Abstract
Objective
Much recent research has focused on nonhormonal treatments for menopausal hot flashes. The purpose of the present study was to determine the effects of 5-Hydroxytroptophan (5-HTP), the immediate precursor of serotonin, upon menopausal hot flashes. Selective, serotonergic, reuptake inhibitors (SSRI’s), which increase the amount of serotonin in the synaptic gap, have shown some promise in the amelioration of hot flashes.
Methods
We administered 5-HTP or placebo, in double-blind fashion, to 24 postmenopausal women reporting frequent hot flashes. Treatment outcome was measured using a miniature, electronic, hot flash recorder.
Results
No significant effects of 150 mg/day 5-HTP upon hot flash frequency were found. The 5-HTP group had 23.8 ± 5.7 (SD) hot flashes/24 hours prior to treatment and 18.5 ± 9.6 at the end of treatment. The placebo group had 18.5 ± 9.6 before treatment and 22.6 ± 12.4 at treatment completion.
Conclusions
At the dose given, 5-HTP does not significantly ameliorate frequency of menopausal hot flashes, as measured objectively with an electronic recorder. Given the small size, this study must be considered preliminary in nature.
Keywords: Hot flash, Serotonin, 5-Hydroxytryptophan (5-HTP), Menopause
1. Introduction
Hot flashes are the most common symptom of menopause and affect millions of women worldwide. Although hormone replacement therapy is the most effective treatment for them, many women presently choose not to receive it due to concerns about its risks. This has stimulated research on nonhormonal treatments [1].
Work conducted in the author’s laboratory has shown that hot flashes are triggered by small elevations in core body temperature acting within a reduced thermoneutral zone [2]. Thus, women with hot flashes both sweat and shiver more readily than women without them.
The reduction in the thermoneutral zone is caused, in part, by elevated brain norepinephrine [3]. This is supported by work showing that clonidine, a α2-adrenergic agonist, reduces brain norepinephrine and hot flash occurrence [4,5]. Conversely, yohimbine, a α2-adrenergic antagonist, elevates brain norepinephrine and provokes hot flashes [4,6].
Animal studies have shown that serotonin in brain generally works in opposite fashion to norepinephrine [3], suggesting that elevation of brain serotonin should ameliorate hot flashes. Indeed, recent studies of selective, serotonergic, reuptake inhibitors (SSRI’s), which increase the amount of serotonin in the synaptic gap, have demonstrated amelioration of hot flashes. For example, a double-blind study of 151 women with hot flashes showed that paroxetine 10 mg/day reduced hot flash frequency by 40.6% compared to 13.7% for placebo and that paroxetine 20 mg reduced hot flash frequency by 51.7% compared to 26.6% for placebo [7]. A double-blind study of 707 women found that desvenlafaxine succinate 100 mg/day reduced hot flash frequency by 64% compared with 29% for placebo [8].
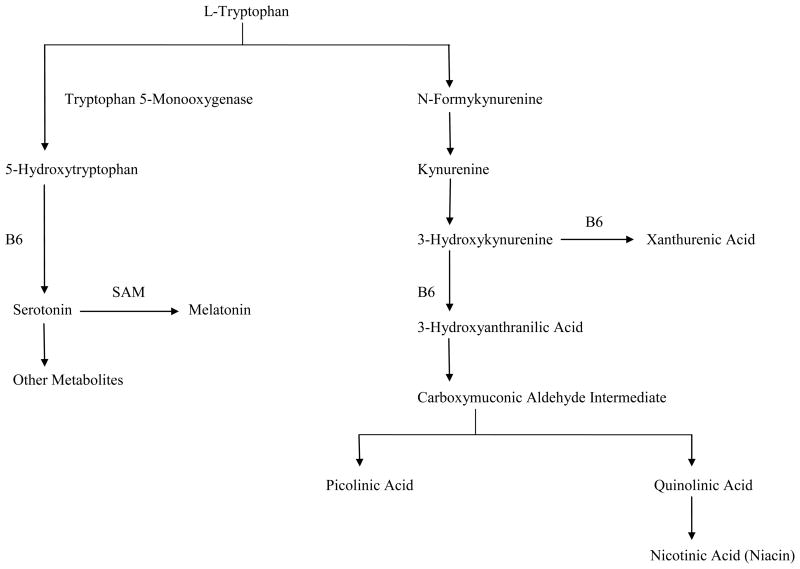
Tryptophan is the amino acid precursor of serotonin. Once in the CNS, tryptophan is converted to 5-Hydroxytryptophan (5-HTP) and then is decarboxylated to serotonin (Fig. 1). The levels of some neurotransmitters can be elevated by increasing the dietary supply of their precursors [9,10]. Oral administration of L-tryptophan produces increases in serotonin synthesis and release in brain. Therefore, in the present investigation, we sought to determine if oral administration of 5-HTP would result in significant declines in hot flash frequency compared to placebo. There have been no prior trials of this type.
Fig. 1.
Pathway of tryptophan metabolism (adapted from Ref. 10).
2. Materials and Methods
Subjects were 24 postmenopausal (12-months amenorrhea) women who reported at least six hot flashes per day. They were recruited using advertisements in local newspapers. They were required to be free of any antidepressant drug, any hot flash treatment drug or supplement (soy, herbs) and to report no history of diabetes, glaucoma, bladder cancer, or cataracts.
Sample size was determined in the following manner:
Based on our previous work [11], we assumed the variability in hot flash frequency to be 25% and the placebo response rate to be a 20% decline from baseline (100%). A power analysis, based on these assumptions (n Query Advisor), showed the power to find a significant difference between the active treatment and placebo would be .99 with n = 24 and P = .05.
5-HTP was purchased from the manufacturer (Orthomolecular Products, Stevens Point, WI, USA) in 50 mg capsules. A certificate of analysis, provided by the manufacturer, demonstrated that the 5-HTP conformed to all FDA specifications. These were rebottled into coded, blinded bottles with identical placebo capsules by Nathan Worthing, R.Ph., Pharm.D. (Clark Professional Pharmacy, Ypsilanti, MI, USA). He retained the code and could be contacted at any time if it was necessary to unblind the study. Subjects were randomly assigned to receive 5-HTP or placebo using a computer-generated code by Dr. Worthing. The dose of 5-HTP was recommended by a physician who is a consultant to NIH and an expert on the use of this compound based on clinical experience.
Hot flashes were assessed using a recorder developed by the author (Flashmark Pro; Pending U.S.A. Patent No. 60,741,3760) [12]. The device counts hot flashes by measuring humidity on the chest. There is no electrical connection to the body. The device contains a humidity sensor, microcontroller, flash memory, and 1.5 volt hearing aid battery. The adhesive collars are made from an FDA-approved material (3M #5122). There are no known risks to this device.
The study was approved by the Medical Institutional Review Board, Human Investigation Committee, Wayne State University School of Medicine, in Detroit, MI, USA.
Subjects were first interviewed by the author, who determined if they met the screening criteria. Written informed consent was obtained using procedures approved by our Institutional Review Board. Subjects were paid $300.00 USD for their participation.
Subjects were then shown the recorder. They were instructed to attach it over the sternum, to remove it prior to bathing, and to reattach it afterwards using circular adhesive collars supplied by us. They were told to wear the recorder continuously except while bathing. Subjects were then sent home for one week.
Subjects then came to the laboratory and returned the recorder. They were given a blinded bottle of capsules to be taken three times per day with meals. After three weeks, subjects returned to the laboratory to receive the recorder for one more week. They continued taking the capsules during this period. At the end of one week, subjects came to the laboratory to return the recorder and pill bottle. We did not query the subjects regarding their hot flashes.
Data were downloaded from the recorder and hot flashes counted using a computer program developed by Kolar Engineering (Royal Oak, MI, USA). A hot flash was defined as a change in relative humidity of at least 3%/minute. We have validated and published these methods [12].
Group differences in age, BMI, and years postmenopause were analyzed using unpaired t-tests. Hot flash frequencies were analyzed using a 2-way (Group X Pre/Post), repeated measures ANOVA. The minimum level of statistical significance for all analyses was P <.05.
3. Results
There were no significant group differences on any demographic variable (Table 1). There were no significant effects whatsoever from the analysis of hot flash frequencies (Table 2). A power analysis performed on the data in Table 2 showed a power of only .21 for the Group x Pre/Post interaction effect. Thus, the effect of 5-HTP upon hot flash frequency relative to that of placebo was very small. No subjects withdrew from the study. It was not necessary to unblind the pill bottles during the conduct of the study.
Table 1.
Demographics of subjects (12 5-HTP, 12 placebo).
| 5-HTP Group | Placebo Group | |
|---|---|---|
| Age (years) | 50.8 ± 2.3 (range=46.0–54.0) | 52.7 ± 4.2 (range=46.0–58.0) |
| BMI | 26.6 ± 4.0 (range=19.3–32.0) | 25.1 ± 4.5 (range=19.3–32.0) |
| Years postmenopause | 5.4 ± 3.3 (range=1.0–11.0) | 7.6 ± 6.2 (range = 1.0–21.0) |
| Natural/surgical menopause | 9/3 | 9/3 |
| Race (Caucasian/African American) | 7/5 | 7/5 |
Table 2.
Number of hot flashes/24 hours (means ± SD).
| 5-HTP Group | Placebo Group | |
|---|---|---|
| Pre-treatment | 23.8 ± 5.7 (range=15.9–33.3) | 22.8 ± 9.8 (range=11.0–37.4) |
| Post-treatment | 18.5 ± 9.6 (range=18.5–9.6) | 22.6 ± 12.4 (range=12.4–3.6) |
4. Discussion
In the present investigation, we failed to find a significant effect of 5-HTP upon menopausal hot flash frequency. Indeed, a power analysis showed that this effect was very small. What could account for this?
All of the studies showing that 5-HTP can elevate brain serotonin were conducted in rats and cats [9]. It is possible that these findings cannot be validly extrapolated to humans. Second, the dose that we administered (150 mg/day) is at the low end of the therapeutic range [9]. We did this to minimize the occurrence of undesirable side effects (e.g., dizziness, sedation, dry mouth). It is possible that the use of a higher dose might have achieved a significant reduction in hot flash frequency.
A strength of the present study was its use of an objective outcome measure, a miniature, electronic hot flash recorder [13]. This device requires no interventions from the subject to log hot flashes. Most hot flash treatment studies have used diary methods as the major outcome measure. There are several problems with diaries. Patient noncompliance and false compliance are major sources of error and bias [14]. Furthermore, hot flashes occurring during sleep are not accurately reported because recall of these events is often poor and most hot flashes do not produce full awakenings [15]. Finally, placebo effects as large as 40–50% occur with self-report [16]. These factors make self-report a problematic measure of therapeutic efficacy.
One study [17] compared subjective hot flash reports (event marker and diary) with a continuous electronic method (sternal skin conductance) during 24-hour ambulatory monitoring. Diary hot flashes were underreported by a factor of over 50%. The probability that a woman would report a hot flash by diary or event marker was 36%–50% during waking and 22%–42% during sleep. A more recent study was conducted in men who had hot flashes due to endocrine treatments for prostate cancer. Hot flashes were recorded using ambulatory monitoring of sternal skin conductance level, an electronic event marker, and twice daily diaries. In agreement with the results cited above [18], diaries yielded the lowest hot flash rate.
The present investigation is clearly limited by its small sample size and, therefore, must be considered preliminary in nature.
5. Conclusions
In conclusion, the present investigation found no significant effect of 150 mg/day of 5-HTP upon hot flash frequency measured with an electronic recorder. Further research using higher doses would be of interest.
Acknowledgments
Funding for this research was supported by grant AG05233 from the National Institute on Aging, National Institute of Health, Bethesda, MD, USA.
Footnotes
Author contributions
Robert R. Freedman has designed the study, performed the literature overview, and has written the manuscript.
There is a pending U.S.A. Patent No. 60,741,376, “Miniature, Hygrometric Hot Flash Recorder,” on the device described herein. Robert R. Freedman is the President and CEO of Biomedical Monitors, LLC, Ann Arbor, MI, USA, the firm that manufactures and sells the recorders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voelker R. NIH panel tries to clear confusion, spur research on managing menopause. JAMA. 2005;293:2329–31. doi: 10.1001/jama.293.19.2329. [DOI] [PubMed] [Google Scholar]
- 2.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 3.Brück K, Zeisberger E. Adaptive changes in thermoregulation and their neuropharmacological basis. In: Schönbaum E, Lomax P, editors. Thermoregulation: Physiology and Biochemistry. New York: Pergamon; 1990. pp. 255–307. [Google Scholar]
- 4.Freedman RR, Woodward S, Sabharwal SC. Adrenergic mechanism in menopausal hot flushes. Obstet Gynecol. 1990;76:573–8. [PubMed] [Google Scholar]
- 5.Schmitt H. The pharmacology of clonidine and related products. In: Gross F, editor. Antihypertensive Agents. New York: Springer-Verlag; 1977. pp. 299–396. [Google Scholar]
- 6.Goldberg M, Robertson D. Yohimbine: A pharmacological probe for study of the α2-adrenoceptor. Pharmacol Rev. 1983;35:143–80. [PubMed] [Google Scholar]
- 7.Stearns V, Slack R, Greep N, Henry-Tilman R, Osborne M, Bunnell C, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919–30. doi: 10.1200/JCO.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 8.Speroff L, Gass M, Constantine G, Olivier S Study 315 Investigators. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms: a randomized controlled trial. Obstet Gynecol. 2008;111:77–87. doi: 10.1097/01.AOG.0000297371.89129.b3. [DOI] [PubMed] [Google Scholar]
- 9.Sandyk R. L-Tryptophan in neuropsychiatric disorders: a review. Intern J Neuroscience. 1992;67:127–44. doi: 10.3109/00207459208994781. [DOI] [PubMed] [Google Scholar]
- 10.Curcio JJ, Kim LS, Wollner D, Pockaj BA. The potential role of 5-Hydroxytryptophan for hot flash reduction: a hypothesis. Altern Med Rev. 2005;10:216–21. [PubMed] [Google Scholar]
- 11.Freedman RR, Dinsay R. Clonidine raises the sweating threshold in symptomatic but not asymptomatic postmenopausal women. Fertil Steril. 2000;74:20–3. doi: 10.1016/s0015-0282(00)00563-x. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR, Wasson S. Miniature, hygrometric hot flash recorder. Fertil Steril. 2007;88:494–96. doi: 10.1016/j.fertnstert.2006.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman RR. Patient satisfaction with miniature, ambulatory, postmenopausal hot flash recorder. The Open Medical Devices Journal. 2009;1:1–2. [Google Scholar]
- 14.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Controlled Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 15.Regestein Q. Editorial. Hot flashes and sleep Menopause. 2006;13:549–52. doi: 10.1097/01.gme.0000227395.30321.ff. [DOI] [PubMed] [Google Scholar]
- 16.Loprinzi CL, Stearns V, Barton D. Centrally active nonhormonal hot flash therapies. Am J Med. 2005;118:118S–23S. doi: 10.1016/j.amjmed.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–26. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 18.Hanisch LJ, Palmer SC, Marcus SC, Hantsoo L, Vaughn DJ, Coyne JC. Comparison of objective and patient-reported hot flash measures in men with prostate cancer. J Support Oncol. 2009;7:131–5. [PubMed] [Google Scholar]