Abstract
GSK3 is one of the few signaling mediators that play central roles in a diverse range of signaling pathways, including those activated by Wnts, hedgehog, growth factors, cytokines, and G protein-coupled ligands. Although the inhibition of GSK3-mediated β-catenin phosphorylation is known to be the key event in Wnt-β-catenin signaling, the mechanisms which underlie this event remain incompletely understood. The recent demonstration of GSK3 involvement in Wnt receptor phosphorylation illustrates the multifaceted roles that GSK3 plays in Wnt-β-catenin signaling. In this review, we will summarize these recent results and offer explanations, hypotheses, and models to reconcile some of these observations.
GSK and Wnt-β-catenin signaling
Glycogen synthase kinase 3 (GSK3), originally identified in 1980 by Embi et al. 1, is highly conserved from yeast to mammals. Mammals express two GSK3 isoforms, α (51 kDa) and β (47 kDa), which are encoded by distinct genes and share 97% amino acid sequence identity within their catalytic domains. However, their sequences differ significantly outside the kinase domain 2. Both GSK3 isoforms appear to be ubiquitously expressed, and they seem to be functionally redundant in some signaling pathways, including Wnt-β-catenin signaling, but they perform distinct functions in others 2, 3. Numerous studies have pointed to an association of GSK3 dysregulation, particularly hyperactivation, with various pathological conditions, including diabetes mellitus, obesity, inflammation, neurological disorders, and tumorigenesis 2, 4, 5. Thus, GSK3 inhibitors comprise a group of potential therapeutics for human diseases.
GSK3 was first implicated in the Wnt-β-catenin signaling pathway (Box 1; Fig 1) owing to the induction of a dorsal-ventral axis duplication phenotype by its dominant negative form in Xenopus laevis embryos 6–8 (axis duplication is associated with Wnt-β-catenin signaling pathway activation). Subsequently, β-catenin was identified as a GSK3 substrate: GSK3-mediated phosphorylation triggers β-catenin destabilization 9, 10. This finding thus established a central role for GSK3 in Wnt-β-catenin signaling. Studies since then have revealed multifaceted roles of this kinase in Wnt signal transduction. Although the detailed mechanisms for GSK3 regulation during Wnt signal transduction remain incompletely understood, it is clear that Wnt-mediated GSK3 regulation does not utilize the same phosphorylation events as in AKT signaling 11, 12 (Box1). Recent advances indicate that GSK3 also plays a positive role in Wnt signal transduction by phosphorylating the Wnt receptors low density lipoprotein receptor-related protein (LRP5/6) and provide new mechanisms for the suppression of GSK3 activity by Wnt in β-catnein stabilization. Furthermore, GSK3 mediates crosstalk between signaling pathways and β-catenin-independent downstream signaling from Wnt.
Text box 1. Phosphorylation-mediated regulation of GSK3.
A distinct feature of GSK3 is its constitutive kinase activity, which is often negatively regulated 4 through posttranslational modifications. Indeed, it is well established that AKT (also called protein kinase B; PKB), which is activated by the phosphatidylinositol 3-kinase (PI3K)-PtdIns(3,4,5)P3 pathway, inhibits GSK3 kinase activity via phosphorylation of Ser-21 in GSK3α) or Ser-9 in GSK3β 73. Structural studies have provided important insights into the regulation of GSK3 by AKT-mediated phosphorylation. The phosphorylated N-terminus becomes a pseudo-substrate, which competes with the priming phosphate for substrate binding, resulting in GSK3 inactivation 74. In addition to AKT, other Ser/Thr kinases, including AGC kinase, p70 ribosomal S6 kinase, p90 ribosomal S6 kinase, and p38 mitogen-activated protein kinase, can also phosphorylate and inhibit GSK3 74.
In many cellular settings, GSK3 exerts a negative effect on substrate-mediated downstream signaling. Accordingly, GSK3 inactivation frequently stimulates many cellular events 74. It is noteworthy that efficient phosphorylation of its substrates can require a priming phosphate, specifically a phosphorylated Ser/Thr residue, often located at the +4 position downstream of the GSK3 phosphorylation site. Therefore, a consensus recognition sequence for GSK3 has been proposed as Ser/Thr-(X-X-X)-pSer/pThr with X being any amino acid 74. Based on this recognition motif, a large number of putative substrates have been predicted by bioinformatics approaches, and some have been validated in vitro 74.
Figure 1. Schematic representation of simplified canonical Wnt signaling pathways.
There are generally two pools of β-catenin in cells. One pool is associated with cadherins, whereas the other is degraded in the absence of Wnt by the β-catenin destruction complex. Wnt binds two cell surface receptors (LRP5/6 and FZD) and leads to phosphorylation at least of Thr-1479 by CKIγ, Ser-1490 by GSK3, and Thr-1493 by yet to be identified CKs on LRP6. These phosphorylation events are required for AXIN recruitment and β-catenin stabilization. Stabilized β-catenin enters the nucleus and activates gene transcription activation. Two of the Wnt antagonists, Dickkopf (DKK) and soluble frizzled-related protein (sFRP), are also shown.
GSK3 in the β-catenin destruction complex
There are generally two pools of β-catenin in cells; one pool is tightly associated with cadherins at cell-cell junctions, and the other is “free” in the cytosol/nucleus (Fig. 1). The latter pool is involved in gene transcription regulation. In the resting state, cytosolic/nuclear β-catenin must be maintained at a very low level through rapid turnover of free β-catenin. This turnover is executed through a multi-protein complex, termed the β-catenin destruction complex, anchored by AXIN1/2 and adenomatous polyposis coli (APC) (Fig. 1). Casein kinase I-alpha (CKIα)) and GSK3, two other important components of this complex, sequentially phosphorylate β-catenin. Hyperphosphorylated β-catenin is then subjected to ubiquitylation by the SKP1–cullin1–F-box (SCFβ-TrCP) E3 ligase complex followed by degradation via the 26S proteasome 13, 14.
Structural studies on some of the β-catenin destruction complex components led to the proposal of the following working model 15,16. The β-catenin destruction complex is assembled through the interactions between APC, AXIN, GSK3 and CK1α). Specifically, APC directly binds AXIN via Ser-Ala-Met-Pro (SAMP) repeat sequences and β-catenin via three 15-amino acid repeats and seven 20-amino acid repeats. AXIN, in addition to binding APC, interacts with GSK3, CKIα) and β-catenin. The complex is stabilized via GSK3- and CKIα)-mediated AXIN phosphorylation. β-catenin enters this complex primarily through its interaction with AXIN and the 15 amino acid repeats in APC. CK1α) phosphorylates Ser-45, which primes the subsequent, sequential, phosphorylation of Thr-41, Ser-37 and Ser-33 by GSK3. After these phosphorylation events, β-catenin can be retained in the complex via its interaction with the 20 amino acid repeats on APC, thereby promoting its recognition by β-TrCP for polyubiquitylation on Lys-19.
The affinity of β-TrCP for β catenin is low; however a newly discovered component of the β-catenin destruction complex, WTX, might facilitate this inefficient process through its interaction with β-TrCP 15–20. WTX forms a complex with β-catenin, AXIN1, β-TrCP and APC, and functionally promotes β-catenin ubiquitylation and degradation. Thus, loss of function mutations in WTX should result in β-catenin stabilization and promote cancer formation. It is, however, intriguing that whereas APC mutations are frequently found in colon cancer, WTX mutations are mainly found in Wilm’s tumors 17, 21. The reasons for this tissue-type specificity remain unknown.
Because GSK3- and CKIα) mediated phosphorylation function as a switch in regulating β-catenin stability, it is reasonable to postulate that Wnt stabilizes β-catenin by regulating the activity of these kinases. Indeed, pharmacologic inhibition of GSK3 activity can lead to β-catenin stabilization and activation of β-catenin and TCF-dependent gene transcription 22–24. Liu et al. (2002) measured β-catenin phosphorylation status upon Wnt treatment and observed no effect on CK1α)-mediated phosphorylation of Ser-45, but strong inhibition of GSK3-mediated phosphorylation of Ser-33, Ser-37, and Thr-41. These results led to the conclusion that Wnt inhibits GSK3-, but not CK1α)-, mediated β-catenin phosphorylation. Thus, how Wnt suppresses GSK3-mediated phosphorylation of β-catenin became a central question in the Wnt-β-catenin signaling field. Although this question continues to remain incompletely resolved and in dispute, recent findings of Wnt-induced LRP5/6 phosphorylation and possible direct inhibition of GSK3 by phosphorylated LRP5/6 shed fresh light on this question.
GSK3 in Wnt-induced phosphorylation of LRP5/6
Nearly a decade ago, the interaction between the C-terminus of LRP5 and AXIN, the first link between a Wnt receptor and any of its intracellular signaling mediators, was discovered in a yeast two hybrid screen 25. The LRP5–AXIN interaction appeared to depend on the LRP5 PPP(S/T)P motif and could be greatly enhanced by the presence of GSK3 25. LRP5 and LRP6, as well as the fly ortholog Arrow, contain multiple copies of this PPP(S/T)P motif (Fig. 1). By using an anti-phospho-specific antibody, He’s group demonstrated that the PPPS/TP motif can be phosphorylated, and importantly, that this phosphorylation could be stimulated by Wnt 26. The same group subsequently showed that GSK3 phosphorylated the Ser/Thr residue in the PPP(S/T)P motif, thus identifying a new role for GSK3 in Wnt signaling 27.
The discovery that Wnt stimulates GSK3-dependent phosphorylation of its co-receptor LRP5/6 instantly provides a mechanism for Wnt to suppress GSK3-mediated β-catenin phosphorylation. In this mechanism, Wnt could divert GSK3 from phosphorylating β-catenin to phosphorylating LRP5/6. However, it was initially suggested that different pools of GSK3 could be used in phosphorylation of LRP5/6 and β-catenin based on the observation that GSK3 did not translocate to the plasma membrane in response to Wnt stimulation. However, recent studies using a more elaborate membrane fractionation approach showed that GSK3 translocated to the plasma membrane, along with AXIN, upon Wnt stimulation 28–30. Because AXIN translocation depends on frizzled (FZD) and dishevelled (DVL), Zeng et al proposed a model which suggested that Wnt could recruit AXIN to the membrane via the interactions between FZD and DVL and between DVL and AXIN 30. There, AXIN-associated GSK3 phosphorylates LRP5/6. The observation that AXIN is required for Wnt-induced LRP5/6 phosphorylation of the PPP(S/T)P motif in LRP6 supports this model 29, 30.
Although this DVL-mediated recruitment of AXIN (Fig 2; direct recruitment model) appears plausible, Wnt does not appear to induce robust DVL membrane translocation in many cells, despite the fact that FZD overexpression can recruit DVL to membranes 28, 30–35 and that FZD might be able to directly interact with DVL 35. It is also not known how Wnt regulates the interaction between FZD and DVL. Could this occur via conformational changes in FZD or modifications of DVL and/or FZD, or production or modifications of signaling mediators such as phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2)?
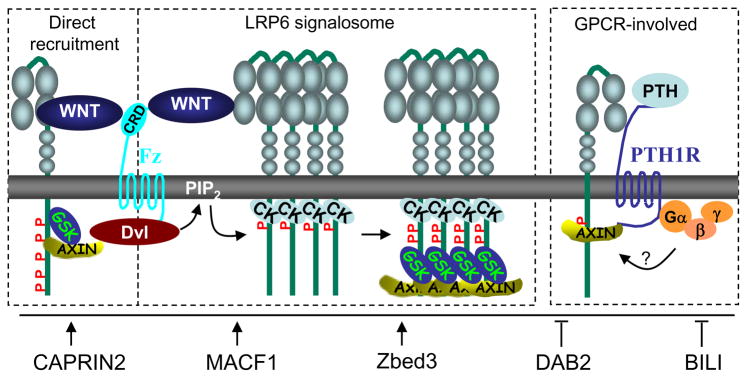
Figure 2. Three major models for the regulation of LRP5/6 phosphorylation.
In the direct inhibition model, GSK3 might be directly inhibited by phosphorylated PPP(S/T)P motifs. GSK3 might be recruited in a manner depending on AXIN, which might be recruited by Wnt via FZD and DVL. In the signalosome model, Wnt induces clustering of LRP6, leading to its phosphorylation by CKIγ and subsequently by GSK3 and recruitment of AXIN. PIP2, whose production is stimulated by Wnt via FZD and DVL, is required for the signalosome formation. GPCRs such as PTH1R might interact with LRP6 and regulates LRP6 phosphorylation and AXIN recruitment. Proteins that were identified recently for promoting or inhibiting LRP6 phosphorylation are listed.
GSK3-dependent PPP(S/T)P phosphorylation is not the only Wnt-induced phosphorylation event on LRP5/6. Niehrs’ group showed that Thr-1479, which is immediately upstream of the first PPP(S/T)P motif of LRP6 (Fig. 1), could be phosphorylated by CKIγ, a protein kinase constitutively associated with LRP6, in response to Wnt signaling, thereby proposing an alternative mechanism by for Wnt-dependent AXIN recruitment 36. Recently, the same group showed that Wnt induced the co-clustering of LRP6 and AXIN to form a structure, referred to as the signalosome (a multi-protein complex essential for signal transduction) 37. Signalosome formation requires FZD and DVL and appears to supersede Thr-1479 phosphorylation by CKIγ, It was also suggested that DVL oligomerization, mediated by the DIX (dishevelled and AXIN) domain, might be involved in signalosome formation 37, 38. Importantly, AXIN seems to interact only with LRP6 in the signalosome. AXIN contains a DIX domain that behaves similarly to the one in DVL 38; therefore it is possible that the AXIN DIX domain might mediate AXIN oligomerization in the signalosome. These observations suggest a model (the signalosome model in Fig. 2) in which Wnt induces the aggregation of LRP6, leading to Thr-1479phosphorylation by LRP6-associated CKIγ in a manner reminiscent of dimerization-mediated growth factor receptor activation. The aggregated receptors have a higher affinity for AXIN, and it is reasonable to hypothesize that AXIN would further recruit GSK3 to phosphorylate LRP6 at Ser-1490 in the first PPP(S/T)P motif and other Ser or Thr residues in the LRP5/6 PPP(S/T)P motifs. Mutational analysis suggests that at least four of these motifs are required for intact signal transduction to elicit β-catenin stabilization 39. The relationship between Thr-1479 phosphorylation and the PPP(S/T)P motif phosphorylation has not been thoroughly examined. The available information suggests that Thr-1479 might be required for GSK3-mediated PPP(S/T)P phosphorylation 37, 40.
There is another question left unaddressed for the LRP aggregation model, which is the initial interaction site for AXIN. AXIN has been assumed to be capable of directly interacting with LRP5/6, but this has not been rigorously examined by using purified recombinant proteins. Although the interaction of the LRP5 intracellular domain with AXIN was initially identified in a yeast two hybrid screen, yeast have GSK3 homologs which could bridge an in vivo LRP5–AXIN interaction. Recent studies indicate that GSK3 can directly interact with the LRP6 intracellular domain 41, 42, suggesting that GSK3 might bridge the interaction between AXIN and LRP6. Of note, although GSK3 does not readily co-immunoprecipitate with the LRP5 intracellular domain, its presence strongly facilitates the co-immunoprecipitation of LRP5 and AXIN 25. Thus, although signaling through LRP5 and LRP6 can achieve the same goals 25, 43, these two receptors might not use the exactly same means.
New players in regulating LRP5/6 phosphorylation
In a kinomic siRNA library screen, we recently identified PI4KII and PIP5KI family lipid kinases as being involved in the regulation of LRP5/6 phosphorylation by Wnt via PtdIns(4,5)P2 production 29. PtdIns(4,5)P2 is required, but not sufficient, for Wnt-induced phosphorylation of both Thr-1479 and Ser-1490 in LRP6. WNT3A is a potent stimulator of PtdIns(4,5)P2 production in a number of mammalian cell lines, and WNT3A-induced production of PtdIns(4,5)P2 requires both FZD and DVL. DVL can directly interact with and activate both PI4KIIα) and PIP5KI via different domains of DVL29, 44. Although it remains uncertain how PtdIns(4,5)P2 regulates LRP6 phosphorylation, PtdIns(4,5)P2 is required for both AXIN–GSK3 membrane translocation and LRP6 aggregation in response to WNT3A. As PtdIns(4,5)P2 can regulate lipid microdomain formation and dynamics and is involved in formation of macromolecular complexes such as clathrin- coated pits in endocytosis, it would be reasonable to speculate that PtdIns(4,5)P2 regulates the formation of LRP6 aggregation in similar ways.
Another new player implicated in regulating Wnt-GSK3-mediated phosphorylation of LRP5/6 is cytoplasmic activation/proliferation-associated protein 2 (CAPRIN2). A yeast two-hybrid screen identified CAPRIN2 as a LRP5-binding protein. It facilitates GSK3-mediated LRP5/6 phosphorylation and enhances the interaction between AXIN and LRP5/6 45. Moreover, CAPRIN2 stimulates Wnt-β-catenin signaling when overexpressed; accordingly, the pathway is inhibited by its siRNA-mediated knockdown. More recently, the same laboratory identified zinc-finger BED domain-containing 3 (ZBED3) as an AXIN-interacting protein46. ZBED3 elicits opposite effects on Wnt-β-catenin signaling than does CAPRIN2. It contains a single PPPSPT motif, and phosphorylation of this motif by GSK3 and CKIα) promotes the ZBED3–AXIN interaction. CAPRIN2 and ZBED3 might modulate Wnt signaling via changes in their expression levels. In addition, CAPRIN2 could be a Wnt signaling mediator if its effects on LRP5/6 phosphorylation are regulated by Wnt-dependent posttranslational modifications or known Wnt signaling mediators such as PtdIns(4,5)P2. It is also interesting to note that there is no human homology of Zbed3.
Among other emerging players in Wnt signaling that affect Wnt-GSK3 mediated phosphorylation of LRP5/6 are MACF1 (microtubule actin cross-linking factor), Bili, and disabled-2 (DAB2). MACF1 interacts with the β-catenin destruction complex and promotes Wnt signaling by facilitating the translocation and subsequent binding of the AXIN complex to LRP6 at the cell membrane 47. Macf1−/− mice are embryonic lethal and show phenotypes similar to those caused by the lack of WNT3 or LRP5/6. Bili, a Band4.1-domain containing protein, was identified in a recent genome-wide RNAi screen in Drosophila melanogaster cells as a negative regulator of Wnt-β-catenin signaling by inhibiting the recruitment of AXIN to LRP6 48. DAB2 is an endocytic adaptor which interacts with both DVL and AXIN and inhibits Wnt-β-catenin signaling 49. It is possible that DAB2 competes with protein phosphatase 1 (PP1) for binding to AXIN, thus preventing AXIN dephosphorylation and subsequent degradation. In addition, DAB2 prevents AXIN from translocating to the plasma membrane, thus inhibiting Wnt-mediated phosphorylation of LRP6 on Ser-1490. Of note, DAB2 is a PtdIns(4,5)P2-binding protein, and the binding sequences for PtdIns(4,5)P2 and AXIN on DAB2 overlap. It would be interesting to determine if PtdIns(4,5)P2 competes with AXIN for binding to DAB2, thus providing a mechanism by which PtdIns(4,5)P2 stimulates Wnt-induced LRP5/6 phosphorylation.
Wnt is not the only ligand that can stimulate GSK3-mediated LRP6 phosphorylation. Parathyroid hormone (PTH) can induce the formation of a complex of LRP6 and PTH G protein-coupled receptor (PTH1R) and stimulate LRP6 phosphorylation at Ser-1490. Such LRP6 phosphorylation is accompanied by rapid recruitment of AXIN to LRP6 and stabilization of β-catenin. Interestingly, further investigation using the LRP5/6 antagonist DKK1 (dickkopf homolog 1) and the FZD antagonist FZD8 cysteine-rich domain suggested that PTH-mediated LRP6 activation is independent of Wnt or FZD signaling. It remains to be determined whether a heterotrimeric G protein, DVL or GSK3 is involved in this PTH-LRP6 signaling model (the GPCR-involved model in Fig. 2) 50.
Suppression of GSK3-mediated β-catenin phosphorylation
A number of models have been proposed to explain how Wnt suppresses GSK3-mediated phosphorylation of β-catenin. These include: Wnt-induced repositioning of GSK3 away from β-catenin, dissociation of GSK3 from AXIN via conformational changes or post-translational modifications, recruitment of GSK3 inhibitory proteins such as FRAT, recruitment or activation of phosphatases, and AXIN degradation (for reviews, see. 15, 51). More recently, compelling evidence suggests that GSK3 is directly inhibited by phosphorylated PPPS/TP sequences 41, 42, 52, 53. Together with the AXIN degradation model, the direct inhibition model appears to be the prevailing view of how Wnt suppresses of GSK3-mediated β-catenin phosphorylation.
Direct inhibition model
The first piece of evidence for the direct inhibition model came from the observation that the LRP6 intracellular tail directly interacts with and inhibits GSK3 41. A similar result was also observed by Cselenyi et al 52. However, the most compelling piece of evidence came from in vitro assays which showed that phosphorylated peptides containing the first LRP6 PPPSP motif can inhibit GSK3-mediated phosphorylation of a β-catenin fragment 42. In addition, the phosphorylated peptides potentiated Xwnt8-induced axis duplication in Xenopus embryos, presumably due to GSK3 inhibition [46, 64]. These results excluded the possibility of substrate competition 41, 52.
The study by Piao et al also suggests that the two Thr residues surrounding the LRP6 PPPSP motif (Fig. 1), when phosphorylated, might also play an important role in GSK3 inhibition [42]. Indeed, Far-Western blotting showed that these Thr residues, when phosphorylated, enhanced the interaction with GSK3. Phosphorylated Ser-1490, which lies within the PPPSP motif, is required for the LRP6–AXIN interaction 26, 27. Based on the Far Western data, it would seem that GSK3 might mediate the recruitment of AXIN to LRP642. Unfortunately, the AXIN used in their study did not encompass all of the sequences required for its recruitment to LRP5/6. Moreover, this model cannot reconcile the findings that AXIN is required for Ser-1490 phosphorylation 29, 30. Thus, how AXIN interacts with LRP5/6 remains an open question. As an alternative model to indirect AXIN recruitment to LRP6, Wu et al proposed a model which suggests that whereas the first PPPSP motif in LRP5/6 is involved in GSK3 inhibition, AXIN interacts with other PPP(S/T)P motifs 53. Because there is a lack of evidence for a direct interaction of phosphorylated Ser within the PPPSP motif with GSK3, which might only be unambiguously determined by structural studies, it is also possible that a certain conformation of phospho-PPP(S/T)P inhibits GSK3. In other words, the direct interacting site might not be limited to the phosphorylated Ser residue. The adjacent proline residues could also be involved; indeed, proline residues can assume trans or cis conformations in polypeptides 54. In addition, phosphorylated PPP(S/T)P motifs might not be the sole mechanism for LRP5/6-mediated GSK3 regulation because the soluble, unphosphorylated LRP6 intracellular domain can still stabilize β-catenin in an overexpression assay 55.
AXIN degradation model
A decade ago, GSK3 was first shown to phosphorylate and stabilize AXIN 56. Thus, Wnt-mediated GSK3 inhibition would presumably lead to AXIN destabilization. Because AXIN is present in cells at the rate limiting level (at least in Xenopus eggs) 57, a reduction in AXIN levels would lead to β-catenin stabilization. In fact, a number of groups later showed that overexpression of activated Wnt receptors or recombinant DVL could trigger AXIN degradation 25, 58, 59. However, Wnt-induced AXIN degradation lags significantly behind β-catenin stabilization in many systems 60, 61 and thus might not be the mechanism responsible for Wnt’s acute response. Moreover, Cseleny et al provided strong evidence for the ability of LRP6 to stabilize β-catenin in the absence of AXIN degradation 52. However, a recent fly genetic study indicates that AXIN degradation remains an important mechanism that probably has an important role in long-term Wnt signaling 62. This genetic study also confirms the importance of APC in AXIN degradation. A more recent study shed new light on the mechanism for the regulation of AXIN stability by demonstrating that the poly-ADP-ribosylating enzymes tankyrase 1 and tankyrase 2 participate in AXIN ubiquitylation 63.
Other GSK3 regulatory mechanisms affecting β-catenin stability
Because FZD shares similar topological features with serpentine G protein coupled receptors, it has been postulated to couple to heterotrimeric G proteins 64. Work using a chimeric receptor consisting of β-adrenergic receptor extracellular and transmembrane domains and FZD intracellular domains suggest that these chimeric receptors might couple to the Goα, Gqα, and Gtα classes of G proteins to stabilize β-catenin 64. More recently, Liu et al showed that FZD receptors are pre-coupled to Goα. Upon FZD activation, Goα rapidly dissociates from FZD; this process correlates with the dissociation of GSK3β from AXIN 60. Depletion of Goα and Gqα with siRNAs inhibits Wnt-induced stabilization of β-catenin. The involvement of Goα downstream of FZD in Wingless to GSK3-β-catenin signaling is also supported by fly genetics analysis 65. However, expression of activated Gqα, which strongly activates phospholipase C (PLC) in all of cells tested so far, failed to stimulate β-catenin stability or β-catenin-TCF-mediated gene transcription in most cell types. By contrast, expression of active Gqα inhibits β-catenin pathways 66.
Very recently, Maher et al revealed a cadherin-dependent mechanism that regulates β-catenin stability via AXIN, APC2 and GSK3β 67. Although they are both localized at cell membranes, this β-catenin-destruction complex is physically distinct from the cadherin–catenin complex. The study also showed that cadherins promote the N-terminal phosphorylation of β-catenin and that cell–cell adhesion increases the turnover of cytosolic β-catenin. However, the mechanisms which underlie these events remain unclear.
Wnt-GSK3 signaling beyond β-catenin
GSK3 has been reported to phosphorylate more than 40 protein substrates 2, 24, pointing to a potential role for GSK3 in other, β-catenin-independent, cellular functions. Indeed, recent studies indicate that Wnt-mediated GSK3 regulation can modulate other downstream signaling events independently of β-catenin.
An example of Wnt-mediated regulation of GSK3 which signals beyond β-catenin is the regulation of mTOR (mammalian target of rapamycin) kinase activity via TSC2 (tuberous sclerosis complex 2). Tuberous sclerosis is an autosomal-dominant syndrome, caused by mutations in either TSC1 or TSC2, which is characterized by the formation of hamartomas in many organs. TSC1 and TSC2 form a complex which regulates the small GTPase RHEB through TSC2-mediated GTPase-activating protein (GAP) activity. RHEB in turn induces mTOR-mediated phosphorylation events which regulate cell growth and survival (see reviews 68, 69). Inoki et al, showed that Wnt can regulate the TSC2-mTOR pathway via GSK3, but independently of β-catenin 70. GSK3 inhibits the mTOR pathway by phosphorylating TSC2; this can be blocked by Wnt treatment. In addition, Wnt signaling components, including DKK1, DVL, AXIN, and GSK3, but not β-catenin, regulate the pathway. The study also provides a large body of evidence for the involvement of this Wnt-GSK3-TSC2-mTOR pathway in the regulation of cell growth and size. Because GSK3-mediated TSC2 phosphorylation depends on priming phosphorylation by AMPK (AMP-regulated protein kinase), a cellular energy sensor that plays an important role in cellular energy homeostasis, the study also characterized the crosstalk between two important cellular signaling pathways.
Another excellent example is the regulation of SMAD1, a mediator of the transforming growth factor beta (TGF-β)-bone morphogenetic protein (BMP) superfamily of ligands, by the sequential phosphorylations of its linker region by mitogen-activated protein kinase (MAPK) and GSK3. These phosphorylations trigger SMAD1 polyubiquitylation and subsequent proteasomal degradation. Importantly, Wnt signaling decreases GSK3-mediated SMAD1 phosphorylation, thus altering GSK3 subcellular localization. This Wnt-GSK3-SMAD signaling pathway plays an important role in epidermal induction by regulating dorsoventral (controlled by BMP) and anteroposterior (by Wnt-GSK3) patterning in Xenopus embryos. GSK3-mediated phosphorylation of SMAD1 also requires a priming phosphate, which is provided by growth factor-dependent MAPK phosphorylation. Therefore, SMAD1 integrates three signaling pathways – Wnt-, growth factor-, and BMP-dependent - during embryonic pattern formation 71.
These findings that Wnt regulates signaling events via GSK3, but not β-catenin, also raise an important question: does Wnt use the same pool of GSK3 in the regulation of β-catenin-dependent and independent signaling? Earlier work by Ding et al suggests that there are at least two pools of GSK3 in cells 11, one associated with AXIN and refractory to phosphorylation by AKT at Ser-9 or Ser-21, and another which can be regulated by AKT. As insulin treatment 11 or overexpression of active AKT 12 fails to stimulate β-catenin-dependent signaling in most cells, the GSK3 pool regulated by AKT might not have a significant role in Wnt-β-catenin signaling. AXIN is a core component in the β-catenin destruction complex; therefore this AXIN-associated pool of GSK3 would need to be suppressed in order to stabilize β-catenin. However, Ding et al also observed that Wnt might not only regulate the AXIN-associated pool of GSK3. Wnt treatment inhibits nearly 50% of the kinase activity of immunoprecipitated GSK3. Based on their co-immunoprecipitation results, the AXIN-associated pool of GSK3 should be far smaller than 50% of the total population. In addition, Wnt-induced inhibition of immunoprecipitated GSK3 completely diminished within 60 min, whereas Wnt-induced β-catenin accumulation peaked at 3 hrs and lasted up to 9 hrs. Thus, Wnt might not only inhibit AXIN-associated GSK3, most likely via the LRP6 mechanism discussed earlier, but also additional pools of GSK3. Although the LRP6 intracellular domain can directly inhibit GSK3 activity in in vitro assays, there is no evidence that LRP6 is able to do so in vivo independently of AXIN; indeed, the existing evidence actually shows the opposite result 29, 30. Furthermore, the phospho-PPPSP motif has a very low affinity for GSK3 42; therefore it is unlikely that LRP6 would recruit multiple GSK3 molecules in the absence of AXIN. The association of AXIN with LRP5/6, directly or indirectly, makes it very difficult to use the direct GSK3 regulation model to explain the Wnt-dependent regulation of the AXIN-free pool of GSK3. Therefore, we hypothesize that the AXIN-independent pool can be further divided, and that Wnt might also regulate one of the AXIN-free pools in a way independent of Ser-9 or Ser-21 phosphorylation or direct LRP5/6 interaction (Fig. 3).
Figure 3. Hypothetical depiction of three GSK pools and their possible involvement in regulation of diverse signaling pathways.
Three pools of GSK3 are hypothesized to exist. One pool is associated with AXIN and probably regulated by LRP5/6. Another pool is regulated by phosphorylation at Ser9 or Ser-27 by the PI3K-AKT pathway. Additionally, there might be an AXIN-independent pool of GSK3, which is also regulated by Wnt. However, the mechanisms by which this pool would be regulated are not clear.
Concluding remarks
GSK3, unlike most other kinases or signaling mediators, is regulated by multiple mechanisms, which can be insulated from each other. This property reflects the fact that GSK3 is involved in numerous cellular processes, often at the intersections that link diverse signaling pathways (Fig. 3). The complexity of GSK3 regulation ensures the flexibility and insulation of certain single pathway and also provides the mechanism for appropriate coordination of multiple pathways.
Since the discovery of the involvement of GSK3 in Wnt signaling, we have come a long way in understanding the mechanisms for its regulation and its roles in Wnt-β-catenin signaling. Many questions, however, remain unanswered. What are the interaction sites for GSK3 and AXIN on LRP5/6? What is the molecular mechanism for LRP5/6-mediated AXIN degradation? Is there a pool of AXIN-free GSK3 that can be regulated by Wnt signaling, and how is it regulated? Which Wnt-regulated pools of GSK3 are involved in the crosstalk with the SMAD and TSC-mTOR pathways? How is Wnt signaling to GSK3 turned off? Given that WNT5A also stimulates PtdIns (4,5)P2 production and, via GSK3, the phosphorylation of receptor tyrosine kinase-like orphan receptor (ROR) 2, a putative WNT5A receptor which mediates β-catenin-independent Wnt signaling 72, it would also be interesting to determine whether WNT5A regulates GSK3 in a manner similar to WNT3A. If so, what are the roles of GSK3 in this β-catenin-independent signaling pathway? In light of the recent advances in genomics, proteomics, imaging, and structural biology, as well as the increasing use of these approaches in combination with genetics and biochemistry, we shall not be surprised to see the resolution of many of these questions in near future.
Text box 2. Overview of the Wnt-β-catenin signaling pathway.
The Wnt family of secretory glycoproteins is one of the major families of developmentally important signaling molecules. They were initially characterized for their roles in regulating embryonic development and tumorigenesis. Studies in the past decade have implicated Wnt signaling in a diverse range of physiological and pathophysiological processes, including bone development, angiogenesis, vasculature remodeling, myogenesis, adipogenesis, stem cell renewal and differentiation, and lipid and glucose metabolism.
The Wnt proteins are defined based on sequence homology to the original Wnt members: mouse WNT1 (originally called int-1) and Drosophila melanogaster Wingless (Wg). Mutations in Wnt genes exhibit a variety of intriguing phenotypes in the mouse, Caenorhabditis elegans, and Drosophila, indicating that Wnt proteins function in diverse developmental processes. Numerous studies have established a canonical signaling pathway that leads to β-catenin stabilization; in this review we refer to this as the Wnt-β-catenin pathway (Fig. 1). In the absence of Wnt, a number of proteins, including AXIN, adenomatous polyposis coli (APC), GSK3, casein kinase Iα (CKIα)), and β-catenin, form a complex (referred to as the β-catenin destruction complex), in which β-catenin is phosphorylated by CKIα) and GSK3. This phosphorylation event targets β-catenin for proteasome-mediated proteolytic degradation. When Wnt proteins bind cell-surface receptors frizzled (FZD) and lipoprotein receptor-related protein (LRP) 5/6, GSK3-dependent β-catenin phosphorylation is suppressed through a mechanism that requires the scaffold protein dishevelled (DVL), and β-catenin is stabilized (Fig. 1). Stabilized β-catenin enters the nucleus and interacts with transcriptional regulators, including lymphoid enhancing factor-1 (LEF1) and T cell factors (TCFs), to activate gene transcription. Mammals express 19 Wnt genes as well as many Wnt antagonists. Please refer to recent reviews 13, 14 and the frequently updated website managed by Roel Nusse’s laboratory (http://www.stanford.edu/~rnusse/wntwindow.html) for details regarding general Wnt signaling mechanisms and functions.
Acknowledgments
We thank Matthew C. Jones for reading and commenting on the manuscript. D.W. is supported by NIH grants (CA139395, CA132317, AR051476). Due to space limitation, we apologize for many references we have to omit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dianqing Wu, Email: dan.wu@yale.edu, Vascular Biology and Therapeutics Program and Department of Pharmacology, Yale University School of Medicine, New Haven, CT, USA, 065202.
Weijun Pan, Email: panw2@mail.nih.gov, Laboratory of Molecular Genetics, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA, 20892.
References
- 1.Embi N, et al. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 2.Wada A. GSK-3 inhibitors and insulin receptor signaling in health, disease, and therapeutics. Front Biosci. 2009;14:1558–1570. doi: 10.2741/3324. [DOI] [PubMed] [Google Scholar]
- 3.Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. 2009;284:9643–9647. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayasam GV, et al. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodgett JR. Physiological roles of glycogen synthase kinase-3: potential as a therapeutic target for diabetes and other disorders. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:281–290. doi: 10.2174/1568008033340153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X, et al. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 7.Pierce SB, Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development (Cambridge, England) 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez I, et al. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peifer M, et al. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- 10.Yost C, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 11.Ding VW, et al. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H, et al. Suppression of glycogen synthase kinase activity is not sufficient for leukemia enhancer factor-1 activation. J Biol Chem. 1999;274:30419–30423. doi: 10.1074/jbc.274.43.30419. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald BT, et al. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 16.Ha NC, et al. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Mol Cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Major MB, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science (New York, NY) 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. Journal of cell science. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, et al. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. Journal of molecular biology. 2006;360:133–144. doi: 10.1016/j.jmb.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, et al. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Molecular cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 21.Nusse R. Cancer. Converging on beta-catenin in Wilms tumor. Science (New York, NY) 2007;316:988–989. doi: 10.1126/science.1143337. [DOI] [PubMed] [Google Scholar]
- 22.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stambolic V, et al. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 24.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 25.Mao J, et al. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 26.Tamai K, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 27.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama N, et al. Abundance, complexation, and trafficking of Wnt/beta-catenin signaling elements in response to Wnt3. J Mol Signal. 2007;2:11. doi: 10.1186/1750-2187-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan W, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng X, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelrod JD, et al. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 33.Cong F, et al. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan WJ, et al. Characterization of function of three domains in dishevelled-1: DEP domain is responsible for membrane translocation of dishevelled-1. Cell Res. 2004;14:324–330. doi: 10.1038/sj.cr.7290232. [DOI] [PubMed] [Google Scholar]
- 35.Wong HC, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson G, et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 37.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz-Romond T, et al. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald BT, et al. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J Biol Chem. 2008;283:16115–16123. doi: 10.1074/jbc.M800327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yum S, et al. The role of the Ser/Thr cluster in the phosphorylation of PPPSP motifs in Wnt coreceptors. Biochem Biophys Res Commun. 2009;381:345–349. doi: 10.1016/j.bbrc.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 41.Mi K, et al. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J Biol Chem. 2006;281:4787–4794. doi: 10.1074/jbc.M508657200. [DOI] [PubMed] [Google Scholar]
- 42.Piao S, et al. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS ONE. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mi K, Johnson GV. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem. 2005;95:328–338. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- 44.Qin Y, et al. Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J Biol Chem. 2009;284:22544–22548. doi: 10.1074/jbc.M109.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Y, et al. Caprin-2 enhances canonical Wnt signaling through regulating LRP5/6 phosphorylation. The Journal of cell biology. 2008;182:865–872. doi: 10.1083/jcb.200803147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T, et al. Identification of zinc-finger BED domain-containing 3 (Zbed3) as a novel Axin-interacting protein that activates Wnt/beta-catenin signaling. The Journal of biological chemistry. 2009;284:6683–6689. doi: 10.1074/jbc.M807753200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen HJ, et al. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes & development. 2006;20:1933–1945. doi: 10.1101/gad.1411206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kategaya LS, et al. Bili inhibits Wnt/beta-catenin signaling by regulating the recruitment of axin to LRP6. PloS one. 2009;4:e6129. doi: 10.1371/journal.pone.0006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, et al. Dab2 stabilizes Axin and attenuates Wnt/beta-catenin signaling by preventing protein phosphatase 1 (PP1)-Axin interactions. Oncogene. 2009;28:2999–3007. doi: 10.1038/onc.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan M, et al. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes & development. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He X, et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 52.Cselenyi CS, et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of {beta}-catenin. Proceedings of the National Academy of Sciences. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu G, et al. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 55.Beagle B, et al. Phosphorylation of PPP(S/T)P motif of the free LRP6 intracellular domain is not required to activate the Wnt/beta-catenin pathway and attenuate GSK3beta activity. J Cell Biochem. 2009 doi: 10.1002/jcb.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto H, et al. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase 3b regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 57.Salic A, et al. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 58.Lee E, et al. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolwinski NS, et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, et al. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 61.Willert K, et al. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takacs CM, et al. Dual positive and negative regulation of wingless signaling by adenomatous polyposis coli. Science. 2008;319:333–336. doi: 10.1126/science.1151232. [DOI] [PubMed] [Google Scholar]
- 63.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009 doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 64.Malbon CC. Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front Biosci. 2004;9:1048–1058. doi: 10.2741/1308. [DOI] [PubMed] [Google Scholar]
- 65.Katanaev VL, et al. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Maye P, et al. Multiple Mechanisms for Wnt11-mediated Repression of the Canonical Wnt Signaling Pathway. J Biol Chem. 2004;279:24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- 67.Maher MT, et al. Activity of the {beta}-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol. 2009 doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 69.Sarbassov DD, et al. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Inoki K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 71.Fuentealba LC, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto H, et al. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 73.Buttrick GJ, Wakefield JG. PI3-K and GSK-3: Akt-ing together with microtubules. Cell Cycle. 2008;7:2621–2625. doi: 10.4161/cc.7.17.6514. [DOI] [PubMed] [Google Scholar]
- 74.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]