Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (Cftr) that impair its role as an apical chloride channel that supports bicarbonate transport1. Patients with CF exhibit retained, thickened mucus that plugs airways and obstructs luminal organs2 as well as numerous other abnormalities that include inflammation of affected organs1, alterations in lipid metabolism3 and insulin resistance4. Here we demonstrate that colonic epithelial cells and lungs from Cftr-deficient mice exhibit a defect in peroxisome proliferator-activated receptor γ (PPARγ) function that contributes to a pathological program of gene expression. Lipidomic analysis of colonic epithelial cells suggests that this defect results in part from reduced levels of the endogenous PPARγ ligand 15-keto-PGE2. Treatment of CFTR-deficient mice with the synthetic PPARγ ligand rosiglitazone (Ro) partially normalizes the altered gene expression pattern associated with Cftr deficiency and reduces disease severity. Ro has no effect on chloride secretion in the colon, but increases expression of carbonic anhydrase 4 and 2, increases bicarbonate secretion and reduces mucus retention. These studies reveal a reversible defect in PPARγ signaling in Cftr-deficient cells that can be pharmacologically corrected to ameliorate the severity of the cystic fibrosis phenotype in mice.
Cftr knock-out (Cftrtm1Unc, hereafter Cftr−/−) mice accumulate mucus in the small bowel and colon and die from intestinal or colonic obstruction within the first 6 weeks of life5. Survival of the Cftr−/− mouse is partially improved by providing a low-residue elemental liquid diet (Peptamen)6 or electrolyte lavage solution (GoLYTELY)7. We performed transcriptome analysis of colonic epithelial cells isolated from wild-type and Cftr−/− mice, maintaining both genotypes on GoLYTELY to exclude secondary consequences of obstruction in the Cftr−/− mice. GeneOntology analysis of genes that were down-regulated in Cftr−/− cells revealed significant enrichment for genes involved in lipid metabolism (Supplementary Fig. 1a)8, and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis suggesting a defect in PPAR–dependent gene expression (p<0.05). A corresponding set of genes was up-regulated in Cftr−/− cells, and these genes were enriched for functional annotations linked to inflammatory responses, despite an absence of inflammatory cells in Cftr−/− colons by standard H&E staining. Analysis of PPAR isoforms in the intestinal tract revealed high levels of PPARγ in the colon of wild-type mice (Fig. 1a). While a previous study reported a decrease in PPARγ expression in the intact colons of Cftr−/− mice9, we found that PPARγ mRNA (Fig. 1a) and protein levels (Fig. 3d) were similar in wild-type and Cftr−/− colonic epithelial cells derived from mice maintained on GoLYTELY. We therefore tested the possibility that administration of a synthetic PPARγ agonist might partially restore the abnormal pattern of gene expression observed in Cftr−/− cells. Consistent with this hypothesis, transcriptome analysis of wild-type and Cftr−/− colonic epithelial cells treated with the synthetic PPARγ agonist rosiglitazone (Ro) revealed that Ro treatment increased the expression of 107 of the 388 transcripts that were down-regulated in Cftr−/− compared to wild-type cells, while reducing the expression of 75 of the 328 up-regulated genes (Supplementary Fig. 1b and Supplementary Tables 1 and 2).
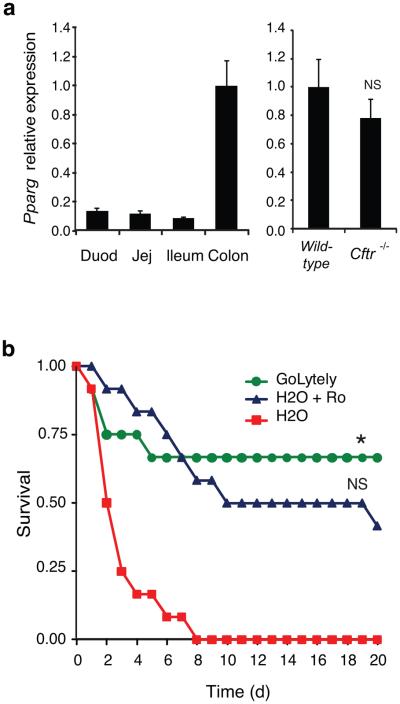
Figure 1. Effect of PPARγ activation on the CF intestinal phenotype in mice.
(a) Left panel; Q-PCR analysis of Pparg mRNA in the indicated regions of the intestinal tract (n=4). Right panel; Q-PCR analysis of Pparg mRNA in colonic epithelial cells from wild-type and Cftr−/− mice (n=8, P=0.17). Error bars represent s.e.m. Expression is normalized to GAPDH and expressed relative to wild-type cells. (b) Kaplan-Meier analysis of 4-week old Cftr−/− mice maintained on GoLYTELY, H2O plus Ro or H2O alone. Log-Rank test, *P<0.001 comparing H2O plus Ro to H2O alone and p<0.60 comparing H2O plus Ro to GoLYTELY (n=12 mice per group).
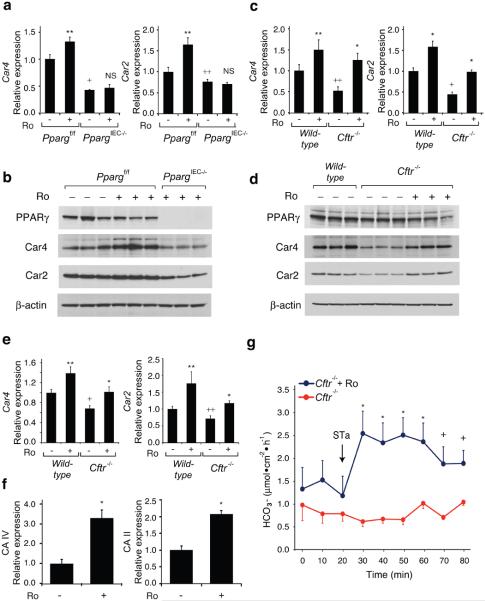
Figure 3. Effect of Ro on Car4 and Car2 expression and bicarbonate transport.
(a) Q-PCR analysis of Car4 and Car2 mRNAs in colonic epithelial cells derived from Ppargf/f and PpargIEC−/− mice treated with Ro or maintained on a control diet (n=6 mice per group). RNA levels are normalized to GAPDH and expressed relative to wild-type cells. (b) Western blot of PPARγ, Car4, Car2 and β-actin proteins using colonic epithelial cell lysates derived from Ppargf/f and PpargIEC−/− mice. (c) Q-PCR analysis Car4 and Car2 mRNAs in colonic epithelial cells derived from wild-type or Cftr−/− mice treated with Ro or maintained on a control diet (n=10 mice per group). In panels a and c, values are presented as means ± s.e.m. For mice treated with Ro, *P<0.01 and **P<0.05 versus untreated mice (similar genotype). For untreated knock-out mice (Cftr−/− or PpargIEC−/−), +P<0.01 and ++P<0.05 versus wild-type or Ppargf/f controls. (d) Western blot of PPARγ, Car4, Car2 and β-actin proteins using colonic epithelial cell lysates derived from wild-type and Cftr−/− mice. (e) Q-PCR analysis of Car4 and Car2 mRNAs in lung derived from wild-type and Cftr−/− mice treated with Ro or control diet (n=6 mice per group). (f) Effect of Ro treatment on expression of the human CA IV and CA II mRNAs in Calu-3 cells (* P<0.01, n=4 replicates). Values are means ± s.e.m. (g) Bicarbonate secretion in response to STa (10−7 M) as measured by pH-stat titration (n=4 mice per group, error bars represent s.e.m., *P<0.01 and +P<0.05).
We investigated whether the effect of Ro treatment could have a functional consequence by randomizing 4-week old Cftr−/− mice to receive either GoLYTELY and standard chow, water and standard chow, or water and standard chow mixed with Ro (20 mg/kg/d). Cftr−/− mice receiving Ro were significantly less likely to suffer from bowel obstruction than mice receiving control chow without Ro, resulting in an increased survival rate (Log-Rank test, p<0.001, Fig. 1b). Several possibilities may account for treatment failure in the 50% of mice going on to develop obstruction, including subclinical obstruction in this subset of mice prior to study onset that would result in reduced feeding behavior and drug consumption. Consistent with this, mortality rates were similar for mice treated with GoLYTELY or Ro.
Quantitative PCR confirmed down-regulation of known PPAR target genes, including Acaa1b, Angptl4, Mgll and Hmgcs2 10-12, in Cftr−/− cells and restoration of their expression by Ro treatment (Fig. 2a and Supplementary Fig. 2a). Conversely, genes up-regulated in Cftr−/− cells, including Cxcl1, Cxcl2, Pap and Reg3g, were suppressed by Ro (Supplementary Fig. 3a). We generated mice lacking PPARγ in the intestinal epithelium to establish the receptor specificity of the effects of Ro in the gastrointestinal tract. We mated mice carrying the exon 2 floxed allele of Pparg with mice in which the villin 1 promoter drives intestinal-epithelial cell (IEC)-specific expression of Cre recombinase13, resulting in more than 95% recombination of the floxed Pparg locus in colonic epithelial cells with a corresponding loss of PPARγ protein (Supplementary Fig. 2b). We refer to Vil1-Cre− Ppargf/f mice as Ppargf/f and Vil1-Cre+ Ppargf/f mice as PpargIEC−/−. Similar mice were previously described and noted to exhibit accumulation of Alcian blue positive mucins in the colon and increased sensitivity to chemical colitis14.
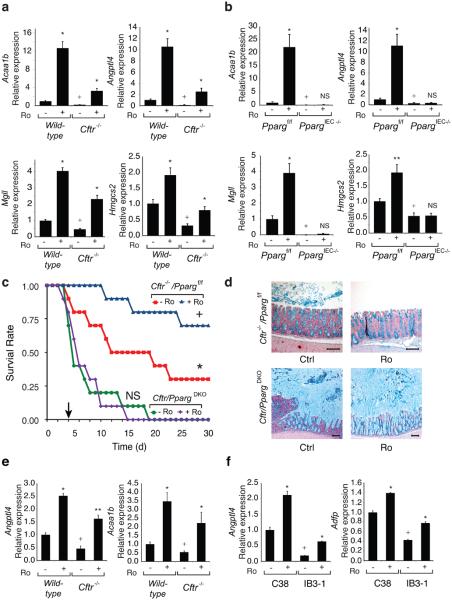
Figure 2. PPARγ function and CFTR intestinal phenotype.
(a) Q-PCR analysis of Acaa1b, Angptl4, Mgll and Hmgcs2 mRNAs in colonic epithelial cells derived from wild-type and Cftr−/− mice treated with Ro (20 mg/kg/d for 5 days) or maintained on a control diet (n=10 mice per group). mRNA levels are normalized to GAPDH and expressed relative to wild-type cells. (b) Q-PCR analysis of mRNAs shown in panel a in Ppargf/f and PpargIEC−/− colonic epithelial cells (n=6 mice per group). In panels a and b, values are means ± s.e.m. For mice treated with Ro, *P<0.01 and **P<0.05 versus untreated mice of the same genotype. For untreated knock-out mice (Cftr−/− or PpargIEC−/−), +P<0.01 and ++P<0.05 versus wild-type or Ppargf/f controls. (c) Kaplan-Meier analysis, of 8-week old Cftr−/−/Ppargf/f and Cftr/PpargDKO mice following removal of GoLYTELY treated with standard or Ro chow (n=10 mice per group) Log-Rank test, *P<0.01 comparing Cftr−/−/Ppargf/f and Cftr/PpargDKO, +P<0.05 comparing Cftr−/−/Ppargf/f and Cftr−/−/Ppargf/f plus Ro, and p=0.85 (ns) comparing Cftr/PpargDKO and Cftr/PpargDKO plus Ro. (d) Effect of genotype and Ro treatment on accumulation of Alcian blue-positive mucins in the right colon. Scale bar, 100 μm. (e) Q-PCR analysis of Acaa1b and Angptl4 mRNAs in lung derived from wild-type and Cftr−/− mice treated with Ro or control diet (n=6 mice per group). (f) Effect of Ro treatment and expression of Angptl4 and Adfp mRNAs in polarized CFTR mutant bronchial epithelial cell line IB3-1 and corrected wild-type C38 cells (n=3 replicates).
Assessment of gene expression in PpargIEC−/− mice demonstrated reduced expression of the PPARγ target genes identified in the Cftr−/− mice and no induction by Ro (Fig. 2b). Conversely, genes up-regulated in the Cftr−/− mice were up-regulated in PpargIEC−/− cells and were not suppressed by Ro. These results confirmed that Ro acted through colonic epithelial cell PPARγ to affect target gene expression (Supplementary Fig. 3b) and suggested that loss of PPARγ activity in colonic epithelial cells contributed to cell-autonomous activation of inflammatory response genes. We crossed Cftr−/− mice with PpargIEC−/− mice to investigate a functional interaction between PPARγ and the CF phenotype. Mice with the combined deletion (Cftr−/− and PpargIEC−/−, hereafter Cftr/PpargDKO) were smaller at age 30-days compared to littermate controls (Cftr−/−/Ppargf/f) in both male and female groups (p<0.001), while there was no weight difference between Ppargf/f control and PpargIEC−/− mice (Supplementary Fig. 4a). Cftr−/− and Cftr/PpargDKO mice demonstrated similar poor survival when switched from GoLYTEY to water at 4-weeks age, as expected. However, when we switched Cftr−/−/Ppargf/f mice and Cftr/PpargDKO mice from GoLYTELY to water at 8-weeks age, Cftr/PpargDKO mice were more prone to death than control Cftr−/−/Ppargf/f (Log-Rank test, p<0.01)(Fig. 2c) and exhibited massive mucus accumulation resulting in bowel obstruction (Fig. 2d and Supplementary Fig. 4b). Ro treatment had no effect on survival of Cftr/PpargDKO mice but prolonged survival of 8-week old Cftr−/−/Ppargf/f mice (Log-Rank test, p<0.05). Furthermore, the ability of Ro to suppress mucus accumulation in Cftr−/−/Ppargf/f mice was absent in Cftr/PpargDKO mice (Fig. 2d), demonstrating that Ro acted through PPARγ expressed in epithelial cells to ameliorate the obstructive phenotype.
Expression levels of PPARγ target genes were also reduced in lungs of Cftr−/− mice, exemplified by Angptl4 and Acaa1b, and were restored by Ro treatment (Fig. 2e). To address whether there is a defect in PPARγ function in human cells bearing mutations that are common causes of CF, we made use of the IB3-1, C38 and S9 cell lines. IB3-1 cells are bronchial epithelial cells derived from a compound heterozygote CF patient (ΔF508/W1282X) expressing only ΔF508 CFTR protein due to instability of the W1282X mutation15. The C38 cell line was derived from IB3-1 cells by transduction with a functional N-terminal truncated CFTR allele that restores chloride secretion, while the S9 cell line was transduced with a full-length version of CFTR. Although these cells are aneuploid and exhibit a number of differences with respect to primary bronchial epithelial cells, they allow a direct determination of CFTR-dependent alterations by comparison to the parental line16. Notably, basal levels of PPARγ target genes, such as Angptl4 and Adfp, were reduced in the IB3-1 cell line compared to the rescued C38 cell line and were induced by Ro (Fig. 2f). Similar results were obtained in S9 cells (Supplementary Fig. 5).
We performed ion transport studies to determine whether Ro treatment ameliorated the colonic phenotype of CFTR mice by affecting chloride secretion. Colonic tissue from Cftr−/− mice or human colonic T84 cells treated with a CFTR inhibitor (CFTRinh-172) demonstrated the expected defect in forskolin- or calcium-dependent stimulated chloride secretion, but this defect was not affected by Ro treatment (Supplementary Fig. 6 and data not shown). We therefore systematically searched for Ro-inducible, PPARγ-dependent genes that might be involved in other types of compensatory ion transport (Fig. 3a, Supplementary Fig. 7). These studies identified carbonic anhydrase 4 and 2 (Car4, Car2) as being of potential interest because they were Ro-inducible in wild-type but not PpargIEC−/− cells and have established roles in bicarbonate production and secretion in the intestine17,18. We confirmed reduced protein levels of Car4 and Car2 in the PpargIEC−/− cells under basal conditions that were increased by Ro treatment in wild-type cells (Fig. 3b). Furthermore, Car4 and Car2 transcript and protein levels were reduced in colonic epithelial cells derived from Cftr−/− mice in comparison to cells derived from wild-type mice, and were induced by treatment with Ro (Fig. 3c, d). Car4 and Car2 mRNA levels were also reduced in lungs of Cftr−/− mice and were inducible by Ro (Fig. 3e). Finally, Ro was capable of inducing the homologous genes (CAIV and CAII) in the human lung epithelial cell line Calu3 (Fig. 3f). We investigated whether increased carbonic anhydrase gene expression correlated with physiologic consequences by measuring bicarbonate secretion induced by the heat-stable enterotoxin of E. coli (STa)19 in colonic tissue from Cftr−/− mice. These studies demonstrated a significant increase in bicarbonate secretion in colonic tissue derived from Ro-treated Cftr−/− compared to untreated control mice (Fig. 3g).
The observation that Cftr−/− colonic epithelial cells exhibit a defect in PPARγ-dependent gene expression but normal levels of PPARγ protein suggested a defect in PPARγ function. We performed chromatin immunoprecipitation (ChIP) experiments to quantify PPARγ occupancy of PPAR response elements in wild-type and Cftr−/− colonic epithelial cells. We evaluated known PPARγ binding sites in the case of the Hmgcs220 and Angptl421 genes. We identified putative PPREs in the Acaa1b, Mgll and Car4 genes by sequence analysis and confirmed that they confer PPARγ-dependent transcriptional responses in enhancer assays (Supplementary Fig. 8). These experiments demonstrated equivalent binding of PPARγ to promoter proximal and intronic elements in the Angptl4, Hmgcs2 and Car4 genes and reduced binding of PPARγ to distal elements in the Acaa1b and Mgll genes in Cftr−/− cells (Fig. 4a). This binding was clearly specific for PPARγ because it was not observed in PpargIEC−/− cells (Fig. 4b). Thus, the expression of PPARγ target genes was reduced in Cftr−/− cells even in cases in which genomic PPARγ binding to response elements was equivalent to that in wild-type cells. We performed ChIP for TRAP220, a nuclear receptor coactivator that is a component of the mediator complex, to confirm a functional defect in DNA-bound PPARγ. Because TRAP220 interacts directly with PPARγ in a ligand-dependent manner through LXXLL nuclear-receptor-interacting domains22,23, its PPAR-dependent interaction with PPRE-elements in vivo provided an assessment of the functional activity state of PPARγ. The binding of TRAP220 was reduced at all PPREs examined in Cftr−/− colonic epithelial cells compared to wild-type cells, including sites at which PPARγ binding itself was equivalent (Fig. 4c). In contrast, binding of TRAP220 to the β-actin promoter was equivalent in both cell types. Evidence that recruitment of TRAP220 to PPREs in wild-type cells was dependent on PPARγ was indicated by the marked reduction of TRAP220 binding to these sites in PPARγIEC−/− cells, with no alteration at the β-actin promoter (Fig. 4d).
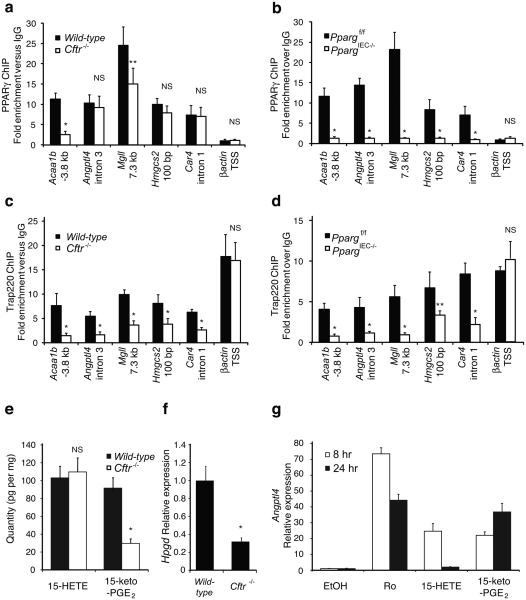
Figure 4. Molecular analysis of PPARγ function in Cftr−/− colonic epithelial cells.
(a) Chromatin immunoprecipitation (ChIP) of PPARγ occupancy of promoter proximal elements (Angptl4, Hmgcs2, and Car4) and distal elements (Acaa1b, and Mgll) in colonic epithelial cells derived from wild-type and Cftr−/− mice. (b) ChIP of PPARγ binding to elements shown in panel a in colonic epithelial cells derived from Ppargf/f and PpargIEC−/− mice. (c) ChIP of Trap220 on PPRE sites shown in panel a in wild-type and Cftr−/− cells demonstrating reduced occupancy in Cftr-deficient cells. (d) ChIP of Trap220 on the PPRE sites shown in panel a in Ppargf/f and PpargIEC−/− mouse colonic epithelial cells demonstrating PPARγ specificity for the displayed sites. Values are means ± s.d. of n=4 technical replicates (* p<0.01, ** p<0.05 for wild-type vs Cftr−/− or Pparf/f vs PpargIEC−/−). Results are representative of 3 independent biological replicates. (e) Quantitative analysis of endogenous 15-HETE and 15-keto-PGE2 levels in Cftr−/− cells (* p<0.002). (f) Q-PCR analysis of Hpgd in colonic epithelial cells derived from wild-type and Cftr−/− mice (n=6 mice per group, error bars represent s.e.m., *P<0.001). (g) Q-PCR analysis of Angptl4 expression in HT-29 colonic epithelial cells treated with 1 uM Ro, 10 μM 15-HETE or 10 μM 15-keto-PGE2.
We utilized both gas chromatography and liquid chromatography mass spectrometry (GC/MS and LC/MS/MS) to quantitatively evaluate levels of fatty acids and eicosanoids present in wild-type and Cftr−/− colonic epithelial cells in vivo. Among the 94 eicosanoid analytes that were quantified, 15-HETE and 15-keto-PGE2 were the two most abundant species present in wild-type cells that are capable of activating PPARγ in the low micromolar range24,25. Although 15-HETE was unchanged, 15-keto-PGE2 was reduced by about 65% in Cftr−/− cells (p<0.002, Fig. 4e). In concert with this finding, expression of 15-hydroxyprostaglandin dehydrogenase (Hpgd), which is required for synthesis of 15-keto-PGE2 from PGE2, was also reduced by 70% in Cftr−/− cells (Fig. 4f). Using induction of Angptl4 expression as a functional assay, 15-keto-PGE2 was found to promote more sustained induced expression than 15-HETE (Fig. 4g).
In concert, these studies build upon prior work suggesting reduced expression and function of PPARγ in colon12 and airway epithelial cells26 in the setting of CFTR-deficiency. Here, we provide evidence for a reversible defect in PPARγ function in Cftr−/− colon and lung that contributes to a pathogenic program of gene expression. Lipidomic analysis suggests that this defect results, at least in part, from a reduction in endogenous PPARγ ligands that include 15-keto-PGE2. The corresponding reduction in Hpgd expression raises the interesting possibility that reduced conversion of PGE2 to 15-keto-PGE2 might contribute to the increased levels of PGE2 observed in CF patients27. The functional defect in PPARγ activity appears to contribute to the intestinal phenotype of Cftr−/− mice based on the ability of Ro to reduce mortality and the increased disease severity in Cftr/PpargDKO mice. Due to the large number of down- and up-regulated genes that are ‘corrected’ in Cftr−/− colonic epithelial cells by Ro treatment it is likely that multiple genes contribute to phenotype attenuation. Mucus accumulation and overexpression of inflammatory response genes are two relevant pathogenic features of CF that are inhibited by Ro in a PPARγ-dependent manner. Previous studies have demonstrated that PPARγ agonists suppress pro-inflammatory mediators and neutrophil recruitment in bronchoalveolar lavage fluid following Psuedomonas aeruginosa infection26. Although inhibition of inflammation is a well-established function of PPARγ in several cell types and tissues, including colon28,29, roles in regulation of mucus have not been previously described. Several mechanisms have been proposed to account for mucus accumulation in CF, including isotonic contraction of the air-surface layer30 and reduced mucus clearance possibly due to defects in bicarbonate transport31. The effects of Ro on bicarbonate secretion and mucus accumulation in the colon are consistent with the hypothesis that luminal bicarbonate plays an important role in the normal transition of mucins from the compacted to expanded state32.
PPARγ ligands have been considered for treatment of CF based on their anti-inflammatory activities33, but clinical efficacy remains to be established. The present studies suggest that additional parameters be considered in the design of clinical trials. The relatively high rate of treatment failure in Cftr−/− mice suggests that appropriate dosing may be critical, as documented in clinical studies of the effect of ibuprofen on neutrophil migration into the lungs of CF patients34. Differences in the effects of 15-keto-PGE2 and 15-HETE on Angptl4 expression raise the possibility that not all PPARγ ligands may be equivalent with respect to restoration of functional defects in the setting of CFTR deficiency. Finally, measurement of levels of 15-keto-PGE2 may provide a biomarker for selecting patients most likely to benefit from PPARγ agonists.
METHODS
Animals
All procedures were approved by the University of California, San Diego IACUC. Mice heterozygous for the S489X (B6.129P2-CFTRtm1Unc, or Cftr+/−) mutation were inbred >10 generations. An electrolyte solution containing polyethylene glycol 3350 (GoLYTELY, Braintree Laboratories) was administered ad libitum to the colony to reduce intestinal obstruction of the Cftr−/− mice5. Four-week old male and female Cftr−/− mice were randomized to 3 groups to receive 1) control rodent chow (ground Harlan 8604) and GoLYTELY, 2) control chow and water or 3) rosiglitzone 20 mg/kg/d in chow and water. For the first 72-hours, all mice were maintained on GoLYTELY until day 0 of study. Mice with signs of distress were euthanized and scored as study-related deaths.
Mice carrying the loxP-targeted PPARγ were described previously35. Ppargf/f mice were crossed with Vil1-Cre mice to generate the intestinal epithelial specific deletion of PPARγ (PpargIEC−/−)13. Heterozygous loxP targeted PPARγ and Cre transgenic mice were backcrossed 8 generations to C57Bl/6. PpargIEC−/− and Cftr−/− mice were mated, and double heterozygotes were backcrossed >8 generations to the original Cftr−/− colony. Ten Cftr/PpargDKO and Cftr−/−/Ppargf/f controls were maintained on GoLYTELY until 8-weeks of age, weaned to water, and assessed for survival with or without treatment with rosiglitazone (20mg/kg/d). For histological analysis, mice were withdrawn from GoLYTELY and the colon isolated on day 4. The tissue was cut longitudinally, fixed in 10% neutral buffered formalin and paraffin embedded. 4 mm sections were cut, deparaffinised with xylene and stained with haematoxylin-eosin, Alcian blue, or PAS.
RNA isolation and quantitative PCR
Colonic epithelial cells were harvested from sibling female wild-type or Cftr−/− and Ppargf/f or PpargIEC−/− mice as described36. Mice were fed control chow or Ro (20mg/kg/d) for five days prior to isolation to ensure adequate drug levels. Total RNA was isolated from intestinal and colonic epithelial cells by TRIzol (Invitrogen) and mRNA enriched by RNeasy column purification (QIAGEN). Following first-strand cDNA synthesis, quantitative PCR was performed with SYBR-GreenER (Invitrogen) using an Applied Biosystems 7300 Real-Time PCR System. Amplified transcripts were normalized to standard housekeeping genes (GAPDH) using the ΔΔCT method as described by the manufacturer.
Western blot
Intestinal epithelial whole-cell extracts were generated in RIPA buffer, quantified by the DC protein assay (BioRad), separated by gel electrophoresis, and transferred to Immobilon-P (Millipore). Antibodies used were anti-PPARγ (C26H12, Cell Signaling), anti-CA II (H-70, Santa Cruz), anti-CA IV (M-50, Santa Cruz), and anti-βactin (AC-15, Sigma-Aldrich). Secondary antibodies were from Jackson Immunoresearch and Dako.
Cell culture
Human lung Calu-3 cells (ATCC) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Hyclone), seeded on 12-mm Millicell-HA inserts (Millipore) and cultured for 21 days. Human colon HT-29 (ATCC) were maintained in McCoy's 5A (Mediatech) with 10% fetal calf serum, seeded in 24-well inserts, starved 12 hours in 0.5% serum and treated with 1 μM Ro, 10 μM 15-HETE, or 10 μM 15-keto-PGE2 (Cayman Chemical). Human bronchial IB3-1 and C-38 cells (ATCC) were maintained in LHC-8 (Invitrogen), seeded on bovine collagen type 1 (BD Biosciences) coated 12-mm Millicell-CM inserts (Millipore) and cultured for 14-21 days at an air-liquid interface (ALI) to achieve differentiation in a 1:1 mixture of bronchial epithelial basal media (BEBM) and DMEM-H (Mediatech) supplemented with BEGM SingleQuots (Lonza)30.
Colonic epithelial ion transport
Proximal colon tissue was removed and placed in cold iso-osmolar solution containing mannitol and indomethacin (10 μM). Tissue was stripped of seromuscular layers and mounted on Ussing chamber inserts with a window area of 0.1 cm2. Experiments were performed under continuous short-circuited conditions (Voltage-Current Clamp, VCC 600; Physiologic Instruments) as previously described19. Measurements were recorded at 5-min periods and the values for 10-min intervals averaged. The rate of luminal bicarbonate secretion is expressed as μmol·cm−2·h−1.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation assay was performed as previously described37. Briefly, primary colonic epithelial cells were isolated by scraping, cross-linked with 1% formaldehyde, lysed, and sonicated to generate DNA fragments of 300-900 nucleotides. Protein-linked DNA was immunoprecipitated with anti-PPARγ (H-100 and E-8, Santa Cruz), anti-Trap220 (C-19, Santa Cruz), or control rabbit or goat IgG (Santa Cruz), reverse cross-linked at 65°C overnight and column purified (QIAGEN). Extracted DNA was amplified by quantitative PCR in quadruplicate replicates and the results normalized to control serum.
Lipidomics analysis
Sample preparation, liquid chromatography mass spectrometry, and gas chromatography mass spectrometry were conducted as previously described with details provided in the supplementary methods38-40.
Statitistical analysis
Standard deviation, standard error, Log-Rank and unpaired two-tailed t-test were performed with SigmaStat (Systat Software). Kaplan-Meier curves were analyzed by Log-Rank test with multiple pair-wise comparisons performed by the Holm-Sidak method. Measurements of multiple samples are presented as means ± s.e.m or ± s.d. as indicated in the figure legends and differences were analyzed for significance by t-test.
Supplementary Material
Acknowledgements
We thank P. Quinton for advice and critical reading of the manuscript. We thank the late J. Isenberg (University of California, San Diego) for providing CFTRtm1Unc mice, R. Sasik for assistance with microarray data analysis, and D. McCole for assistance with chloride transport studies. Microarray analysis was performed at the Biogem Core Facility of the University of California, San Diego (G. Hardiman, Director) and histopathology was performed by the UCSD Histopathology Core Facility (N. Varki, Director). These studies were supported by NIH grants P01DK074868, GM 069338-03, DK063491 to C.K.G. and E.D; NIH DK007202 and FDHN FFTA to G.S.H.
Footnotes
Competing financial interests
The authors declare no competing financial interests
References
- 1.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009 doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 2.Zuelzer WW, Newton WA., Jr. The pathogenesis of fibrocystic disease of the pancreas; a study of 36 cases with special reference to the pulmonary lesions. Pediatrics. 1949;4:53–69. [PubMed] [Google Scholar]
- 3.Freedman SD, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 4.Hardin DS, LeBlanc A, Lukenbough S, Seilheimer DK. Insulin resistance is associated with decreased clinical status in cystic fibrosis. J Pediatr. 1997;130:948–956. doi: 10.1016/s0022-3476(97)70282-8. [DOI] [PubMed] [Google Scholar]
- 5.Snouwaert JN, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 6.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol. 1995;269:L625–630. doi: 10.1152/ajplung.1995.269.5.L625. [DOI] [PubMed] [Google Scholar]
- 7.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- 8.Creating the gene ontology resource: design and implementation. Genome Res. 2001;11:1425–1433. doi: 10.1101/gr.180801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ollero M, et al. Decreased expression of peroxisome proliferator activated receptor gamma in cftr−/− mice. J Cell Physiol. 2004;200:235–244. doi: 10.1002/jcp.20020. [DOI] [PubMed] [Google Scholar]
- 10.Yu S, et al. Human peroxisome proliferator-activated receptor alpha (PPARalpha) supports the induction of peroxisome proliferation in PPARalpha-deficient mouse liver. J Biol Chem. 2001;276:42485–42491. doi: 10.1074/jbc.M106480200. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana K, et al. Gene expression profiling of potential peroxisome proliferator-activated receptor (PPAR) target genes in human hepatoblastoma cell lines inducibly expressing different PPAR isoforms. Nucl Recept. 2005;3:3. doi: 10.1186/1478-1336-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lytle C, et al. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis. 2005;11:231–243. doi: 10.1097/01.mib.0000160805.46235.eb. [DOI] [PubMed] [Google Scholar]
- 13.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 14.Adachi M, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–1113. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamosh A, Rosenstein BJ, Cutting GR. CFTR nonsense mutations G542X and W1282X associated with severe reduction of CFTR mRNA in nasal epithelial cells. Hum Mol Genet. 1992;1:542–544. doi: 10.1093/hmg/1.7.542. [DOI] [PubMed] [Google Scholar]
- 16.Zeitlin PL, et al. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 17.Leppilampi M, et al. Carbonic anhydrase isozyme-II-deficient mice lack the duodenal bicarbonate secretory response to prostaglandin E2. Proc Natl Acad Sci U S A. 2005;102:15247–15252. doi: 10.1073/pnas.0508007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurtrie HL, et al. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 19.Sellers ZM, et al. Heat-stable enterotoxin of Escherichia coli stimulates a non-CFTR-mediated duodenal bicarbonate secretory pathway. Am J Physiol Gastrointest Liver Physiol. 2005;288:G654–663. doi: 10.1152/ajpgi.00386.2004. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez JC, Gil-Gomez G, Hegardt FG, Haro D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J Biol Chem. 1994;269:18767–18772. [PubMed] [Google Scholar]
- 21.Mandard S, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–34420. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 22.Ge K, et al. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature. 2002;417:563–567. doi: 10.1038/417563a. [DOI] [PubMed] [Google Scholar]
- 23.Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci U S A. 1998;95:7939–7944. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wigren J, et al. Differential recruitment of the coactivator proteins CREB-binding protein and steroid receptor coactivator-1 to peroxisome proliferator-activated receptor gamma/9-cis-retinoic acid receptor heterodimers by ligands present in oxidized low-density lipoprotein. J Endocrinol. 2003;177:207–214. doi: 10.1677/joe.0.1770207. [DOI] [PubMed] [Google Scholar]
- 25.Chou WL, et al. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2007;282:18162–18172. doi: 10.1074/jbc.M702289200. [DOI] [PubMed] [Google Scholar]
- 26.Perez A, et al. Peroxisome proliferator-activated receptor-gamma in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;295:L303–313. doi: 10.1152/ajplung.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucidi V, Ciabattoni G, Bella S, Barnes PJ, Montuschi P. Exhaled 8-isoprostane and prostaglandin E(2) in patients with stable and unstable cystic fibrosis. Free Radic Biol Med. 2008;45:913–919. doi: 10.1016/j.freeradbiomed.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Su CG, et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis JD, et al. An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol. 2001;96:3323–3328. doi: 10.1111/j.1572-0241.2001.05333.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 31.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 33.Nichols DP, Konstan MW, Chmiel JF. Anti-inflammatory therapies for cystic fibrosis-related lung disease. Clin Rev Allergy Immunol. 2008;35:135–153. doi: 10.1007/s12016-008-8081-2. [DOI] [PubMed] [Google Scholar]
- 34.Konstan MW, et al. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J Pharmacol Exp Ther. 2003;306:1086–1091. doi: 10.1124/jpet.103.052449. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama TE, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogler G, et al. Establishment of long-term primary cultures of human small and large intestinal epithelial cells. Lab Invest. 1998;78:889–890. [PubMed] [Google Scholar]
- 37.Ogawa S, et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J Biol Chem. 2009;284:21599–21612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarini S, Gijon MA, Folco G, Murphy RC. Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte-macrophage colony-stimulating factor and formyl-methionyl-leucyl-phenylalanine. J Biol Chem. 2006;281:10134–10142. doi: 10.1074/jbc.M510783200. [DOI] [PubMed] [Google Scholar]
- 40.Quehenberger O, Armando A, Dumlao D, Stephens DL, Dennis EA. Lipidomics analysis of essential fatty acids in macrophages. Prostaglandins Leukot Essent Fatty Acids. 2008;79:123–129. doi: 10.1016/j.plefa.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.