Abstract
For patients with the most severe manifestations of lower extremity arterial occlusive disease, bypass surgery using autogenous vein has been the most durable reconstruction. However the incidence of bypass graft stenosis and graft failure remains substantial and wholesale improvements in patency are lacking. One potential explanation is that stenosis arises not only from over exuberant intimal hyperplasia but also due to insufficient adaptation or remodeling of the vein to the arterial environment. Although in vivo human studies are difficult to conduct, recent advances in imaging technology have made possible a more comprehensive structural examination of vein bypass maturation. This review summarizes recent translational efforts to understand the structural and functional properties of human vein grafts and places it within the context of the rich existing literature of vein graft failure.
Introduction
With the changing demographics of an aging population and a near-epidemic of diabetes mellitus, the prevalence of peripheral arterial disease (PAD) now approaches between 9–12 million Americans.[1, 2] Among established treatments for advanced PAD, the autogenous vein bypass graft has been both the clinical standard and the most comprehensively studied revascularization strategy. Nevertheless, 30–50% of saphenous vein grafts fail during the intermediate period from 1–18 months postimplantation. This period is the most active from a biologic standpoint and the culprit lesion is intimal hyperplasia (IH). It is clear that IH is universal to all implanted vein grafts but why it becomes pernicious and progresses to clinical stenosis at some locations in some veins while others are relatively spared is not clear. In addition, all vein grafts undergo structural remodeling of the lumen and wall to some extent during this same time period. Long-term patency of the vein graft likely depends on adequate adaptation into the arterial environment as well as a relative moderation in the development of IH.
For well over 50 years surgeon-scientists have studied histopathological changes in the vein after implantation, developed animal models, documented efficacy of vein bypass, and with the aid of modern biology techniques, elucidated molecular signaling pathways involved in IH. With the later, came the potential of genetic engineering of the vein graft and the promise of improving patency through novel therapeutics with specific molecular stratagies.[3–5] For example, the PRoject of Ex-Vivo graft ENgineering via Transfection III (PREVENT III) was a phase III study employing an anti-sense DNA decoy molecule to the transcription factor E2F. This randomized, placebo-controlled, double blinded, trial was specifically powered to detect a significant difference in vein graft patency in a cohort of over 1400 patients with critical limb ischemia (CLI). In this contemporary study, the overall one-year primary patency rate was 61% - a value that has not changed for the past several decades.[6] While this trial ultimately failed to meet its primary efficacy endpoint, it underscored our lack of understanding of the pathophysiology of vein graft failure perhaps by underestimating the complexity and redundancy in the molecular elements contributing to IH or neglecting other potential variables such as remodeling.[4, 6]
Advances in imaging technology during the last decade such as high resolution ultrasound, 3-diminsional magnetic resonance imaging (3D-MRI), and intravascular ultrasound have greatly facilitated our ability to study human vein bypass grafts in vivo.[7, 8] These modalities have the ability to reliably resolve sub millimeter structures.[8–10] Serial changes in the vein graft structure and function can be quantified and patterns of normal and abnormal adaptation be discerned. Beyond luminal changes, wall thickness can now be determined and mathematical models constructed building on the rich existing experimental data compiled from animal and histomorphological studies from the last 4 decades.
Herein, recent advances to better understand the pathogenesis and subsequent evolution of vein graft failure are outlined. Importantly, in vivo observations are emphasized and placed within a historical and a clinical context whenever possible.
The human saphenous vein and the universal response to injury
The normal in vivo appearance of the human saphenous vein is a light blue, thin walled structure that easily distends with minimal pressure. Unfortunately, many veins used in bypass grafting have pre-existing lesions such as endothelial damage, medial hypertrophy, or intimal thickening which may give them a slightly sclerotic appearance.[11, 12] This creates considerable variability in the normal (useable) range of vein wall thickness from 180 to 650 µm.[13] While severe changes render the vein unusable, the impact of subtle pre-bypass morphological changes on the future development of vein graft stenosis is not known, though decreased compliance has been shown to be associated with early vein graft failure.[14] The focal nature of vein graft stenosis detected by surveillance ultrasound studies, suggests that predisposing lesions may exist in these areas.[15]
Intimal hyperplasia was first described in veins by the Nobel prize winning physician Alex Carrel in 1906 and is generally regarded as the universal injury response to a blood vessel: regardless of the injury or the vessel.[16] It is thought to be due to a proliferation of smooth muscle cells (SMC) which have undergone a phenotypic switch from a quiescent, contractile phenotype to that of a synthetic migratory phenotype. The source the SMCs which contribute to the intimal hyperplasia has long been thought to be from the media of the vessel wall but the identification of graft-extrinsic cells such as peri-adventitial fibroblast or circulating precursor cells has challenged this concept.[17–22] Production of extracellular fibrous matrix and ground substance by synthetic SMCs leads to a progressive increase in intimal and medial fibrosis, reduction in cellularity, and overall stiffening of the vein graft which may subsequently limit the graft’s ability to properly remodel in the arterial circulation. The endothelial cells play a key role in regulation of intimal growth by a number of tonic growth-inhibitory mechanisms.[23, 24] Endothelial cell loss or damage markedly attenuates these growth modulating effects.
Biological mediators are also involved.[25] Liberated growth factors from platelets lining the injured vessel wall, infiltration of inflammatory cells through the permeable EC layer, circulating inflammatory molecules, and endothelial dysfunction are likely operative.[26–28] The platelet-endothelium interaction is emphasized by the effectiveness of platelet inhibitors in preventing the early and late occlusion of vein grafts.[29, 30]
The harvesting and preparation of the saphenous vein for bypass surgery affords ample opportunity to impart injury. Mechanical manipulation, valve lysis, pressure distension, ischemia/reperfusion, devascularization and denervation, and transposition into the arterial environment contribute to the injury of the vein.[31–33] A number of intra-operative techniques have been employed in an attempt to mitigate the venous injury. These include no-touch harvesting technique, various storage solutions such as the University of Wisconsin Solution, and placing veins in the in situ configuration to theoretically decrease manipulation and warm-ischemia time.[34–38] In general, it is felt that storage in a papaverine-treated, tissue culture solution, gentle harvesting, and the use of controlled distension decreases endothelial injury and possibly subsequent IH formation.[39]
The causal relationship between arterial hemodynamics and IH is well established and, in a sense, fulfills Koch’s postulates. For example, Brody demonstrated that veins transposed into the arterial circulation developed IH whereas those dissected and re-anastomosed to the venous circulation did not.[40] Experimental vein grafts explanted from the arterial circulation and subsequently transferred back to the venous circulation demonstrated regression of IH.[41–44]Finally, vein grafts placed in a low flow, high resistance, environment developed an exuberant IH response that regressed once placed into a normal flow environment.[45] These experiments suggest that the cyclic mechanical forces of the arterial circulation are sufficient and necessary for the development IH rather than simply the injury of dissection.
Evidence from animal models consistently show that the IH thickness of a vein graft varies inversely proportional to the magnitude of shear stress across the endothelial cell surface.[45–53] In one study, vein grafts exposed to 50% less shear stress had a 63% thicker intimal layer after 4 weeks.[48] Vein grafts placed in conditions of high shear stress demonstrated more endothelium dependent relaxation suggesting that higher shear stress favorably improved endothelial function, possibly through increased nitric oxide (NO) production.[47, 54, 55]
The kinetics of IH development have also been studied in the rabbit and canine vein graft models.[22, 56] There is an initial burst of endothelial and smooth muscle cell proliferation occurring within 1 week following implantation and a return to the baseline quiescent state by week 12. More recently, mathematical relationships of the temporal sequence of the development of IH as a function of shear stress and time, h(t, τ)=ho+(RL[1−e−A(t−t*)])/(1+BτC), where h is the intimal thickness, t is time, τ is shear stress, ho is the intimal thickness at the time of implantation, RL is the initial lumen radius, and t* is the time when intimal thickness begins to change. A, B, C are experimentally derived constants and therefore unique to the model used.[57, 58] The application of mathematical models of this type, validated in human vein grafts, would be of great benefit in the determination of efficacy of new therapeutics or biologics developed to inhibit IH.
The importance of vein graft caliber regulation
While much attention has been given to IH as the primary mode of failure for vein grafts, relatively limited information is available on the role remodeling plays in the failing vein graft.[6, 7, 10, 59, 60] Remodeling is defined as a dynamic structural and biochemical adaptation within the vein graft that results in long-term changes in lumen caliber, wall thickness, composition of matrix proteins, and endothelial cell reactivity.[7, 61–67] Remodeling is dependent on an interaction between locally and remotely generated growth factors, vasoactive substances, and hemodynamic stimuli.[22, 68]
It is well known that arteries undergo compensatory remodeling in response to hemodynamic forces.[69, 70] [71] [72–74] [75] The resulting change in lumen caliber or wall thickness is thought to maintain these forces at a biologically preset value that promotes maximum efficiency of blood transport.[69, 70] Between the predominant hemodynamic forces, wall tension and shear stress, the later appears to be the dominant of the two in regulating lumen caliber.[76] While blood flow is proportional to the product of the vessel’s radius and the pressure gradient across the vessel, shear stress is proportional to the quotient of blood flow and lumen radius. Shear stress is the frictional force per unit area tangential to the vessel wall and therefore “sensed” by endothelial cells. Endothelial cells are the recognized biosensor of the vessel and align themselves along the direction of shear stress. Steady laminar shear stress has a healthy trophic effect on the endothelial cells by inhibiting leukocyte adhesion, promoting vasodilator production such as NO[77] and prostacyclin[78], limiting SMC hyperplasia[79], and decreasing platelet aggregation[80]. Conversely, low shear stress and turbulence associated with complex geometries such as anastomosis and valves, has the opposite effect.[81] Removal of the endothelium abolishes flow-dependent vasodilation.[82]
A vein implanted into the arterial circulation will experience a several-fold increase in both shear stress and wall tension and several lines of evidence argue that hemodynamic regulation of venous structures exists.[74, 83–85] In a rabbit jugular vein – carotid artery interposition model, the lumen radius increased 57% over a 24 week study period: a magnitude sufficient to reduce the initially high shear stress down to that of a normal rabbit carotid artery.[56] The remarkable rapidity in which the vein remodels after implantation in the arterial circulation was exemplified in a canine model whereby maximal diameter was attained in just 7 days.[22]
The ability for human saphenous vein grafts to dilate substantially-and sometimes pathologically-after implantation has been known from autopsy studies for nearly 50 years.[86] Fillinger et al demonstrated that final lumen diameter attained at 12 months was a function of the initial lumen diameter and shear stress.[87] While vein grafts which had the highest initial shear stress tended to be smaller at the time of implantation, they underwent greater lumen dilation then those with lower initial shear stress. This observation is important to consider when choosing to use or discard a relatively small vein as it is well known that the luminal size of the vein is of primary importance for subsequent patency.[88, 89] However a small vein, free of pre-existing pathology, that is compliant and distends easily will likely dilate and function well as a bypass graft.
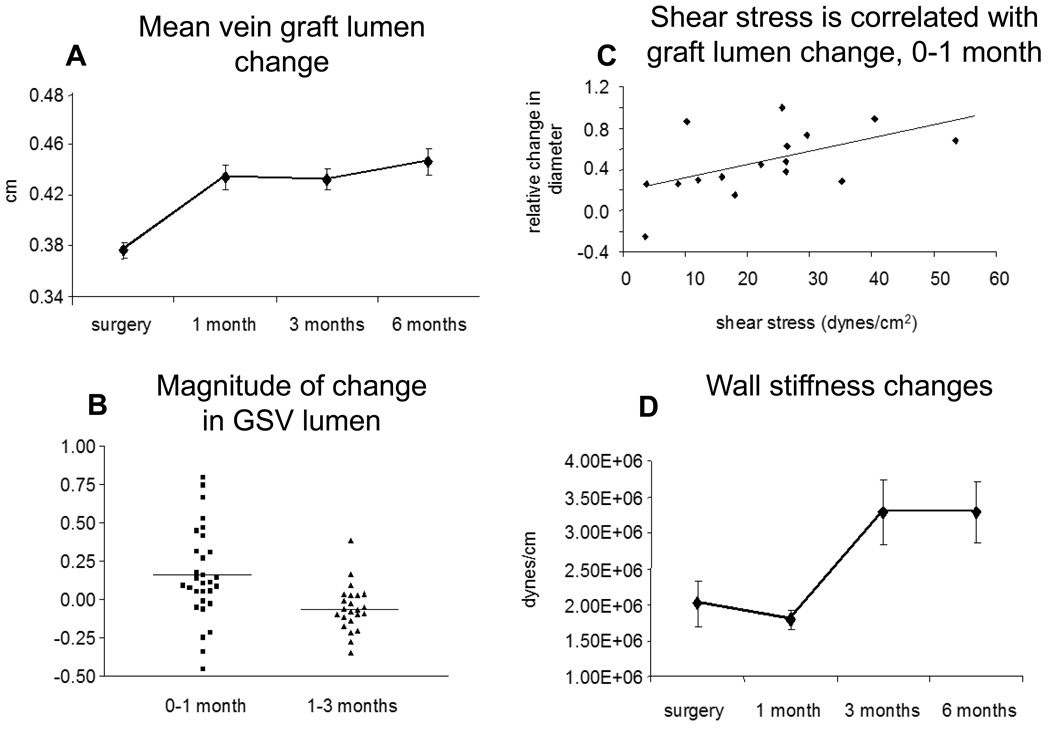
More recently, quantification of human vein graft lumen remodeling has been assessed using high resolution ultrasound. It has been shown that the vein graft lumen, on average, dilates about 22% over the first 6 months of implantation, Figure 1a.[10] The most pronounced period of luminal change was during the first 30 days. However, considerable variability existed in luminal dilation (range −31 to +64%) as evidence by the fact that a substantial minority (28%) of the vein grafts actually decreased in size during this time frame, Figure 1b. Importantly, loss of vein graft lumen size-negative remodeling- during the first month was associated with earlier loss of primary patency compared to those veins which underwent luminal dilation. Therefore it appears that the ability of vein grafts to adequately dilate in the early period of implantation is critical to ensure long-term patency. Not surprisingly, shear stress correlated with early vein graft lumen remodeling, Figure 1c.[10] Those with higher initial shear stress dilated to a greater extent than those with less.
Figure 1.
While the mean bypass graft lumen increases about 22% over the first 6 months following implantation, A, considerable variability exists between individual grafts, B. Early luminal remodeling is correlated with initial shear stress at the time of implantation, C. Temporally distinct from luminal remodeling, the vein graft significantly stiffens between 1 and 3 months following implantation, D. Adapted from reference 10.
Taken together, these data indicate that laminar shear stress acting through endothelium-dependent mechanisms, not only attenuates the development of over-exuberant IH, but also influences vein graft lumen caliber regulation; perhaps, through common mediators such as NO, prostanoids, or hyperpolarizing factors.[22, 50, 54, 55, 90]
The influence of inflammation
It has long been recognized that the implantation of a vein in the arterial circulation is accompanied by a local inflammatory response within the vein graft wall.[27, 91] But the effects of systemic inflammation on the incidence of vein graft stenosis is unknown and has only recently been investigated in a cohort of patients undergoing lower extremity revascularization exclusively with autogenous constructs.[92, 93] In this study, 3 biomarkers of inflammation, high sensitivity C reactive protein (hs-CRP), serum amyloid A (SAA), and fibrinogen, were examined. While all three were all associated with the severity of PAD at the time of presentation, diabetes, and renal failure, only hs-CRP was found to be independently associated with adverse events following bypass surgery, most of which were vein graft related.[92]
Among all inflammatory markers, the high sensitivity CRP test (hsCRP) is the most validated, most widely available, and the only test endorsed by the American Heart Association (AHA) and Center for Disease Control as a cardiovascular risk marker.[94] The AHA has determined the hs-CRP test is appropriate for patients with intermediate risk of cardiovascular events and that values of 0–1 mg/L, 1–3 mg/L, and >3 mg/L would place patients in low, medium and high risk categories respectively. However, PAD is considered to be a coronary heart disease equivalent and these patients are already considered high risk. Indeed, patients undergoing lower extremity bypass surgery have a median hsCRP was 3.25 mg/L and therefore the AHA guidelines would not appropriate for this population.[92] Rather, a cutoff value of hsCRP at 5 mg/L has more potent discriminating ability with respect to adverse outcomes then hsCRP placed in tertiles, according to the AHA recommendations, or another cutoff value.
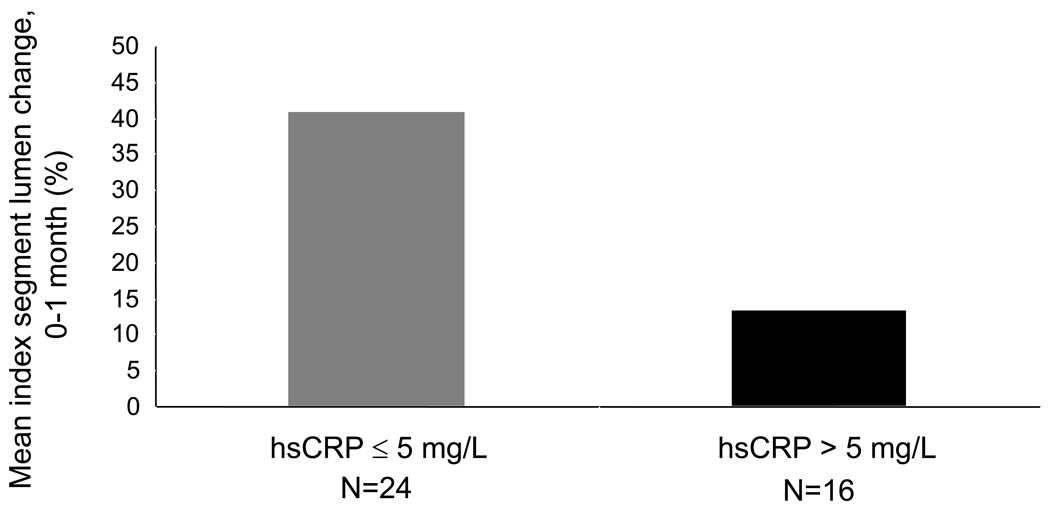
It is still unclear whether CRP is simply a marker of inflammation or a biomodulator of vein graft biology. However, vein grafts in patients with high CRP do not dilate as much as would be expected to shear stress.[93] Dichotomizing patients undergoing bypass surgery with autogenous vein by preoperative plasma CRP concentration, individuals with high CRP levels (>5 mg/L) had significantly less vein graft luminal dilation in the first month after surgery compared to patients with CRP below 5 mg/L, 10% vs. 37%, Figure 2.[93] Further, the significant positive correlation between shear stress and vein graft luminal remodeling was no longer present in the individuals with higher levels of CRP. Hence, patients with high levels of systemic inflammation have impaired ability to positively remodel vein grafts, possibly by impairing endothelial function. These studies are ongoing and the contention that inflammation per se, can be used as a surrogate biomarker for graft-specific failure remains to be proven.
Figure 2.
Vein graft lumen change (percent) from implantation to 1 month in a population undergoing lower extremity bypass grafting for arterial occlusive disease. Luminal measurements were made with high resolution M-mode ultrasound at the same location of the vein graft for the operative and the 1 month assessment. By dichotomizing the population by baseline plasma CRP levels above and below 5 mg/L, disparate early luminal remodeling patterns of the vein graft become apparent, 37% vs 10 %, P=.0072. Thus, patients with high levels of systemic inflammation have impaired ability to positively remodel vein grafts, adapted from reference 93.
However, many lines of evidence suggest that systemic inflammation impairs endothelial function in patients with PAD with significant clinical consequences. C-reactive protein has been shown to be associated with impaired brachial artery flow mediated, endothelium dependent, vasodilation (FMD) in patients with PAD.[95][96][97] Further, in a cohort of patients undergoing vascular surgery, impaired FMD has been shown to be independently associated with adverse 30-day cardiovascular outcomes.[98, 99] Whether or not intensive peri-operative reduction of inflammation such as with high-dose statin therapy would improve clinical outcomes in this population, remains to be studied.
Endothelial Function
Animal studies have demonstrated that veins are capable of relaxation and contraction to vasoactive mediators but to a lesser extent than a similarly sized arteries.[100–104]The thinner media or the histologic arrangement of SMCs and fibrous protein may be responsible. Vascular responses of the human GSV to vasoactive mediators has been studied using Acetylcholine (Ach) or the calcium ionophore A23187 to elicit endothelium-dependent vasodilation.[105–107] In general, the GSV can generate as much contractile force in response to phenylephrine as the internal mammary artery-about 8–10 grams. However, precontracted GSVs exhibit variable relaxation with Ach and a more pronounced relaxation to A23187.[105, 106]
However, the underlying hypothesis that vein graft endothelial function is a critical regulator of final luminal caliber, and ultimately patency, remains to be proven. Veins excised during redo coronary bypass grafting [108] or during revision of lower extremity vein graft stenosis[109] have demonstrated altered smooth muscle cell contractility and the absence of relaxation to Ach.[105, 108, 109] However, these were diseased specimens and the clinical relevance is uncertain. Ku et al studied functioning vein grafts with patent lumens that were 7 months to 12 years in age excised from patients undergoing heart transplantation.[110] Several important observations are noteworthy from this study. First, all vein grafts exhibited vasorelaxation to Ach though considerable variability, −9 to −65% of the pre-contracted state, existed between different grafts. Second, there was significant variability even within a single vein graft segment indicating local heterogeneity in NO production along the course of the vein graft. And third, vein grafts with the most pronounced IH exhibited the least amount of endothelium dependent relaxation. These observations suggest that a functional endothelial lining is regenerated in human vein grafts, that it is not constant along the length of the vein graft, and that IH is at least associated with endothelial impairment.[111]
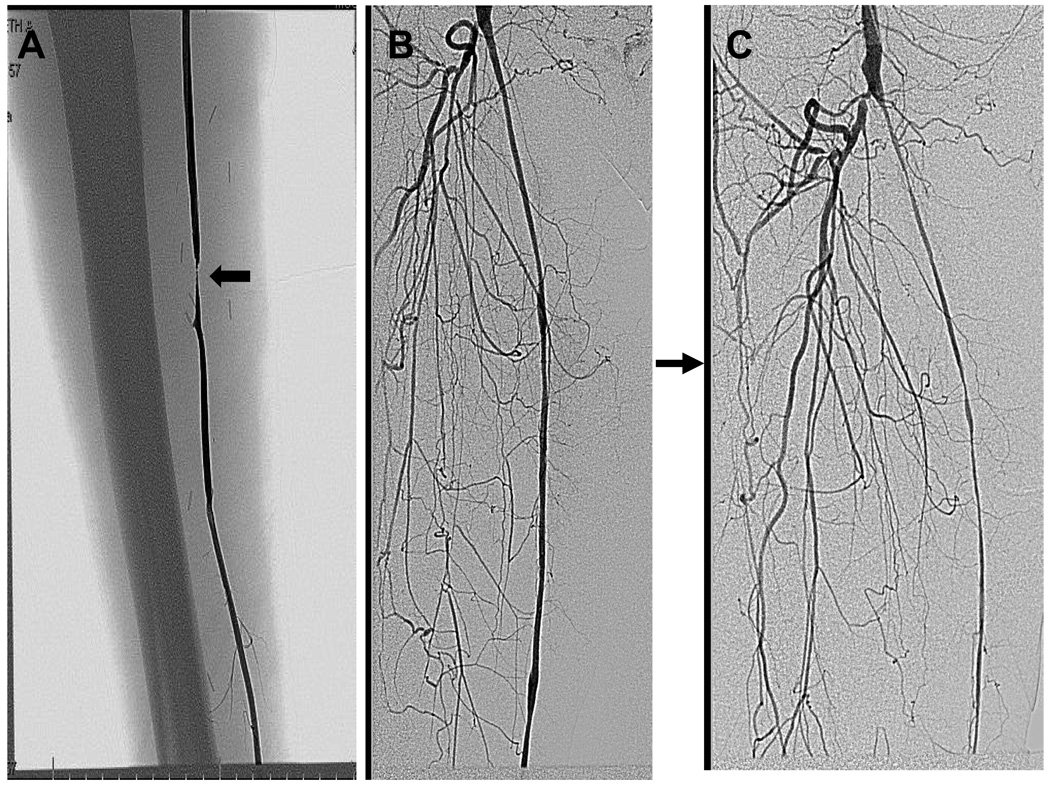
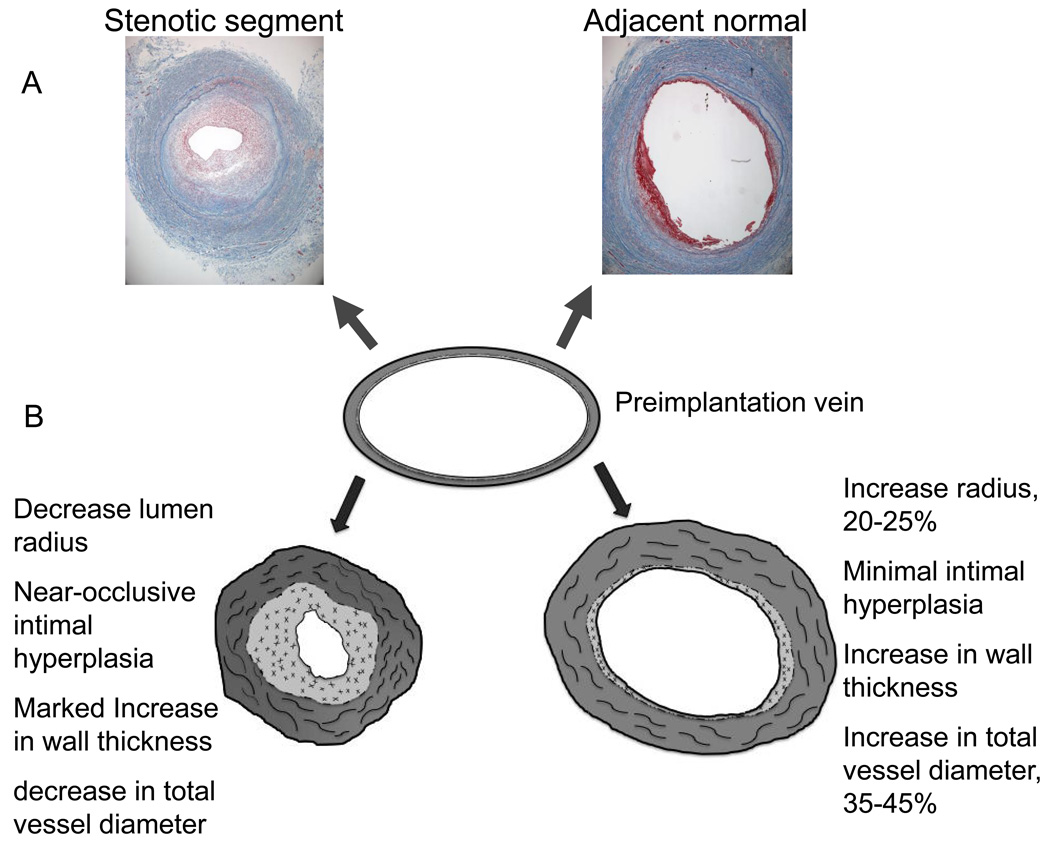
These findings may be one explanation of the focal nature of most vein graft lesions. While diffuse IH occlusive disease may occur in approximately 12% vein grafts, the majority of culprit lesions are focal and often perianastomotic, Figure 3.[15, 16, 35] A focal deficiency in NO from either pre-existing lesions, primary endothelial injury, or turbulent flow with altered shear stress, may result in an area of the graft that fails to sufficiently remodel.[31, 33, 112–114] The cumulative result of the relative deficiency of NO would be increased IH,[79, 115] decreased remodeling,[116] and possibly an increase in collagen production[117, 118] thereby stiffening the vein graft and further reducing the ability to dilate. Hence, the wall would thicken disproportionately to the lumen dilatation producing the clinically familiar focal stenosis within a segment of the vein graft, Figure 4.
Figure 3.
Modes of vein graft failure. While the majority of intimal hyperplasia in vein grafts is focal, a substantial minority (12%) is diffuse. Panel A represents a 6 month old vein graft in a 67 year old white man that developed a mid graft stenosis (arrow) that was successfully treated with a vein patch angioplasty. Panel B represents a 4 month old vein graft in a 77 year Black women undergoing angiography for contralateral limb ischemia. Three months later, she developed diffuse intimal hyperplasia which progressed to vein graft occlusion.
Figure 4.
Histologic sections (10X) of an 8 month old vein graft which developed a focal mid-graft stenosis and underwent open revision with a short interposition graft. The vein was of uniform size and caliber at the time of implantation. The sections were taken approximately 2 cm from one another. The area of stenosis has developed marked intimal hyperplasia and has a smaller area circumscribed by the internal elastic laminae as well as decreased total vessel diameter indicative of negative remodeling of the entire vein graft, A. Theoretical normal and abnormal adaptation patterns of a human lower extremity vein grafts, B. Normally the lumen and wall area increase in the early post-implantation period to produce a lumen diameter to wall thickness ratio of about 7.
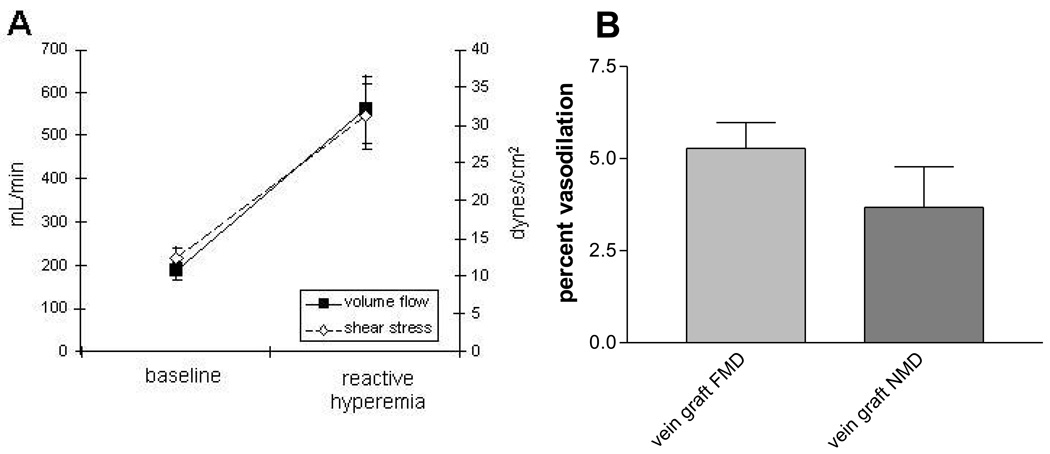
Detection of the more physiologically relevant flow stimulated endothelial function has recently been tested in vivo in a cohort of patients with mature (>1 year since implantation) femoro-popliteal vein grafts that have not underwent revision. The technique was similar to the one employed to test brachial artery FMD which is known to be endothelium dependent and mediated by NO.[119] Assessment of endothelial function by brachial artery flow mediated dilation is a well established research tool which measures small changes in brachial artery diameter in response to an increase in blood flow. By applying an occlusive blood pressure cuff over the proximal calf for 5 minutes, a hyperemic response (increase in blood flow and shear stress) was stimulated in the leg upon its release, figure 5. High resolution ultrasound and post-processing imaging software was then used to carefully measure vein graft blood flow, shear stress, and lumen diameter before and after the hyperemic response and therefore calculate flow mediated dilation (FMD) in the vein graft. Hyperemia resulted in a 233% increase in volumetric flow through the vein graft above baseline in this experiment.[120] This study showed for the first time that vein grafts did dilate to an increase in blood flow. Vein graft FMD was 5.28% ± 3.1% (range 1.99–9.36%), for the entire cohort, Figure 5. The specificity of NO as the mediator of this response was established by intravenously administering the NO inhibitor NG L-monomethyl arginine (LNMMA), during a subsequent test which abolished the FMD response in all vein grafts.
Figure 5.
Flow mediated vasodilation in mature human saphenous vein grafts demonstrates a functional endothelium. Application of an occlusive blood pressure cuff (220 mmHg) to the proximal calf of a cohort of patients undergoing femoro-popliteal bypass grafts for 5 minutes produces an increase in blood flow and shear stress within the graft, A. In this cohort, flow mediated, endothelium dependent vasodilation was 5.3% and nitroglycerin mediated, endothelium independent (0.4 mg sublingual nitroglycerin) dilation was 3.7%.
These studies demonstrate that vein grafts have a biologically active endothelial layer which can be assessed by non-invasive techniques. The relationship of endothelial cell function, the extent and timing of its recovery of function, and patency of the vein graft remains to be seen and is an area of ongoing investigation. It is provocative to speculate that pharmacologic adjuncts, provided locally or remotely, to improve endothelial function and NO availability may decrease IH, improve remodeling, and increase vein graft patency.
The vein graft wall
While shear stress is the dominant regulator of lumen caliber, wall tension is the more critical determinant of wall thickness. Animal models indicate that there is a structurally optimal lumen radius/wall thickness ratio to support arterial pressure with minimal wall stress. Indeed, there is a remarkable consistency of tension per lamellar unit of the aortic media across diverse mammalian species from the mouse to man.[121] In a rabbit vein graft model the ratio of lumen radius to wall thickness (ri/h), initially very high at the time of implantation, approached that of the normal carotid artery by 24 weeks.[56] In humans, vein graft lumen diameter to wall thickness ratio, about 9:1 at the time of implantation, is reduced to 7.4 at 6 months, a value close to that of the superficial femoral artery,Table 1.[122]
Table 1.
Remodeling of Human Saphenous Vein Graft, 0–6 months
| Implantation | 6 mo. | SFA | |
|---|---|---|---|
| LD (mm) | 3.7 | 4.5 | 6.8 |
| Stiffness (Mdynes/cm) |
2.0 | 3.3 | 1.7 |
| Wall thickness (µm) | 410 | 610 | 870 |
| LD/WT | 9.0 | 7.4 | 7.8 |
LD lumen diameter, WT wall thickness
Until recently, accurate in vivo measurement of the vein graft wall has been elusive. However, high resolution, 3-D MRI with superior signal to noise ratio, is capable of resolving structures as small as 300 microns-within the range of the vein graft wall.[8] By simultaneously measuring wall thickness and lumen radius at 1 and 6 months post-implantation, a very strong correlation existed between luminal enlargement and total vessel enlargement.[120] The equation ΔLD = .62(ΔOD) −.02, where LD is lumen diameter and OD is total vein graft diameter, relates changes in luminal diameter to changes in total vessel diameter. This linear regression equation relates what should happen to the lumen of a normal patent bypass graft given a measurable change in the vein graft wall. For example, a 22% increase in the lumen diameter should be accompanied by a 35% increase in the outer diameter of the vein graft. A slope substantially less then .62 would indicate excessive thickening of the wall or insufficient lumen dilation.
Systematic studies of human vein grafts with aid of modern high resolution imaging technology provide insight into vein adaptation in the arterial environment. It has become clear that vein remodeling constitutes changes in luminal and total vessel diameter. The forces and flows responsible for these changes are becoming clear but other factors are involved as well. As can be seen in figure 4, segments of a vein graft with near occlusive IH also had a smaller total vessel diameter compared with a normal adjacent segment. Therefore, in this particular case, the stenosis was due to a combination of negative remodeling and IH. Further studies are needed in this important area.
Clinical science
Translational advances notwithstanding, there has been much improvement in the clinical sciences of vein graft outcomes. Hypothesis-driven, multi-centered trials[6], national data bases with quality measures,[123] and comparative effectiveness trials[124] have replaced single-center retrospective studies and promise to be more reflective of real world practice. These trials, when carefully conducted, can potentially bridge the bench to bedside translational gulf and careful observations of the results can often be hypothesis-generating. For example, PREVENT III alone has resulted in 10 published manuscripts of outcomes in patients with CLI examining quality measures, resource utilization, racial disparities, technical considerations, and risk estimation scores.[6, 89, 125–132]
Long-term results from the BASIL trial emphasize that the more durable reconstruction with a vein graft translates into improved outcomes compared to less invasive alternatives.[124, 133] However, technical factors such as smaller diameter and longer bypass grafts experience more graft related events (GRE) and GREs result in decreased quality of life (QoL) and increased resource utilization emphasizing the importance of patency as an outcome measure.[89, 128] A priori identification of vulnerable grafts through the use of biochemical and imaging biomarkers may provide an opportunity for targeted therapy or at the very least identify patients who would benefit from a more intensive surveillance program. Surrogate endpoints such as novel methods of detection of vein endothelial health (function), IH kinetics, and normal and abnormal remodeling patterns will improve our understanding of the mechanisms of action for the next biologic or new clinical entity to emerge to improve vein graft patency.
Results from clinical trials may have significant biologic implications. For example, gender and racial disparity, often overlooked in single-centered studies due to small sample size, can be examined in multi-centered cohorts. While it is well known that PAD disproportionately affects black patients, less is known about graft specific outcomes. In the PREVENT III database, patients with black race and female gender were at increased risk of graft occlusion and had inferior limb salvage but there were no differences in primary patency compared with white patients.[125] This finding suggests that the disease process is more aggressive, possibly more diffuse, and therefore lower rates of graft salvage by revisions once IH stenosis developed or surgeons were quicker to abandon the graft. An altered biologic healing response to the implanted graft, possibly through increased inflammation, altered thrombogenicity, or differential endothelial cell responses may be responsible.[134–138] Thus observations from clinical trials such as these can directly stimulate basic scientific efforts.
Conclusion
Beyond the risk of limb loss, decreased graft patency results in increased resource utilization[128] and significantly impacts quality of life.[127] Our current pharmacopoeia has not made any significant impact on the natural history of vein graft stenosis emphasizing the need for a better mechanistic understanding of graft failure.[139] Animal models and mathematical constructs should incorporate structural as well as functional changes of the maturing vein graft. Understanding the relative contributions of IH and remodeling are not simply academic as molecular therapeutics targeted at IH will likely not improve remodeling. Multidisciplinary translational research programs leveraging recent advances in biomedical imaging, molecular technology, and genetic engineering will likely dramatically increase our understanding of this vexing problem.
Acknowledgment
I would like to thank Michael Belkin MD and Michael S. Conte, MD for continued support and mentorship.
Supported by funding from the National Heart, Lung, and Blood Institute R01 HL75771, K23 HL 92163, and the American Vascular Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competition of interest: none
References
- 1.Rosamond W, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. Jama. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JA, et al. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103(12):1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS, et al. Genetic interventions for vein bypass graft disease: a review. J Vasc Surg. 2002;36(5):1040–1052. doi: 10.1067/mva.2002.129112. [DOI] [PubMed] [Google Scholar]
- 5.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98(1):82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 6.Conte MS, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 7.Kaneda H, et al. Mechanisms of lumen narrowing of saphenous vein bypass grafts 12 months after implantation: an intravascular ultrasound study. Am Heart J. 2006;151(3):726–729. doi: 10.1016/j.ahj.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Mitsouras D, et al. High-resolution peripheral vein bypass graft wall studies using high sampling efficiency inner volume 3D FSE. Magn Reson Med. 2008;59(3):650–654. doi: 10.1002/mrm.21359. [DOI] [PubMed] [Google Scholar]
- 9.Rybicki FJ, et al. Lower extremity peripheral vein bypass graft wall thickness changes demonstrated at 1 and 6 months after surgery with ultra-high spatial resolution black blood inner volume three-dimensional fast spin echo magnetic resonance imaging. Int J Cardiovasc Imaging. 2008;24(5):529–533. doi: 10.1007/s10554-007-9287-8. [DOI] [PubMed] [Google Scholar]
- 10.Owens CD, et al. Early biomechanical changes in lower extremity vein grafts--distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;44(4):740–746. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 12.Milroy CM, et al. Histological appearances of the long saphenous vein. J Pathol. 1989;159(4):311–316. doi: 10.1002/path.1711590408. [DOI] [PubMed] [Google Scholar]
- 13.Canham PB, Finlay HM, Boughner DR. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc Res. 1997;34(3):557–567. doi: 10.1016/s0008-6363(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 14.Davies AH, et al. Vein compliance: a preoperative indicator of vein morphology and of veins at risk of vascular graft stenosis. Br J Surg. 1992;79(10):1019–1021. doi: 10.1002/bjs.1800791011. [DOI] [PubMed] [Google Scholar]
- 15.Mills JL, Fujitani RM, Taylor SM. The characteristics and anatomic distribution of lesions that cause reversed vein graft failure: a five-year prospective study. J Vasc Surg. 1993;17(1):195–204. doi: 10.1067/mva.1993.42796. discussion 204-6. [DOI] [PubMed] [Google Scholar]
- 16.Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9(1):7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, et al. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95(12):2684–2693. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, et al. Origin of extracellular matrix synthesis during coronary repair. Circulation. 1997;95(4):997–1006. doi: 10.1161/01.cir.95.4.997. [DOI] [PubMed] [Google Scholar]
- 19.Sartore S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 20.Zalewski A, Shi Y, Johnson AG. Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts, and beyond? Circ Res. 2002;91(8):652–655. doi: 10.1161/01.res.0000038996.97287.9a. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, et al. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24(3):470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 22.Kalra M, Miller VM. Early remodeling of saphenous vein grafts: proliferation, migration and apoptosis of adventitial and medial cells occur simultaneously with changes in graft diameter and blood flow. J Vasc Res. 2000;37(6):576–584. doi: 10.1159/000054091. [DOI] [PubMed] [Google Scholar]
- 23.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007;45 Suppl A:A64–A73. doi: 10.1016/j.jvs.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Davies MG, et al. The integrity of experimental vein graft endothelium--implications on the etiology of early graft failure. Eur J Vasc Surg. 1993;7(2):156–165. doi: 10.1016/s0950-821x(05)80756-x. [DOI] [PubMed] [Google Scholar]
- 25.Bratt J, Palmblad J. Cytokine-induced neutrophil-mediated injury of human endothelial cells. J Immunol. 1997;159(2):912–918. [PubMed] [Google Scholar]
- 26.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84(7):2068–2101. [PubMed] [Google Scholar]
- 27.Eslami MH, et al. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001;34(5):923–929. doi: 10.1067/mva.2001.118590. [DOI] [PubMed] [Google Scholar]
- 28.Gangadharan SP, et al. Monocyte adhesion to balloon-injured arteries: the influence of endothelial cell seeding. J Vasc Surg. 2001;33(6):1247–1254. doi: 10.1067/mva.2001.114211. [DOI] [PubMed] [Google Scholar]
- 29.Chesebro JH, et al. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med. 1982;307(2):73–78. doi: 10.1056/NEJM198207083070201. [DOI] [PubMed] [Google Scholar]
- 30.Chesebro JH, et al. Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations. N Engl J Med. 1984;310(4):209–214. doi: 10.1056/NEJM198401263100401. [DOI] [PubMed] [Google Scholar]
- 31.Tsui JC, et al. Localization of nitric oxide synthase in saphenous vein grafts harvested with a novel "no-touch" technique: potential role of nitric oxide contribution to improved early graft patency rates. J Vasc Surg. 2002;35(2):356–362. doi: 10.1067/mva.2002.121072. [DOI] [PubMed] [Google Scholar]
- 32.Quist WC, LoGerfo FW. Prevention of smooth muscle cell phenotypic modulation in vein grafts: a histomorphometric study. J Vasc Surg. 1992;16(2):225–231. [PubMed] [Google Scholar]
- 33.Hinokiyama K, et al. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg. 2006;82(4):1458–1464. doi: 10.1016/j.athoracsur.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 34.Odashiro T, et al. Comparison of endothelial function between in situ and reversed vein graft: differences in endothelium-dependent responses. Surgery. 1995;117(2):179–188. doi: 10.1016/s0039-6060(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 35.Bandyk DF, et al. Monitoring functional patency of in situ saphenous vein bypasses: the impact of a surveillance protocol and elective revision. J Vasc Surg. 1989;9(2):286–296. [PubMed] [Google Scholar]
- 36.Leather RP, et al. Resurrection of the in situ saphenous vein bypass. 1000 cases later. Ann Surg. 1988;208(4):435–442. doi: 10.1097/00000658-198810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fogle MA, et al. A comparison of in situ and reversed saphenous vein grafts for infrainguinal reconstruction. J Vasc Surg. 1987;5(1):46–52. [PubMed] [Google Scholar]
- 38.Haudenschild C, et al. Protection of endothelium in vessel segments excised for grafting. Circulation. 1981;64(2 Pt 2):II101–II107. [PubMed] [Google Scholar]
- 39.Davies MG, Hagen PO. Influence of perioperative storage solutions on long-term vein graft function and morphology. Ann Vasc Surg. 1994;8(2):150–157. doi: 10.1007/BF02018863. [DOI] [PubMed] [Google Scholar]
- 40.Brody WR, Angeli WW, Kosek JC. Histologic fate of the venous coronary artery bypass in dogs. Am J Pathol. 1972;66(1):111–130. [PMC free article] [PubMed] [Google Scholar]
- 41.Davies MG, et al. Regression of intimal hyperplasia with restoration of endothelium-dependent relaxing factor-mediated relaxation in experimental vein grafts. Surgery. 1993;114(2):258–270. discussion 270-1. [PubMed] [Google Scholar]
- 42.Fann JI, et al. The reversibility of canine vein-graft arterialization. Circulation. 1990;82 5 Suppl:IV9–IV18. [PubMed] [Google Scholar]
- 43.Sterpetti AV, et al. Progression and regression of myointimal hyperplasia in experimental vein grafts depends on platelet-derived growth factor and basic fibroblastic growth factor production. J Vasc Surg. 1996;23(4):568–575. doi: 10.1016/s0741-5214(96)80034-6. [DOI] [PubMed] [Google Scholar]
- 44.Davies MG, et al. Time course of the regression of intimal hyperplasia in experimental vein grafts. Cardiovasc Pathol. 1999;8(3):161–168. doi: 10.1016/s1054-8807(98)00029-5. [DOI] [PubMed] [Google Scholar]
- 45.Morinaga K, et al. Development and regression of intimal thickening of arterially transplanted autologous vein grafts in dogs. J Vasc Surg. 1987;5(5):719–730. doi: 10.1067/mva.1987.avs0050719. [DOI] [PubMed] [Google Scholar]
- 46.Morinaga K, et al. Effect of wall shear stress on intimal thickening of arterially transplanted autogenous veins in dogs. J Vasc Surg. 1985;2(3):430–433. [PubMed] [Google Scholar]
- 47.Cambria RA, et al. Chronic changes in blood flow alter endothelium-dependent responses in autogenous vein grafts in dogs. J Vasc Surg. 1994;20(5):765–773. doi: 10.1016/s0741-5214(94)70164-4. [DOI] [PubMed] [Google Scholar]
- 48.Galt SW, et al. Differential response of arteries and vein grafts to blood flow reduction. J Vasc Surg. 1993;17(3):563–570. [PubMed] [Google Scholar]
- 49.Kohler TR, et al. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ Res. 1991;69(6):1557–1565. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- 50.Kraiss LW, et al. Shear stress regulates smooth muscle proliferation and neointimal thickening in porous polytetrafluoroethylene grafts. Arterioscler Thromb. 1991;11(6):1844–1852. doi: 10.1161/01.atv.11.6.1844. [DOI] [PubMed] [Google Scholar]
- 51.Meyerson SL, et al. The effects of extremely low shear stress on cellular proliferation and neointimal thickening in the failing bypass graft. J Vasc Surg. 2001;34(1):90–97. doi: 10.1067/mva.2001.114819. [DOI] [PubMed] [Google Scholar]
- 52.Geary RL, et al. Time course of flow-induced smooth muscle cell proliferation and intimal thickening in endothelialized baboon vascular grafts. Circ Res. 1994;74(1):14–23. doi: 10.1161/01.res.74.1.14. [DOI] [PubMed] [Google Scholar]
- 53.Dobrin PB, Littooy FN, Endean ED. Mechanical factors predisposing to intimal hyperplasia and medial thickening in autogenous vein grafts. Surgery. 1989;105(3):393–400. [PubMed] [Google Scholar]
- 54.Dimmeler S, et al. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83(3):334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 55.Fisslthaler B, et al. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168(1):81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 56.Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987;5(1):126–136. [PubMed] [Google Scholar]
- 57.Tran-Son-Tay R, et al. An experiment-based model of vein graft remodeling induced by shear stress. Ann Biomed Eng. 2008;36(7):1083–1091. doi: 10.1007/s10439-008-9495-y. [DOI] [PubMed] [Google Scholar]
- 58.Tran-Son-Tay R, et al. A model of vein graft intimal hyperplasia; Conf Proc IEEE Eng Med Biol Soc, 2007; 2007. pp. 5807–5810. [DOI] [PubMed] [Google Scholar]
- 59.Wong AP, et al. Expansive remodeling in venous bypass grafts: novel implications for vein graft disease. Atherosclerosis. 2008;196(2):580–589. doi: 10.1016/j.atherosclerosis.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 60.Ge J, et al. Does remodeling occur in the diseased human saphenous vein bypass grafts? An intravascular ultrasound study. Int J Card Imaging. 1999;15(4):295–300. doi: 10.1023/a:1006125205217. [DOI] [PubMed] [Google Scholar]
- 61.Nishioka T, et al. Absence of focal compensatory enlargement or constriction in diseased human coronary saphenous vein bypass grafts. An intravascular ultrasound study. Circulation. 1996;93(4):683–690. doi: 10.1161/01.cir.93.4.683. [DOI] [PubMed] [Google Scholar]
- 62.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 63.O'Brien JE, Jr, et al. Early injury to the media after saphenous vein grafting. Ann Thorac Surg. 1998;65(5):1273–1278. doi: 10.1016/s0003-4975(98)00175-1. [DOI] [PubMed] [Google Scholar]
- 64.Hong MK, et al. Intravascular ultrasound assessment of the presence of vascular remodeling in diseased human saphenous vein bypass grafts. Am J Cardiol. 1999;84(9):992–998. doi: 10.1016/s0002-9149(99)00486-5. [DOI] [PubMed] [Google Scholar]
- 65.Canos DA, et al. Clinical, angiographic, and intravascular ultrasound characteristics of early saphenous vein graft failure. J Am Coll Cardiol. 2004;44(1):53–56. doi: 10.1016/j.jacc.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 66.Mendelsohn FO, et al. In vivo assessment by intravascular ultrasound of enlargement in saphenous vein bypass grafts. Am J Cardiol. 1995;76(14):1066–1069. doi: 10.1016/s0002-9149(99)80299-9. [DOI] [PubMed] [Google Scholar]
- 67.Leotta DF, et al. Remodeling in peripheral vein graft revisions: serial study with three-dimensional ultrasound imaging. J Vasc Surg. 2003;37(4):798–807. doi: 10.1067/mva.2003.137. [DOI] [PubMed] [Google Scholar]
- 68.Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vasc Med. 2008;13(1):63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- 69.Rodbard S. Vascular caliber. Cardiology. 1975;60(1):4–49. doi: 10.1159/000169701. [DOI] [PubMed] [Google Scholar]
- 70.Murray CD. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc Natl Acad Sci U S A. 1926;12(3):207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vita JA, et al. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation. 2008;117(24):3126–3133. doi: 10.1161/CIRCULATIONAHA.108.778472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zarins CK, et al. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5(3):413–420. [PubMed] [Google Scholar]
- 73.Glagov S, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 74.Back MR, et al. Expected flow parameters within hemodialysis access and selection for remedial intervention of nonmaturing conduits. Vasc Endovascular Surg. 2008;42(2):150–158. doi: 10.1177/1538574407312648. [DOI] [PubMed] [Google Scholar]
- 75.Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89(6):2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- 76.Skelly CL, et al. The hemodynamics of vein grafts: measurement and meaning. Ann Vasc Surg. 2001;15(1):110–122. doi: 10.1007/s100160010019. [DOI] [PubMed] [Google Scholar]
- 77.Buga GM, et al. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 78.Frangos JA, et al. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 79.Garanich JS, Pahakis M, Tarbell JM. Shear stress inhibits smooth muscle cell migration via nitric oxide-mediated downregulation of matrix metalloproteinase-2 activity. Am J Physiol Heart Circ Physiol. 2005;288(5):H2244–H2252. doi: 10.1152/ajpheart.00428.2003. [DOI] [PubMed] [Google Scholar]
- 80.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol. 1993;264(1 Pt 2):H150–H156. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- 82.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 83.Stanley JC, Ernst CB, Fry WJ. Fate of 100 aortorenal vein grafts: characteristics of late graft expansion, aneurysmal dilatation, and stenosis. Surgery. 1973;74(6):931–944. [PubMed] [Google Scholar]
- 84.Upchurch GR, Jr, et al. Improved graft patency and altered remodeling in infrainguinal vein graft reconstruction for aneurysmal versus occlusive disease. J Vasc Surg. 1999;29(6):1022–1030. doi: 10.1016/s0741-5214(99)70243-0. [DOI] [PubMed] [Google Scholar]
- 85.Dean RH, et al. Saphenous vein aortorenal bypass grafts: Serial arteriographic study. Ann Surg. 1974;180(4):469–478. doi: 10.1097/00000658-197410000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szilagyi DE, Smith RF, Elliott JP. Venous Autografts In Femoropopliteal Arterioplasty. Observations In The Treatment Of Occlusive Disease. Arch Surg. 1964;89:113–125. doi: 10.1001/archsurg.1964.01320010115011. [DOI] [PubMed] [Google Scholar]
- 87.Fillinger MF, et al. Vein adaptation to the hemodynamic environment of infrainguinal grafts. J Vasc Surg. 1994;19(6):970–978. doi: 10.1016/s0741-5214(94)70208-x. discussion 978-9. [DOI] [PubMed] [Google Scholar]
- 88.Idu MM, et al. Factors influencing the development of vein-graft stenosis and their significance for clinical management. Eur J Vasc Endovasc Surg. 1999;17(1):15–21. doi: 10.1053/ejvs.1998.0676. [DOI] [PubMed] [Google Scholar]
- 89.Schanzer A, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46(6):1180–1190. doi: 10.1016/j.jvs.2007.08.033. discussion 1190. [DOI] [PubMed] [Google Scholar]
- 90.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;92(11):3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 91.Ozaki CK. Cytokines and the early vein graft: strategies to enhance durability. J Vasc Surg. 2007;45 Suppl A:A92–A98. doi: 10.1016/j.jvs.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Owens CD, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45(1):2–9. doi: 10.1016/j.jvs.2006.08.048. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Owens CD, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47(6):1235–1242. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fortmann SP, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the population science discussion group. Circulation. 2004;110(25):e554–e559. doi: 10.1161/01.CIR.0000148982.95775.BF. [DOI] [PubMed] [Google Scholar]
- 95.Brevetti G, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38(2):374–379. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 96.Fichtlscherer S, et al. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102(9):1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 97.Brevetti G, et al. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108(17):2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 98.Gokce N, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41(10):1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 99.Gokce N, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105(13):1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 100.Seidel CL, LaRochelle J. Venous and arterial endothelia: different dilator abilities in dog vessels. Circ Res. 1987;60(4):626–630. doi: 10.1161/01.res.60.4.626. [DOI] [PubMed] [Google Scholar]
- 101.Ignarro LJ, Buga GM, Chaudhuri G. EDRF generation and release from perfused bovine pulmonary artery and vein. Eur J Pharmacol. 1988;149(1–2):79–88. doi: 10.1016/0014-2999(88)90045-3. [DOI] [PubMed] [Google Scholar]
- 102.Vanhoutte PM, Miller VM. Heterogeneity of endothelium-dependent responses in mammalian blood vessels. J Cardiovasc Pharmacol. 1985;7 Suppl 3:S12–S23. doi: 10.1097/00005344-198500073-00002. [DOI] [PubMed] [Google Scholar]
- 103.Rubanyi GM, Vanhoutte PM. Heterogeneity of endothelium-dependent responses to acetylcholine in canine femoral arteries and veins. Separation of the role played by endothelial and smooth muscle cells. Blood Vessels. 1988;25(2):75–81. [PubMed] [Google Scholar]
- 104.De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- 105.Luscher TF, et al. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988;319(8):462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz LB, et al. Saphenous vein endothelium-dependent relaxation in patients with peripheral vascular disease. Ann Vasc Surg. 1992;6(5):425–432. doi: 10.1007/BF02006997. [DOI] [PubMed] [Google Scholar]
- 107.Ignarro LJ, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cross KS, et al. Long-term human vein graft contractility and morphology: a functional and histopathological study of retrieved coronary vein grafts. Br J Surg. 1994;81(5):699–705. doi: 10.1002/bjs.1800810524. [DOI] [PubMed] [Google Scholar]
- 109.Park TC, et al. Human saphenous vein grafts explanted from the arterial circulation demonstrate altered smooth-muscle and endothelial responses. J Vasc Surg. 1993;18(1):61–68. doi: 10.1067/mva.1993.42071. discussion 68-9. [DOI] [PubMed] [Google Scholar]
- 110.Ku DD, Caulfield JB, Kirklin JK. Endothelium-dependent responses in long-term human coronary artery bypass grafts. Circulation. 1991;83(2):402–411. doi: 10.1161/01.cir.83.2.402. [DOI] [PubMed] [Google Scholar]
- 111.Davies MG, Berkowitz DE, Hagen PO. Constitutive nitric oxide synthase is expressed and nitric oxide-mediated relaxation is preserved in retrieved human aortocoronary vein grafts. J Surg Res. 1995;58(6):732–738. doi: 10.1006/jsre.1995.1116. [DOI] [PubMed] [Google Scholar]
- 112.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292(3):H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 113.Dashwood MR, et al. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80(3):939–944. doi: 10.1016/j.athoracsur.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 114.Sasaki Y, et al. Role of endothelial cell denudation and smooth muscle cell dedifferentiation in neointimal formation of human vein grafts after coronary artery bypass grafting: therapeutic implications. Heart. 2000;83(1):69–75. doi: 10.1136/heart.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palumbo R, et al. Shear stress downregulation of platelet-derived growth factor receptor-beta and matrix metalloprotease-2 is associated with inhibition of smooth muscle cell invasion and migration. Circulation. 2000;102(2):225–230. doi: 10.1161/01.cir.102.2.225. [DOI] [PubMed] [Google Scholar]
- 116.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 117.Rizvi MA, Myers PR. Nitric oxide modulates basal and endothelin-induced coronary artery vascular smooth muscle cell proliferation and collagen levels. J Mol Cell Cardiol. 1997;29(7):1779–1789. doi: 10.1006/jmcc.1996.0480. [DOI] [PubMed] [Google Scholar]
- 118.Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995;76(2):305–309. doi: 10.1161/01.res.76.2.305. [DOI] [PubMed] [Google Scholar]
- 119.Corretti MC, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 120.Owens CD, Whitmore A, Mitsourus D, Kim JM, Rybicki F, Conte MS. Radial Wall Stress is the Primary Biomechanical Force Determining Wall Thickness In Human Lower Extremity Vein Grafts: Assessment of Wall Characteristics and Remodeling Patterns Using Ultra-High Resolution Magnetic Resonance Imaging (MRI); Society for Vascular Surgery Vascular Annual Meeting; San Diego, CA: 2008. [Google Scholar]
- 121.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20(1):99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 122.Jacot JG, et al. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. J Vasc Surg. 2004;39(3):547–555. doi: 10.1016/j.jvs.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 123.Hutter MM, et al. Comparison of risk-adjusted 30-day postoperative mortality and morbidity in Department of Veterans Affairs hospitals and selected university medical centers: vascular surgical operations in men. J Am Coll Surg. 2007;204(6):1115–1126. doi: 10.1016/j.jamcollsurg.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 124.Adam DJ, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen LL, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119(1):123–130. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nguyen LL, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007;46(6):1191–1197. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nguyen LL, et al. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44(5):977–983. doi: 10.1016/j.jvs.2006.07.015. discussion 983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen LL, et al. Resource utilization in the treatment of critical limb ischemia: The effect of tissue loss, comorbidities, and graft-related events. J Vasc Surg. 2006;44(5):971–975. doi: 10.1016/j.jvs.2006.07.035. discussion 975-6. [DOI] [PubMed] [Google Scholar]
- 129.Berceli SA, et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. J Vasc Surg. 2007;46(6):1173–1179. doi: 10.1016/j.jvs.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 130.Schanzer A, et al. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(4):774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 131.Schanzer A, et al. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48(6):1464–1471. doi: 10.1016/j.jvs.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Conte MS, et al. Risk factors, medical therapies and perioperative events in limb salvage surgery: observations from the PREVENT III multicenter trial. J Vasc Surg. 2005;42(3):456–464. doi: 10.1016/j.jvs.2005.05.001. discussion 464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bradbury AW. Long-Term (5-Year) Results Of BASIL Trial Show "Open Surgery First" To Be Superior To "Endovascular Treatment First" For Critical Limb Ischemia. [cited 2009 June 27];2008 http://www.veithsymposium.org/pdf/aim/1579.pdf.
- 134.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 135.Grubbs AL, Anstadt MP, Ergul A. Saphenous vein endothelin system expression and activity in African American patients. Arterioscler Thromb Vasc Biol. 2002;22(7):1122–1127. doi: 10.1161/01.atv.0000023160.67766.f0. [DOI] [PubMed] [Google Scholar]
- 136.Albert MA, Ridker PM. Inflammatory biomarkers in African Americans: a potential link to accelerated atherosclerosis. Rev Cardiovasc Med. 2004;5 Suppl 3:S22–S27. [PubMed] [Google Scholar]
- 137.Khera A, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3):464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 138.Geary RL, et al. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27(1):96–106. doi: 10.1016/s0741-5214(98)70296-4. discussion 106-8. [DOI] [PubMed] [Google Scholar]
- 139.Conte MS, et al. Impact of increasing comorbidity on infrainguinal reconstruction: a 20-year perspective. Ann Surg. 2001;233(3):445–452. doi: 10.1097/00000658-200103000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]