Abstract
Background & Aims
Porphyria cutanea tarda (PCT) is the most common of the human porphyrias and results from an acquired deficiency of hepatic uroporphyrinogen decarboxylase (UROD). Some susceptibility factors have been identified; we examined associations among multiple factors in a large cohort of patients.
Methods
Multiple known or suspected susceptibility factors and demographic and clinical features of 143 patients (mean age 52 years, 66% male, 88% Caucasian) with documented PCT (mean onset at 41±8.8 yrs) were tabulated; associations were examined by contingency tables, classification and regression tree (CART) analysis and logistic regression.
Results
The most common susceptibility factors for PCT were ethanol use (87%), smoking (81%), chronic hepatitis C virus (HCV) infection (69%), and HFE mutations (53%; 6% C282Y/C282Y and 8% C282Y/H63D). Of those who underwent hepatic biopsy or ultrasound, 56% had evidence of hepatic steatosis. Of those with PCT, 66% of females took estrogen, 8% were diabetic, 13% had human immunodeficiency virus (HIV) infection, and 17% had inherited uroporphyrinogen decarboxylase (UROD) deficiency (determined by low erythrocyte UROD activity). HCV infection in patients with PCT was significantly associated with other behavior-related factors such as ethanol use (odds ratio [OR] 6.3) and smoking (OR 11.9).
Conclusions
Susceptibility factors for PCT were similar to previous studies; most patients had 3 or more. Associations between PCT and HCV, ethanol or smoking could be accounted for by a history of multiple substance abuse; other factors are distributed more randomly amongpatients.
INTRODUCTION
Porphyria cutanea tarda (PCT) is an iron-related disorder that results from decreased activity of hepatic uroporphyrinogen decarboxylase (UROD), the fifth enzyme of the heme synthetic pathway. This condition develops when hepatic UROD activity becomes less than ~20% of normal, porphyrins accumulate in the liver and are then transported to the skin where they are photoactivated by long-wave ultraviolet light, forming activated oxygen species that cause characteristic skin fragility and blistering. Cutaneous lesions are found on sun exposed areas such as the dorsa of the hands, forearms, face, neck and feet1. Massive accumulation of porphyrins in the liver also is associated with low-grade hepatocellular damage. Certain susceptibility factors themselves cause liver damage, and risks of progressive liver disease, cirrhosis and hepatocellular carcinoma are increased 2, 3.
A number of genetic and environmental susceptibility factors are known or strongly suspected to be important in PCT, and causative mechanisms have been proposed for most 1. For example, HFE mutations, as found in hemochromatosis, and alcohol can increase hepatic iron. Alcohol, hepatitis C and estrogens may increase oxidative stress and the production of reactive oxygen species in hepatocytes. Smoking and ethanol also induce cytochrome P450 enzymes, including CYP1A2, which is essential for causing uroporphyria in rodent models 4, 5 and may contribute to production of a recently characterized UROD inhibitor 1, 6. Susceptibility factors have been described individually in most case series, and associations among them have been given little attention.
We hypothesized that some of the multiple susceptibility factors in PCT might be interrelated and therefore not randomly distributed among patients with this condition in this geographic area. Information on 143 patients with well documented PCT was compiled, including 39 patients reported previously by Egger et al 7, which was a sufficiently large number to investigate associations among susceptibility factors.
METHODS
Medical records and reports from the Porphyria Laboratory were available from 143 patients with well documented PCT seen at the University of Texas Medical Branch (UTMB) during the past 20 years. All patients presented initially with typical blistering lesions on sun exposed skin, most commonly on the dorsa of the hands, forearms and face. PCT was documented by substantial increases in urinary or plasma uroporphyrin (octacarboxyl porphyrin) and heptacarboxyl porphyrin. When measured, increases in plasma total porphyrins (with a characteristic fluorescence emission spectrum at neutral pH) and fecal isocoproporphyrins were noted. Recurrences were documented by reappearance of skin lesions and elevations in urine or plasma porphyrins. The study was approved by the UTMB Institutional Review Board.
Current and past use of alcohol, estrogen and smoking was recorded as positive or negative; details regarding quantities and duration were often not described in the medical records. Treatment, usually by phlebotomy, recurrences and concurrent medical diagnoses were recorded. Information was recorded on data collection forms and entered into a database for analysis. Because hepatic steatosis has been suggested to increase susceptibility for PCT 8, liver biopsy and hepatic ultrasound results were examined, when available. Routine laboratory testing was performed by the UTMB Hospital clinical laboratories. Anti-HCV (EIA, Abbott Laboratories, Chicago, IL) was detected by a second-generation enzyme-linked immunoabsorbent assay and HCV infection was confirmed in 51% of cases by HCV RNA as detected by polymerase chain reaction (PCR). No patient found to have HCV antibody was negative for HCV RNA. HIV infection was detected by finding antibody to HIV and confirmed by demonstrating circulating HIV RNA. Erythrocyte UROD activity was measured using whole blood lysates and values less than 33 nmol/ml erythrocytes/hr were considered to indicate an inherited partial deficiency of UROD 7.
Data were summarized as means and standard deviations. Means of continuous data were compared using student’s two-group t-test and categorical data using χ2. Statistical associations among these factors and demographic characteristics were analyzed by contingency tables, classification and regression tree (CART) analysis and multiple logistic regression.
RESULTS
The majority of the patients were Caucasian (88%), with African Americans and Hispanics accounting for only 4% and 6% respectively. Males comprised 66% of all patients, and predominated in all racial and ethnic categories. Mean onset of symptoms was in the fourth decade of life in both men and women (mean 41 years; range 18–72). The mean age at the time of initial visit to this medical center was 52.2 years (range 33–86). Body mass index (BMI) assessed in 89 patients was 25.3 ± 3.9 (mean ± SD, range 17–36.7) and was not significantly different in males (25.1 ± 3.5, 17–35.3) and females (25.8 ± 5, 18–36). A family history of PCT was reported in 9.8% (including 3 siblings in the same family), blistering skin lesions in 9.6%, iron overload in 8.3% and liver disease in 19% of the 143 patients.
Susceptibility factors were considerably more prevalent in this group of 143 patients than in the general population, as shown in Table 1. These included several related to health behaviors and life style (ethanol use and smoking, and HCV and HIV infections, which were usually associated with a history of intravenous drug use), a class of prescription medication (estrogens) and genetic or metabolic traits (HFE mutations, inherited deficiency of UROD as measured in erythrocytes, hepatic steatosis and diabetes mellitus). HCV genotype was assessed in 40 patients; 31 were genotype 1, 6 genotype 2, 2 genotype 3 and 1 genotype 4, which is similar to the distribution of genotypes in HCV-infected patients without PCT seen at UTMB.
Table 1.
Known or suspected susceptibility factors identified among 143 patients with porphyria cutanea tarda .
| Susceptibility factors | Cases studied |
Cases affected | Prevalence in US |
Ref | |
|---|---|---|---|---|---|
| (n) | (n) | (%) | (%) | ||
| Health behavior-related | |||||
| Alcohol use | 131 | 115 | 87.7 | 25 | 19 |
| Smoking | 121 | 98 | 80.9 | 24.5 | 20 |
| Hepatitis C infection | 135 | 93 | 68.8 | 1.6 | 16 |
| HIV | 95 | 12 | 12.6 | 0.3 | 21 |
| Prescribed drugs | |||||
| Estrogen use (in females) | 47 | 31 | 65.9 | ? | |
| Genetic or metabolic | |||||
| Low erythrocyte UROD | 106 | 18 | 16.9 | ? | |
| HFE mutations | 98 | 50 | 51.0 | 18 | 22 |
| Diabetes mellitus | 111 | 8 | 7.2 | 6.5 | 23 |
| Hepatic steatosis (liver biopsy) | 28 | 9 | 32.1 | 17–33 | 24 |
The prevalence of diabetes mellitus was 7.2% in this series, which is not very different from the prevalence of diabetes mellitus in the US population (Table 1) and less than the 17% recently reported in PCT patients in Sweden 8. We examined evidence for steatosis, which may be associated with diabetes mellitus, in the 63 patients in this series who underwent liver biopsy, hepatic ultrasound or both. Such evidence was found by one or both methods in 35 (56%). Of the 60 patients who underwent ultrasound, 32 (54%) had increased echogenicity, suggesting steatosis. Of the 28 patients who underwent biopsy, 7 had histological evidence of mild steatosis (affecting 5–15% of hepatocytes), 2 had moderate macrovesicular steatosis (affecting 16–30% of hepatocytes, and none had severe steatosis. Steatosis observed by biopsy often correlated with increased echogenicity by ultrasound in the 19 patients who underwent both procedures, but given the small numbers the agreement was only slight (κ = 0.38). Steatosis by ultrasound was significantly associated with alcohol intake in the 57 patients with known alcohol status who underwent ultrasound (p<0.0003), but there was no such association in the 28 who underwent liver biopsy.
Scoring of multiple susceptibility factors
A simple scoring method was used to illustrate the multiplicity of factors usually found in individual patients. Each coexisting susceptibility factor, including heterozygosity for an HFE mutation, was scored as one; homozygous (C282Y/C282Y or H63D/H63D) or compound heterozygous (C282Y/H63D) genotypes were scored as two, as in the previous study of Egger et al 7, since these are more likely to be associated with iron overload and have been more strongly associated with PCT 9, 10. Iron status before treatment could often not be assessed because treatment had already been started in many patients before full evaluation for susceptibility factors. Whether this or another system of scoring can predict initial occurrence or recurrences of PCT has not been studied.
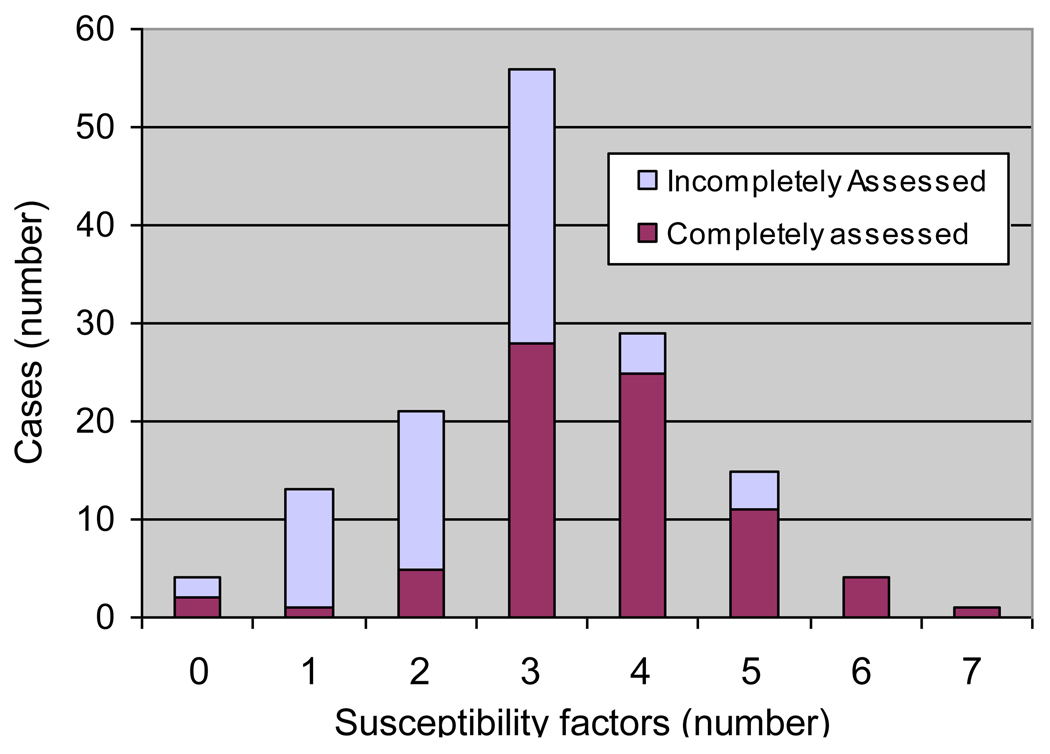
Multiple susceptibility factors were indeed found in the great majority of patients. As shown in Figure 1, 75% of patients had 3 or more of these susceptibility factors with a maximum of 7 factors found in 1 patient. As expected in a retrospective study of patients seen over many years, information was incomplete for some patients, and those with fewer identified susceptibility factors were more likely to have missing information due to incomplete assessment (Figure 1).
Figure1.
Varying numbers of coexisting susceptibility factors in 143 patients with porphyria cutanea tarda. Patients who were assessed for all the susceptibility factors listed in Table 1 (solid portions of bars) had more identified factors than those who were incompletely assessed (open portions of bars).
Analyses of associations
Contingency tables were prepared to look for evidence for associations among known or suspected susceptibility factors and clinical characteristics. As shown in Table 2, HCV infection chosen as the target variable was positively associated with alcohol use, smoking, and male gender, and negatively with HIV infection. There was no significant association of HCV infection with diabetes mellitus (Table 2). Age of onset of symptoms was 40.6 ± 7.5 vs. 42.5 ± 10.8 years, and age when first seen 51.2 ± 6.7 vs. 52.4 ± 11.8 years in those with and without HCV infection, respectively, which were not significant differences. With other factors as target variables, estrogen use was associated with Caucasian race (OR 6.4) and absence of HCV infection (OR 4.2), and diabetes with inherited UROD deficiency (OR 8.9, all p<0.001).
Table 2.
Clinical features and susceptibility factors in 135 patients with PCT as related to HCV infection as the target variable.
| Clinical features and susceptibility factors |
Total studied |
Affected | HCV positive |
HCV negative |
p |
|---|---|---|---|---|---|
| (n) | (percent of those affected) | ||||
| Sex (M) | 135 | 93 | 80.7 | 19.3 | 0.0001 |
| Alcohol use | 128 | 112 | 75.0 | 25.0 | <0.0001 |
| Smoking | 118 | 96 | 80.2 | 19.8 | <0.0001 |
| HIV infection | 95 | 12 | 33.3 | 66.7 | 0.017 |
| Estrogen use | 45 | 29 | 41.4 | 58.6 | 0.75 |
| HFE mutations | 89 | 50 | 68.0 | 32.0 | 0.82 |
| Low erythrocyte UROD | 105 | 18 | 61.1 | 39.9 | 0.58 |
| Diabetes mellitus | 104 | 8 | 75 | 25 | 0.99 |
| Obesity (BMI ≥25) | 81 | 39 | 66.7 | 33.3 | 0.63 |
| Steatosis (liver biopsy) | 19 | 6 | 83 | 17 | 0.31 |
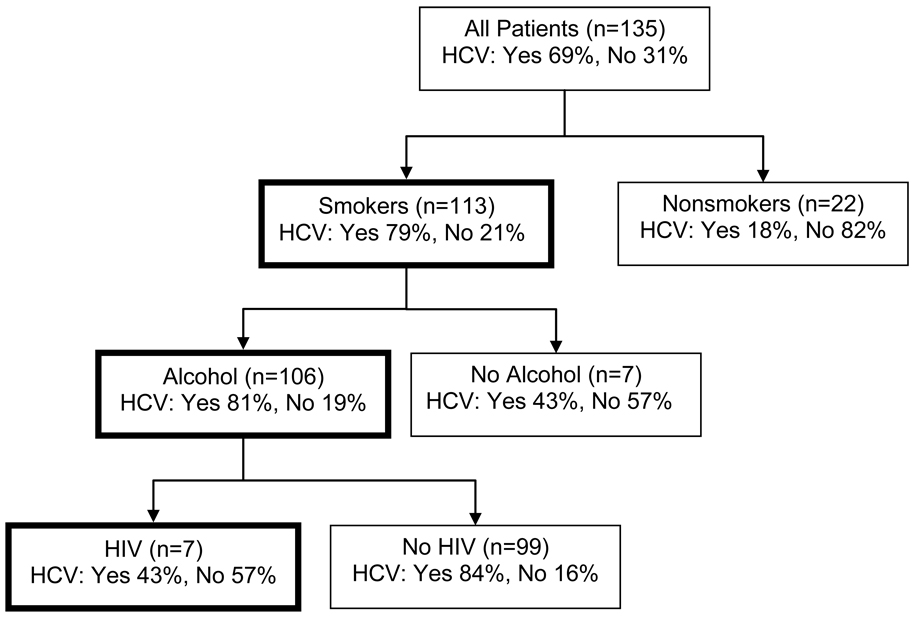
The summary detail of the CART analysis, with HCV infection as the target variable and other susceptibility factors as predictors (Figure 2), revealed associations of HCV infection with smoking, alcohol use, and absence of HIV infection.
Figure 2.
Classification and regression tree (CART) exploration of associations among susceptibility factors in porphyria cutanea tarda. HCV infection was used as the target variable and the predictive contributions of smoking, alcohol use and HIV infection are shown.
In the logistic regression analysis of binary variables, HCV infection (present vs absent) was statistically significantly associated with the explanatory variables of alcohol use (OR, 6.3; P = .02), smoking (OR, 11.9; P = .007), HIV infection (OR, 4.9; P = .02), and Caucasian race (OR, 16.8; 95% confidence interval, 6.8 – 41.4; P = .0001). No other significant associations among susceptibility factors were found.
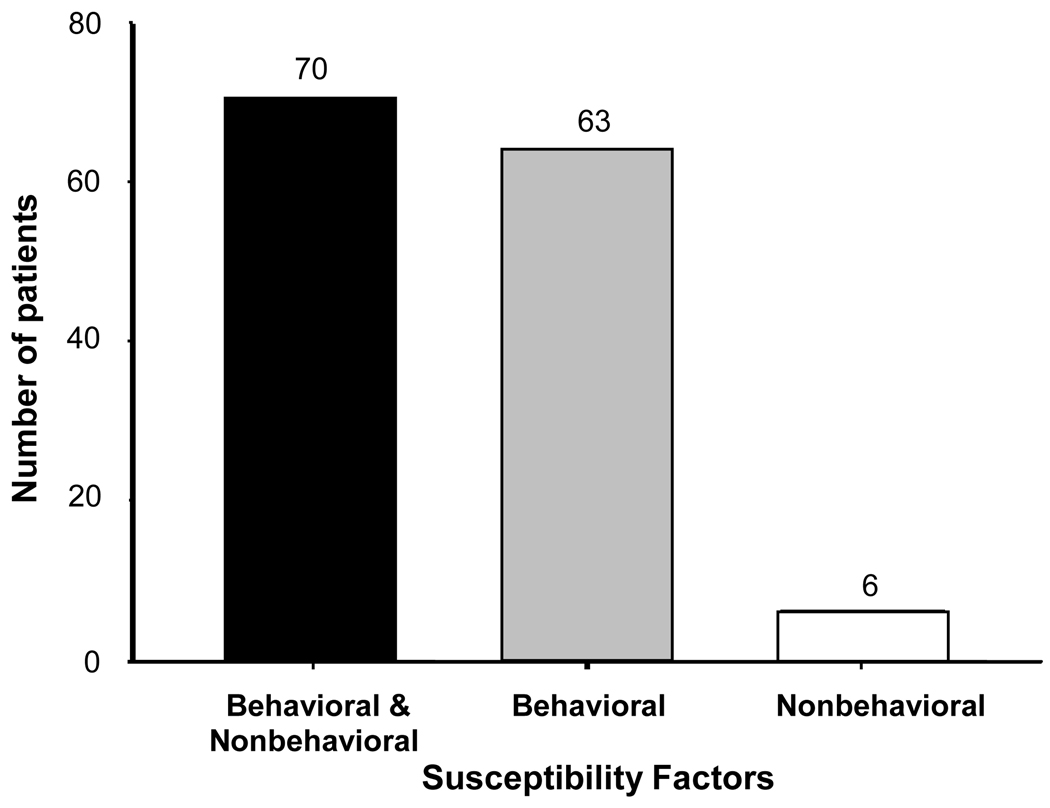
The 3 susceptibility factors that were clustered in this patient population by these analyses, namely alcohol use, smoking and hepatitis C infection, as well as HIV infection, commonly reflect high risk behavior. HIV infection was more prevalent in patients with hepatitis C than in the general population, but was even more prevalent in those without hepatitis C (Table 2), which suggests these are separate susceptibility factors. As shown in Figure 3, these behavior-related factors (one or more) were combined with one or more others (estrogen use, inherited UROD deficiency, or HFE mutations) in 70 patients, were present without other factors in 63 patients, and were absent in only 6 patients.
Figure 3.
Frequencies of susceptibility factors related to behavior among 139 patients with porphyria cutanea tarda. Risk factors both related and unrelated to behavior were present in 70 patients (solid bar), only behavior-related risk factors (alcohol use, smoking, hepatitis C and HIV infection) in 63 (gray bar), and only those not related to behavior (HFE mutations, decreased erythrocyte UROD activity and estrogen use) in 6 (open bar).
Treatment and relapse
Patients were advised to stop alcohol, smoking, and estrogen. Repeated phlebotomy until the serum ferritin concentration is reduced to near the lower limit of normal is the preferred therapy at this center, and was prescribed for 89% of patients. Complete remission, with decreases in plasma porphyrin levels to within or close to the normal range and resolution of skin symptoms was achieved in 93% of those treated by phlebotomy. However, 35% experienced one or more later recurrences and responded to another series of phlebotomies. Low-dose hydroxychloroquine or chloroquine was prescribed for 16 patients when phlebotomy was poorly tolerated or was relatively contraindicated by concurrent conditions (e.g. anemia, coronary artery disease, severe pulmonary disease or complications of HIV infection), or when repeated visits to a blood bank were not feasible. This treatment was successful in 7 of 8 patients for whom responses were recorded. A prospective study comparing these treatments is in progress. We advised treating PCT rather than hepatitis C initially because blistering skin lesions are usually most symptomatic, treatment of PCT is more often effective and usually requires less time, anemia during treatment of HCV makes it difficult to treat PCT concurrently, and HCV treatment may be more effective after iron reduction.
DISCUSSION
PCT, the most common of the human porphyrias, is an iron-related disorder and the only type of porphyria that can, and indeed usually does, develop in the absence of a mutation of a heme biosynthetic pathway enzyme. Therefore, it is primarily an acquired disorder for which susceptibility is determined by a number of known factors as well as others that remain to be identified. PCT can be regarded as an uncommon hepato-cutaneous manifestation of common disorders such as alcoholic liver disease, chronic hepatitis C and hemochromatosis 11–13. PCT occurs worldwide, but there are geographic differences in incidence and in the proportion of patients with given susceptibility factors such as HCV infection 14, as illustrated by the representative case series compiled in Table 3. These differences may reflect geographic variations in genetic makeup, health behavior and exposure to viral infections and environmental agents.
Table 3.
Prevalence of susceptibility factors in representative series of patients with porphyria cutanea tarda from different geographic areas.
| Series (year) | Case s |
HCV | Ethanol | Smoking | HIV | Estrogen* | HFE† | Hepatic steatosis |
Diabetes mellitus |
|---|---|---|---|---|---|---|---|---|---|
| (n) | (percent) | ||||||||
| North America (U.S.) | |||||||||
| This series | 143 | 69 | 88 | 81 | 13 | 66 | 51 | 25 | 8.1 |
| Egger et al. (2002) 7 | 39‡ | 74 | 79 | 86 | 25 | 73 | 65 | - § | - |
| Bulaj et al. (2000) 9 | 108 | 59 | 46 | - | - | 63 | 63 | - | - |
| Bonkovsky et al. (1998) 15 | 70 | 56 | 90 | - | - | 46.6 | 73 | - | - |
| Europe | |||||||||
| Sweden (2005) 8 | 84 | 19 | 25 | - | - | 55 | 24 | - | 17 |
| Hungary (2004) 25 | 50 | 44 | 66 | - | - | 60 | 50 | - | - |
| Germany (2001) 26 | 190 | 15 | - | - | - | - | 69 | - | - |
| Bulgaria (1999) 27 | 48 | - | - | - | - | - | 22.9 | - | - |
| France (1998) 28 | 124 | 21 | 73 | - | - | 36.5 | - | - | - |
| U.K. (1997) 29 | 41 | - | - | - | - | - | 37 | - | - |
| Scotland (1996) 30 | 12 | 91.6 | 33.3 | - | 8.3 | - | - | - | - |
| Spain (1993) 31 | 100 | 79 | 71 | - | - | - | - | - | - |
| Italy (1992) 11 | 74 | 76 | 38 | - | - | - | 52.9 | - | - |
| Australia(1998) 32 | 27 | 25.9 | - | - | - | - | 44.4 | - | - |
| South America | |||||||||
| Argentina (2005) 33 | 1000 | 35.2 | 42.3 | - | 6.2 | 28.7 | 53.3 | - | - |
| Brazil (2000) 34 | 23 | 65.2 | 73.9 | - | - | - | 43.5 | - | - |
Use among females
C282Y and H63D mutations
Also included in the present series.
Not reported
In this case series, the largest yet reported in North America, the average age of onset of PCT was in the fourth decade of life in both men and women, and the disease was more common in Caucasians than other ethnic groups. These features are consistent with previous reports from North America 9, 15. The proportion of African Americans and Hispanics was much less than would be expected based on the ethnic distribution of patients at this medical center. PCT may be less symptomatic and less often recognized in African Americans due to greater skin pigmentation. However, the lower proportion of Hispanics is unexplained. Ethnic disparities in ethanol use, smoking, hepatitis C 16 and HFE mutations 17, 18 have been little studied in this and other geographic regions. Unidentified genetic susceptibility factors for this disease may also vary across major ethnic groups.
Factors known or suspected to be related to susceptibility for PCT were much more prevalent in this large group of patients than has been observed in the general US population (Table 1), but comparable to other large case series of this disease from North America and, with some exceptions, from other regions in the world (Table 3). Especially prevalent were alcohol use (in 88%), smoking (81%), hepatitis C (69%), HFE mutations (51%) and estrogen use (in 66% of females). Less commonly observed were HIV infection (in 13%) and inherited UROD deficiency (17% had subnormal erythrocyte UROD). Prevalence of hepatic steatosis was 32.1% as determined by liver biopsy and 54% as suggested by increased echogenicity on ultrasound, which might be greater than that expected in the general population (Table 1).
As shown in Figure 1, multiple susceptibility factors were found in individual patients. Data was more commonly incomplete in this retrospective study in patients with fewer identified susceptibility factors (Figure 1). For example, results for HCV and HFE mutations were available only for patients seen after these testing methods became available in 1990 and 1995, respectively. Alcohol intake was often intermittent and difficult to quantify from the clinical records. Therefore, an even greater multiplicity of factors would have been evident if all patients had been completely evaluated. A thorough identification of known susceptibility factors is important clinically, because management of PCT may be affected, and some themselves cause liver disease and other medical problems.
Most other case series have reported the frequency of one or a few susceptibility factors in PCT (Table 3), but did not describe multiple factors or address associations among them. Our current data set was large enough to meaningfully assess associations among multiple factors. We found significant associations between HCV infection and other behavior-related factors, namely alcohol use and smoking, and with male sex and Caucasian race, which indicates that behavior-related factors are especially important contributors to development of PCT in our population. HIV infection was more common than expected in PCT patients with hepatitis C and even more so in those without this infection. An observed association between Caucasian race and estrogen use might reflect ethnic differences in use of prescription drugs. Estrogen use was more prevalent in the absence of HCV infection, both of which were associated with female sex. An association between diabetes mellitus and decreased erythrocyte UROD activity in this patient group is unexplained, and deserves further study. Hepatic steatosis, which was assessed incompletely in this series, may result from other factors, such as alcohol and diabetes mellitus 8. Steatosis, as assessed by ultrasound, was significantly associated with alcohol use in this study.
Our findings support the view that in most patients with PCT a multiplicity of susceptibility factors is needed to produce sufficient oxidative stress in hepatocytes to generate a UROD inhibitor 6. Some of the most prevalent factors in our patient population are associated with each other and can be related to life style and behaviors such as substance abuse. PCT patients without behavior-related factors may be particularly likely to have inborn susceptibility factors that are not presently known. It will be of interest to determine if clustering of particular susceptibility factors is observed in PCT patients in other geographic areas, and how associated factors act together to cause hepatic UROD deficiency.
Acknowledgments
Grant support: This study was supported in part by grants R21 DK073093 from the National Institutes of Health, R01 FD002604 from the U.S. Food and Drug Administration Office of Rare Diseases and a General Clinical Research Center grant MO1-RR000073 from the National Center for Research Resources of the National Institutes of Health.
Abbreviations
- BMI
body mass index
- CART
classification and regression tree
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- OR
odds ratio
- PCT
porphyria cutanea tarda
- UROD
uroporphyrinogen decarboxylase
- US
United States
- UTMB
University of Texas Medical Branch
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors have no conflicts to disclose
Writing assistance: none
Author involvement: Sajid Jalil was involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. James Grady was involved in study concept and design; analysis and interpretation of data; statistical analysis; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Chul Lee was involved in acquisition of data; technical support; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Karl Anderson was involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; administrative support; study supervision.
References
- 1.Elder GH. Porphyria cutanea tarda and related disorders. Chapter 88. In: Kadish KM, Smith K, Guilard R, editors. Porphyrin Handbook, Part II. Volume 14. San Diego: Academic Press; 2003. pp. 67–92. [Google Scholar]
- 2.Gisbert JP, Garcia-Buey L, Alonso A, Rubio S, Hernandez A, Pajares JM, Garcia-Diez A, Moreno-Otero R. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689–692. doi: 10.1097/01.meg.0000108318.52416.c9. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KE. The porphyrias. Chapter 72. In: Boyer T, Wright T, Manns M, editors. Zakim and Boyer’s Hepatology: A Textbook of Liver Diseases. Philadelphia: Elsevier; 2006. pp. 1391–1432. [Google Scholar]
- 4.Sinclair PR, Gorman N, Walton HS, Bement WJ, Dalton TP, Sinclair JF, Smith AG, Nebert DW. CYP1A2 is essential in murine uroporphyria caused by hexachlorobenzene and iron. Toxicol Appl Pharmacol. 2000;162:60–67. doi: 10.1006/taap.1999.8832. [DOI] [PubMed] [Google Scholar]
- 5.Smith AG, Clothier B, Carthew P, Childs NL, Sinclair PR, Nebert DW, Dalton TP. Protection of the Cyp1A2(−/−) null mouse against uroporphyria and hepatic injury following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2001;173:89–98. doi: 10.1006/taap.2001.9167. [DOI] [PubMed] [Google Scholar]
- 6.Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2007;104:5079–5084. doi: 10.1073/pnas.0700547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002;47:419–426. doi: 10.1023/a:1013746828074. [DOI] [PubMed] [Google Scholar]
- 8.Rossmann-Ringdahl I, Olsson R. Porphyria cutanea tarda in a Swedish population: risk factors and complications. Acta Derm Venereol. 2005;85:337–341. doi: 10.1080/00015550510033688. [DOI] [PubMed] [Google Scholar]
- 9.Bulaj ZJ, Phillips JD, Ajioka RS, Franklin MR, Griffen LM, Guinee DJ, Edwards CQ, Kushner JP. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood. 2000;95:1565–1571. [PubMed] [Google Scholar]
- 10.Chiaverini C, Halimi G, Ouzan D, Halfon P, Ortonne JP, Lacour JP. Porphyria cutanea tarda, C282Y, H63D and S65C HFE gene mutations and hepatitis C infection: a study from southern France. Dermatology. 2003;206:212–216. doi: 10.1159/000068895. [DOI] [PubMed] [Google Scholar]
- 11.Fargion S, Piperno A, Cappellini MD, Sampietro M, Fracanzani AL, Romano R, Caldarelli R, Marcelli R, Vecchi L, Fiorelli G. Hepatitis-C virus and porphyria cutanea tarda - evidence of a strong association. Hepatology. 1992;16:1322–1326. doi: 10.1002/hep.1840160603. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 13.Bonkovsky HL, Lambrecht RW, Shan Y. Iron as a co-morbid factor in nonhemochromatotic liver disease. Alcohol. 2003;30:137–144. doi: 10.1016/s0741-8329(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620–627. doi: 10.1016/s0168-8278(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 15.Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, Obando J, Di Bisceglie A, Tattrie C, Tortorelli K, LeClair P, Mercurio MG, Lambrecht RW. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27:1661–1669. doi: 10.1002/hep.510270627. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cogswell ME, Gallagher ML, Steinberg KK, Caudill Ph DS, Looker AC, Bowman BA, Gunter EW, Franks AL, Satten GA, Khoury MJ, Grummer-Strawn LM. HFE genotype and transferrin saturation in the United States. Genet Med. 2003;5:304–310. doi: 10.1097/01.GIM.0000076976.08421.AB. [DOI] [PubMed] [Google Scholar]
- 18.Acton RT, Barton JC, Snively BM, McLaren CE, Adams PC, Harris EL, Speechley MR, McLaren GD, Dawkins FW, Leiendecker-Foster C, Holup JL, Balasubramanyam A. Geographic and racial/ethnic differences in HFE mutation frequencies in the Hemochromatosis and Iron Overload Screening (HEIRS) Study. Ethn Dis. 2006;16:815–821. [PubMed] [Google Scholar]
- 19.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Curtis Ellison R. Alcohol consumption and the prevalence of the Metabolic Syndrome in the US.: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2954–2959. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 20.Lee DJ, Fleming LE, Arheart KL, LeBlanc WG, Caban AJ, Chung-Bridges K, Christ SL, McCollister KE, Pitman T. Smoking rate trends in U.S. occupational groups: the 1987 to 2004 National Health Interview Survey. J Occup Environ Med. 2007;49:75–81. doi: 10.1097/JOM.0b013e31802ec68c. [DOI] [PubMed] [Google Scholar]
- 21.Karon JM, Rosenberg PS, McQuillan G, Khare M, Gwinn M, Petersen LR. Prevalence of HIV infection in the United States, 1984 to 1992. Jama. 1996;276:126–131. [PubMed] [Google Scholar]
- 22.Steinberg KK, Cogswell ME, Chang JC, Caudill SP, McQuillan GM, Bowman BA, Grummer-Strawn LM, Sampson EJ, Khoury MJ, Gallagher ML. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. Jama. 2001;285:2216–2222. doi: 10.1001/jama.285.17.2216. [DOI] [PubMed] [Google Scholar]
- 23.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 24.Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124:248–250. doi: 10.1053/gast.2003.50032. [DOI] [PubMed] [Google Scholar]
- 25.Nagy Z, Koszo F, Par A, Emri G, Horkay I, Horanyi M, Karadi O, Rumi G, Jr, Morvay M, Varga V, Dobozy A, Mozsik G. Hemochromatosis (HFE) gene mutations and hepatitis C virus infection as risk factors for porphyria cutanea tarda in Hungarian patients. Liver Int. 2004;24:16–20. doi: 10.1111/j.1478-3231.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 26.Tannapfel A, Stolzel U, Kostler E, Melz S, Richter M, Keim V, Schuppan D, Wittekind C. C282Y and H63D mutation of the hemochromatosis gene in German porphyria cutanea tarda patients. Virchows Arch. 2001;439:1–5. doi: 10.1007/s004280100401. [DOI] [PubMed] [Google Scholar]
- 27.Ivanova A, von Ahsen N, Adjarov D, Krastev Z, Oellerich M, Wieland E. C282Y and H63D mutations in the HFE gene are not associated with porphyria cutanea tarda in Bulgaria [letter] Hepatology. 1999;30:1531–1532. doi: 10.1002/hep.510300626. [DOI] [PubMed] [Google Scholar]
- 28.Lamoril J, Andant C, Bogard C, Puy H, Gouya L, Pawlotsky JM, Da Silva V, Soule JC, Deybach JC, Nordmann Y. Epidemiology of hepatitis C and G in sporadic and familial porphyria cutanea tarda. Hepatology. 1998;27:848–852. doi: 10.1002/hep.510270329. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AG, Whatley SD, Nicklin S, Worwood M, Pointon JJ, Stone C, Elder GH. The frequency of hemochromatosis-associated alleles is increased in British patients with sporadic porphyria cutanea tarda. Hepatology. 1997;25:159–161. doi: 10.1002/hep.510250129. [DOI] [PubMed] [Google Scholar]
- 30.Hussain I, Hepburn NC, Jones A, O'Rourke K, Hayes PC. The association of hepatitis C viral infection with porphyria cutanea tarda in the Lothian region of Scotland. Clin Exp Dermatol. 1996;21:283–285. doi: 10.1111/j.1365-2230.1996.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 31.Herrero C, Vicente A, Bruguera M, Ercilla MG, Barrera JM, Vidal J, Teres J, Mascaro JM, Rodes J. Is hepatitis C virus infection a trigger of porphyria cutanea tarda? Lancet. 1993;341:788–789. doi: 10.1016/0140-6736(93)90562-u. [DOI] [PubMed] [Google Scholar]
- 32.Stuart KA, Busfield F, Jazwinska EC, Gibson P, Butterworth LA, Cooksley WG, Powell LW, Crawford DH. The C282Y mutation in the haemochromatosis gene (HFE) and hepatitis C virus infection are independent cofactors for porphyria cutanea tarda in Australian patients. J Hepatol. 1998;28:404–409. doi: 10.1016/s0168-8278(98)80313-9. [DOI] [PubMed] [Google Scholar]
- 33.Mendez M, Rossetti MV, Del CBAM, Parera VE. The role of inherited and acquired factors in the development of porphyria cutanea tarda in the Argentinean population. J Am Acad Dermatol. 2005;52:417–424. doi: 10.1016/j.jaad.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Martinelli AL, Zago MA, Roselino AM, Filho AB, Villanova MG, Secaf M, Tavella MH, Ramalho LN, Zucoloto S, Franco RF. Porphyria cutanea tarda in Brazilian patients: association with hemochromatosis C282Y mutation and hepatitis C virus infection. Am J Gastroenterol. 2000;95:3516–3521. doi: 10.1111/j.1572-0241.2000.03369.x. [DOI] [PubMed] [Google Scholar]