Abstract
Flavin-containing monooxygenase (FMO) expression in male mouse liver is altered after 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure or castration. Because TCDD is slowly eliminated from the body, we examined hepatic Fmo mRNA alterations for up to 32 days following 10 or 64 µg/kg TCDD exposure by oral gavage in male C57BL/6J mice. Fmo2 mRNA was significantly induced at 1, 4, and 8 days whereas Fmo3 mRNA was also induced at 32 days relative to controls. Fmo3 mRNA levels exhibited a dose-dependent increase at 4, 8, and 32 days after exposure; Fmo1, Fmo4, and Fmo5 mRNA did not exhibit clear trends. Because castration alone also increased Fmo2, Fmo3, and Fmo4 mRNA we examined the combined effects of castration and TCDD treatment on FMO expression. A greater than additive effect was observed with Fmo2 and Fmo3 mRNA expression. Fmo2 mRNA exhibited a 3–5 fold increase after castration or 10 µg/kg TCDD exposure by oral gavage, whereas an approximately 20-fold increase was observed between the sham-castrated control and castrated TCDD-treated mice. Similarly, treatment with 10 µg/kg TCDD alone increased Fmo3 mRNA 130- and 180-fold in the sham-castrated and castrated mice compared to their controls respectively, whereas, Fmo3 mRNA increased approximately 1900-fold between the sham control and castrated TCDD-treated mice. An increase in hepatic Fmo3 protein in TCDD treated mice was observed by immunoblotting and assaying methionine S-oxidase activity. Collectively, these results provide evidence for isoform distinct time-, dose-, and castration-dependent effects of TCDD on FMO expression and suggest cross-talk between TCDD and testosterone signal transduction pathways.
Keywords: Flavin-containing monooxygenase; aryl-hydrocarbon receptor; 2,3,7,8,-tetrachlorodibenzo-p-dioxin; testosterone; castration
1. Introduction
Flavin-containing monooxygenases (FMOs) are microsomal enzymes that catalyze the addition of oxygen to many sulfur-, nitrogen-, phosphorus-, and selenium-containing compounds including pesticides, therapeutics, and dietary substances. Generally, FMO oxidation acts as a detoxification pathway; however, specific substrates are bioactivated into more reactive and deleterious compounds. There are five active and expressed FMO isoforms (FMO1-5) and several pseudogenes in humans and mice [1,2]. Importantly, FMO polymorphisms have been correlated to the human disease trimethylaminuria and to the efficacy of pharmaceuticals [3]. In contrast to their prominent role in xenobiotic metabolism, a clear physiological role for FMOs has yet to be established [4].
FMO isoform distribution is species-, tissue-, gender- and age-dependent. FMOs are regulated by many endogenous factors; including sex and stress steroids, nutritional status, and circadian rhythms [5–8]. Hepatic FMO3 expression is gender-dependent in mice and dogs but this dependency is not observed in other mouse tissues nor in human, rabbit, or rat liver [9,10]. Testosterone represses the expression of FMO3, and to a lesser degree FMO1, in male mouse liver; whereas females exhibit high levels of FMO3 and FMO1 [6]. In mice, castration increases FMO3 and FMO1 levels [6]. In contrast, FMO activity is reduced in rats after castration [11]. FMO3 expression increased and FMO1 levels remained unchanged in male rat liver during sexual maturation (3–11 weeks) a period of increased testosterone secretion [12]. These data suggest that testosterone effects FMO expression differently in mice and rats. Estrogen and progesterone have also been implicated in the modification of FMO expression in various species and tissues [7,13,14].
Despite extensive endogenous regulation, FMOs were previously designated as non-inducible by xenobiotics. Recent studies have shown hepatic Fmo mRNA or protein expression is altered due to pharmaceuticals like rifampin in primary human hepatocytes, and to disease states such as streptozotocin-induced diabetes in rats, and inflammation models in mice and rats [15–18]. Additional studies have shown hepatic Fmo mRNA or enzymatic activity changes by several aryl-hydrocarbon receptor (AHR) ligands, including 3-methylcholanthrene and 3,3'-diindolylmethane in rats, β-naphthoflavone in mice, and 2,3,7,8,-tetrachlorodibenzo-p-dioxin (TCDD) in mice and rats [19–23].
TCDD, a ubiquitous environmental contaminant, is a model compound for a range of chemicals that act through the AHR and the aryl hydrocarbon nuclear translocator. TCDD exposure causes liver toxicity and other deleterious effects [24]. There has been extensive characterization of TCDD-induced xenobiotic metabolizing enzymes, such as cytochrome P450s, UDP-glucuronosyltransferases, and glutathione-S-transferases [25]. In contrast, TCDD regulation of Fmo expression has only been recently discovered and is not fully characterized.
Hepatic Fmo2 and Fmo3 induction was detected in an AHR-dependent manner by microarray in male mice 19 h after 1000 µg/kg TCDD was administered by gavage [22]. Notably, TCDD was able to overcome testosterone repression of Fmo3 mRNA expression in male mouse liver. Further studies showed 24 h after i.p. injections of 30 µg/kg TCDD hepatic Fmo1, Fmo2, and Fmo3 mRNA were upregulated and Fmo5 mRNA was downregulated in male mice [26]. Fmo1 and Fmo2 mRNA were not induced in female mice. In male mice, TCDD exposure from 0.1–10 µg/kg, induced hepatic Fmo2 and Fmo3 mRNA in a dose-dependent fashion [26]. These data suggest complex regulation for Fmos in response to AHR ligands.
This study sought to further characterize the induction of Fmo mRNA for up to 32 days post-TCDD treatment to determine the duration and maximum of mRNA alterations. To our knowledge, no previous study has examined Fmo mRNA levels beyond 24 h. Doses were based on a dose response study in which 10 µg/kg was able to cause maximal transcription of known target genes and 64 µg/kg was able to cause toxic endpoints such as hepatomegaly, hydropic degeneration, and inflammation [24]. Additionally, we examined the combined effects of TCDD and castration because of reported gender-dependent TCDD alterations in mouse hepatic Fmo mRNA [26].
2. Materials and methods
2.1. Materials
β-Nicotinamide adenine dinucleotide phosphate reduced tetrasodium salt (NADPH), methionine, methionine sulfoxide, and 1-fluoro-2,4-dinitrobenzene were obtained from Sigma-Aldrich Chemicals (St. Louis, MO). TCDD was purchased from Cambridge Isotope Laboratories (Woburn, MA). Human cDNA-expressed FMO3 and FMO5 and anti-human FMO3 and FMO5 antibodies were purchased from BD Gentest (Woburn, MA). Rabbit anti-rat FMO4 antibody was previously characterized [27]. Goat anti-rabbit secondary antibody was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
2.2. Animals and treatments
For the time course experiment, C57BL/6J male mouse liver sections from mice that were administered TCDD (10 or 64 µg/kg) or corn oil alone by oral gavage were obtained from Dr. Chris Bradfield’s laboratory [24]. For the castration experiment, male C57BL/6J mice (5–6months old; The Jackson Laboratory, Bar Harbor, ME) were maintained on a 12 h light/dark cycle with food (5015 Mouse Diet, PMI Nutrition International, Brentwood, MO) and tap water available ad libitum. Procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison. Male mice were castrated via the scrotal route under isoflurane anesthesia. Mice were sham castrated via a scrotal incision and wound closure. Mice were given 14 days for recovery after surgery. Then, TCDD (10 µg/kg in corn oil) or vehicle (corn oil) was administered by oral gavage. Four days post treatment mice were euthanized by CO2 overdose and the livers were removed. The four day post-TCDD time point was chosen based on preliminary observations illustrating significant Fmo3 mRNA induction. For mRNA analysis, liver sections of approximately 100 mg were placed in RNAlater (Qiagen, Valencia, CA) overnight and the following day the excess RNAlater was removed. The rest of each liver was snap frozen for immunoblotting and enzymatic activity assays.
2.3. RNA Isolation, cDNA synthesis, and real time qPCR
Frozen hepatic samples were homogenized with a pestle and passed through a Qiashedder column (Qiagen) according to the manufacturer’s instructions. Total RNA was isolated with the Qiagen RNAeasy Mini kit (Qiagen) by the manufacturer’s instructions. RNA purity was assessed in 10 mM tris pH 7.5 and all samples exhibited a 260/280 ratio greater than 1.8. The RNA samples were determined to be of adequate quality (mean RIN=7.5) on a Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was synthesized as previously described [28]. cDNA was diluted in H2O and stored at −80°C until used. Real-time qPCR primers were from mRNA sequences reported in the Entrez database (National Center for Biotechnology Information) and were selected using Primer3 software [29]. Primer sequences and accession numbers are listed in Table 1. Real-time qPCR was performed as previously described [30]. A melting curve was performed to confirm the formation of a single amplicon. Changes in relative gene expression were calculated using the comparative threshold (Ct) cycle. Crossing points were determined using the Roche Light Cycler software version 3.5.3. The housekeeping gene peptidyl prolyl isomerase a (Ppia) did not exhibit a noticeable difference between control and treated samples and the data was normalized to this gene. The data was normalized by calculating the difference between Ct for the housekeeping gene and the Fmo genes (ΔCt). The difference between the control and treated animals was calculated 2-(ΔCt TCDD – ΔCt control).
Table 1.
FMO Primers for real-time qPCR
| Gene | Forward/reverse primer | Accession number |
Position | Product size (bp) |
|---|---|---|---|---|
| FMO1 | 5'-CGATTCCTCTGGGTGAAGAAG-3' | NM_010231 | 102-122 | 144 |
| 5'-TTACGTTGGAGCAAGGGATG-3' | 245-226 | |||
| FMO2 | 5'-ACTCAGAGCAACGGAAAGGAG-3' | NM_018881 | 432-452 | 102 |
| 5'-ACCTGGGAATGACTTGAGTGG-3' | 533-513 | |||
| FMO3 | 5'-CAGCATTTACCAATCGGTCTTC-3' | NM_008030 | 193-214 | 102 |
| 5'-TTGCTGTGATGCATGAAGTTG-3' | 294-274 | |||
| FMO4 | 5'-TCCTGAGCCCACATTTACCTC-3' | NM_144878 | 478-498 | 139 |
| 5'-CCAGTGTTTCCAAGACCAACC-3' | 616-596 | |||
| FMO5 | 5'-CATCCAACAGTGAATGATGACC-3' | NM_010232 | 931-952 | 114 |
| 5'-CCTGGAGCCATCCTCAAATAC-3' | 1044-1024 | |||
| CYP1A1 | 5'-CCTCCGTTACCTGCCTAACTC-3' | NM_001136059.1 | 830-850 | 149 |
| 5'-AATGCTCAATGAGGCTGTCTG-3' | 958-978 |
2.4. Immunoblotting
Hepatic samples were snap frozen and stored at −80°C. Tissue was made into “washed” microsomes [31]. Protein concentration was determined by an average of three separate bicinchoninic acid protein assays (Thermo Scientific, Waltham, MA). Hepatic microsomal proteins were separated by SDS-PAGE on a precast Criterion 12.5% resolving gel (Bio-Rad, Los Angeles, CA). Each gel contained Kaldiescope molecular weight standards (Bio-Rad) and a protein standard with cDNA-expressed human FMO3, FMO5, (BD Gentest) or rat kidney microsomes. Samples were run on the gel and transferred to nitrocellulose as previously described [32]. Transfer efficiency and loading were checked by Ponceau S staining (Sigma-Aldrich) with a visual assessment of staining amounts. The following antibodies were used for detection anti-human FMO3 (BD Gentest), anti-human FMO5 (BD Gentest), rabbit anti-rat FMO4 [27] followed by goat anti-rabbit secondary (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Membranes were incubated with enhanced chemiluminescence (ThermoScientific) and transferred to film. Quantitation was performed by obtaining the volume (optical density×mm2) of each band with Quantity One software (Molecular Dynamics). To our knowledge, antibodies that specifically recognize mouse FMO1 and FMO2 are not commercially available.
2.5. Enzymatic Activity
Methionine S-oxidase activity was correlated to FMO3 protein levels in male and female dog, rat, and rabbit liver and kidney [9]. Low levels of endogenous methioinine S-oxidase activity were observed in male mouse liver and were not correlated to FMO3 protein, which has extremely low expression [9]. The correlation of FMO3 protein and methionine S-oxidase in other tissues, including female mouse liver, suggests this assay would measure increases in FMO3. Methionine S-oxidase activity assays and HPLC analysis were conducted in the same manner as describe previously [32]. Briefly, assays were performed at 37°C with constant shaking. Liver microsomes (0.25 mg), NADPH (final concentration 2mM), and buffer (10 mM potassium phosphate, 1 mM EDTA, pH 7.6) were pre-incubated for 5 min. Control incubations were run without NADPH. The reaction (final reaction volume 0.25 ml) was started with the addition of methionine (final concentration 10 mM) and incubated for 10 min at 37°C. The enzymatic reaction was stopped with the addition of cold ethanol (0.25 ml). Methionine reaction aliquots (350 µl) were derivatized by the addition of 9 µl of 1-fluoro-2, 4-dinitrobenzene (10% v/v in ethanol) and 6 µl of 1 M NaHCO3 and incubated for 30 min at 37°C and then in the dark at room temperature for 6 h. HPLC analysis of the reactions were carried out using a Beckman System Gold 125 Solvent Module equipped with a Beckman Ultrasphere column (4.6 mm×25 cm) and a Beckman System Gold Detector 166. The system was run at 1 ml/min with the detector set at 360 nm. Quantitation of the methionine sulfoxide was carried out by comparing peak areas, corrected for the nonenzymatic activity, to a standard curve. The standard curve was of chemically synthesized methionine sulfoxide (Sigma) and was derivatized and run on the HPLC at the same time as the assay. The correlation coefficient of the standard curve was excellent (r > 0.99).
2.6. Statistical Analysis
Statistical analysis of data for each treatment was performed with a Kruskal-Wallis test (Mstat, http://www.mcardle.wisc.edu/mstat/). Results were considered significant if p<0.05.
3. Results
3.1. Time course of FMO mRNA changes after TCDD exposure
The time points of 1, 4, 8, and 32 days were chosen to examine sustained Fmo mRNA alterations following 10 or 64µg/kg TCDD. Compared to time-matched controls Fmo2 mRNA was significantly induced by 10 and 64 µg/kg TCDD on 1, 4, and 8 days after exposure (Fig. 1). Fmo2 mRNA was increased 3–5 fold 4 and 8 days after exposure to 10 or 64 µg/kg TCDD. Fmo3 mRNA was significantly increased 1, 4, 8 and 32 days after 10 or 64 µg/kg TCDD exposure compared to time-matched controls. The largest Fmo3 induction was 4 days after the exposure, with 230- and 1200-fold increase for the 10 and 64µg/kg TCDD doses respectively. Fmo3 was the only FMO isoform to show a dose-dependent induction. At 4, 8, and 32 days post-TCDD, Fmo3 mRNA was significantly higher in mouse liver following 64 µg/kg TCDD than following 10 µg/kg TCDD (Fig. 1). Fmo1, Fmo4, and Fmo5 mRNA were also examined but trends in TCDD regulation were not as clear (Fig.1). Cyp1a1 mRNA expression was significantly increased at all time points after 10 and 64 µg/kg TCDD exposure compared to time matched controls. The Cyp1a1 mRNA expression levels were not significantly different between the two doses.
Figure 1.
Timecourse of Fmo mRNA levels in adult male mouse liver in vehicle and TCDD treated animals. Adult male mice were dosed with 10 µg/kg TCDD, 64 µg/kg TCDD, or corn oil alone and euthanized 1, 4, 8, or 32 days later. Livers were removed and mRNA levels were measured by real-time qPCR as described in Materials and Methods. mRNA levels are expressed relative to time-matched control. Cyp1a1 mRNA was measured as a positive control. *, p<0.05 compared to time-matched control; **, p<0.01 compared to time-matched control; ‡, p<0.05 compared alternate dose at that time point; plotted bars represent mean ± SD; n=3–4 mice in treated groups and 3–8 mice in control groups.
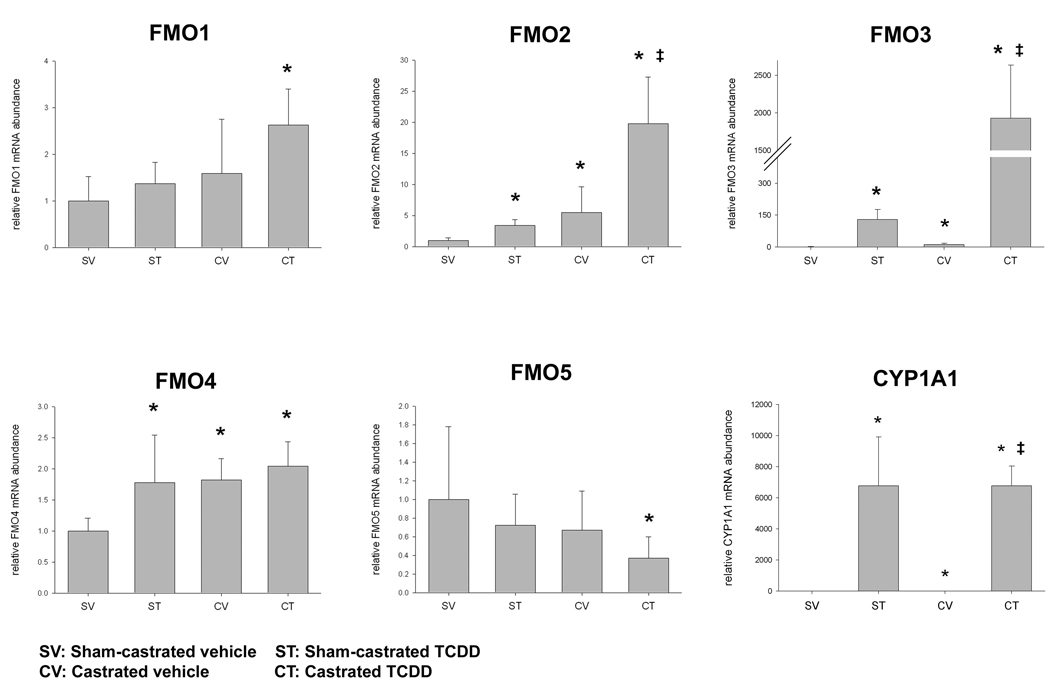
3.2. FMO mRNA levels after castration and TCDD exposure
Fmo expression changes due to castration and TCDD treatment were examined in male mouse liver at four days after 10 µg/kg TCDD exposure (Fig. 2). Castration increased Fmo3, Fmo2, and Fmo4 mRNA 11-,5.4-, 1.8-fold respectively in comparison to sham-operated controls.
Figure 2.
Fmo mRNA levels in the livers of adult male mice after castration and TCCD treatment. Mice were sham-castrated or castrated and allowed 2 weeks for recovery. Mice received vehicle or TCDD (10 µg/kg) and livers were collected 4 days later. mRNA levels were measured by real-time qPCR as described in Materials and Methods. Cyp1a1 mRNA was measured as a positive control. *, p<0.05 compared to SV; ‡, p<0.05 compared to CV; plotted bars represent mean ± SD; n=3–5 mice per group.
Fmo2 and Fmo3 mRNAs were induced by TCDD in intact and castrated mice. TCDD treatment increased Fmo2 mRNA in sham-castrated and castrated mice approximately 3–4 fold relative to the respective controls. Fmo3 mRNA increased after TCDD treatment approximately 130- and 180-fold in sham-castrated and castrated mice respectively, relative to their respective vehicle controls. The change in mRNA expression between the sham-castrated control and the castrated TCDD treated was approximately 20-fold for Fmo2 and 1900-fold for Fmo3 mRNA.
Fmo4 mRNA was significantly induced by TCDD in sham-castrated mice 1.8-fold but not in the castrated mice. There was a 2-fold increase between the sham-castrated control and castrated TCDD treated mice. Significant changes in Fmo1 and Fmo5 mRNA expression were not observed due to TCDD treatment alone. Fmo1 mRNA was increased 2.6–fold and Fmo5 mRNA was decreased 0.37–fold between the sham-castrated control and castrated TCDD treated mice. Thus, Fmo1, Fmo2, and Fmo3 mRNA exhibited a greater than additive response to castration and TCDD.
Significant increases in Cyp1a1 mRNA expression was observed in the 10 µg/kg TCDD samples (Fig. 2). We also observed a 2-fold increase in Cyp1a1 mRNA between the sham-castrated and castrated mice that received vehicle.
3.3. FMO activity and protein levels after castration and TCDD exposure
Liver microsomal enzymatic activity of the oxidation of methionine to methionine sulfoxide was increased by TCDD treatment (Fig. 3). Castration alone did not cause a significant increase in the methionine S-oxidase activity. TCDD treatment caused a 1.3-fold increase in activity in sham-castrated and castrated animals and a 1.7-fold induction between the sham-castrated control and castrated TCDD treated mice.
Figure 3.
Methionine S-oxidase activities of mouse liver microsomes were determined as described in Materials and Methods section. Incubations contained 10 mM methionine, 2 mM NADPH, and 0.25 mg microsomal protein. Reaction time was 10 min with a 5 min pre-incubation before the addition of substrate. Mice were sham-castrated (S) or castrated (C) and allowed two weeks for recovery. Then, mice received vehicle (V) or 10 µg/kg TCDD (T) and were euthanized four days later. *, p<0.05 compared to SV: **, p<0.01 compared to SV; ‡, p<0.05 compared to CV; plotted bars represent mean ± SD; n=4–5 mice per group.
Immunoblotting for Fmo3 revealed a large induction of Fmo3 protein after TCDD treatment (Fig. 4). It was difficult to quantify the change in Fmo3 protein abundance due to TCDD because it was close to non-detectable in vehicle control samples. However; even without quantification the differences between vehicle and TCCD treated samples is clearly evident. Quantification of the protein values of TCDD treated samples from the sham-castrated and castrated animals showed a 1.6-fold increase but it did not reach significance (p=0.07). Immunobloting for Fmo4 and Fmo5 was also performed but did not exhibit clear trends (data not shown).
Figure 4.
Immunoblot for FMO3 protein in adult the livers of adult male mice after castration and TCCD treatment. 50, 25, 10, or 5 fmol of human cDNA-expressed FMO3 (BD Gentest), determined by manufacturer’s FAD content, and 10 µg microsomal protein were resolved on an SDS polyacrylamide gel, transferred to nitrocellulose and incubated with rabbit polyclonal anti-human FMO3 as described in Materials and Methods. Mice were sham-castrated (S) or castrated (C) and received vehicle (V) or 10 µg/kg TCDD (T). The 4 treatment groups are shown. n=5 mice per group. Protein load and transfer efficiency were visually assessed by Ponceau S staining of the membrane.
4. Discussion
FMOs are important enzymes in the bioactivation and detoxification of xenobiotics; yet, the regulation of FMOs is not well understood. This study sought to further characterize the time-, dose-, and castration-dependent response of FMOs to TCDD treatment.
TCDD is resistant to metabolic processing and thus causes sustained gene alterations. For example, TCDD-dependent induction of Cyp1a1 and Cyp1a2 mRNA persist at least 64 days after treatment [24]. Hepatic levels of TCDD in mice after receiving 10 µg/kg by oral dose were maximal at 72 h and decreased 50% between 72 and 168 h [33]. The distribution and excretion of TCDD in C56BL/6J mice has been described and TCDD was detected in the liver at 42 days after exposure [34]. Thus, it is not surprising to see the largest induction of Fmo2 and Fmo3 at 4 days post-TCDD and to have Fmo3 mRNA induction remaining at 32 days. Changes in Fmo1, Fmo2, and Fmo3 mRNA in male mouse liver after treatment with AHR ligands have been observed previously [21,22,26] but, to our knowledge, this is the first examination of TCDD induced alterations in these genes beyond 24 h.
Known TCDD target genes, such as Cyp1a1 and Cyp1a2, exhibit maximal induction at 10 µg/kg in male mice [24]. TCDD exposure from 0.1–10 µg/kg, induced hepatic Fmo2 and Fmo3 mRNA levels at 24 h in a dose-dependent fashion in male mice [26]. In this study, Fmo3 mRNA exhibited a greater induction after 64 µg/kg TCDD in comparison to 10 µg/kg TCDD but Fmo2 mRNA did not exhibit a difference between the two doses. Perhaps, the dose dependent induction of Fmo3 mRNA at high doses is due to the initial low expression level and hormonal repression. It is also possible the change in Fmo3 mRNA expression in mice that received 64 µg/kg TCDD is due to direct induction through the AHR and indirect induction through other TCDD induced cellular changes. The differences between the two doses is only observed at later time points after indirect mechanisms would have time to occur. A possible mechanism may be TCDD-induced depression of testosterone levels which has been previously observed in rats two days after administration of 15 µg/kg TCDD [35].
Previously, significant changes were observed for Fmo1 and Fmo5 mRNA in male mouse liver after 30 µg/kg TCDD exposure [26]. We observed similar trends but the change in mRNA abundance in this study did not reach significance. In agreement with our data, other mouse liver microarray studies also only reported Fmo2 and Fmo3 mRNA as upregulated by AHR ligands and did not report changes in Fmo1, Fmo4, or Fmo5 mRNA [21,22]. Regardless, the differences in dose, route of exposure, and the time points studied after exposure could explain the variability of the results among the different reported studies.
It was previously reported that FMO3, and to a lesser extent FMO1, are repressed by testosterone in male mouse liver whereas FMO5 is not affected [6]. We observed increased Fmo3, Fmo2, and Fmo4 mRNA after castration. This suggests that Fmo2 and Fmo4 mRNA may be weakly repressed by androgens. To our knowledge, a gender difference has not been reported for FMO2 or FMO4 in mice. Mouse hepatic Fmo2 and Fmo4 mRNA levels are low and these proteins have few known isoform-specific substrates; thus, if a small gender difference exists it may not be easily observed [36]. Further studies are necessary to determine if the observed changes in Fmo mRNA after castration are androgen-related.
Our results show a greater than additive effect of castration and TCDD treatment on Fmo1, Fmo2, and Fmo3 mRNA. If the effect of testosterone and TCDD were independent, we would expect the fold change after castration and TCDD exposure to be additive but we observed greater than additive changes for Fmo mRNAs, suggesting possible cross-talk between the androgen receptor and TCDD signal transduction pathways. Previous studies have reported cross-talk between androgen receptor and AHR in other tissues. In prostate cancer cells, testosterone treatment inhibited the induction of CYP1A1 by TCDD [37,38]. In mouse prostate development, TCDD exerts both androgenic and anti-androgenic effects [39]. Additionally, inclusion of TCDD blocked androgen-dependent cell proliferation in cell culture [40]. A mechanism for the cross-talk was proposed where the androgen receptor and the AHR form a complex, altering transcription [41]. Further studies showed that activated AHR decreased the protein level of androgen receptor through an increase in targeted protein degradation through an E3 ubiquitin ligase complex in prostate cancer cells [42]. The AHR pathway is known to be involved in cross-talk with estrogen signaling and pathways essential to cycle regulation, apoptosis, and cellular kinases [42,43].
The large change in Fmo2 and Fmo3 mRNA in male mouse after TCDD exposure could be utilized to further characterize FMO activity. FMO2 is active in mice [44] but inactive in Caucasian and Asian human subjects due to a mutation [45]. Approximately 26% and 5% African and Hispanic Americans express active FMO2, respectively [46,47]. In sub-Saharan Africa, there are populations where almost 50% of individuals express active FMO2 and it was estimated that approximately 220 million people world wide express the active enzyme [48]. This allele may increase the risk of pulmonary damage due to thiourea exposure because thioureas are activated by FMO2 to reactive sulfenic acids [49]. Additional substrates for the active FMO2 include the antitubercular drugs ethionamide and thiacetazone, which may be metabolized differently in individuals with active FMO2 [50]. FMO3 is highly expressed in human liver and is the predominant isoform in human drug metabolism. FMO3 substrates include important therapeutics such as amphetamine, clozapine, tamoxifen, ethionamide and thiacetazone [4]. FMO3 is the FMO isoform that has exhibited the lowest Km values with methionine, S-allyl-cysteine, and S-(1,2-dichlorovinyl)-L-cysteine, a metabolite of trichloroethylene [51,52]. Thus, the effect of TCDD on FMO2 and FMO3 expression could alter an organism’s response to many therapeutics or chemicals and provide an opportunity for further characterization.
Protein levels are not always well correlated to mRNA levels due to the complex relationship between transcription and translation, differences in stability between mRNA and protein, and the cellular localization and molecular associations of proteins. FMO protein levels did not correlate to mRNA levels in human liver [53]. As we seek to understand the physiological implications of mRNA changes, a relationship to protein content and activity must be established. Previously, large increases in TCDD-induced Fmo mRNA resulted in smaller (~1.4) fold-increases in methimazole activity which is metabolized by multiple FMO isoforms [26]. Although our observed 1.7-fold increase in methionine S-oxidase activity was not large, it suggests an increase in FMO3 after TCDD exposure. Methionine S-oxidase activity has been previously observed in untreated male mouse liver [9] and is likely due to metabolism by FMO1 or FMO4, which are expressed in male mouse liver [6,27,51,54]. It is likely the observed 1.7-fold increase in activity is from the change in FMO3 level. Thus, the activity of newly expressed FMO3 protein is about equivalent to activity of FMO1 and FMO4 combined. In this light, a 1.7-fold change in activity suggests a significant increase in the amount of protein. To our knowledge this was the first detection of an increase in FMO3 protein between vehicle and TCDD treated mice by immunoblotting. We did not expect the change in protein to be as great as the change in mRNA. In addition to the normal difficulty in predicting protein level from mRNA level, TCDD causes an increase in many genes; thus, the molecular machinery used to make protein may be unable to translate the additional mRNA into protein. It is also possible the enzymatic activity increases less than the protein because of limited incorporation of FAD into the FMO3 active site.
Determination of Fmo expression in response to xenobiotics and signal transduction pathways is necessary to understand their endogenous regulation and xenobiotic response. About 85% of the gene response to TCDD is species-specific between rats and mice [55] and the effect of TCDD on FMO expression in humans is unknown. Further analysis of the promoter regions and DNA regulatory elements may provide insight into how transcription factors control the species-, tissue-, and gender-dependent expression of FMOs. In addition, experiments on changes to FMO metabolism and protein levels in relation to mRNA expression will provide valuable data on the physiological effects of these alterations. Changes in FMO expression level could have profound effects on the metabolism of therapeutics and may alter an organism’s response to xenobiotic exposure. An endogenous role for FMOs has yet to be determined and it is unlikely that these enzymes evolved to metabolize the known FMO substrates. FMO induction, especially chronic FMO induction, may alter metabolic homeostatisis and have an impact on the organism.
Acknowledgements
This research is supported by the National Institute of Health Grants T32-ES-007015 and RO1 DK044295.
Abbreviations
- FMO
flavin-containing monooxygenase
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- AHR
Aryl hydrocarbon receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rachel M. Novick, Email: novick@wisc.edu.
Chad M. Vezina, Email: cmvezina@wisc.edu.
Adnan A. Elfarra, Email: elfarraa@svm.vetmed.wisc.edu.
References
- 1.Lawton MP, Cashman JR, Cresteil T, Dolphin CT, Elfarra AA, Hines RN, et al. A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch Biochem Biophys. 1994;308:254–257. doi: 10.1006/abbi.1994.1035. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez D, Janmohamed A, Chandan P, Phillips IR, Shephard EA. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: identification of novel gene and pseudogene clusters. Pharmacogenetics. 2004;14:117–130. doi: 10.1097/00008571-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Phillips IR, Shephard EA. Flavin-containing monooxygenases: mutations, disease and drug response. Trends Pharmacol Sci. 2008;29:294–301. doi: 10.1016/j.tips.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixit A, Roche TE. Spectrophotometric assay of the flavin-containing monooxygenase and changes in its activity in female mouse liver with nutritional and diurnal conditions. Arch Biochem Biophys. 1984;233:50–63. doi: 10.1016/0003-9861(84)90600-3. [DOI] [PubMed] [Google Scholar]
- 6.Falls JG, Ryu DY, Cao Y, Levi PE, Hodgson E. Regulation of mouse liver flavin-containing monooxygenases 1 and 3 by sex steroids. Arch Biochem Biophys. 1997;342:212–223. doi: 10.1006/abbi.1997.9965. [DOI] [PubMed] [Google Scholar]
- 7.Coecke S, Debast G, Phillips IR, Vercruysse A, Shephard EA, Rogiers V. Hormonal regulation of microsomal flavin-containing monooxygenase activity by sex steroids and growth hormone in co-cultured adult male rat hepatocytes. Biochem Pharmacol. 1998;56:1047–1051. doi: 10.1016/s0006-2952(98)00104-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen QM, Alexander D, Sun H, Xie L, Lin Y, Terrand J, et al. Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: induction of antiapoptosis, antioxidant, and detoxification genes. Mol Pharmacol. 2005;67:1861–1873. doi: 10.1124/mol.104.003814. [DOI] [PubMed] [Google Scholar]
- 9.Ripp SL, Itagaki K, Philpot RM, Elfarra AA. Species and sex differences in expression of flavin-containing monooxygenase form 3 in liver and kidney microsomes. Drug Metab Dispos. 1999;27:46–52. [PubMed] [Google Scholar]
- 10.Lickteig AJ, Riley IR, Melton RJ, Reitz BA, Fischer HD, Stevens JC. Expression and characterization of functional dog flavin-containing monooxygenase 3. Drug Metab Dispos. 2009;37:1987–1990. doi: 10.1124/dmd.109.027714. [DOI] [PubMed] [Google Scholar]
- 11.Lemoine A, Williams DE, Cresteil T, Leroux JP. Hormonal regulation of microsomal flavin-containing monooxygenase: tissue-dependent expression and substrate specificity. Mol Pharmacol. 1991;40:211–217. [PubMed] [Google Scholar]
- 12.Lattard V, Lachuer J, Buronfosse T, Garnier F, Benoit E. Physiological factors affecting the expression of FMO1 and FMO3 in the rat liver and kidney. Biochem Pharmacol. 2002;63:1453–1464. doi: 10.1016/s0006-2952(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee MY, Clark JE, Williams DE. Induction of flavin-containing monooxygenase (FMO B) in rabbit lung and kidney by sex steroids and glucocorticoids. Arch Biochem Biophys. 1993;302:332–336. doi: 10.1006/abbi.1993.1219. [DOI] [PubMed] [Google Scholar]
- 14.Miller MM, James RA, Richer JK, Gordon DF, Wood WM, Horwitz KB. Progesterone regulated expression of flavin-containing monooxygenase 5 by the B-isoform of progesterone receptors: implications for tamoxifen carcinogenicity. J Clin Endocrinol Metab. 1997;82:2956–2961. doi: 10.1210/jcem.82.9.4239. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Chaluvadi MR, Reddy R, Motika MS, Richardson TA, Cashman JR, et al. Hepatic flavin-containing monooxygenase gene regulation in different mouse inflammation models. Drug Metab Dispos. 2009;37:462–468. doi: 10.1124/dmd.108.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Shankar K, Ronis MJ, Mehendale HM. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J Pharmacol Exp Ther. 2000;294:473–479. [PubMed] [Google Scholar]
- 17.Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849–857. [PubMed] [Google Scholar]
- 18.Park CS, Baek HM, Chung WG, Lee KH, Ryu SD, Cha YN. Suppression of flavin-containing monooxygenase by overproduced nitric oxide in rat liver. Mol Pharmacol. 1999;56:507–514. doi: 10.1124/mol.56.3.507. [DOI] [PubMed] [Google Scholar]
- 19.Chung WG, Park CS, Roh HK, Cha YN. Induction of flavin-containing monooxygenase (FMO1) by a polycyclic aromatic hydrocarbon, 3-methylcholanthrene, in rat liver. Mol Cells. 1997;7:738–741. [PubMed] [Google Scholar]
- 20.Katchamart S, Stresser DM, Dehal SS, Kupfer D, Williams DE. Concurrent flavin-containing monooxygenase down-regulation and cytochrome P-450 induction by dietary indoles in rat: implications for drug-drug interaction. Drug Metab Dispos. 2000;28:930–936. [PubMed] [Google Scholar]
- 21.Patel RD, Hollingshead BD, Omiecinski CJ, Perdew GH. Aryl-hydrocarbon receptor activation regulates constitutive androstane receptor levels in murine and human liver. Hepatology. 2007;46:209–218. doi: 10.1002/hep.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol. 2006;69:140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 23.Ovando BJ, Vezina CM, McGarrigle BP, Olson JR. Hepatic gene downregulation following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;94:428–438. doi: 10.1093/toxsci/kfl111. [DOI] [PubMed] [Google Scholar]
- 24.Hayes KR, Zastrow GM, Nukaya M, Pande K, Glover E, Maufort JP, et al. Hepatic transcriptional networks induced by exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem Res Toxicol. 2007;20:1573–1581. doi: 10.1021/tx7003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitlock JP, Jr, Chichester CH, Bedgood RM, Okino ST, Ko HP, Ma Q, et al. Induction of drug-metabolizing enzymes by dioxin. Drug Metab Rev. 1997;29:1107–1127. doi: 10.3109/03602539709002245. [DOI] [PubMed] [Google Scholar]
- 26.Celius T, Roblin S, Harper PA, Matthews J, Boutros PC, Pohjanvirta R, et al. Aryl hydrocarbon receptor (AHR)-dependent induction of flavin-containing monooxygenase mRNAs in mouse liver. Drug Metab Dispos. 2008;36:2499–2505. doi: 10.1124/dmd.108.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novick RM, Mitzey AM, Brownfield MS, Elfarra AA. Differential localization of flavin-containing monooxygenase (FMO) isoforms 1, 3, and 4 in rat liver and kidney and evidence for expression of FMO4 in mouse, rat, and human liver and kidney microsomes. J Pharmacol Exp Ther. 2009;329:1148–1155. doi: 10.1124/jpet.109.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, et al. Retinoic acid induces prostatic bud formation. Dev Dyn. 2008;237:1321–1333. doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 30.Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Tox Sci. 2002;68:479–487. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 31.Sausen PJ, Elfarra AA. Cysteine conjugate S-oxidase. Characterization of a novel enzymatic activity in rat hepatic and renal microsomes. J Biol Chem. 1990;265:6139–6145. [PubMed] [Google Scholar]
- 32.Novick RM, Elfarra AA. Purification and Characterization of Flavin-Containing Monooxygenase Isoform 3 from Rat Kidney Microsomes. Drug Metab Dispos. 2008;36:2468–2474. doi: 10.1124/dmd.108.021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boverhof DR, Burgoon LD, Tashiro C, Sharratt B, Chittim B, Harkema JR, et al. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci. 2006;94:398–416. doi: 10.1093/toxsci/kfl100. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaum LS. Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab Dispos. 1986;14:34–40. [PubMed] [Google Scholar]
- 35.Moore RW, Potter CL, Theobald HM, Robinson JA, Peterson RE. Androgenic deficiency in male rats treated with 2,3,7,8,-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1985;79:99–111. doi: 10.1016/0041-008x(85)90372-2. [DOI] [PubMed] [Google Scholar]
- 36.Janmohamed A, Hernandez D, Phillips IR, Shephard EA. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos) Biochem Pharmacol. 2004;68:73–83. doi: 10.1016/j.bcp.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 37.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Cross-talk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem Biophys Res Commun. 1999;256:462–468. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- 38.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Comparative effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on MCF-7, RL95-2, and LNCaP cells: role of target steroid hormones in cellular responsiveness to CYP1A1 induction. Mol Cell Biol Res Commun. 2000;4:174–180. doi: 10.1006/mcbr.2001.0275. [DOI] [PubMed] [Google Scholar]
- 39.Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68:479–487. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 40.Barnes-Ellerbe S, Knudsen KE, Puga A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol Pharmacol. 2004;66:502–511. doi: 10.1124/mol.104.000356. [DOI] [PubMed] [Google Scholar]
- 41.Sanada N, Gotoh Y, Shimazawa R, Klinge CM, Kizu R. Repression of activated aryl hydrocarbon receptor-induced transcriptional activation by 5alpha-dihydrotestosterone in human prostate cancer LNCaP and human breast cancer T47D cells. J Pharmacol Sci. 2009;109:380–387. doi: 10.1254/jphs.08328fp. [DOI] [PubMed] [Google Scholar]
- 42.Ohtake F, Fujii-Kuriyama Y, Kato S. AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions. Biochem Pharmacol. 2009;77:474–484. doi: 10.1016/j.bcp.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 43.Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karoly ED, Rose RL. Sequencing, expression, and characterization of cDNA expressed flavin-containing monooxygenase 2 from mouse. J Biochem Mol Toxicol. 2001;15:300–308. doi: 10.1002/jbt.10009. [DOI] [PubMed] [Google Scholar]
- 45.Dolphin CT, Beckett DJ, Janmohamed A, Cullingford TE, Smith RL, Shephard EA, Phillips IR. The flavin-containing monooxygenase 2 gene (FMO2) of humans, but not of other primates, encodes a truncated, nonfunctional protein. J Biol Chem. 1998;273:30599–30607. doi: 10.1074/jbc.273.46.30599. [DOI] [PubMed] [Google Scholar]
- 46.Whetstine JR, Yueh MF, McCarver DG, Williams DE, Park CS, Kang JH, et al. Ethnic differences in human flavin-containing monooxygenase 2 (FMO2) polymorphisms: detection of expressed protein in African-Americans. Toxicol Appl Pharmacol. 2000;168:216–224. doi: 10.1006/taap.2000.9050. [DOI] [PubMed] [Google Scholar]
- 47.Krueger SK, Williams DE, Yueh MF, Martin SR, Hines RN, Raucy JL, et al. Genetic polymorphisms of flavin-containing monooxygenase (FMO) Drug Metab Rev. 2002;34:523–532. doi: 10.1081/dmr-120005653. [DOI] [PubMed] [Google Scholar]
- 48.Veeramah KR, Thomas MG, Weale ME, Zeitlyn D, Tarekegn A, Bekele E, Mendell NR, Shephard EA, Bradman N, Phillips IR. The potentially deleterious functional variant flavin-containing monooxygenase 2*1 is at high frequency throughout sub-Saharan Africa. Pharmacogenet Genomics. 2008;18:877–886. doi: 10.1097/FPC.0b013e3283097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson MC, Krueger SK, Stevens JF, Williams DE. Human flavin-containing monooxygenase form 2 S-oxygenation: sulfenic acid formation from thioureas and oxidation of glutathione. Chem Res Toxicol. 2004;17:633–640. doi: 10.1021/tx034253s. [DOI] [PubMed] [Google Scholar]
- 50.Francois AA, Nishida CR, de Montellano PR, Phillips IR, Shephard EA. Human flavin-containing monooxygenase 2.1 catalyzes oxygenation of the antitubercular drugs thiacetazone and ethionamide. Drug Metab Dispos. 2009;37:178–186. doi: 10.1124/dmd.108.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krause RJ, Ripp SL, Sausen PJ, Overby LH, Philpot RM, Elfarra AA. Characterization of the methionine S-oxidase activity of rat liver and kidney microsomes: immunochemical and kinetic evidence for FMO3 being the major catalyst. Arch Biochem Biophys. 1996;333:109–116. doi: 10.1006/abbi.1996.0370. [DOI] [PubMed] [Google Scholar]
- 52.Ripp SL, Overby LH, Philpot RM, Elfarra AA. Oxidation of cysteine S-conjugates by rabbit liver microsomes and cDNA-expressed flavin-containing mono-oxygenases: studies with S-(1,2-dichlorovinyl)-L-cysteine, S-(1,2,2-trichlorovinyl)-L-cysteine, Sallyl-L-cysteine, and S-benzyl-L-cysteine. Mol Pharmacol. 1997;51:507–515. [PubMed] [Google Scholar]
- 53.Overby LH, Carver GC, Philpot RM. Quantitation and kinetic properties of hepatic microsomal and recombinant flavin-containing monooxygenases 3 and 5 from humans. Chem Biol Interact. 1997;106:29–45. doi: 10.1016/s0009-2797(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 54.Ripp SL, Itagaki K, Philpot RM, Elfarra AA. Methionine S-oxidation in human and rabbit liver microsomes: evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3. Arch Biochem Biophys. 1999;367:322–332. doi: 10.1006/abbi.1999.1247. [DOI] [PubMed] [Google Scholar]
- 55.Boutros PC, Yan R, Moffat ID, Pohjanvirta R, Okey AB. Transcriptomic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver: comparison of rat and mouse. BMC Genomics. 2008;9:419. doi: 10.1186/1471-2164-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]