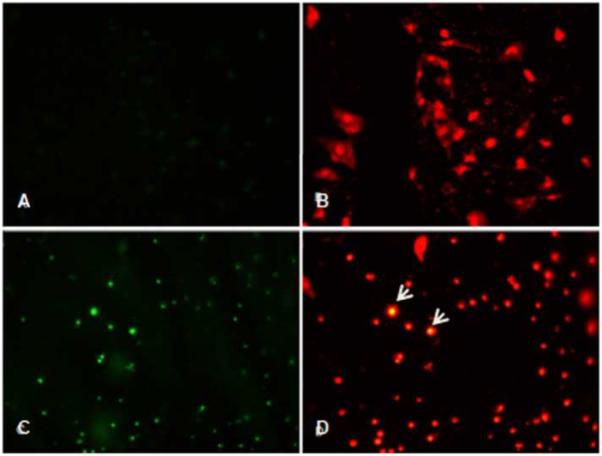
EGCG-mediated apoptosis assessed by TUNEL staining. Apoptotic nuclei are detected with green fluorescence and all nuclei stained with propidium iodide (red fluorescence) in ELT3 cells at 0μM (A, B) and 100μM(C, D) EGCG. Arrows indicate prominent apoptotic nuclei that are double stained. Magnification 200×
EGCG Reduced Fibroid Tumor Size and Weight in the Nude Mice Model
To validate the antitumor effects of EGCG in vivo, 1.25 mg EGCG/mouse was administered orally on daily basis, dispensed in their drinking water. The average water consumption was tested prior to beginning the experiment and was estimated about 3ml/mouse/day. EGCG was dissolved into autoclaved water based on the amount of daily consumption and replaced every other day. All animals developed visible tumors after inoculation of ELT3 cells and appeared otherwise healthy. Figure 5 shows representative mice with tumors in control group (Figure 5 A, C) and EGCG group (Figure 5 B, D) at 4 weeks and 8 post treatment. The progression of tumor size was slow in the EGCG treated group. In the control animals, the tumor volume reached 46±5 mm3 and 288±57 mm3 at 4 weeks and 8 weeks post ELT3 cells inoculation, respectively. On the other hand, in the EGCG treated group, tumors were significantly smaller, 31±5 mm3 and 129±54 mm3 (p<0.05), at the same time points (Figure 6A). One mouse in the EGCG treated group showed no tumor at the end of 8th week. The difference in tumor size was consistent with the tumor weight when the animals were sacrificed at 4 weeks and 8. Tumor weight in EGCG treated group was 0.0036 ± 0.0027g and 0.11 ± 0.04g, versus 0.077± 0.0045 and 0.29 ± 0.06g in water-fed group 4 weeks and 8 post-treatment, respectively (p<0.05). Tumor weight in EGCG treated group was significantly less than those of water-fed group after 4 weeks or 8 weeks of treatment (p<0.05) (Figure 6B).
Figure 5.
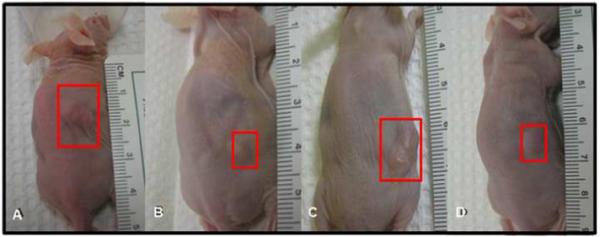
Effect of oral administration of EGCG on the growth of ELT3 cells established tumor in nude mice. Photographs show representative mice with tumors from each group at 4 and 8 weeks post-treatment. (A) water-fed for 4 weeks, (B) EGCG treated for 4 weeks, (C) water-fed for 8weeks, (D) EGCG treated for 8 weeks.
Figure 6.
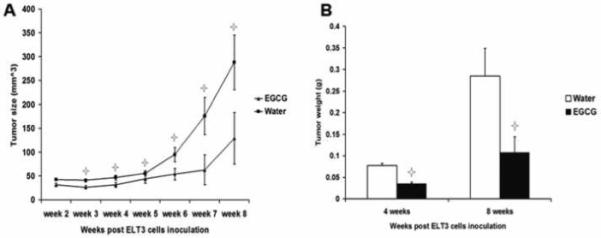
Effect of oral administration of EGCG on the growth of ELT3 cells established tumor in nude mice. Tumor volume measured and calculated from each group weekly post-treatment (A). Tumor weight measured at 4 and 8 weeks post-treatment (B). Values shown are mean ± SD (bars). Asterisks indicate significant change (P<0.05) compared with control.
EGCG Decreases Protein Expression of PCNA and Cdk4 in Fibroid Tumor Tissues
Effects of EGCG treatment on PCNA and Cdk4 expression in fibroid tumors tissues retrieved from ELT3 cells based-nude mice model were assessed by western blot analysis of samples collected at 4 weeks and 8 weeks post-treatment. The results revealed that 1.25mg/mouse/day of EGCG oral consumption significantly decreased PCNA (Figure 7) and Cdk4 (Figure 8) protein expression compared to water-fed control group at 4 and 8 weeks post-treatment (p<0.05).
Figure 7.
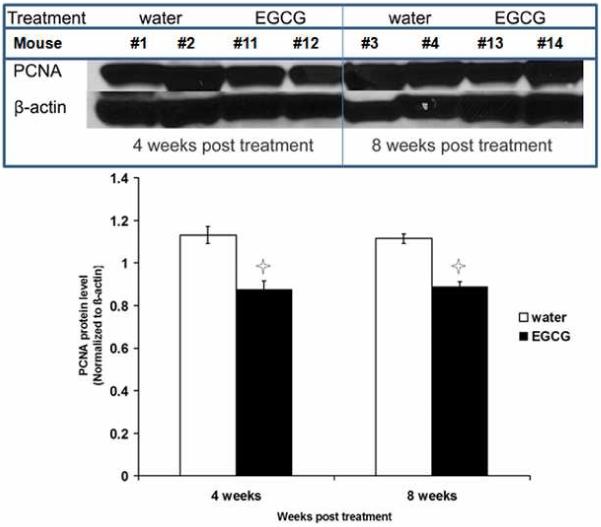
Effect of oral consumption of EGCG on PCNA protein expression in ELT3 cells established tumors. Tumor tissue from individual mice sacrificed at 4 and 8 weeks post-treatment were used per lane in western blot analysis. Equal loading was confirmed by re-probing the membrane and with β-actin. Asterisks indicate significant (P<0.05) difference compared with control.
Figure 8.
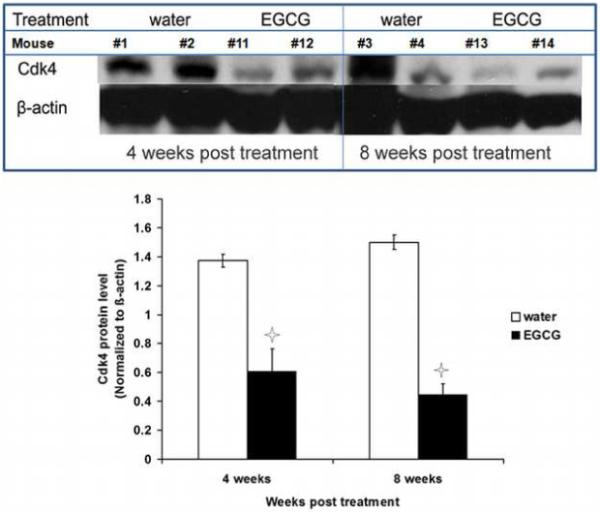
Effect of oral consumption of EGCG on Cdk4 protein expression in ELT3 cells established tumors. Tumor tissues from individual mice sacrificed at 4 and 8 weeks post-treatment were used per lane in western blot analysis. Equal loading was confirmed by stripping the membrane and re-probing with ß-actin. Asterisks indicate significant (P<0.05) difference compared with control.
Immunohistochemical Staining Reveals Decreased PCNA Expression in Fibroid Tumors from EGCG-treated Mice
The inhibitory effect of EGCG on ELT3 cells-based fibroid tumors was confirmed by PCNA immunohistochemical staining. EGCG treated nude mice showed decreased in number of PCNA positive cells at 4 and 8 weeks, compared to the water-fed control. The percentage of positive cells for PCNA was 72.88% ± 4.21% and 58.69% ± 3.55% per high power field in water-fed control and EGCG treated at 4 weeks, respectively (Figure 9 A, B). The number of positive cells for PCNA was 99.6% ± 8.77% and 71.3% ± 9.38% per high power field in the control group and EGCG treated group at 8 weeks, respectively (Figure 9 C, D). This difference was statistically significant (p<0.05) at both 4 and 8 weeks post-treatment compared to vehicle treated control group (Figure 9 E).
Figure 9.
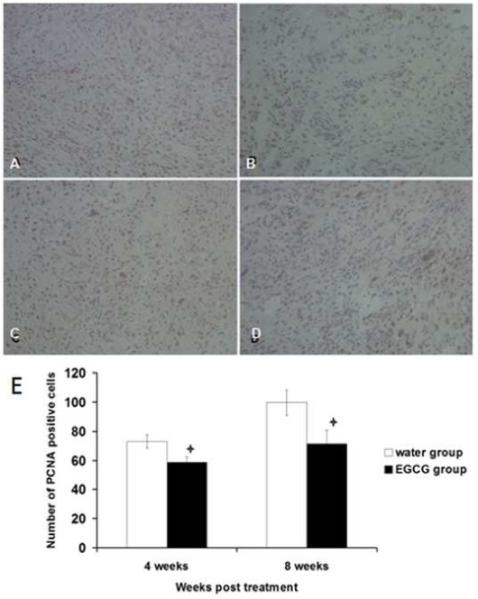
Effect of EGCG on PCNA expression by ELT3 Cells in vivo detected using immuno-histochemical staining. Representative fields from each group, at 4 and 8 weeks post-treatment. (A) water-fed control after 4 weeks, (B) EGCG treated after 4 weeks, (C) water-fed control for 8weeks, (D) EGCG treated for 8 weeks. Positive cells were brown. Magnification 200×. E) Effect of EGCG on PCNA expression by ELT3 cells in vivo at 4 and 8 weeks post-treatment. The positive cells were counted from 4 fields per each slide randomly. Asterisk indicate significance (P<0.05) compared with control.
COMMENTS
Here we report for the first time that EGCG has robust inhibitory effects on ELT3 cells in vitro and in a nude mouse model. The increased efficacy of EGCG towards inhibition of ELT3 cells growth in vitro was dose-dependent and was mediated through apoptosis and inhibition of proliferation at various EGCG concentrations. Recent studies have demonstrated a wide variety of positive effects of EGCG on diverse physiological and pathological situations. Several mechanisms for EGCG bioactivities have been proposed. For example the anti-tumor effects of EGCG seem to be mediated through decreasing the activities of Cdk2 and Cdk4;20 inducing apoptosis in a variety of tumor cells21-23; as well as blocking telomerase.24 EGCG also inhibits matrix metalloproteinases, in particular MMP-2 and MMP-9, which along with their antiangiogenic properties can block tumor cells invasion.25-28 Actually, EGCG modulate tumor cell proliferation through multiple signal pathways.29 We have recently reported higher expression of Catechol-O-methyltransferase (COMT) in human uterine fibroid tissues compared to adjacent normal myometrium.30-32 Additionally we reported that higher incidence of functional high activity COMT polymorphic markers might explain the increased prevalence of uterine fibroids in African Americans.30 We have also reported that selective COMT inhibitors are able to inhibit proliferation in both human and rat uterine leiomyoma cells.30 Interestingly, the steric structure of EGCG enable it to exert a strong inhibition of COMT activity as a substrate or by tightly binding with COMT.33-35 We propose that the strong anti-leiomyoma effects of EGCG could conceivable be mediated via COMT inhibition.
Although EGCG appears to affect many biological processes of various cell types, the dose and action of orally administered EGCG might differ from direct effects on cells in vitro. The great majority of EGCG in vitro studies, including ours, have shown in vitro activities at dosages from 10-100 μM, substantially higher than those obtainable in vivo. These results do not reflect typical catechin level found in animal or human plasma. Although there was a remarkable dose-dependent antiproliferation effect of EGCG on ELT3 cells in vitro, the in vivo procedure might not be as clear-cut due to the individual differences in ingestion, absorption and metabolism. That is why the evaluation of EGCG utility against fibroid tumors in vivo in an animal model was critical. Several animal studies have been done on metabolism and pharmacokinetic profile of EGCG in vivo. After intragastric administration of EGCG in rats at dose of 75mg/kg, the plasma bioavailability of EGCG was about 1.6%, while high levels were observed in the intestine and kidney.36 In the mice model, after administration of EGCG intragastrically at 163.8 μmol/kg, the plasma bioavailability of EGCG was about 26.5% after one dose consumption, higher than previously reported in rats.37 In humans, the maximum plasma concentration of EGCG was 77.9 ng/ml, after oral administration of a single dose of EGCG (2mg/kg).38 Other studies also found that in healthy individual, the plasma level of EGCG reached at 0.1-5.0 μM after moderate green tea consumption.37-39 Since catechins are relatively unstable and differ quantitatively during experiments, it is difficult to compare the relation of ingested dose and biological effect between studies. Nevertheless, several animal experiments have shown promising antitumor effects of orally administered EGCG or other green tea extracts.24,40,41 Theoretically, higher plasma level of EGCG might be reached by taking high dose of EGCG, however, studies showed that consumption of higher level of EGCG dose did not necessarily give substantially higher plasma concentration.41,42 It is highly encouraging that in our work, a relatively modest dose of EGCG of 1.25mg/mouse/day delivered in drinking water was successful in inducing a dramatic and sustained reduction in fibroid tumor size up to 8 weeks of treatment. The EGCG is a nontoxic, natural anticancer agent, and it appears to be safe and typically associated with minimal innocent side effects. Recently, healthy individuals who consumed decaffeinated, purified 800mg of EGCG daily for 4 weeks reported mild side effects such as upset stomach, nausea, abdominal pain, headache, and dizziness.43 A higher oral dose of 1600mg EGCG was also used without reported toxicity.44 In our studies, there was no observed liver or kidney tissue changes as examined by H&E staining in EGCG treated group after 8 weeks treatment (data not shown).
On the basis of the present study, we conclude that EGCG and green tea extract may have potential as oral agents for prevention or treatment of uterine leiomyoma. EGCG might be particularly useful for long term use in women low fibroid tumor burden to arrest tumor progression and avoid the development of severe symptoms necessitating major surgery. Further evaluation of EGCG in well designed clinical trials in women with uterine fibroids is warranted.
Acknowledgments
Financial support for Study: RCMI grant G12 RR03032 and NIH/NICHD 1 R01 HD046228 to AA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONDENSATION
Epigallocatechin gallate (EGCG), a green tea extract, inhibits uterine leiomyoma cell proliferation in vitro and in nude mice providing potential treatment for human uterine leiomyoma.
REFERENCES
- 1.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21(3):334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71(10):1397–421. doi: 10.1016/j.bcp.2006.02.009. Review. [DOI] [PubMed] [Google Scholar]
- 3.Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769–775. doi: 10.1006/pmed.1997.0242. [DOI] [PubMed] [Google Scholar]
- 4.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Waladkhani AR, Clemens MR. Effect of dietary phytochemicals on cancer development. Int J Mol Med. 1998;1:747–753. doi: 10.3892/ijmm.1.4.747. [DOI] [PubMed] [Google Scholar]
- 6.Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- 7.Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, Blot WJ, Fraumeni JF., Jr. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997;70:255–258. doi: 10.1002/(sici)1097-0215(19970127)70:3<255::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339–344. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 9.Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–8. doi: 10.1016/S0140-6736(00)03622-9. Review. [DOI] [PubMed] [Google Scholar]
- 10.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–8. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 11.Parker WH. Uterine myomas: management. Fertil Steril. 2007;88(2):255–71. doi: 10.1016/j.fertnstert.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 12.Salama SA, Kamel M, Christman G, Wang HQ, Fouad HM, Al-Hendy A. Gene therapy of uterine leiomyoma: adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells in vitro and in a nude mouse model. Gynecol Obstet Invest. 2007;63(2):61–70. doi: 10.1159/000095627. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update. 2006;12(4):385–400. doi: 10.1093/humupd/dml015. [DOI] [PubMed] [Google Scholar]
- 14.Deeba N, Naghma Khan Syed, Afaq Farrukh, Mukhtar Hasan. Chemoprevention of Prostate Cancer through Dietary Agents: Progress and Promise. Cancer Epidemiology Biomarkers & Prevention. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 15.Mukhtar H, Ahmad N. Green tea in chemoprevention of cancer. Toxicol Sci. 1999;52(2 Suppl):111–7. doi: 10.1093/toxsci/52.2.111. [DOI] [PubMed] [Google Scholar]
- 16.Bonovas S, Tsantes A, Drosos T, Sitaras NM. Cancer chemoprevention: a summary of the current evidence. Anticancer Res. 2008;28(3B):1857–66. [PubMed] [Google Scholar]
- 17.Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C. Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am J Pathol. 1995;146(6):1568–79. [PMC free article] [PubMed] [Google Scholar]
- 18.Azab SS, Salama SA, Abdel-Naim AB, Khalifa AE, El-Demerdash E, Al-Hendy A. 2-Methoxyestradiol and multidrug resistance: can 2-methoxyestradiol chemosensitize resistant breast cancer cells? Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9898-3. [DOI] [PubMed] [Google Scholar]
- 19.Salama SA, Nasr AB, Dubey RZK, Al-Hendy Estrogen metabolite 2-methoxyestradiol induces apoptosis and inhibits cell proliferation and collagen production in rat and human leiomyoma cells: a potential medicinal treatment for uterine fibroids. J Soc Gynecol Investig. 2006;13(8):542–50. doi: 10.1016/j.jsgi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (−)-epigallocatechin-3-gallate. J Cell Biochem. 1999;75(1):1–12. [PubMed] [Google Scholar]
- 21.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst (Bethesda) 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Zhao DY, Elliott S, Zhao W, Curiel TJ, Beckman BS, Burow ME. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int J Oncol. 2007;31(4):705–11. [PubMed] [Google Scholar]
- 24.Naasani I, Oh-Hashi F, Oh-Hara T, Feng WY, Johnston J, Chan K, Tsuruo T. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003;63(4):824–30. [PubMed] [Google Scholar]
- 25.Garbisa S, Biggin S, Cavallarin N, Sartor L, Benelli R, Albini A. Tumor invasion: molecular shears blunted by green tea [letter] Nat Med. 1999;5:1216. doi: 10.1038/15145. [DOI] [PubMed] [Google Scholar]
- 26.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 27.Jung YD, Kim MS, Shin BA, et al. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–50. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–9. [PubMed] [Google Scholar]
- 29.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting Multiple Signaling Pathways by Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate. Cancer Res. 2006;66(5):2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 30.Al-Hendy A, Salama SA. Catechol-O-Methyltransferase polymorphism is associated with increased uterine leiomyoma risk in risk in different ethnic groups. J Soc Gynecol Invest. 2006;13(2):136–144. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Salama SA, Ho S, Wang HQ, Tenhunen J, Tilgmann C, Al_Hendy A. Hormonal regulation of catechol-O-methyl transferase activity in women with uterine leiomyomas. Fertility and Sterility. 2006;86(1):259–262. doi: 10.1016/j.fertnstert.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 32.Othman E, Al-Hendy Molecular genetics racial disparities of uterine leiomyomas. Best Pract Res Clin Obstet Gynecol. 2008;22(4):589–601. doi: 10.1016/j.bpobgyn.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69(10):1523–31. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Zhu BT, Shim JY, Nagai M, Bai HW. Molecular modelling study of the mechanism of high-potency inhibition of human catechol-O-methyltransferase by (−)-epigallocatechin-3-O-gallate. Xenobiotica. 2008;38(2):130–46. doi: 10.1080/00498250701744641. [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos. 2003;31(5):572–9. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 36.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 37.Yang CS. Inhibition of carcinogenesis by tea. Nature. 1997;389(6647):134–5. doi: 10.1038/38154. [DOI] [PubMed] [Google Scholar]
- 38.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7(4):351–4. [PubMed] [Google Scholar]
- 39.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130(8S Suppl):2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 40.Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M, Mukhtar H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res. 2007;13(5):1611–9. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 41.Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006;34(1):8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 42.Goodin MG, Rosenberg RJ. Epigallocatechin gallate modulates CYP450 isoforms in the female Swiss-Webster mouse. Toxicol Sci. 2003;76(2):262–70. doi: 10.1093/toxsci/kfh001. Epub 2003 Nov 4. [DOI] [PubMed] [Google Scholar]
- 43.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9(9):3312–9. [PubMed] [Google Scholar]
- 44.Ullmann U, Haller J, Decourt JP, Girault J, Richard-Caudron AS, Pineau B. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003;31(2):88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]


