Abstract
Turmeric (Curcuma longa L., Zingiberaceae) rhizomes contain two classes of secondary metabolites, curcuminoids and the less well-studied essential oils. Having previously identified potent anti-arthritic effects of the curcuminoids in turmeric extracts in an animal model of rheumatoid arthritis (RA), studies were undertaken to determine whether the turmeric essential oils (TEO) were also joint protective using the same experimental model. Crude or refined TEO extracts dramatically inhibited joint swelling (90-100% inhibition) in female rats with streptococcal cell wall (SCW)-induced arthritis when extracts were administered via intraperitoneal injection to maximize uniform delivery. However, this anti-arthritic effect was accompanied by significant morbidity and mortality. Oral administration of a 20-fold higher dose TEO was non-toxic, but only mildly joint-protective (20% inhibition). These results do not support the isolated use of TEO for arthritis treatment, but, instead, identify potential safety concerns in vertebrates exposed to TEO.
Keywords: Curcuma longa L., turmeric, essential oil, arthritis, rheumatoid arthritis, liver, anemia
INTRODUCTION
Essential oils are complex mixtures of volatile terpenes selected throughout evolution to protect plants from external threats by various means including mimicking endogenous substrates in herbivores (1). While biologic and potentially medicinal effects of these essential oils are thus to be anticipated, these compounds are often less well studied than other classes of secondary plant metabolites, with polyphenols being a prominent example. Such is the case for turmeric (Curcuma longa L., Zingiberaceae), a medicinal botanical whose rhizome contains two major classes of secondary metabolites, the phenolic curcuminoids and the hydrophobic essential oils (2). Many laboratories, including our own (3, 4), have demonstrated potent and physiologically important effects of the curcuminoids (5). For example, we have elucidated a profound anti-arthritic effect of curcuminoid-containing turmeric extracts due to their ability to inhibit nuclear factor-κB (NF-κB) activation in experimental rheumatoid arthritis, thus blocking multiple downstream signaling pathways critical to joint inflammation, including cyclo-oxygenase (COX)-stimulated prostaglandin-E2 (PGE2) production (3). Far less studied, and indeed, often discarded in the preparation of turmeric dietary supplements (AR), are turmeric's essential oils.
Reported biologic properties of the multi-component essential oils of turmeric include antifungal (6), mosquitocidal (7), antivenom (8), antibacterial and antioxidant properties (9, 10). Medicinal effects of TEO in vertebrates have also been reported for stroke and diabetes: a single acute dose of TEO (250-500 mg/kg oral or ip) was neuroprotective in rats subjected to occlusive or embolic strokes (11-13), while chronic TEO dietary supplementation (≥620 mg/kg/d) normalized serum glucose in diabetic mice (14, 15).
Major compounds present in turmeric essential oils (TEO) include ar-turmerone, α-turmerone and β-turmerone. The relative proportions of these terpenoids in TEO extracts vary depending on the rhizome's geographic origin and method of extraction (9, 16, 17). In isolation, these compounds have demonstrated bioactivity in vitro, including cytotoxic and apoptotic effects in various cells (18, 19); binding to the nuclear receptor, peroxisome proliferator-activated receptor γ (PPAR-γ) (14); inhibition of inducible PGE2 and NO production (2, 20); and inhibition of platelet aggregation (21).
Emerging in vitro evidence from our laboratories and others suggests that crude, multi-component hexane extracts of TEO may be more bioactive than any of their individual fractions or components (2, 9, 11). For example, when our laboratories isolated and compared the efficacy of 8 distinct fractions of a crude TEO extract vs. the extract itself in blocking COX-mediated PGE2 production in vitro, the potency of the crude TEO extract exceeded that of each of its fractions (2). Moreover, the crude essential oils had the same potency as turmeric's other secondary metabolites, the curcuminoids, in this in vitro screening assay. Thus, we have postulated that the essential oils of turmeric may also have significant medicinal anti-arthritic effects in vivo--independent of those attributed to turmeric's polyphenols—that result from synergistic actions of the oil's various constituents.
To test this postulate, in vivo studies described herein were undertaken to determine whether the essential oils of turmeric are indeed effective anti-arthritic agents using an animal model of rheumatoid arthritis (RA), streptococcal cell wall (SCW)-induced arthritis. Because we previously used this model to characterize anti-inflammatory properties of essential-oil free, curcuminoid-containing turmeric extracts (3, 4), these experiments also allowed us to compare the relative in vivo anti-inflammatory efficacy of each of turmeric's two major classes of secondary metabolites. Moreover, our isolation and in vivo testing of two complex TEO extracts, a crude hexane extract and a curcuminoid-stripped, refined fraction of the crude TEO extract, also allowed for clarification of the independent role of the essential oils (vs. curcuminoids) in preventing intra-articular inflammation.
MATERIALS AND METHODS
Turmeric Essential Oil Preparation
Turmeric essential oil (TEO) extracts were isolated from dry turmeric rhizome powder as described in detail in United States Patent 7,205,011. In brief, a crude TEO extract was prepared from 1 kg dry Curcuma longa L., Zingiberaceae rhizome powder (San Francisco Herb, SF, CA) by n-hexane extraction at room temperature for 24 hours. The resultant mixture was filtered, the filtrate was combined with an n-hexane wash of the marc, and the solvent was stripped under vacuum for 24 hours to obtain a crude turmeric oil extract (yield, 3.7%). HPLC analysis revealed minor curcuminoid contamination of this essential oil-enriched extract. The crude TEO (36g) was processed further by silca gel column chromatography. Elution was by the gravity method. The column was eluted with n-hexane (100%). Fractions (25-30 mL each) were collected until the color of the eluant changed from colorless to yellow to colorless again. The column was then eluted with a mixture of n-hexane and ethyl acetate (17:1 v/v) for a total of 15 L. The column was finally leached with 100% ethyl acetate (1 L) and elution was discontinued. The solvent from the 15 L fraction was stripped off under vacuum using a rotary evaporator, and the resulting refined TEO was then left under vacuum for a minimum of 24 hrs (66-74% yield, by weight).
Chemical Analysis of Turmeric Essential Oil Extract
The major components of crude and refined TEO extracts were characterized chemically by GC-MS and HPLC. Identification of the three major constituents of the essential oils was achieved by comparison of their mass spectral fragmentation patterns (NIST08 database/WorkStation data system) and with literature reports (22, 23). GC–MS data were recorded with a Varian Saturn 2100T (Palo Alto, CA). The gas chromatograph was fitted with a Chrompack capillary column (CP Sil 8 CB; 30 m × 0.25 mm). Operating conditions: column oven temperature programmed at 80 °C for 5 min and then to 280 °C at 10 °C/min; injector/transfer line/trap temperatures 250/250/200, respectively; electron voltage, 50–80 eV. UHP helium was used as the carrier gas at a flow rate of 1.2 mL/min. Each sample (1 mg) was dissolved in CH2Cl2 (0.5 mL) and injected (1 μL) directly into the chromatograph.
The HPLC system for extract analysis was composed of the following: Agilent 1100 series with a quaternary pump, a degasser, a thermostated column compartment, a thermostated autosampler, a diode array detector and ChemStation for LC 3D, Rev. A.09.03 (1417) software for system control and data acquisition (Agilent Technologies Inc., Palo Alto, CA). The samples (20 μL) were injected onto a Synergy, 4m, Hydro-RP 250 × 4.6 mm column coupled with SecurityGuard AJO-4287 guard column (Phenomenex, Torrance, CA). The mobile phases used were as follows: A: 500 μL acetic acid/L of Nanopure water; B: 500 μL acetic acid/L of acetonitrile. The organic mobile phase was filtered through a 0.2 μm PTFE membrane filter and the water based mobile phase was filtered through a 0.2 μm cellulose nitrate. The mobile phases were thoroughly degassed before analysis and throughout the analysis. The eluent was monitored at 425 nm, 370 nm, 280 nm and 250 nm; the flow rate was 1 mL/min. For HPLC analysis, triplicates of approx. 1-2 mg sample were weighed using analytical balance, dissolved in a small volume of dichloromethane then completed to volume with methanol (5:95) with final concentration of approximately 1 ppm. Solutions were transferred into an amber autosampler vials and 3 × 20 μL was injected onto the HPLC column.
SCW-induced Arthritis and Dosing Regimes
All animal experiments were performed in compliance with the University of Arizona Institutional Animal Use and Care Committee (IACUC). To induce arthritis, female Lewis rats (110 g, Harlan, Indianapolis, IN) were injected ip with peptidoglycan-polysaccharide polymers (25 μg rhamnose/g body weight) isolated from the sonicated cell wall of Group A Streptococcus pyogenes (SCW) (Lee Laboratories, Grayson, GA) or normal saline (vehicle) (3, 24). Intraperitoneal (ip) or oral (po) administration of TEO extracts or the appropriate vehicle (0.5 μL DMSO/g for ip dosing or 0.5 μL 8% saccharine in DMSO/g for oral dosing) began 4 days before, or 3 days after, SCW injection, as indicated (4). Daily dosing continued until acute inflammation subsided (10 days post-SCW), at which time frequency was decreased to 5 days/week. Oral dosing was maintained at this level until the end of the experimental period, while ip dosing during the final week decreased again to thrice weekly due to increasing evidence of toxicity. Treatment groups for arthritis experiments thus included 1) 2 normal animals treated with vehicle; 2) normal animals treated with TEO extracts; 3) SCW-injected arthritic animals treated with vehicle; and 4) SCW-injected arthritic animals treated with TEO extracts.
Joint inflammation
Joint inflammation was determined in a blinded fashion by daily assessment of arthritic index (AI) in each distal limb using standard criteria (3,24) (0 = normal; 1 = slight erythema and edema; 2 = increased edema with loss of landmarks; 3 = marked edema; 4 = marked edema with ankylosis on flexion, for a total possible score of 16/animal).
Real time RT PCR
RNA was isolated from hind ankle joints as previously described (3). Equal amounts of RNA were combined from 3 (non-arthritic) or 3 (arthritic) joints/treatment group to make one sample. Three such pooled RNA samples/treatment group (i.e. total of 9-12 joints analyzed per group) were used for analysis of articular mRNA expression using real-time RT-PCR TaqMan® analysis and previously described methods (3). Rat specific primers for interleukin 1 beta (Il-1ß) (Rn00580432_m1), monocyte-chemotactic protein 1 (MCP-1) (Rn00580555_m1), growth-related oncogene/keratinocyte chemoattractant (GRO/KC) (Rn00578225_m1), cyclooxygenase 2 (COX-2) Rn00568225-m1), mannan-binding lectin serine peptidase 1 (Rn00434830_m1), properdin (Rn01526084_g1), and an 18S primer (as an internal control; Hs99999901_s1) were obtained from Applied Biosystems (Foster City, CA). Data, normalized to the endogenous reference (18S RNA), were analyzed using the comparative CT method as previously described (3).
Toxicity
To monitor for possible toxic effects of TEO treatments in normal or SCW animals, daily weights and mortality were assessed. Liver and renal function were monitored by assay of alanine aminotransferase (ALT) and creatinine levels in blood samples obtained at the indicated times after injection of SCW (or vehicle) using a Hemagen Diagnostics Endocheck Plus Chemistry Analyzer (Columbia, MD). Circulating white blood cell counts, hematocrit and platelet counts in whole blood were also determined using a Hemavet 880 analyzer (CDC Technologies, Oxford, Conn.). Necropsies, including histologic assessment of all organs, were performed in a blinded fashion by a veterinary pathologist. Occult blood in stools was assessed by Hemocult testing (VWR, West Chester, PA).
Histology
Liver tissue specimens were fixed in 10% formalin and subsequently embedded in paraffin. SCW-induced hepatic granuloma formation at day 28 was assessed in H&E stained liver sections using standard criteria and blinded analysis (3, 24).
Statistical Analysis
Values are presented as mean ± SEM except where indicated. Accumulative effects of treatment on joint swelling were determined by assessing area under the curve (AUC) for each animal (25). Statistical significance was determined by ANOVA with post hoc testing, Student's t-test or by Fisher's exact test, as indicated, using InStat software (GraphPad Software, San Diego, CA).
RESULTS AND DISCUSSION
Isolation and Characterization of major components in TEO Extract
HPLC analysis of the crude hexane extract prepared from powdered turmeric rhizomes (3.7% yield) revealed slight contamination of the oils by the 3 major curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) with retention times less than 25 minutes, as well as the presence of 3 major peaks with retention time more than 25 minutes. Column fractionation of the crude TEO extract resulted in a second, refined extract (66% yield). In the refined TEO, the relative ratios and content of the 3 major terpenoids were retained, while curcuminoids where no longer detectable (Figures S1 and Figure S2). GC-MS data (the retention time order, the MS fragmentation pattern) of these 3 major peaks were compared with NIST08 library and with those published of turmeric essential oils (22, 23). This resulted in the identification of the three major HPLC peaks as: ar-turmerone, α-turmerone and β-turmerone (Figure S1 A-G). These two experimental TEO extracts were then tested in vivo.
Antiarthritic Effects of Intraperitoneal TEO Extracts
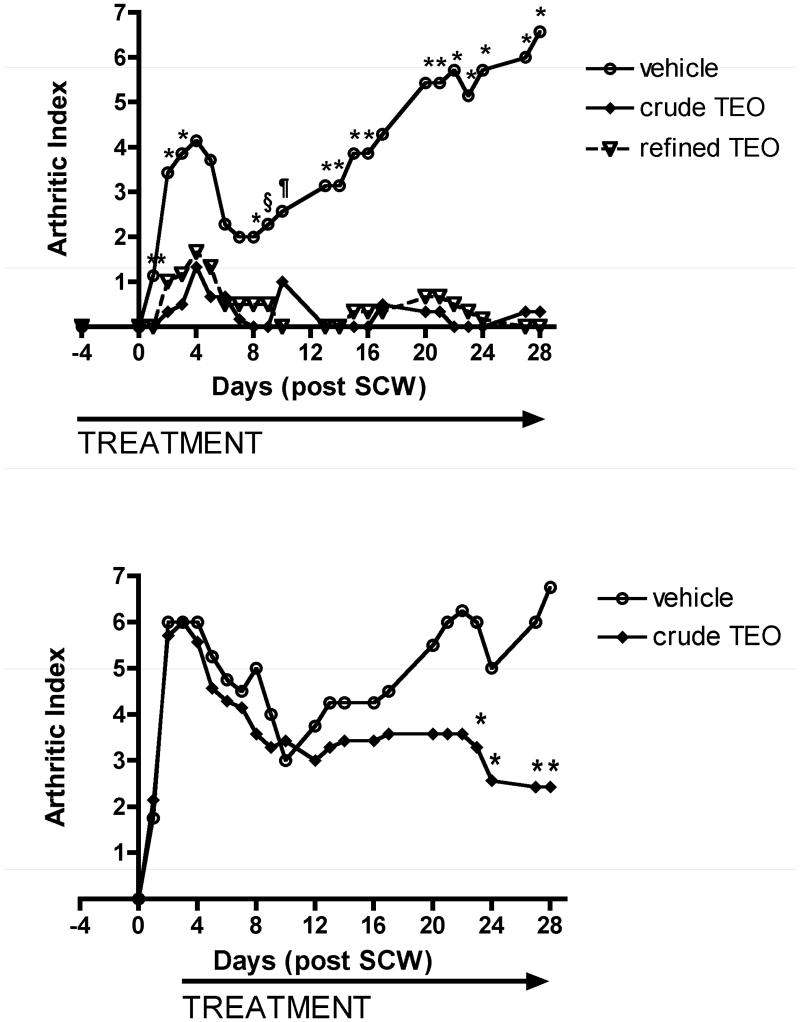
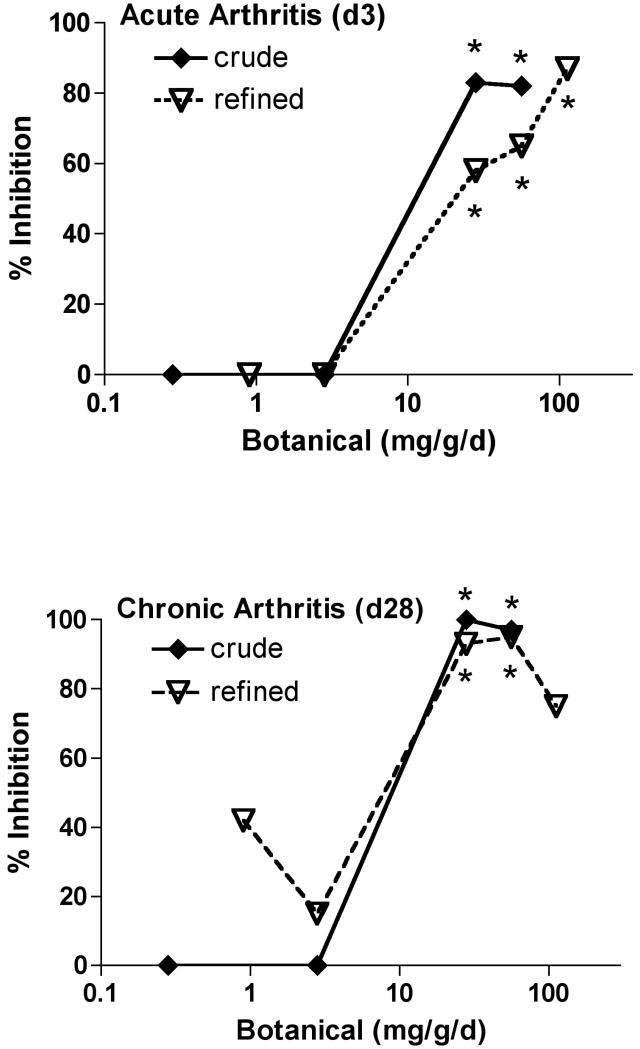
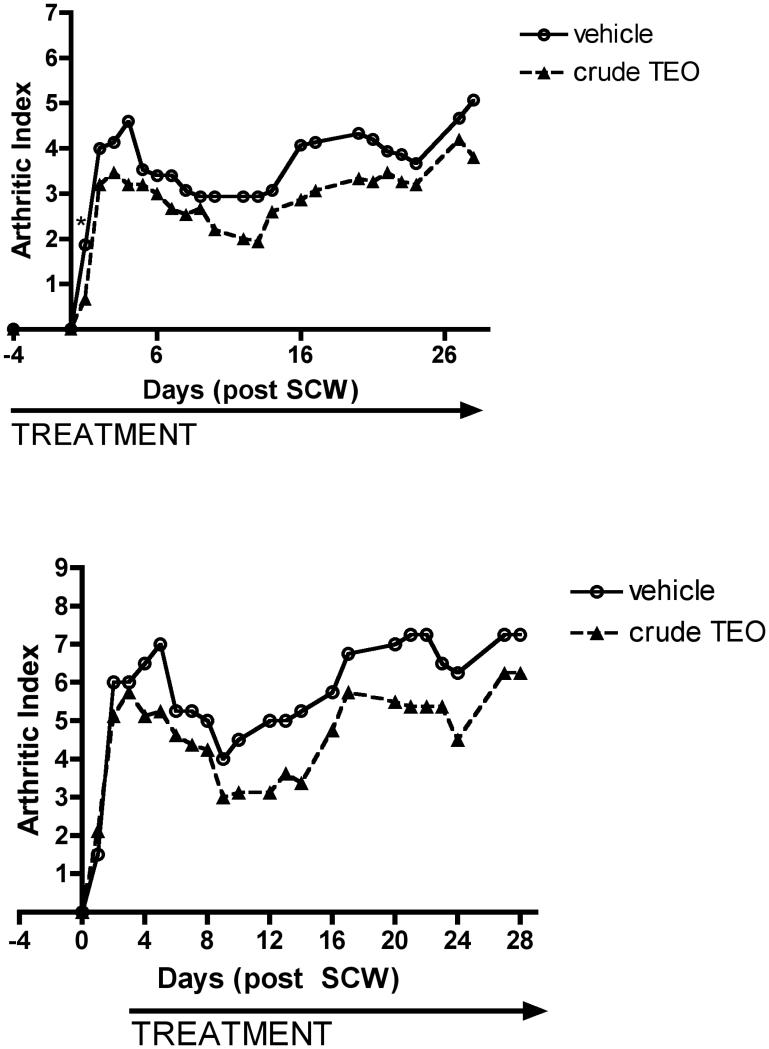
To assure uniform delivery of the two experimental extracts while establishing and comparing their anti-arthritic bioactivity, TEO extracts were administered ip to maximize delivery and eliminating any potential confounding effects of differential gastrointestinal absorption. Doses of the crude TEO were normalized based on the 66% yield of the refined extract and are expressed as mg equivalents of refined TEO (e.g. 86 mg crude × 0.66 = 56 mg equivalents of refined). Using this approximation, differences in bioactivity for a given normalized dose of crude vs. refined TEO can be attributed to the additional components present only in the crude extract. Treatment with either TEO extract (56 mg equivalents of refined/kg/d) beginning prior to SCW injection, profoundly inhibited joint swelling (95-100% inhibition) in animals with SCW-induced arthritis (Figure 1A). This marked degree of inhibition by turmeric's essential oils is comparable to that previously demonstrated by our laboratories for an equivalent dose of turmeric's other secondary metabolites, the curcuminoids (3). Indeed, this extract dose was specifically chosen as a starting point for determining TEO's anti-arthritic efficacy based on our previous findings with the curcuminoids. When start of TEO treatment was delayed until after the peak in acute inflammation (day 3), the essential oils were also protective (Figure 1B), although to a lesser degree (64%). These findings are again consistent with results we obtained previously in this model when evaluating curcuminoid-only turmeric extracts (3). Dose-dependent inhibition of arthritis was similar for the crude vs. refined TEO (ID50 approximately 10 mg equivalents of refined TEO/kg/d) during the initial acute phase (day 3) of joint swelling (Figure 2A), as well as the later, chronic phase of articular inflammation (day 28) when erosion of articular cartilage and bone occurs (Figure 2B). These data suggest that additional components present only in the crude TEO, including, but not limited to, the very low levels of curcuminoids, were not likely to have additive or synergistic anti-arthritic effects with turmeric's purified essential oils. In totality, then, our data suggest that each class of secondary metabolites in turmeric, the essential oils and the curcuminoids, can act independently and with similar efficacy and potency to block arthritis using this experimental model and mode of delivery (ip).
Figure 1.
Effects of ip turmeric essential oil (TEO) extracts on joint inflammation. Female Lewis rats were injected on day 0 with vehicle or streptococcal cell wall (SCW, 25 ug/g body weight) to induce arthritis. Joint swelling in limbs was assessed at indicated times and expressed as mean arthritic index (AI) (scale 0-3/limb for total possible score of 16/animal) with statistical significance determined by analysis of variance with post hoc testing or Mann Whitney (n = 4-7 animals/group). A. Intraperitoneal treatment with crude or refined TEO (normalized to 56 mg refined TEO/g) or vehicle was begun 4 days prior to SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 3-5 days per week. * p < 0.05 for refined or crude TEO (vs. vehicle). ** p < 0.10 for refined or crude TEO (vs. vehicle). § p < 0.05 for crude TEO only (vs. vehicle), ¶ p < 0.05 for refined TEO only (vs. vehicle). B. Intraperitoneal treatment with crude TEO (normalized to 56 mg refined TEO/g) or vehicle was delayed until 3 days after SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 3-5 days per week. * p < 0.05 for crude TEO (vs. vehicle).
Figure 2.
Dose-dependent inhibition of joint swelling by ip TEO extracts. Female Lewis rats were injected on day 0 with vehicle or streptococcal cell wall (SCW, 25 μg/g body weight) to induce arthritis. Intraperitoneal treatment with crude or refined TEO (with indicated extract doses, beginning as low as 0.28 mg/kg/d, normalized to refined TEO), or with vehicle, was begun 4 days prior to SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 3-5 days per week. Arthritic index was assessed in TEO-treated and vehicle-injected animals and is expressed here as percent inhibition relative to controls (n = 5-30/group) with * p < 0.01 vs. untreated. A. TEO inhibition of initial, transient joint swelling (day 3). B. TEO inhibition of joint swelling during chronic, joint-destructive phase (day 28).
In addition to examining the clinical endpoint of joint swelling, inhibitory effects of TEO on articular inflammation were confirmed at the molecular level by assessing the activation of genes critical to articular inflammation. The early induction of chemokines responsible for the intra-articular recruitment of inflammatory cells (the neutrophil chemokine, GRO/KC, and monocyte chemokine, MCP-1), and of key inflammatory cytokines (IL-1beta), was prevented by TEO treatment. Similarly, activation of an additional arm of the innate immune response, antibody-independent complement pathways (induced expression of mannose binding lectin and properdin) (26), and the inducible expression of other downstream mediators of joint inflammation, such as COX-2, was blocked in the joints of SCW-injected animals treated with TEO (Table 1).
TABLE 1.
Effects of Crude TEO (56 mg/kg/d) on Articular Gene Expression
| NON-ARTHRITIC | ARTHRITIC | ||||
|---|---|---|---|---|---|
| Gene Type | Gene | vehicle | TEO | SCW | SCW + TEO |
| Cytokine | IL-1beta | 1.4 ± 0.3 | 1.6 ± 0.3 | 21.1 + 6.5a | 2.7 ± 0.3b |
| Chemokines | GRO/KC | 1.0 ± 0.1 | 1.6 ± 0.8 | 54.9 + 11.9c | 1.7 + 0.6d |
| MCP-1 | 1.4 ± 0.6 | 0.7 + 0.3 | 12.0 + 7.9 | 0.4 + 0.1 | |
| Complement | properdin | 1.0 ± 0.1 | 1.0 ± 0.3 | 8.1 ± 2.8a | 1.0 ± 0.1b |
| cascade | MBL serine peptidase | 0.9 ± 0.1 | 1.2 ± 0.2 | 6.5 ± 2.0a | 0.9 ± 0.2b |
| Arachidonic acid metabolism |
COX-2 | 1.6 ± 0.3 | 1.4 ± 0.6 | 10.3 ± 3.2a | 1.9 ± 0.2b |
Values are expressed as fold change from normal vehicle-treated animals (mean ± SEM for n = 3 samples/group with 3 joints combined per sample).
p < 0.05 vs. vehicle.
p < 0.01 vs. SCW.
p < 0.001 vs. vehicle.
p < 0.001 vs. SCW.
Extra-articular Anti-inflammatory Effects of Intraperitoneal TEO Extracts
Anti-inflammatory effects of TEO were not limited to the joints. TEO also blocked SCW-induced leukocytosis and the granulomatous inflammatory response that occurs at sites of hepatic SCW deposition (4,24) (Table 2). As with arthritis inhibition, these extra-articular anti-inflammatory effects were dose-dependent, occurring only with TEO doses ≥ 28 mg/kg/day (data not shown). However, unlike the equipotent effects of refined and crude TEO on articular inflammation, the crude TEO was more effective in blocking nonarticular inflammation (Table 2), suggesting that the additional components present only in the crude extract were also bioactive with independent and/or synergistic anti-inflammatory effects.
Table 2.
Screening for TEO Toxicity in Normal and Arthritic Animals
| Non-arthritic Animals |
Arthritic Animals |
|||||
|---|---|---|---|---|---|---|
| vehicle | crude | refined | SCW + vehicle | SCW + crude | SCW + refined | |
| Hepatic Granulomas (% incidence) | 68% (34/50) | 15% (4/26)a | 41% (7/17) | |||
| WBC (K/μl) | 7.2 ± 0.4 | 10.1 ± 1.2 | 7.4 ± 0.5 | 23.7 ± 2.5b | 13.6 ± 1.5c,d | 18.0 ± 1.4b,e |
| ALT (U/L) | 16.0 ± 1.5 | 40.3 + 13.7 | 24.6 ± 2.0 | 9.5 ± 1.0 | 64.6 ± 41.3 | 12.9 ± 1.4 |
| ALT (% > upper range of normal) | 0% (0/23) | 22% (4/18)c | 10% (3/29) | 0% (0/25) | 20% (3/15)c | 0% (0/21) |
| Hct (%) | 41.8 ± 0.8 | 31.3 ± 3.2f | 36.1 ± 1.4f | 32.3 ± 1.2b | 33.7 ± 2.3c | 32.1 ± 1.4b |
| Creatinine (mg/dL) | 0.18 ± 0.01 | 0.21 ± 0.02 | 0.22 ± 0.02 | 0.17 ± 0.02 | 0.15 ± 0.01 | 0.015 ± 0.01 |
Data, obtained from animals surviving one month of treatment with ≥ 28 mg/kg/d TEO (or vehicle) (n = 15-29/group), are expressed as mean ± SEM with statistical significance determined by ANOVA with post-hoc testing of Fisher's test, as appropriate.
p < 0.0001 vs. (SCW + vehicle),
p < 0.001 vs vehicle,
p < 0.05 vs. vehicle,
p < 0.01 vs. (SCW + vehicle),
p < 0.01 vs. (SCW + vehicle),
p < 0.01 vs. vehicle.
Toxicity Associated with Intraperitoneal TEO Extracts
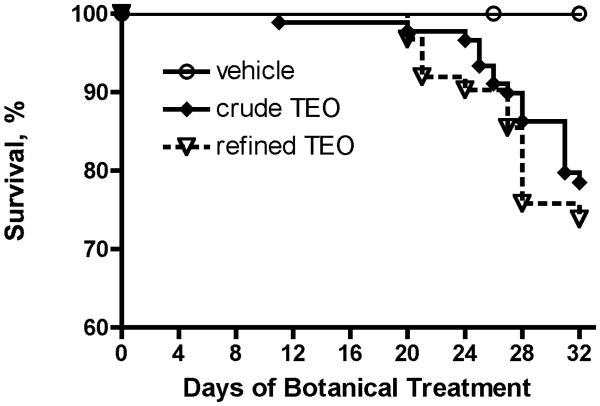
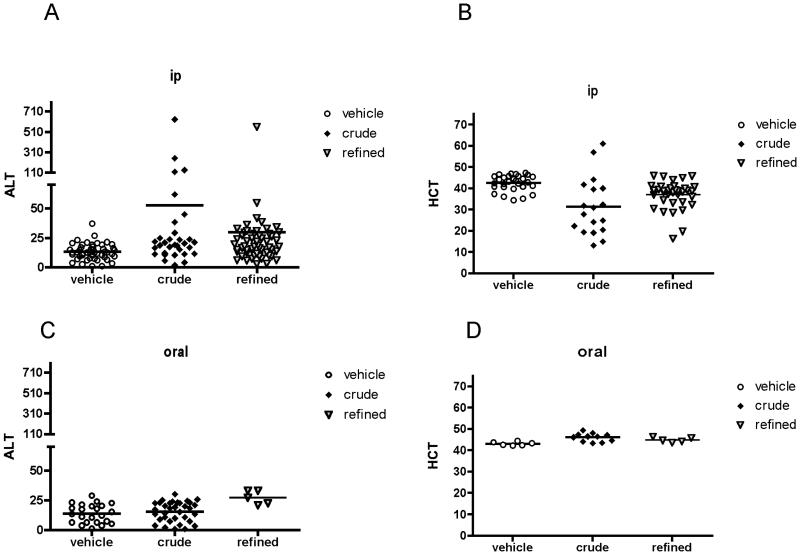
Joint-protective and/or anti-inflammatory effects of ip TEO were associated with an extremely high rate of mortality. Significant numbers of normal and SCW-injected animals treated ip with ≥ 28 mg/kg/d TEO began to die after 2 weeks of treatment (Figure 3), resulting in a mortality rate of 20% (crude) or 36% (refined) by the end of the month-long experimental period. In contrast, no deaths occurred in animals (control or SCW-injected) treated with vehicle or lower TEO doses (≤ 2.8 mg/kg/d) that were without evidence of anti-inflammatory effects (data not shown). Notable findings in those animals that survived one month of ip treatment with ≥ 28 mg/kg/d TEO included elevations in serum alanine aminotransferase (ALT), consistent with hepatocellular damage, that was more notable in crude TEO survivors (Table 2 and Figure 4A). In addition, control animals treated with either TEO were anemic (Table 2 and Figure 4B), while the anemia that occurs in SCW-injected animals, which is typically reversed with effective anti-arthritic treatment (3) neither improved nor worsened with anti-inflammatory doses of TEO (Table 2). In contrast to these adverse effects, there was no evidence of damage to the kidneys (Table 2), a primary site of clearance of terpenes and their metabolites (27), in surviving animals.
Figure 3.
Mortality in Animals Treated with ip TEO. Survival in all animals (SCW or control) treated ip with ≥ 28 mg/mg/d refined (n = 62) or crude TEO (n = 90) vs. vehicle (n = 86) was analyzed by log-rank using Kaplan Meier survival curves. Mortality was significantly (p < 0.0001) increased in animals treated ip with either crude or refined TEO extract as compared with vehicle.
Figure 4.
Hepatotoxicity and anemia associated with ip vs. oral TEO. A. Effects of ip treatment (≥ 26 mg/kg/d) with crude TEO (n = 32) or refined TEO (n = 54) vs. vehicle alone (n = 48) on serum levels of alanine aminotransferase (ALT) were determined in SCW or control animals surviving 23-32 days of treatment. Means are not statistically different by ANOVA. B. Effects of ip treatment with crude TEO (n = 18) or refined TEO (n = 37) vs. vehicle alone (n = 29) on hemacrit (HCT) were determined in control animals surviving 23-32 days of treatment. p < 0.001, crude vs. vehicle. p <0.01, refined vs. vehicle. C. Effects of oral treatment (> 560 mg/kg/d) with crude TEO (n = 34) or refined TEO (n = 5) vs. vehicle alone (n = 23) on serum levels of alanine aminotransferase (ALT) were determined in SCW or control animals surviving 28-32 days of treatment. p <0.01, refined vs. vehicle. D. Effects of oral treatment (> 560 mg/kg/d) with crude TEO (n = 11) or refined TEO (n = 5) vs. vehicle alone (n = 6) on HCT were determined in control animals surviving 28-32 days of treatment. p <0.01, crude vs. vehicle.
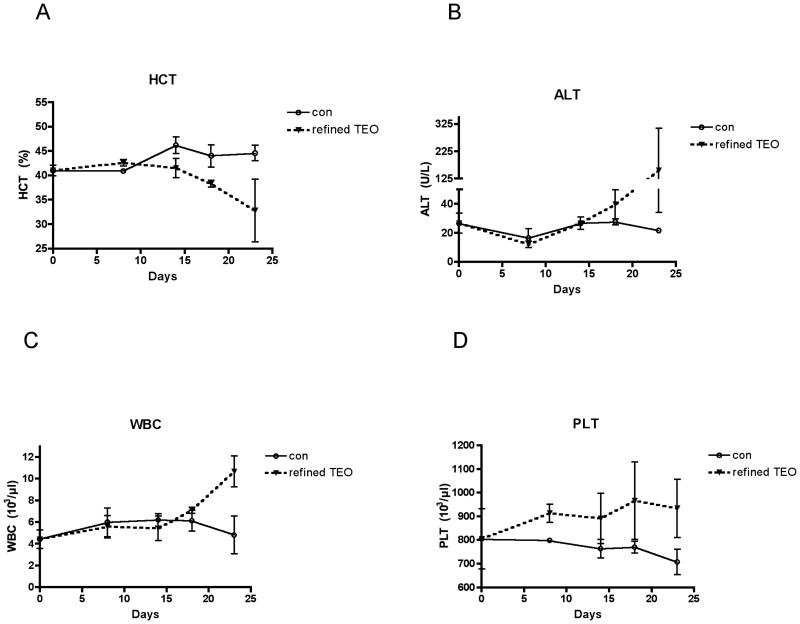
To eliminate survivor bias and identify the time of onset of TEO-induced anemia and hepatotoxicity, toxicity screening was performed as a function of time in control animals treated with the TEO extract causing the highest mortality (refined TEO, 56 mg/kg/d ip). Anemia (Figure 5A) and elevations in ALT (Figure 5B) began at 2 weeks, a time co-incident with the onset of mortality in arthritis treatment experiments (Figure 3), thus suggesting possible causality. While elevations in circulating leukocytes (WBC) (Table 2) and platelets (PLT) (data not shown) were not detected in non-arthritic animals surviving one month of TEO treatment, both of these indicators of systemic inflammation began to increase after 2 weeks of TEO administration (Figure 5C&D), consistent with an inflammatory response to ip administration of the essential oils.
Figure 5.
Time course of adverse effects of ip treatment with refined TEO on liver and hematopoetic parameters. In normal female Lewis rats, the effect of daily ip treatment with 28 mg/kg/d refined TEO vs. vehicle was determined over a 23-day period. Animals (n = 3 vehicle and n = 4 TEO) were sacrificed at the indicated times for assessment of hematologic parameters (hematocrit [HCT], total white blood cell [WBC], and platelet [PLT] counts) and liver function (serum levels of alanine aminotransferase) as well as gross necropsy which was notable for peritonitis, beginning as early as day 8. Results are presented as mean ± SEM.
Evidence of occult gastrointestinal bleeding as a cause of anemia was sought in animals treated with ≥ 28 mg/kg/d TEO vs. vehicle. On random screening, occult blood was documented in the stools of 38% of crude TEO-treated animals (n = 16, p < 0.05 vs. vehicle [9% incidence, n = 22]) and 19% of those treated with refined TEO (n= 32, p > 0.05 [non-significant]). Necropsies performed after 2-4 weeks of treatment with TEO ≥ 28 mg/kg/d (n = 2, crude TEO; n = 4, refined TEO) were notable for signs of mild to moderate peritonitis, including evidence of a small intestine perforation in one crude TEO treated animal. In contrast, vehicle-treated animals (n = 2) had no peritonitis and necropsies were only notable for granulomatous lesions in the liver in those animals also injected with SCW. There was no evidence of renal toxicity on necropsy. In sum, effective anti-arthritic doses of both TEOs, when administered ip, were also associated with peritonitis, gastrointestinal bleeding with anemia, and hepatocellular damage.
Oral TEO Extract Dosing: Toxicity and Anti-inflammatory Effects
The above findings beg the question of whether the toxic effects of both TEOs were specific to their ip route of administration. Additionally, it is possible that the anti-inflammatory effects of TEO when administered ip were non-specific and simply due to TEO-induced peritonitis inducing tolerance to the SCW (28). To address both questions, oral TEO dosing studies were undertaken. TEO doses (560 mg/kg/d) 20-fold higher than the lowest effective ip doses of crude or refined TEO were arbitrarily chosen for testing since minimal information is available regarding oral absorption and bio-disposition of essential oils or their terpene components (27). No mortality (data not shown), evidence of hepatocellular damage (Figure 4C), anemia (Figure 4D), or occult gastrointestinal bleeding (n = 4-5 animals screened per treatment) occurred after one month of daily oral administration of crude or refined TEO. No peritonitis was seen on necropsy (n = 1 animal per treatment). However, proteus bacteria was detected in the abdominal cavity of the crude TEO treated anima examined, suggesting the possibility of an otherwise undetected intestinal perforation vs. accidental specimen contamination.
Having verified the relative absence of toxicity with oral (vs. ip) administration of TEOs, the oral anti-inflammatory activity of crude TEO, the extract having the greatest range of articular and extra-articular anti-inflammatory effects when administered ip, was assessed in the SCW model. Oral treatment with crude TEO (520 mg/kg/d), begun prior to SCW administration (Figure 6A), or after the peak of acute joint inflammation (Figure 6B), decreased joint swelling at every time point. This anti-inflammatory effect, when analyzed on a daily basis, was only statistically significant at the start of pre-treatment (Figure 6A, day 1). However, assessment of joint inflammation by analysis of the area under the curve (AUC) during the entire course of TEO treatment demonstrated a relatively modest but statistically significant 21% inhibition of joint swelling in response to early or delayed oral crude TEO treatment (p < 0.02 vs. vehicle). In contrast to this joint protective effect of oral crude TEO, and in contradistinction to the articular and extra-articular anti-inflammatory effects of this extract when administered ip, SCW-induced leukocytosis or hepatic granulomatous inflammation were unaltered by oral crude TEO (data not shown).
Figure 6.
Effects of oral crude TEO extract on joint inflammation. Female Lewis rats were injected on day 0 with vehicle or streptococcal cell wall (SCW, 25 μg/g body weight) to induce arthritis. Joint swelling in limbs was assessed at indicated times and expressed as mean arthritic index (AI) (scale 0-3/limb for total possible score of 16/animal) (n = 4-8 animals/group). A. Oral treatment with crude TEO (normalized to 560 mg refined TEO/kg/d) or vehicle was begun 4 days prior to SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 5 days per week. * p < 0.05 for crude TEO (vs. vehicle). B. Oral treatment with crude TEO (normalized to 560 mg refined TEO/kg/d) or vehicle was delayed until 3 days after SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 5 days per week.
In sum, oral crude TEO, at a dose that would correspond to 5000 mg/d in humans after correcting for body surface area (29), has a modest anti-inflammatory effect in rats that was limited to the joints and appeared to be specific in that it was not associated with evidence of significant concurrent toxicity. However, the clinical relevance of this finding is unclear given the magnitude of the dose required to achieve a relatively modest effect. One limitation of this study, then, is the absence of an oral dose response. However, reported side effects in 2 of 9 human subjects treated orally with a 50-fold lower dose of TEO in a prior human trial, including possible reactivation of tuberculosis (17), calls into question the safety and practicality of administering TEO doses higher than, or indeed equivalent to, those tested here.
Not specifically tested in these studies are possible additive or synergistic joint-protective effects of TEO when combined with curcuminoids, the other class of turmeric rhizome secondary metabolites. Previous reports of enhanced bioavailability of curcumin when administered orally with TEO terpenes (30) suggest that the combined administration of these two secondary metabolites of turmeric may be clinically useful due to matrix effects independent of any separate biological activity of the essential oils. However, it must also be stated that the safety of long term use of dietary supplements containing higher doses of TEO and/or curcuminoids than those typically consumed with culinary intake (e.g. 4 mg/kg/d curcuminoids or TEO in a typical Indian diet, given the 3% yield of curcuminoids (4) or TEO from dried rhizome), remains to be investigated. The toxic effects of turmeric essential oils when administered ip in these studies should remain a cautionary finding.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Office of Dietary Supplements (ODS) and the National Center for Complementary and Alternative Medicine (NCCAM of the NIH (5 P50 AT000474). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS or NIH.
ABBREVIATIONS USED
- ALT
serum alanine aminotransferase
- ANOVA
analysis of variance
- COX
cyclooxygenase
- d
day
- GRO/KC
growth-related oncogene/keratinocyte chemoattractant
- HCT
hematocrit
- HPLC
high pressure liquid chromatography
- ip
intraperitoneal
- MCP-1
monocyte-chemotactic protein
- NF-κB
nuclear factor-κB
- PGE2
prostaglandin E2
- PLT
platelet
- RA
rheumatoid arthritis
- SCW
streptococcal cell wall
- SEM
standard error of the mean
- TEO
turmeric essential oils
- TNF
tumor necrosis factor
- WBC
white blood cell
Footnotes
Supporting Information Available: HPLC chromatograms and other chemical analyses identifying major components of the experimental TEO extracts are available in Supplemental Figures 1 and 2. This information is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Pichersky E, Gang DR. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci. 2000;5:439–445. doi: 10.1016/s1360-1385(00)01741-6. [DOI] [PubMed] [Google Scholar]
- 2.Lantz RC, Chen GJ, Solyom AM, Jolad SD, Timmermann BN. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005;12:445–452. doi: 10.1016/j.phymed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz RC, Jolad SD, Solyom AM, Kiela PR, Timmermann BN. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452–3464. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 4.Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solyom AM, Timmermann BN. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J. Altern. Complement. Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 6.Apisariyakul A, Vanittanakom N, Buddhasukh D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae) J. Ethnopharmacol. 1995;49:163–169. doi: 10.1016/0378-8741(95)01320-2. [DOI] [PubMed] [Google Scholar]
- 7.Roth GN, Chandra A, Nair MG. Novel bioactivities of Curcuma longa constituents. J. Nat. Prod. 1998;61:542–545. doi: 10.1021/np970459f. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira LA, Henriques OB, Andreoni AA, Vital GR, Campos MM, Habermehl GG, de Moraes VL. Antivenom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae)[erratum appears in Toxicon 1992 Dec;30(12):1637] Toxicon. 1992;30:1211–1218. doi: 10.1016/0041-0101(92)90437-a. [DOI] [PubMed] [Google Scholar]
- 9.Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J. Agric. Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 10.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z. Naturforsch. [C] 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 11.Rathore P, Dohare P, Varma S, Ray A, Sharma U, Jagannathan NR, Ray M. Curcuma oil: reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008;33:1672–1682. doi: 10.1007/s11064-007-9515-6. [DOI] [PubMed] [Google Scholar]
- 12.Dohare P, Garg P, Sharma U, Jagannathan NR, Ray M. Neuroprotective efficacy and therapeutic window of curcuma oil: in rat embolic stroke model. BMC Complem. Altern. Med. 2008;8:55. doi: 10.1186/1472-6882-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohare P, Varma S, Ray M. Curcuma oil modulates the nitric oxide system response to cerebral ischemia/reperfusion injury. Nitric Oxide. 2008;19:1–11. doi: 10.1016/j.niox.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005;53:959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- 15.Honda S, Aoki F, Tanaka H, Kishida H, Nishiyama T, Okada S, Matsumoto I, Abe K, Mae T. Effects of ingested turmeric oleoresin on glucose and lipid metabolisms in obese diabetic mice: a DNA microarray study. J. Agric. Food Chem. 2006;54:9055–9062. doi: 10.1021/jf061788t. [DOI] [PubMed] [Google Scholar]
- 16.Jain V, Prasad V, Pal R, Singh S. Standardization and stability studies of neuroprotective lipid soluble fraction obtained from Curcuma longa. J. Pharm. Biomed. Anal. 2007;44:1079–1086. doi: 10.1016/j.jpba.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Joshi J, Ghaisas S, Vaidya A, Vaidya R, Kamat DV, Bhagwat AN, Bhide S. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J. Assoc. Physicians India. 2003;51:1055–1060. [PubMed] [Google Scholar]
- 18.Ji M, Choi J, Lee J, Lee Y. Induction of apoptosis by ar-turmerone on various cell lines. Int. J. Mol. Med. 2004;14:253–256. [PubMed] [Google Scholar]
- 19.Aratanechemuge Y, Komiya T, Moteki H, Katsuzaki H, Imai K, Hibasami H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int. J. Mol. Med. 2002;9:481–484. [PubMed] [Google Scholar]
- 20.Hong CH, Noh MS, Lee WY, Lee SK. Inhibitory effects of natural sesquiterpenoids isolated from the rhizomes of Curcuma zedoaria on prostaglandin E2 and nitric oxide production. Planta Med. 2002;68:545–547. doi: 10.1055/s-2002-32560. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour. Technol. 2006;97:1372–1376. doi: 10.1016/j.biortech.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Qin NY, Yang FQ, Wang YT, Li SP. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J. Pharm.Biomed. Anal. 2007;43:486–492. doi: 10.1016/j.jpba.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 23.Garg SN, Bansal RP, Gupta MM, Kumar S. Variation in the rhizome essential oil and curcumin contents and oil quality in the land races of turmeric Curcuma longa of North Indian plains. Flavour Fragr. J. 1999;14:315–318. [Google Scholar]
- 24.Funk JL, Chen J, Downey KJ, Davee SM, Stafford G. Blockade of parathyroid hormone-related protein prevents joint destruction and granuloma formation in streptococcal cell wall-induced arthritis. Arthritis Rheum. 2003;48:1721–1731. doi: 10.1002/art.10985. [DOI] [PubMed] [Google Scholar]
- 25.Bendele AM, Chlipala ES, Scherrer J, Frazier J, Sennello G, Rich WJ, Edwards CK., 3rd Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 2000;43:2648–2659. doi: 10.1002/1529-0131(200012)43:12<2648::AID-ANR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Solomon S, Kassahn D, Illges H. The role of the complement and the Fc gamma R system in the pathogenesis of arthritis. Arthritis Res. Ther. 2005;7:129–135. doi: 10.1186/ar1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohlert C, van Rensen I, Marz R, Schindler G, Graefe EU, Veit M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000;66:495–505. doi: 10.1055/s-2000-8616. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi KS, Flavell RA. Shielding the double-edged sword: negative regulation of the innate immune system. J. Leukoc. Biol. 2004;75:428–433. doi: 10.1189/jlb.0703321. [DOI] [PubMed] [Google Scholar]
- 29.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 30.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95® CG (Biocurcumax™), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008;70:445–449. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.