A variety of mechanisms have been proposed for detecting inorganic ions using magnetic resonance imaging (MRI) contrast agents. In the most widely explored approach, analyte binding to a paramagnetic metal chelate withdraws ligand(s), increasing the number (q) of inner sphere sites available for water coordination at the paramagnetic center. As a consequence, the longitudinal relaxivity (r1) of protons on exchangeable solvent molecules in the complex is similarly increased.[1] Here we introduce a qualitatively new strategy, in which the analyte completely releases the paramagnetic ion from a polydentate chelating ligand to form the aqua (aq) complex, which serves as an efficient MRI contrast agent with sharply different characteristics from the chelate. In this facile and flexible approach, changes in r1 can be tuned by the choice of the metal chelate complex (Fig. 1a). Measurement of the effect by MRI can then be used to determine the analyte concentration with spatiotemporal resolution.
Figure 1.
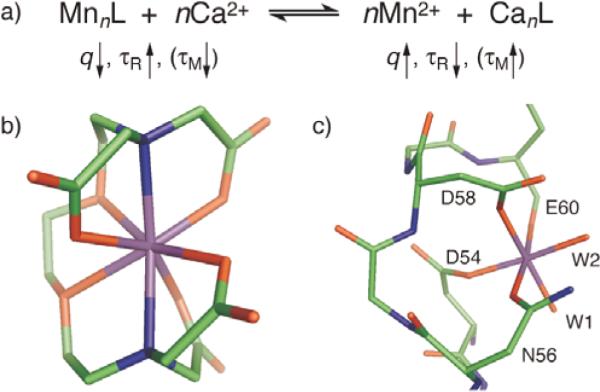
Mechanism of MRI calcium sensors based on displacement of Mn2+ from chelated complexes. a) Upon addition of excess Ca2+, Mn2+ ions are released from ligand molecules (L) including EGTA, BAPTA, and calmodulin (CaM). Dissociated Mn2+ ions have higher q, lower τR, and, for L = CaM, higher τM, than chelated manganese, all factors that influence longitudinal relaxivity. b) In complex with EGTA, Mn2+ (purple) is octacoordinate with q = 0.[3] c) The crystal structure of Mn2+ in complex with a canonical calcium binding site, loop 2 of calbindin,[4] shows two bound waters (W1 and W2); this binding loop has 71% sequence identity to the corresponding seven residues in the first EF hand of CaM, suggesting that Mn2+ ions in complex with CaM may similarly exhibit q = 2.
We tested the applicability of the paramagnetic metal displacement strategy to sense calcium ions by MRI. We chose the relatively labile manganese(II) ion as the paramagnetic reporter because aqueous Mn2+ is widely used as a T1 contrast agent for in vivo neuroimaging in animals.[2] Manganese complexes were assembled with ethylene glycol bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and calmodulin (CaM), all well-studied metal-binding molecules with selective affinity for Ca2+ ions. Structures of Mn2+ associated with EGTA[3] and with a protein domain closely related to the four calcium-binding regions of CaM[4] are shown in Figs. 1b and 1c. They suggest the presence of zero to two coordinated water molecules per Mn2+ ion. In contrast, Mn2+ (aq) is coordinated by six water molecules.[5] These differences in q, combined with rotational correlation time (τR) effects and the time constant for water exchange (τM, where q ≠ 0), contribute to relaxivity changes upon displacement of Mn2+ bound by EGTA, BAPTA, or CaM, upon addition of Ca2+.
Mixtures containing 100 μM Mn2+ in 1:1 stoichiometry with EGTA or BAPTA, or in 4:1 stoichiometry with CaM, which has four metal-binding sites, were imaged in the presence of 0–20 mM CaCl2, using spin echo pulse sequences in a 4.7 T MRI scanner at room temperature (22 °C). In all cases, progressive changes in MRI signal were observed as Ca2+ concentrations increased (Fig. 2a). Controls lacking Mn2+ or chelators showed no significant changes (see Supporting Information). Intensity data were analyzed to determine relaxivity as a function of Ca2+ concentration for each chelator (Fig. 2b). As [Ca2+] increased, values of r1 increased from 1.7 to 5.2 mM−1s−1 for EGTA and from 1.3 to 6.2 mM−1s−1 for BAPTA (estimated errors 10%). For CaM, r1 decreased from 10.5 to 5.5 mM−1s−1 with increasing [Ca2+]. Changes in the transverse relaxation rate (r2) were also observed (Fig. 2c). Addition of Ca2+ to Mn-EGTA or Mn-BAPTA produced r2 increases from 19 or 8.6 mM−1s−1, respectively, to 92 or 98 mM−1s−1. Addition of Ca2+ to Mn4CaM induced a modest drop in apparent transverse relaxivity, from 110 to 98 mM−1s−1. All three ligands showed substantial calcium-dependent changes in r2/r1 (see Supporting Information), suggesting that these reagents and their derivatives can be used to probe concentration-independent “ratiometric” quantification of [Ca2+] or other divalent cations.[6]
Figure 2.
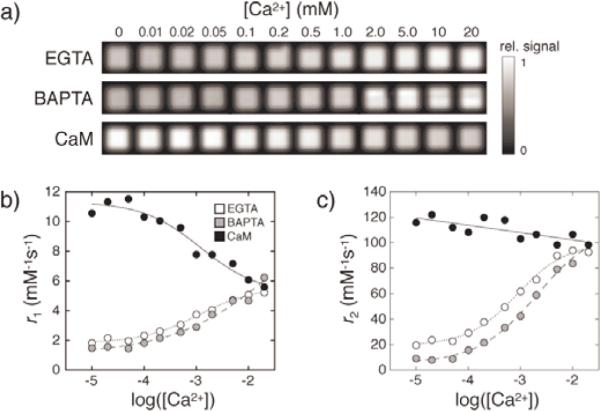
MRI and relaxivity changes upon paramagnetic ment from chelators. a) Progressive T1-weighted MRI signal changes observed as a function of [Ca2+] added to mixtures of 100 μM Mn2+ with 100 μM EGTA, 100 μM BAPTA, or 25 μM CaM, all in MOPS, pH 7. Intensity changes were normalized to the maximal MRI signal across the titration range. b) Longitudinal relaxivities (r1) computed from T1-weighted data, as a function of calcium concentration for Mn-EGTA (open circles), Mn-BAPTA (gray circles), and Mn4CaM (black circles). Fitted curves are shown for visualization purposes. c) Transverse relaxivity (r2) for the same samples.
The titration curves produced by adding Ca2+ to Mn2+ mixtures with EGTA, BAPTA, or CaM converged on relaxivity values similar to those measured using Mn2+ (aq) complexes (r1 = 5.6 ± 0.1 and r2 = 98 ± 3 mM−1s−1), supporting the conclusion that the relaxation changes were caused by the Ca2+-dependent Mn2+ displacement mechanism. A 20 mM Ca2+ concentration brought about almost complete Mn2+ dissociation in all cases. This result is in reasonable agreement with reported values of Kd(Mn2+)/Kd(Ca2+) for EGTA of 10−1.3 and for BAPTA of 10−2.[7, 8] The Ca2+-induced r1 increases observed for Mn-EGTA and Mn-BAPTA were also consistent with the anticipated effects of increasing q of Mn2+ from zero to six following displacement of the ion from the chelators. Relaxivity of Mn-EGTA and Mn-BAPTA complexes measured in the absence of calcium was comparable to outer sphere relaxivities reported at different fields for other Mn2+ complexes having q = 0.[9–11] Ironically, the low relaxivities and relatively labile nature of many q = 0 Mn2+ complexes have historically been considered a limitation to their use as contrast agents in clinical MRI, but these very properties have been exploited here to enable calcium sensing based on displacement of Mn2+ from Mn-EGTA and Mn-BAPTA.
The structure and nuclear magnetic relaxation properties of Mn4CaM have not been previously studied, but inner sphere contributions to r1 of the CaM complex can be estimated, in conjunction with experimentally justified values for τM and τR (see Supporting Information). The modeling studies indicate that the T1 relaxivity per bound water (r1/q) for Mn4CaM is approximately tenfold higher than for Mn2+ (aq). Both a short τM, as generally reported for q ≠ 0 Mn2+ complexes,[5] and long τR for Mn4CaM probably account for its strong relaxation effects, and counterbalance its reduced q, compared to Mn2+ (aq). The shape of the CaM titration curve thus reflects multiple determinants of r1, in addition to contributions of Mn2+/Ca2+ exchange at all of the four protein metal-binding sites. The CaM titration data were well approximated (r = 0.98) by a monophasic binding curve with a Hill coefficient of 0.8, suggesting that metal substitution at each site was approximately independent and identical.
To explore the analyte selectivity, we measured relaxivity changes of Mn2+ complexes in the presence of diamagnetic competitor ions. Excesses of Zn2+, Mg2+, or K+ (each 1 mM) were added to 100 μM Mn2+ in stoichiometric mixtures with metal-binding sites in EGTA, BAPTA, or CaM. As predicted by reported affinity constants[7, 8] and the Irving-Williams series, 1 mM Zn2+ produced strong r1 changes in Mn-EGTA and Mn-BAPTA and abolished calcium sensitivity, but the more biologically relevant competitors Mg2+ and K+ had little effect (see Supporting Information). Calcium-induced longitudinal relaxation (T1) changes on Mn4CaM persisted in the presence of the competitor ions, but both Zn2+ and Mg2+ partially attenuated calcium-dependent effects. Competitive effects on calcium-dependent transverse relaxation (T2) changes followed a similar pattern for all three Mn2+ complexes. As an additional test, we examined the calcium-induced relaxation changes induced in artificial cerebrospinal fluid (aCSF), a biomimetic solution approximating the interstitial environment of the brain. The para-magnetic Mn2+ displacement effect for EGTA and BAPTA was only minimally perturbed by the presence of competing solution components. For Mn4CaM, Ca2+-dependent r1 changes were reduced and occurred at higher [Ca2+], perhaps reflecting the relatively high concentration (0.8 mM) of Mg2+ in aCSF.
These results indicate that the Mn2+ displacement mechanism for measuring Ca2+ or other ion concentrations by MRI provides strong contrast changes and could be useful for applications in solution, as well as in relatively simple naturalistic environments where few extraneous metal ligands are present.[12] A particular strength of the approach is the potential for ratiometric measurements. An obvious limitation in complex contexts is the possibility that paramagnetic metal ions, once released from the chelators, might diffuse away or be sequestered by binding partners unassociated with a sensing mechanism. This problem could be particularly serious where reversible or dynamic measurements are desired. A possible solution would be to use steady-state infusion or dialysis methods to maintain the concentration of paramagnetic complexes to a fixed level, ensuring that any analyte-induced metal dissociation and MRI contrast changes are reversed over time. In cases where the displaced paramagnetic ion is relatively nontoxic, these approaches might conceivably be applied in live animals. We note that the sensitivity range of the complexes studied here is ideally suited to detecting millimolar calcium concentrations present in biological fluids and extracellular compartments like the cerebral interstitium.[13] In the future, extensions of the paramagnetic metal displacement mechanism might include sensing of other ions, such as Zn2+ or Mg2+, or design of novel paramagnetic ion/chelator pairs, for example based on engineered CaM variants, with improved selectivity and sensitivity for detecting analytes by MRI.
Experimental Section
EGTA and BAPTA complexes were assembled from commercially available compounds. CaM was purified from recombinant bacteria. MRI data were acquired on an Avance 4.7 T scanner. Data processing and relaxivity modelling were performed using Matlab. Detailed protocols are available as Supporting Information.
Supplementary Material
Acknowledgments
This research was funded by NIH grant DP2-OD2441 (New Innovator Award) to AJ, NIH grant R01-GM65519 to SJL, and a grant from the McGovern Institute Neurotechnology Program to AJ and SJL.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org
References
- [1].Li W, Fraser SE, Meade TJ. J. Am. Chem. Soc. 1999;121:1413. [Google Scholar]
- [2].Koretsky AP, Silva AC. NMR Biomed. 2004;17:527. doi: 10.1002/nbm.940. [DOI] [PubMed] [Google Scholar]
- [3].Schauer CK, Anderson OP. Acta Cryst. C. 1988;44:981. [Google Scholar]
- [4].Andersson M, Malmendal A, Linse S, Ivarsson I, Forsen S, Svensson LA. Protein Sci. 1997;6:1139. doi: 10.1002/pro.5560060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lauffer RB. Chem Rev. 1987;87:901. [Google Scholar]
- [6].Aime S, Fedeli F, Sanino A, Terreno E. J Am Chem Soc. 2006;128:11326. doi: 10.1021/ja062387x. [DOI] [PubMed] [Google Scholar]
- [7].Cheng KL, Ueno K, Imamura T, editors. CRC Handbook of Organic Analytical Reagents. CRC Press; Boca Raton, FL: 1982. [Google Scholar]
- [8].Yuchi A, Tanaka A, Hirai M, Yasui T, Wada H, Nakagawa G. Bull. Chem. Soc. Jpn. 1993;66:3377. [Google Scholar]
- [9].Elizondo G, Fretz CJ, Stark DD, Rocklage SM, Quay SC, Worah D, Tsang YM, Chen MC, Ferrucci JT. Radiology. 1991;178:73. doi: 10.1148/radiology.178.1.1898538. [DOI] [PubMed] [Google Scholar]
- [10].Troughton JS, Greenfield MT, Greenwood JM, Dumas S, Wiethoff AJ, Wang J, Spiller M, McMurry TJ, Caravan P. Inorg Chem. 2004;43:6313. doi: 10.1021/ic049559g. [DOI] [PubMed] [Google Scholar]
- [11].Wang S, Westmoreland TD. Inorg Chem. 2009;48:719. doi: 10.1021/ic8003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Manz B, Hillgartner M, Zimmermann H, Zimmermann D, Volke F, Zimmermann U. Eur Biophys J. 2004;33:50. doi: 10.1007/s00249-003-0341-8. [DOI] [PubMed] [Google Scholar]
- [13].Angelovski G, Fouskova P, Mamedov I, Canals S, Toth E, Logothetis NK. Chembiochem. 2008;9:1729. doi: 10.1002/cbic.200800165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


