Abstract
Although it is well known that an excess of iodide suppresses thyroid function and blood flow in vivo, the underlying molecular mechanisms are not fully known. The functional effect of iodide occurs at multiple steps, which include inhibition of sodium/iodide symporter (NIS) expression, transient block of organification, and inhibition of hormonal release. The vascular effect likely involves suppression of the vascular endothelial growth factor (VEGF) gene. In this report, we show that excess iodide coordinately suppresses the expression of the NIS and VEGF genes in FRTL-5 thyroid cells. We also demonstrate that the mechanism of iodide suppression of NIS gene expression is transcriptional, which is synergized by the addition of thyroglobulin. Based on the findings of reporter gene assays and electrophoretic gel mobility shift analysis, we also report two novel DNA binding proteins that responded specifically to iodide and modulated NIS promoter activity. The results suggest that excess iodide affects thyroid vascular function in addition to iodide uptake. This study provides additional insights into the mechanism of action of excess iodide on thyroid function.
Keywords: Iodide, Thyroid, NIS, VEGF, Thyroglobulin
Introduction
Iodide (I−), the negatively charged (anionic) form of the iodine atom, is essential for the synthesis of thyroid hormones [1]. It is transported from the bloodstream into thyroid follicular cells by the sodium/iodide symporter (NIS) [2] and is eventually incorporated into thyroglobulin (Tg). Iodide transport is induced by thyrotropin (TSH) [3,4] and suppressed by numerous factors, including an excess of iodide and follicular Tg [5]. An excess of iodide leads to various functional outcomes, ranging from hyperthyroidism to hypothyroidism, depending on the status of the thyroid gland.
In healthy individuals, excess iodide should theoretically lead to an increased production of thyroid hormones; however, the production of thyroid hormones is blocked by two key mechanisms. Primarily, an excess of iodide blocks the release of thyroid hormones, leading to a rapid decline in their circulating levels [6]. In addition, an excess of iodide also inhibits organification and thus the synthesis of thyroid hormones, a process known as the Wolff-Chaikoff effect [7]. This transient inhibition is caused by a high intracellular concentration of iodide, but when this intracellular concentration becomes too low to maintain the inhibitory effect, thyroid hormone synthesis resumes, a process known as escape from the Wolff-Chaikoff effect [8].
Excess iodide has also been shown to decrease vascularity of the thyroid gland [9,10,11,12], so much so that most endocrine surgeons administer Lugol’s solution [a mixture of molecular iodine (I2) and potassium iodide (KI) in distilled water] to prepare patients with Graves’ disease for thyroidectomy [10,11]. Vascular endothelial growth factor (VEGF) is produced by thyroid cells upon stimulation with TSH or antibodies that stimulate the TSH receptor [13,14,15]. However, the overall mechanism by which excess iodide modulates thyroid blood flow remains unexplained.
In this study, we show that an excess of iodide coordinately suppresses NIS and VEGF gene expression in a dose- and time-dependent manner in vitro, and that the suppression of NIS gene expression appears to be caused by the decreased binding of transcriptional factors to the NIS promoter region.
Materials and Methods
Culture and treatment of FRTL-5 thyroid cells
Rat FRTL-5 thyroid cells (ATCC CRL8305) were provided by Interthyr Corporation (Athens, OH, USA) and maintained as previously reported [5,16]. To the culture medium was directly added sodium iodide or sodium chloride at a concentration range of 0.01–10 mM and highly purified bovine Tg at 10 mg/ml as previously described [5,17].
Iodide uptake
Iodide uptake was measured in FRTL-5 thyroid cells as described previously [17]. Briefly, cells grown in 24-well plates were washed with Hanks’ Balanced Salt Solution (HBSS) containing HEPES buffer. Then, 500 µl of HBSS containing 0.1 µCi carrier-free 125I (theoretical specific activity 17.4 Ci/mg; NEN Life Science Products, Boston, MA, USA) was added to each well and incubated at 37 °C for 40 min. Cells were washed with Dulbecco’s phosphate buffered saline and lysed with ice-cold ethanol by placing them at −20 °C for 20 min before measurement of specific radioactivity.
RNA isolation and Northern blot analysis
Total RNA was purified and Northern blot analysis was performed as previously described [5,16]. The VEGF probe was prepared by RT-PCR using the following primers: 5’-ACAGAAGGGGAGCAGAAA-3’ and 5’-GAGGTCTAGTTCCCGAAA-3’. First strand complementary DNA (cDNA) was synthesized using an Advantage RT-for-PCR kit (Clontech, Palo Alto, CA, USA). cDNA amplification was performed using the touchdown PCR procedure [5,16] with Pfu DNA polymerase (Stratagene, La Jolla, CA, USA). Probes for β-actin and NIS were as previously described [17].
Transfection and analysis of NIS promoter activity
FRTL-5 cells grown in 6-well plates were transfected with 1 µg of plasmid DNA containing the reporter gene constructs using Lipofectamine Plus (Invitrogen, Carlsbad, CA, USA) as described [5,16,18]. After transfection, the cells were incubated for 4 h at 37 °C in a 5% CO2 incubator before the addition of 4 ml of complete medium with serum. Fresh medium was added after 24 h, with or without iodide, and reporter activity was measured 36 h later. The activity of the secreted form of human alkaline phosphatase (SEAP) was measured using the Great EscAPe™ SEAP Reporter System Chemiluminescence Detection kit (Clontech) as reported [18].
Preparation of nuclear proteins and electrophoretic gel mobility shift analysis (EMSA)
Preparation of nuclear proteins and EMSA were performed as previously described [5,16]. EMSA was carried out using 3 µg of nuclear extract. A radiolabeled double-stranded DNA probe (50,000 cpm) was added and the samples were incubated for 20 min at 4 °C. Mixtures were analyzed on 5% or 2.5% native polyacrylamide gels.
Western blot analysis
Samples were prepared and Western blot analysis was performed as previously described [16,17]. Anti-NIS antibody was kindly provided by Dr. N. Carrasco (Albert Einstein College of Medicine, Bronx, NY, USA); donkey anti-rabbit IgG was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) as was the molecular weight marker kit. An ECL system (Amersham Pharmacia Biotech, Arlington Heights, IL, USA) was used for detection. Densitometric analysis of intensity was performed using ImageJ: NIH image processing software (Bethesda, MD, USA).
Statistical analysis
Differences among groups were assessed using the non-parametric Wilcoxon signed-rank test.
Results
Excess iodide suppresses NIS expression in a dose-dependent manner
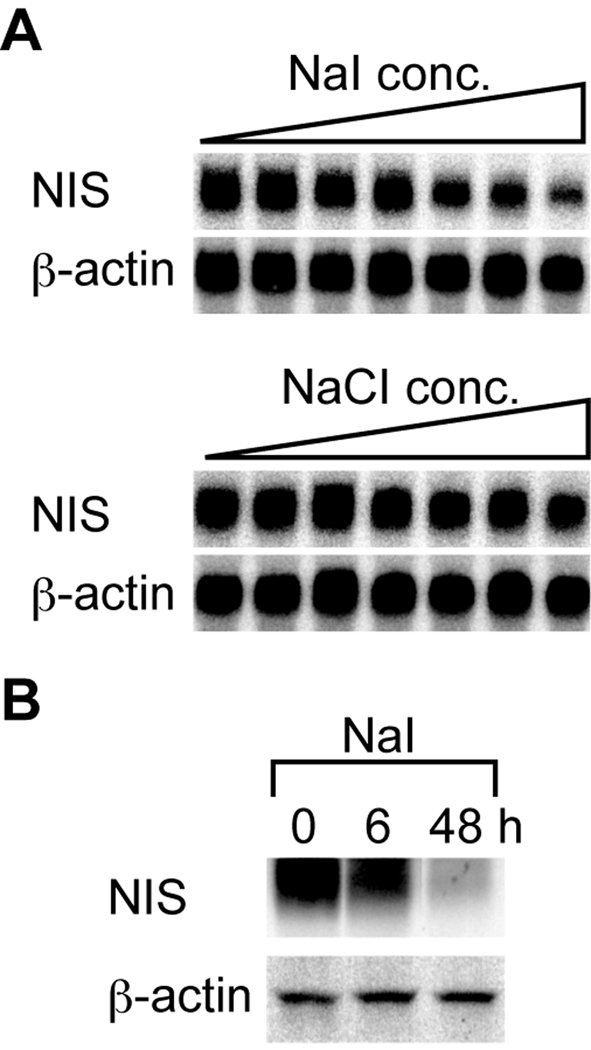
We first investigated the changes in NIS gene expression in rat FRTL-5 thyroid cells. Northern blot analysis showed that iodide suppressed the transcription of the NIS gene in a concentration-dependent manner, while chloride had no effect (Fig. 1A). The decrease in NIS mRNA level was associated with a decrease in NIS protein level, as assessed by Western blot analysis (Fig. 1B). NIS protein reportedly shows a smear on Western blotting due to its glycosylation [17,19]. The level of NIS protein decreased to 65% of the baseline level at 6 h and to 25% at 48 h following iodide addition (densitometric analysis by ImageJ; Fig. 1B). The NIS mRNA level started decreasing 3 h after the addition of iodide and reached its lowest level at 24 h (Fig. 4A). In these conditions, iodide suppressed the uptake of radioactive iodine by FRTL-5 cells in a dose-dependent manner, while chloride had no effect at equivalent concentrations (Supplementary Fig. 1), indicating that this suppression is iodide-specific.
Fig. 1.
Iodide suppression of NIS expression at the mRNA and protein levels in FRTL-5 thyroid cells. FRTL-5 cells were treated with either sodium chloride (NaCl) or sodium iodide (NaI) for 48 h. The final concentrations added to the cells were 0, 0.1, 1, 2.5, 5, 7.5, and 10 mM. NIS and β-actin were detected by Northern hybridization. (B) FRTL-5 cells were treated with 10 mM NaI for 6 or 48 h. NIS protein level was measured by Western blotting. Actin was used as a control.
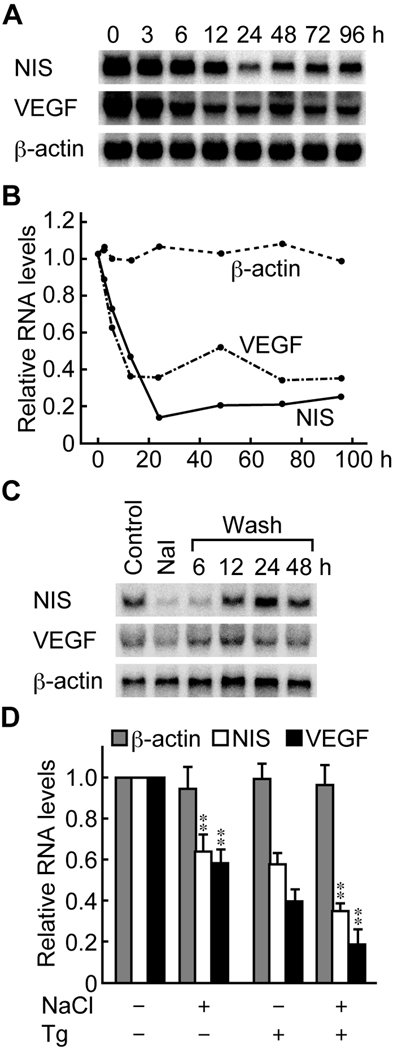
Fig. 4.
Iodide and thyroglobulin (Tg) suppression of the expression of NIS and VEGF. Iodide suppressed the expression of NIS and VEGF at the mRNA level. A representative image of a Northern blot is shown in (A). The results of densitometric measurements are expressed relative to control in (B). (C) NIS and VEGF mRNA expression was restored by the removal of iodide from the medium. (D) Iodide and Tg synergistically suppressed the expression of NIS and VEGF. The results of densitometric measurements of different experiments are expressed as mean ± SD relative to control. Significant decreases are noted: ** p < 0.01.
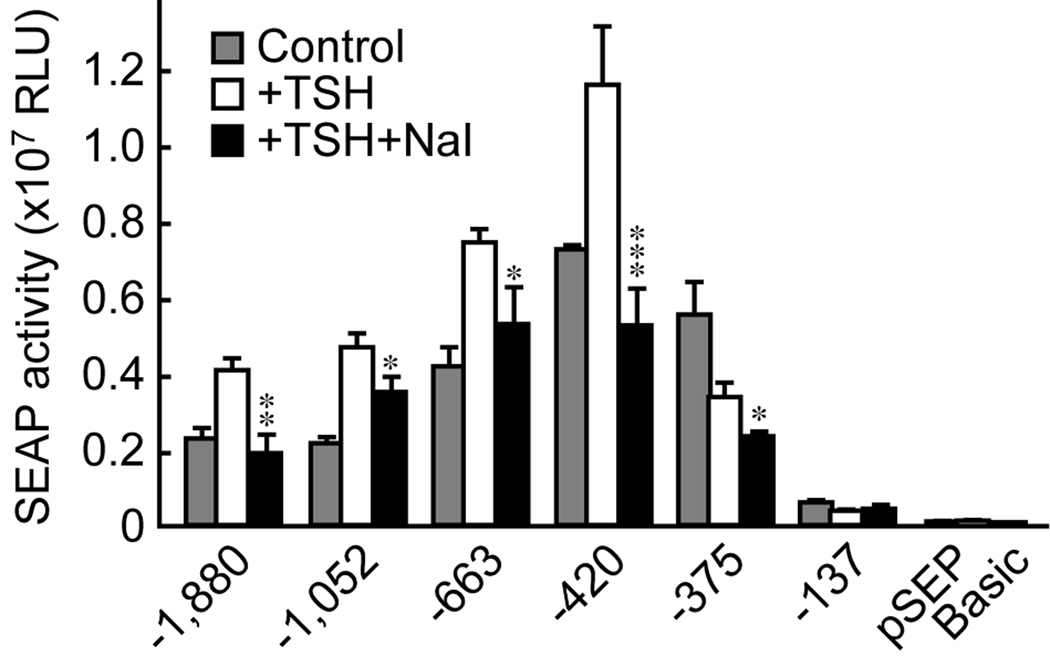
Excess iodide suppresses NIS promoter activity
Next, we studied the NIS promoter activity to identify the regions influenced by iodide. The basal activity of the NIS promoter was highest when using the −420 reporter gene construct rather than when using the longer (−663, −1052, and −1,880) or shorter (−137) constructs (Fig. 2). Addition of TSH to the culture medium increased NIS promoter activity for constructs containing −1,880 to −420 bp from the transcription start site, but not for the −375 and −137 bp constructs. Addition of iodide to the TSH-containing medium annulled the effect of TSH on the NIS promoter (Fig. 2). This effect was observed for all of the constructs from −1,880 to −375 bp. Notably, iodide still suppressed NIS promoter activity in the −375 bp construct, whose activity was not increased by TSH (Fig. 2). The activity of the pSEAP-control, which contained both the promoter and the enhancer, was unchanged (data not shown). Overall, these data indicate that iodide dominates the TSH-induced increase in NIS promoter activity, acting independently of the action of TSH/cAMP.
Fig. 2.
Iodide suppression of NIS promoter activity in FRTL-5 thyroid cells. Iodide suppressed the NIS promoter activity induced by thyrotropin (TSH). Results are expressed as mean ± SD from three different experiments performed in triplicate. Significant decreases are noted: * p < 0.05; ** p < 0.01; *** p < 0.001.
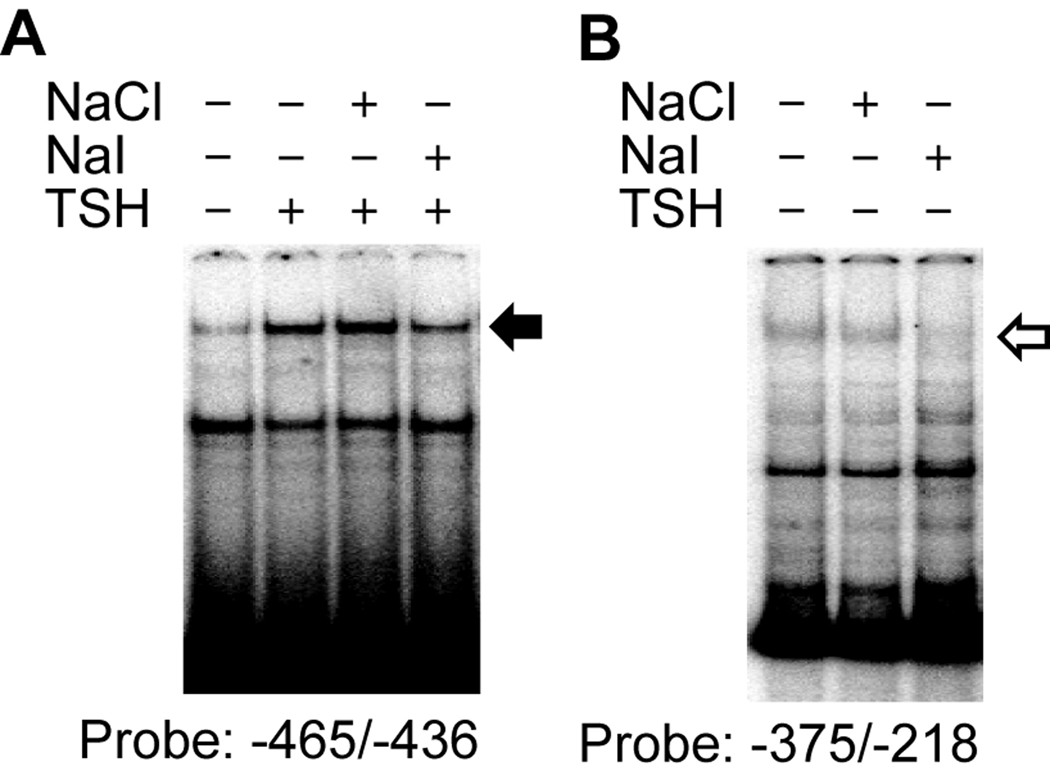
To identify the factors that suppress NIS promoter activity upon iodide stimulation, we performed EMSA. We used a 30-bp oligonucleotide probe from −466 to −436 bp of the NIS 5’ flanking region, and identified a DNA binding complex that was induced by TSH and was specifically suppressed by iodide (Fig. 3A, solid arrow). Using another oligonucleotide probe from −375 to −218 bp of the NIS 5’ promoter sequence, we identified a second DNA binding protein that was decreased by iodide in the absence of TSH (Fig. 3B, open arrow). Although the protein components of these two complexes are unknown at present, these results indicate the existence of factors that respond specifically to iodide and modulate NIS promoter activity.
Fig. 3.
Iodide suppression of DNA binding of nuclear protein to the NIS promoter in FRTL-5 thyroid cells. (A) The effect of iodide on DNA binding activity in the NIS 5’-flanking region was assessed by electrophoretic gel mobility shift analysis (EMSA). In (A), a solid arrow identifies a complex increased by thyrotropin (TSH), but decreased by sodium iodide (NaI). In (B), an open arrow identifies a complex decreased by NaI in the absence of TSH.
Iodide and Tg synergistically suppress the expression of the NIS and VEGF genes
VEGF is produced by thyroid cells upon stimulation with TSH or anti-TSHR stimulatory autoantibodies [13,14,15]. We compared the kinetics of gene expression of NIS and VEGF following iodide stimulation in FRTL-5 thyroid cells. Excess iodide decreased the RNA level of both NIS and VEGF (Fig. 4A and 4B). Their expression level started to decrease 6 h after iodide stimulation, reached a nadir 24 h poststimulation, and remained low thereafter (Fig. 4B). Removal of iodide from the culture medium restored the gene expression of NIS and VEGF, which was apparent 6 h after the cells were washed (Fig. 4C), consistent with the transient nature of iodide suppression [8].
Considering the findings that follicular Tg regulates the gene expression of NIS and VEGF in FRTL-5 thyroid cells [17], as well as other thyroidal genes [5], we next assessed whether Tg synergizes the action of iodide in mediating the suppression of the NIS and VEGF genes. Suppression of NIS and VEGF RNA levels was significantly stronger when both Tg and iodide were added to the culture medium (Fig. 4D), highlighting the contribution of both molecules to thyroidal autoregulation.
Discussion
An excess of iodide has a profound influence on the thyroid gland [7,8,9,10,20]. In vitro studies using rat FRTL-5 thyroid cells have long shown that an excess of iodide suppresses the uptake of radioactive iodide [21]. Here, we showed that an excess of iodide decreases NIS gene expression at the transcriptional level. We further demonstrated that suppression of NIS promoter activity is associated with an iodide-specific decrease in the binding of transcription factors to DNA. In particular, we identified a TSH-inducible DNA binding protein using an oligonucleotide probe (−466 to −436 bp) from the NIS 5’-flanking region, which might correspond to the SP1 element reported by Xu et al. [22]. Considering our previous report that iodide decreases the expression of the p50/p65 NFκB heterodimer and class I major histocompatibility complex [23], it appears reasonable to postulate that an excess of iodide suppresses NIS gene expression by activating transcription factors that modulate gene expression.
NIS regulation is complex, occurring at the transcriptional, translational, and post-translational levels [24], and thus there is often a discrepancy between NIS mRNA and protein levels [17,25]. We also showed that the suppression of NIS mRNA required relatively higher iodide concentrations than the suppression of iodide transport, in keeping with a previous study [26]. The investigators of this previous study showed that NIS mRNA level in FRTL-5 cells was not decreased by 1 mM iodide, despite the fact that the same concentration decreased both NIS protein level and iodide uptake. A possible interpretation of these findings is that an elevated serum concentration of iodide suppresses iodide uptake at the post-translational level, and that NIS is suppressed only at the transcriptional level once the thyroidal concentration of iodide reaches a very high level. It should be noted that iodide transport is still observed even when the concentration of cytoplasmic iodide is 2 mM [27]. This suggests that thyroid cells may be capable of accumulating a high concentration of iodide, and the intracellular concentration of iodide may reach the mM level. In keeping with this outlook, it is possible for such a condition to exist during long-term treatment with iodide for iodine therapy.
Another interesting finding of the present study was the inhibition of VEGF transcription by iodide. The thyroid gland is a highly vascularized tissue in which a fine capillary network surrounds each follicle [28]. The function of the thyroid gland is linked to the control of the vascular network and, as noted earlier, iodide is used to suppress thyroid function as well as blood flow in patients [9,10,11,12]. A high concentration of iodide decreases the blood flow in the thyroid gland as well as vascular permeability by its paracrine action [13,14,15]. We have shown in this study that excess iodide suppresses VEGF gene expression, as has recently been reported in human follicular cells [29], suggesting a role of VEGF in the reduction of blood flow. In addition, we also report here that iodide simultaneously suppresses NIS and VEGF transcription, suggesting an effect on both thyrocytes and endothelial cells. This coordinate regulation of gene expression might be mediated by changes in common transcription factors that respond to iodide and modulate both NIS and VEGF transcription. Further studies focused on the analysis of VEGF promoter activity in response to iodide are necessary to prove this hypothesis.
We previously reported that physiological concentrations of follicular Tg strongly suppress NIS expression and iodide uptake [17]. Specifically, Tg suppresses critical transcription factors in thyroid physiology, thus inhibiting essential genes for thyroid hormonogenesis, such as Tg, thyroperoxidase, the TSH receptor, and NIS [5,17]. In this study, we showed that Tg and iodide exert an additive effect on the suppression of NIS and VEGF mRNA expression, suggesting the existence of different transcription factors responsive to Tg and iodide.
In summary, these results refine our understanding of the mechanisms underlying iodide homeostasis in the thyroid gland [30], a process highly regulated by iodide at the transcriptional level, and can be used to improve the treatment of thyroid cancer using radioactive iodine [31]. The present results also support the hypothesis that both Tg and iodide coordinately regulate follicular function by regulating gene expression in the thyroid gland [5,17,32].
Supplementary Material
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research by the Ministry of Education, Culture, Sport, Science, and Technology of Japan (21591187 to K.S.), and in part by an NIH grant (DK55670 to P.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kohn LD, Saji M, Kosugi M, Ban T, Giuliani C, Hidaka A, Shimura H, Shimura Y, Okajima F. The synthesis and secretion of thyroid hormones: regulation by multiple hormones and signals which can be subverted by autoantibodies to the thyrotropin receptor. Thyroid Diseases: Basic Science, Pathology, Clinical and Laboratory Diagnosis. 1993:59–118. [Google Scholar]
- 2.Dai G, Levy O, Carrasco N. Carrasco, Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 3.Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam. Horm. 1995;50:287–384. doi: 10.1016/s0083-6729(08)60658-5. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SJ, Philp NJ, Ambesi-Impiombato FS, Grollman EF. Thyrotropin-stimulated iodide transport mediated by adenosine 3',5'-monophosphate and dependent on protein synthesis. Endocrinology. 1984;114:1099–1107. doi: 10.1210/endo-114-4-1099. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Lavaroni S, Mori A, Ohta M, Saito J, Pietrarelli M, Singer DS, Kimura S, Katoh R, Kawaoi A, Kohn LD. Autoregulation of thyroid-specific gene transcription by thyroglobulin. Proc. Natl. Acad. Sci. U S A. 1998;95:8251–8256. doi: 10.1073/pnas.95.14.8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerson CH, Anderson AJ, Howard WJ, Utiger RD. Serum thyroxine and triiodothyronine concentrations during iodide treatment of hyperthyroidism. J. Clin. Endocrinol. Metab. 1975;40:33–36. doi: 10.1210/jcem-40-1-33. [DOI] [PubMed] [Google Scholar]
- 7.Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J. Biol. Chem. 1948;174:555–564. [PubMed] [Google Scholar]
- 8.Wolff J, Chaikoff IL, Goldberg RC, Meier JR. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45:504–513. doi: 10.1210/endo-45-5-504. [DOI] [PubMed] [Google Scholar]
- 9.Arntzenius AB, Smit LJ, Schipper J, van der Heide D, Meinders AE. Inverse relation between iodine intake and thyroid blood flow: color Doppler flow imaging in euthyroid humans. J. Clin. Endocrinol. Metab. 1991;73:1051–1055. doi: 10.1210/jcem-73-5-1051. [DOI] [PubMed] [Google Scholar]
- 10.Chang DC, Wheeler MH, Woodcock JP, Curley I, Lazarus JR, Fung H, John R, Hall R, McGregor AM. The effect of preoperative Lugol's iodine on thyroid blood flow in patients with Graves' hyperthyroidism. Surgery. 1987;102:1055–1061. [PubMed] [Google Scholar]
- 11.Marigold JH, Morgan AK, Earle DJ, Young AE, Croft DN. Lugol's iodine: its effect on thyroid blood flow in patients with thyrotoxicosis. Br. J. Surg. 1985;72:45–47. doi: 10.1002/bjs.1800720118. [DOI] [PubMed] [Google Scholar]
- 12.Rangaswamy M, Padhy AK, Gopinath PG, Shukla NK, Gupta K, Kapoor MM. Effect of Lugol's iodine on the vascularity of thyroid gland in hyperthyroidism. Nucl. Med. Commun. 1989;10:679–684. doi: 10.1097/00006231-198909000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Miyakawa M, Onoda N, Demura H, Yamashita T, Miura M, Kasajima T, Yamazaki K, Obara T. Increased concentration of vascular endothelial growth factor/vascular permeability factor in cyst fluid of enlarging and recurrent thyroid nodules. J. Clin. Endocrinol. Metab. 1997;82:1968–1973. doi: 10.1210/jcem.82.6.3989. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Yamazaki K, Shizume K, Kanaji Y, Obara T, Ohsumi K, Demura H, Yamaguchi S, Shibuya M. Stimulation by thyroid-stimulating hormone and Grave's immunoglobulin G of vascular endothelial growth factor mRNA expression in human thyroid follicles in vitro and flt mRNA expression in the rat thyroid in vivo. J. Clin. Invest. 1995;96:1295–1302. doi: 10.1172/JCI118164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, Garcia YK, Min YD, Grossman RF, Siperstein AE, Clark OH. Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J. Clin. Endocrinol. Metab. 1997;82:3741–3747. doi: 10.1210/jcem.82.11.4340. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, Krause PR, Kohn LD. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc. Natl. Acad. Sci. USA. 1999;96:2285–2290. doi: 10.1073/pnas.96.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki K, Mori A, Saito J, Moriyama E, Ullianich L, Kohn LD. Follicular thyroglobulin suppresses iodide uptake by suppressing expression of the sodium/iodide symporter gene. Endocrinology. 1999;140:5422–5430. doi: 10.1210/endo.140.11.7124. [DOI] [PubMed] [Google Scholar]
- 18.Mori-Aoki A, Pietrarelli M, Nakazato M, Caturegli P, Kohn LD, Suzuki K. Class II transactivator suppresses transcription of thyroid-specific genes. Biochem. Biophys. Res. Commun. 2000;278:58–62. doi: 10.1006/bbrc.2000.3769. [DOI] [PubMed] [Google Scholar]
- 19.Levy O, De la Vieja A, Ginter CS, Riedel C, Dai G, Carrasco N. N-linked glycosylation of the thyroid Na+/I− symporter (NIS). Implications for its secondary structure model. J. Biol. Chem. 1998;273:22657–22663. doi: 10.1074/jbc.273.35.22657. [DOI] [PubMed] [Google Scholar]
- 20.Braverman LE, Ingbar SH. Changes in thyroidal function during adaptation to large doses of iodide. J. Clin. Invest. 1963;42:1216–1231. doi: 10.1172/JCI104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grollman EF, Smolar A, Ommaya A, Tombaccini D, Santisteban P. Iodine suppression of iodide uptake in FRTL-5 thyroid cells. Endocrinology. 1986;118:2477–2482. doi: 10.1210/endo-118-6-2477. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Kogai T, Brent GA, Hershman JM. A GC box in the human sodium iodide symporter gene promoter is essential for full activity. Thyroid. 2002;12:107–114. doi: 10.1089/105072502753522338. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi SI, Shong M, Giuliani C, Napolitano G, Saji M, Montani V, Suzuki K, Singer DS, Kohn LD. Iodide suppression of major histocompatibility class I gene expression in thyroid cells involves enhancer A and the transcription factor NF-kappa B. Mol. Endocrinol. 1998;12:19–33. doi: 10.1210/mend.12.1.0052. [DOI] [PubMed] [Google Scholar]
- 24.Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr. Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 25.Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138:2227–2232. doi: 10.1210/endo.138.6.5189. [DOI] [PubMed] [Google Scholar]
- 26.Eng PH, Cardona GR, Previti MC, Chin WW, Braverman LE. Regulation of the sodium iodide symporter by iodide in FRTL-5 cells. Eur. J. Endocrinol. 2001;144:139–144. doi: 10.1530/eje.0.1440139. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida A, Taniguchi S, Hisatome I, Royaux IE, Green ED, Kohn LD, Suzuki K. Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J. Clin. Endocrinol. Metab. 2002;87:3356–3361. doi: 10.1210/jcem.87.7.8679. [DOI] [PubMed] [Google Scholar]
- 28.Fujita H, Murakami T. Scanning electron microscopy on the distribution of the minute blood vessels in the thyroiod gland of the dog, rat and rhesus monkey. Arch. Histol. Jpn. 1974;36:181–188. doi: 10.1679/aohc1950.36.181. [DOI] [PubMed] [Google Scholar]
- 29.Yamada E, Yamazaki K, Takano K, Obara T, Sato K. Iodide inhibits vascular endothelial growth factor-A expression in cultured human thyroid follicles: a microarray search for effects of thyrotropin and iodide on angiogenesis factors. Thyroid. 2006;16:545–554. doi: 10.1089/thy.2006.16.545. [DOI] [PubMed] [Google Scholar]
- 30.Bizhanova A, Kopp P. Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology. 2009;150:1084–1090. doi: 10.1210/en.2008-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr. Relat. Cancer. 2006;13:797–826. doi: 10.1677/erc.1.01143. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Kohn LD. Differential regulation of apical and basal iodide transporters in the thyroid by thyroglobulin. J. Endocrinol. 2006;189:247–255. doi: 10.1677/joe.1.06677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.