Abstract
The classic view that the role of immune cells in cancer is primarily one of tumor rejection has been supplanted by a more complex view of leukocytes having both pro-and anti-tumor properties. This shift is due to the now well recognized capabilities of several myeloid cell types that foster pro-tumor programming of premalignant tissue, as well as the discovery that subsets of leukocytes also suppress development and effector functions of lymphocytes important for mediating anti-tumor immunity. In this review, we focus on the underappreciated role that T lymphocytes play in promoting tumor development. This includes, in addition to the role of T regulatory cells, a role for natural killer T cells and CD4+ T helper cells in suppressing anti-tumor immunity and promoting cancer growth and metastasis.
Keywords: Lymphocytes, Inflammation, Cancer, Tumors
INTRODUCTION
Leukocyte infiltration into developing tumors is now considered one of the hallmarks of cancer development (1). It is thought that the initial immune response to an early neoplasm mirrors the response to acute tissue injury, with sequential infiltration by various myeloid populations leading to eventual infiltration by lymphocytes (2). However, as the kinetics of tumor development and the neoplastic cells themselves alter the local immune microenvironment, making inferences between an immune response to injury/infection and tumor development is difficult. Regardless, if clearance of the would-be cancer cells is not achieved and the initial acute inflammatory response fails to resolve, there inevitably results a state of chronic inflammation within the local tissue. It is now well established that chronic inflammation fosters early cancer development through a number of mechanisms mediated primarily by myeloid-lineage cells, including tumor-associated macrophages, immature myeloid cells that can possess suppressive activity, and Tie2-expressing monocytes (3, 4). The immune microenvironment of a neoplastic tissue encompasses not only the composition of infiltrating leukocytes, but also the bioeffector function of these cells within the tissue. Thus, both the presence of a cell within a tumor and expression of tissue-specific cytokines, chemokines and other immune mediators profoundly influence whether an anti-tumor or pro-tumor immune response is elicited (4, 5).
Although merely responding to tissue damage in the form of inflammatory cues, tumor-infiltrating myeloid cells rapidly respond to soluble and insoluble signals emanating from the neoplastic microenvironment. Responses take the form of dramatically altered gene expression programs that alter bioeffector functions of the immune cells. These often result in increased expression of factors/mediators that enhance growth and survival of neoplastic cells, as well as activating and sustaining angiogenic responses, furthering tissue remodeling, and squelching anti-tumor immune programs (4). Chronic inflammation in tissue resulting from infection or autoimmune disease can also alter the risk of cancer development by providing an environment permissive for initiated preneoplastic cell survival and subsequent proliferation, as well as through production of DNA damaging compounds such as reactive oxygen and nitrogen species that increase mutation frequency (6). While all of these aspects of solid tumor development are susceptible to regulation by infiltrating immune cells, in the context of this review, we will focus on aspects of carcinogenesis regulated by infiltrating lymphocytes, as mechanisms regulated by myeloid cells have been reviewed elsewhere (5–9).
T lymphocytes
T cells develop in the thymus from a common lymphoid progenitor and are defined by expression of a T cell receptor (TCR) that is responsible for recognizing antigens presented by the major histocompatibility complex (MHC) family of genes (also called human leukocyte antigen or HLA). T cells are classically divided into either CD8+ cytotoxic lymphocytes (CTL) or CD4+ T helper (TH) cells that recognize peptides presented by MHCI or MHCII, respectively (Fig. 1). TH cells are further divided into interferon (IFN–γ and tumor necrosis factor (TNF)-α expressing TH1 cells and interleukin (IL)-4, IL-5 and IL-13 expressing TH2 cells. This simplified view of the T cell compartment has been expanded upon by the identification of a range of additional subtypes, including T follicular helper cells (TFH), IL-17 expressing TH cells (TH17), and regulatory T cells (Treg) (10). Paralleling these subtypes in the CD4+ T cell compartment, type 1, type 2, and type 17 CD8+ T cells (TC1, TC2, TC17), as well as regulatory CD8+ cells, have all been described (11–13). There also exist two ‘innate-like’ T cell subsets that can be activated either by cytokines or TCR stimulation. Natural killer T (NKT) cells recognize glycolipids presented by the non-classical MHC molecule CD1d (14), while γδ T cells are not MHC restricted and recognize a diverse range of molecules, including soluble non-protein antigens (15). All of these T lymphocyte subsets have been examined for their role in tumor development and anti-tumor immunity, each with unique roles in directing the immune response.
Figure 1. T cell lineages and subsets.
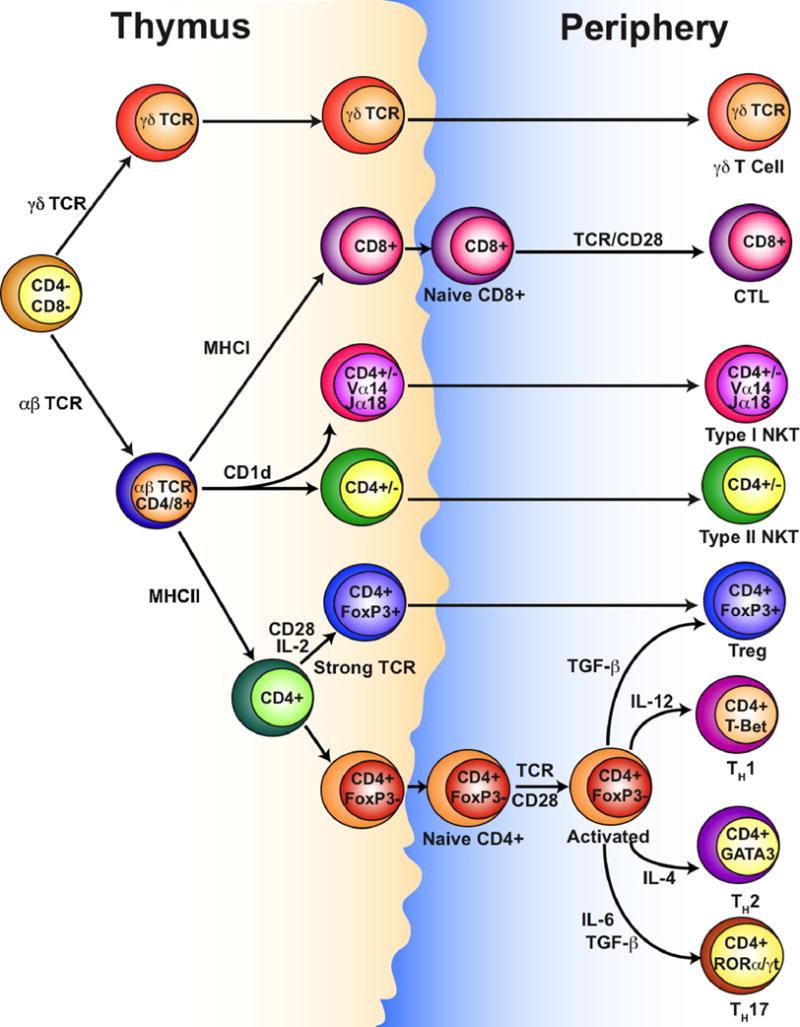
Successful rearrangement and expression of a TCR determines lineage commitment between γδ and αβ T cells. Recognition by αβ T cells of MHCI, MHCII, or CD1d drives CD8+, CD4+ or NKT cell development, respectively. Strong recognition of peptide:MHCII complexes by CD4+ cells drives natural TReg cell development in the thymus, otherwise CD4+ T cells differentiate into TH1, TH2, TH17 or inducible TReg cells following activation in the periphery, with polarization directed by IL-4, IL-6, IL-12 and TGF-β. Type I NKT cells are defined by expression of specific α-chain regions (Vα14-Jα18 in mice, Vα24-Jα18 in humans), but the reason for functional differences between type I and type II NKT cells is unclear.
Cytotoxic T lymphocytes
Mice harboring specific immune-based genetic deficiencies are more susceptible to formation of carcinogen-induced sarcomas, and depending on the specific defect, are also more prone to develop certain spontaneous tumors and lymphomas (16, 17). The ability of immune-deficient mice to reject and/or inhibit the growth of many, but not all cell lines is also impaired. Numerous studies have shown that, due to their ability to produce IFNγ and directly kill target cells, both CD8+ CTLs and natural killer (NK) cells are the critical mediators of the anti-tumor response (16). γδ T cells, which share characteristics with both CTLs and NK cells, are also involved in the anti-tumor response in epithelial tissues such as the skin (18), where they can be the dominant T cell population (15). The relative importance of CTLs, NK cells, and γδ T cells is highly dependent upon the cancer model being used. Even in the skin, genetic-deficiency of αβ T cells increases sarcoma formation following administration of methylcholantrene (MCA), but not 7,12-dimethybenz[a]anthracene (DMBA) or 12-O-tetradecanoylphorbol-13-acetate (TPA) (18), while the absence of CD8+ T cells does not influence the development of neoplasms in a mouse model of de novo squamous carcinogenesis, e.g., K14-HPV16 mice (19).
Epidemiological studies of cancer incidence in acquired immune deficiency syndrome (AIDS) and organ transplant patients reveal that the relative risk (RR) for cancer development varies considerably depending upon organ site and cancer etiology (17, 20) where viral-associated cancers, in particular Human Herpes Virus 8-associated Kaposi’s sarcoma, Epstein-Barr virus-associated Non-Hodgkin’s lymphoma and HPV-associated squamous carcinoma, are elevated in immune suppressed individuals due largely to lack of ability to provide protection against viral infections or viral reactivation in the absence of T cells (21). That said, some cancer types occur with increased frequency in selected groups of immune compromised patients for reasons unrelated to immune suppression for example, chronic exposure to carcinogens (tobacco) for thoracic malignancies, whereas head and neck, esophageal, gastrointestinal and pancreatic cancers are increased in liver transplant patients associated with prior history of alcohol (and tobacco) use (22, 23). On the other hand, the RR for the most common non-viral associated solid tumors of epithelial origin, including breast and prostate, are decreased in immune suppressed patients, with some of these having a RR less than 1.0 (17, 20).
Examination of T cell infiltrates in tumors reveals cells that can display activation markers and are able to recognize tumor antigens (16), indicating that some tumors are indeed immunogenic and can induce an anti-tumor immune response. Tumor antigens encompass both neo and overexpressed antigens, e.g., c-myc, HER-2/neu and p53 (24), as well as host/stromal cell-derived antigens unique to individual tumors. Clinical studies have even reported accumulation of autoantibodies against extracellular matrix (ECM) components, including anti-collagen type I, III and V, as well as anti-fibronectin antibodies that accumulate in lung cancer and nasopharyngeal patients (25). How then do neoplastic cells, expressing mutant proteins in an inflammatory microenvironment that seemingly engenders a robust T cell response, avoid killing by cytotoxic cells? Importantly, lymphocytes do not act in isolation, and their effector functions are largely dependent upon the release of cytokines and binding of inhibitory and activating receptors to ligands expressed by other leukocytes, stromal cells, and even neoplastic cells. For example, NK cells are potent regulators of CD8+ T cell responses through their release of IFNγ, which provides a maturation signal for tissue resident DCs and assists in CD8+ T cell effector function; meanwhile, cytokines released by mature DCs and activated T cells are important for promoting NK cell effector function (26). These interweaving regulatory pathways are necessary to initiate, direct, maintain and eventually shutdown an appropriate immune response. Such pathways are also necessary to prevent an inappropriate immune response: despite central tolerance through negative selection of self-reactive lymphocytes, peripheral tolerance mediated by cytokines, inhibitory receptors and immune regulatory cell types is necessary to prevent autoimmune disorders. As cancer cells are largely recognized as ‘self’, it is not surprising they utilize similar mechanisms that effectively dampen anti-tumor immunity; thus, T lymphocytes are intimately involved in regulating both pro- and anti-tumor immunity and chronic inflammation within the tumor microenvironment.
Regulatory T cells
Since the rediscovery of suppressor T cells as CD4+CD25+ regulatory T (TReg) cells, and their further characterization as cells expressing glucocorticoid-induced tumor necrosis factor receptor (GITR), cytotoxic T-lymphocyte antigen (CTLA)-4, and uniquely, the transcription factor forkhead box P3 (FoxP3), this T cell subset has become an intense focus of cancer research. TReg cells can develop in the thymus or can be converted in the periphery by exposure to transforming growth factor (TGF)-β (27). These ‘natural’ and ‘inducible’ TReg cells, respectively, utilize the same mechanisms to mediate immune suppression and may perform overlapping functions (28). A number of regulatory T cells that do not conform to the CD4+CD25+FoxP3+ phenotype have also been described, but these remain poorly characterized (29) and evidence of a role for these cells in cancer has so far been limited to the isolation of IL-10 producing CD8+ T cells from human ovarian tumors (30). Immune suppression of CD4+ and CD8+ T cell responses by TReg cells is mediated both in the lymphoid organs where T cells activation occurs, and in the tissues (31). Interestingly, in a pancreatic islet allograft model, TReg cells were shown to enter the inflamed tissue and then migrate to the lymph nodes, where these sequential series of steps was found to be associated with increasing graft survival time (32).
While a host of molecules have been described as important for mediating TReg cell suppression, these molecules have been broadly classified as acting in one of four ways (33): i. cytokine inhibition, such as with TGF-β, IL-35 and IL-10; ii. direct cytolysis of effector T cells through perforin and granzyme; iii. metabolic disruption, such as IL-2 deprivation and cyclic adenosine monophosphate (cAMP) transfer; and iv. inhibition of DC function, such as through binding of CTLA-4 to CD80/86 and the induction of indoleamine 2,3-dioxygenase (IDO). These mechanisms appear to have overlapping but non-redundant roles, with the degree of importance being tissue and model dependent (34). This may be significant in cancer, as CD4+CD25high tumor infiltrating T lymphocytes from patients with head and neck squamous cell carcinoma (HNSCC) were found to mediate suppression through IL-10 and TGF-β, while the same population from peripheral blood did not express these cytokines and were less able to suppress proliferation (35). A role for IL-10 and TGF-β has also been revealed in mice following injection of a fibrosarcoma cell line (36), while a role for perforin- and granzyme B-dependent killing of NK and CD8+ T cells was found in mice after injection of a lymphoma cell line (37). Other than these studies, the mechanisms by which TReg cells mediate suppression in cancer has been largely ignored. Instead, research has been focused on correlating TReg cell infiltration with prognosis and attempts to deplete TReg cells with anti-CD25 antibody based therapies (29).
Suppression by T cells in solid tumors was first suggested by Fujimoto et al. (38), cumulating in work by North and colleagues, who showed that the ability to reject a 2nd subcutaneous injection of a fibrosarcoma cell line was inversely correlated to increased suppressor activity of CD3+CD4+ cells over time (39, 40). These findings have since been expanded to show that depletion of CD25+ cells, which are largely CD4+FoxP3+, reduces tumor growth of some tumor cell lines (29), as well as MCA-induced fibrosarcomas (41, 42). Infiltration of TReg cells into the tumor is observed in all of these models, while an increased percentage of TReg cells in the periphery is observed only in some instances. In one study using a fibrosarcoma cell line, depletion of CD4+ cells resulted in CD8+ T cell-dependent tumor regression in 50% of the mice (36), that was increased to 100% following either CD4+ or CD25+ cell depletion when another fibrosarcoma-derived cell line expressing a strong antigen was used (36). Together with other studies, experimental systems such as these indicate that initiation of a CD8+ T cell response is possible during tumor development, depending on the immunogenicity of the tumor antigens involved, but that local and/or systemic immune suppression by TReg cells can limit their effectiveness. Unfortunately, these results have been limited in scope to transplantable tumor models representing few tumor types, and thus further investigation is required to determine whether TReg-dependent immune suppression is applicable to a wide range of spontaneous tumors, as might be expected.
Increased number of tumor-infiltrating FoxP3+ cells is associated with poor prognosis in several cancers, including hepatocellular carcinoma (43), ovarian carcinoma (41), pancreatic ductal carcinoma (44), cervical cancer (45), non-small cell lung carcinoma (46), HNSCC (35), and breast cancer (47, 48); with varying degrees of prognostic value regarding patient outcome. As with some mouse models, increased frequency of TReg cells in peripheral blood of some cancer patients has been reported (49). Peripheral blood TReg cells from patients with ovarian cancer displayed equal suppressive capacity compared to tumor-derived TReg cells (41). This contrasts with the study of patients with HNSCC (35), highlighting the potential for differences in the immune response based on cancer type and/or etiology.
Attempts to translate TReg research into the clinic have focused around CD25+ cell depletion using denileukin diftitox. Known commercially as Ontak, this compound composed of IL-2 fused to a portion of diphtheria toxin has been approved for treating CD25+ cutaneous T-cell leukemia and lymphoma (29). Ontak administration has been demonstrated to reduce the numbers of peripheral TReg cells and improve T cell activation in a small number of patients with either lung, ovarian, breast, or renal cancer; either alone or in combination with DC-based vaccination (50, 51). Other small studies have confirmed these findings, but as before, objective clinical responses were rare. Furthermore, a larger study with NSCLC patients observed no objective clinical responses, and almost half of the patients suffered side effects usually associated with IL-2 immunotherapy (52). Dosage optimization thus remains an issue, as does route of administration, as intratumoral injection of anti-CD25 antibodies demonstrating efficacy in mouse models (36). Determination of which cancer types are most suitable for TReg depletion is also required, as the ability of CD25+ cell depletion to improve anti-tumor immunity is known to depend on the tumor type (53). Finally, combinatorial therapies, such as CD25+ cell depletion with blockage of immunosuppressive molecules such as CTLA-4 and programmed death (PD)-1 (54), has great potential to overcome immune suppression within the tumor, although the induction of autoimmune diseases will likely remain an issue for patients.
T helper cells
Lineage commitment between CD4+ and CD8+ T lymphocytes occurs during development within the thymus. Further differentiation of the CD4+ lineage, with the exception of natural TReg cell development, requires T cell activation through MHCII and co-stimulatory molecules, as well as cytokine dependent signaling that is responsible for directing the cell towards a particular lineage (Fig. 1). Classical differentiation of CD4+ T cells into TH1 and TH2 cells, mediated by IL-12 and IL-4 respectively, was recently updated to a include a new TH17 lineage (10, 55). TH17 cells are induced by a combination of IL-6 and TGF-β and mediate their effects through secretion of IL-17(A), IL-17F, IL-21 and IL-22 (56). Many of these cytokines increase the severity of autoimmune diseases in mouse models by promoting inflammation, but also appear to be functionally important in protecting against some extracellular pathogens, possibly by mediating leukocyte recruitment through the induction of chemokine expression (57, 58).
TH17 cells have been observed in patients with ovarian (59), prostate (60) and gastric cancer (61), and high numbers of IL-17 producing cells in hepatocellular carcinoma patients is an indicator of poor prognosis (62). In mice, transgenic expression of IL-17 by cell lines increases tumor growth by promoting angiogenesis (63, 64), knockdown of the IL-17 receptor in 4T1 mammary carinoma cells reduced survival and tumor growth (65), and IL-17 depletion delays development of chemically-induced papillomas (66). This effect appears to be mediated by a pathway of IL-17 inducing IL-6 production by both neoplastic and stromal cells, that in turn leads to activation of Stat3 (67). Not only is Stat3 involved in upregulating genes that promote tumor growth and immune suppression (68, 69), but it also mediates expression of IL-17 (70), potentially leading to a dangerous feedback loop (Fig. 2).
Figure 2. T cell-derived cytokines regulate pro- and anti-tumor immunity.
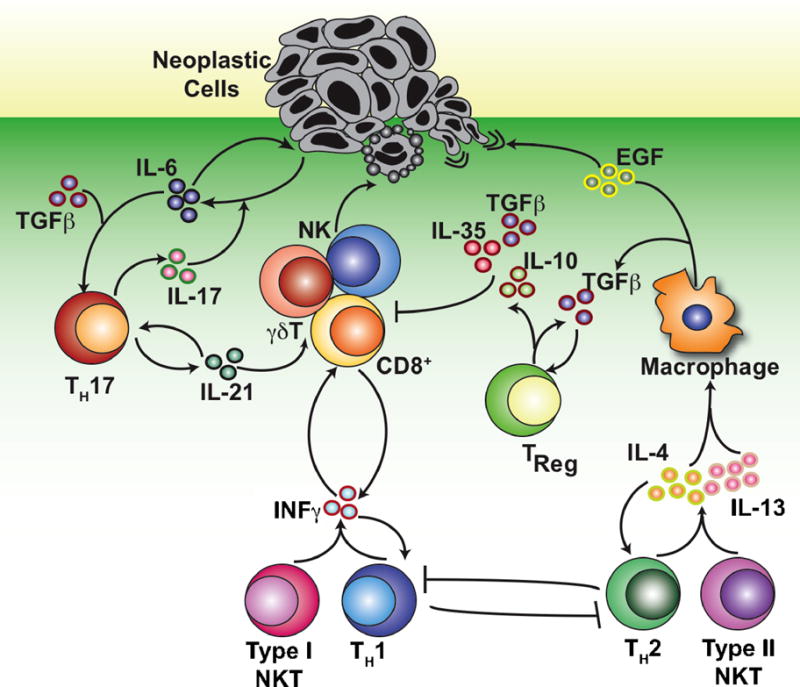
NK cells, γδ T cells, and CD8+ CTLs mediate anti-tumor immunity by inducing cell death in neoplastic cells. The cytotoxic effector functions of these cells are supported by IFNγ released from TH1 and type I NKT cells, as well as by self-production of IFNγ that further drives TH1 polarization. TH2 polarization opposes TH1 polarization, and the release of IL-4 and IL-13 by both TH2 and type II NKT cells can direct macrophages towards an M2 phenotype. Macrophages polarized by IL-4 promote metastasis through the release of EGF, while production of TGFβ suppresses the immune response directly, or indirectly through promotion of TReg development. In the presence of IL-6, TGFβ can also promote TH17 polarization. IL-17 induces the production of IL-6 by tumor cells, which both promotes tumor cell growth and further drives TH17 polarization, while IL-21 has been shown to enhance CTL effector function. Multiple cell types and pathways have been omitted for clarity.
However, as with inflammation in general, IL-17-dependent inflammation may have both positive and negative effects on tumor growth, depending on the tumor model. MC38 colon cancer cell growth is enhanced in IL-17-deficient mice (71), while adoptive transfer of in vitro polarized TH17 cells specific for a B16 melanoma antigen can induce tumor regression (72). This effect appears to depend upon IFNγ, as blocking antibodies preventing TH17 cell transfer from causing tumor regression (72), while reduced frequency of IFNγ producing cells were observed in IL-17-deficient tumors (71). Adding to the confusion is that both IL-21 and IL-22 activate Stat3, and IL-21 can promote Th17 differentiation (56). Meanwhile, CD8+ T cells primed in vitro in the presence of IL-21 provide a more robust anti-tumor response upon adoptive transfer, and IL-21 therapy is currently in clinical trials for cancer treatment (73).
Not surprisingly, CD4+ T cell-deficiency (and elimination of TH1, TH2, TH17 and most TReg cell populations) has differential effects in different mouse tumor models (19, 74–77). In general, TH1 polarization is related to anti-tumor effects, while TH2 polarization is thought to promote tumor formation (20, 78, 79). Direct targeting of TH1 development and effector functions, through IL-12 and IFNγ respectively, clearly indicate a role for TH1 in tumor rejection (80). Genetic deficiency in IFNγ or IFNγ receptor 1, or anti-IFNγ antibody treatment, increases MCA-induced sarcomas (81, 82), with loss of IFNγ also shown to increase the rate of spontaneous lymphomas and lung adenocarcinomas (83). Similarly, IL-12 genetic deficiency increases the frequency of chemically-induced sarcomas (84) and papillomas (85), while exogenous IL-12 treatment has the opposite effect (86). Notably, IL-12 dependent rejection of a sarcoma cell was blocked by administration of neutralizing anti-IFNγ antibodies (87). IFNγ production is not limited to TH1 cells however, and production by CD8+ T cells, γδ T cells, NK cells, and NKT cells is also dependent upon IL-12 (88). In one study, loss of IFNγ expression by γδ T cells was found to account for the increase in MCA-induced carcinogenesis in IFNγ-deficient mice (89). Thus, at least in the skin where a higher percentage of γδ T cells are found, the importance of TH1 cells may be minimal.
Perhaps the best evidence for a specific role for TH1 polarization is in mice deficient for signal transducers and activators of transcription protein 6 (STAT6), which display increased TH1 polarization due to the block in IL-4 signaling (16). These mice were able to reject tumors formed through injection of a mastocytoma cell line that grew permissively in normal mice (90) and were more resistant to growth of the 4T1 mammary carcinoma cell line (91). Cells from the lymph node of tumor bearing STAT6-deficient mice produced more IFNγ following secondary stimulation (90), while splenocytes from these mice displayed increased killing against the cell lines (90, 91). STAT1-deficient mice meanwhile display increased TH2 polarization due to the block in IFNγ signaling, and are more susceptible to tumor development (92). These results are consistent with the notion that TH1 polarization increases IFNγ production, leading to more robust anti-tumor immunity through improved CTL responses. Importantly, direct effects of IFNγ on inhibiting proliferation, promoting apoptosis, and inhibiting angiogenesis have been observed, and loss of sensitivity to IFNγ reduces the immunogenicity of tumors (88). Intriguingly, IFNγ has been found to increase expression of MHCI on MCA-induced sarcomas, thereby improving CTL killing (93), suggesting an addition mechanism by which IFNγ production by TH1 and other lymphocytes may assist anti-tumor immunity.
By virtue of reduced IFNγ production, TH2 polarization is likely to be detrimental to the anti-tumor response. TH2 polarization is dependent upon, and leads to, production of IL-4. This differs from TH1 and TH17 polarization, which are not induced by their respective cytokines, although these are involved in lineage stabilization (10, 55). In addition to reducing TH1 polarization, IL-4 may have direct immunosuppressive effects on CD8+ T cells, as in vitro activation of naïve CD8+ T cells in the presence of IL-4 reduces effectiveness of adoptive transfer of tumor-specific transgenic T cells (94, 95). It should be noted however, that these cells, termed TC2 (as opposed to TC1 CD8+ T cells activated in the presence of IL-12), were still able to improve survival from B16 melanoma cells in the lung when transferred in greater quantities (95). Subsequent work by the same group showed that IL-4 and IL-5 expression by the adoptively transferred TC2 cells was important in mediating this effect (96). Recombinant expression of IL-4 by several tumor lines also greatly improves clearance (97), and one study found that ovalbumin (OVA)-specific TH2 polarized cells helped clear B16 melanoma lung colonies expressing OVA through recruitment of eosinophils (98). Is IL-4 production, and by extension TH2 polarization, therefore protective? Probably not, since the increased anti-tumor response does not depend upon endogenous IL-4, but instead upon an effective TH1 and CTL response by infiltrating leukocytes (96, 99). By promoting inflammation and leukocyte recruitment, IL-4 production does appear to improve anti-tumor immunity. However, this protective effect is time-dependent, with later stage tumors more resistant to the adoptive transfer of IL-4 producing cells CD8+ cells (95). Adoptive transfer of IL-4 producing TH2 cells, or administration of IL-4 intravenously, also increases lung colonization of B16 melanoma cells injected intravenously (100). Thus, while IL-4 has the potential to improve anti-tumor immunity, its use may be limited therapeutically to inducing acute inflammation prior to the development of a chronically inflamed tumor microenvironment, with a TH2 type response itself being detrimental for anti-tumor immunity.
Also produced by TH2 cells, IL-13 affects the immune response in many of the same ways as IL-4 through activation of Stat6 (101). As with IL-4, IL-13 expression by tumors can improve the anti-tumor response (102), while tumor inhibiting endogenous IL-13 can prevent recurrence (103). T cells do not express the type II IL-4 receptor necessary for binding to IL-13 however, and IL-13 instead appears to inhibit the CTL response indirectly by increasing TGF-β production by myeloid cells in the tumor (104). Notably however, the source of this IL-13 did not appear to be TH2 cells in this model, but instead NKT cells (103). Both IL-4 and IL-13 are considered to be the inducers of the M2 phenotype in monocytes/macrophages, reducing inflammatory cytokine expression and increasing expression of immune suppressive cytokines, resulting in indirect immunosuppression (7, 105). We have recently reported that IL-4 also affects late-stage mammary cancer development by mediating the activity of tumor-associated macrophages (TAMs) (77). In the MMTV-PyMT mouse model of mammary carcinogenesis, lung metastasis was dramatically reduced by genetic deficiency of either CD4 or IL-4Rα (which would prevent IL-4 and IL-13 signaling). In vitro, IL-4 expression by TH2 cells was found to increase expression of epidermal growth factor (EGF) by TAMs, enhancing the ability of neoplastic cells to extravasate into the circulation. IL-4 and IL-13 may also affect tumor growth independent of their effects on the immune system, as many cell types express the type II IL-4 receptor that binds both IL-4 and IL-13 (106). This has not been addressed by many studies, but IL-4 has been shown to reduce angiogenesis by inhibiting endothelial cell migration (107, 108). IL-4 and IL-13 also inhibit proliferation of several epithelial cancers, although IL-13 can also promote proliferation and/or inhibit apoptosis of some hematological malignancies (101) as well as breast carcinomas in some models (109).
Natural Killer T cells
NKT cells play a key regulatory role in directing a TH1 or TH2 polarized immune response through the rapid production of IFNγ, TNFα, IL-4, and IL-13 following stimulation. This is observed in mice deficient for NKT cells, as infections in these mice, particularly bacterial or parasitic, are often more severe (110). As with conventional CTL and TH cells, NKT cells develop in the thymus and express the α and β chains of the TCR. Instead of recognizing a peptide presented by MHC class I or II molecules however, the TCR expressed by NKT cells recognizes glycolipid antigens presented by CD1d, a non-classical member of the MHC family (14). Type I, or invariant, NKT cells (iNKT) express a specific alpha chain variable (V) and joining (J) region (Vα14-Jα18 in mice, Vα24-Jα18 in humans) in combination with a limited number of β chains, and were identified for their ability to recognize α-galactosylceramide (α-GalCer). Type II NKT cells, while also recognizing CD1d, express a variety of αβ TCR chains and are activated by glycolipids that remain poorly defined.
Interest in targeting NKT cells for anti-cancer therapy began with the discovery that treatment with α-GalCer increased the survival time of mice injected with B16 melanoma cells (111). The anti-tumor effects of α-GalCer (112) and IL-12 treatment (113) were subsequently found to depend upon the presence of iNKT cells. Jα18-deficient mice, that lack iNKT cells, were also found to be more susceptible to MCA-induced fibrosarcomas (84). Although capable of directly lysing tumor cells in a perforin-dependent manner, a series of studies by Godfrey and colleagues utilizing MCA-induced fibrosarcomas demonstrated that both NKT-dependent immune surveillance and protection provided by IL-12/α-GalCer therapy was dependent upon IFNγ production by NKT cells, leading to CTL-dependent and NK-dependent anti-tumor responses, respectively (84, 114, 115). It has yet to be determined if these same mechanisms are at play in solid tumors of epithelial origin or in models of de novo cancer development.
In addition to possible direct effects of IFNγ production by NKT cells on CTL and NK cells, α-GalCer treatment has been shown to act as an adjuvant through NKT-dependent DC activation and IL-12 production (116, 117). Pulsing DCs in vitro with α-GalCer prior to adoptive transfer also more effectively prevents liver metastasis of B16 melanoma cells than injection of α-GalCer (118), possibly by improving long-term IFNγ production and limiting TH2 cytokine expression (119, 120). Unfortunately, despite these successes in murine tumor transplantation models, injection of α-GalCer, α-GalCer pulsed DCs, or transfer of α-GalCer activated NKT cells all proved ineffectual in early clinical trials in patients with a range of cancer types, even though NKT activation was evident (121).
Using transplantable sarcoma models in which immune surveillance was evident, work by Berzofsky and colleagues found increased resistance of CD1d-deficient mice to tumor development (122). This was due to the absence of IL-13 producing NKT cells in these mice (103), which were responsible for promoting TGFβ production by splenic CD11b+Gr-1+ immature myeloid cells in a model using NIH/3T3-derived cell lines (104). By comparing Jα18-deficient (lacking type I iNKT cells) to CD1d-deficient mice (lacking both type I and type II NKT cells), they concluded that type II NKT cells were responsible for inhibiting immune surveillance in several models where CD25-depletion had no effect (123). Importantly, in agreement with other studies, α-GalCer treatment increased protection in these models (124). Meanwhile, stimulation of at least a portion of type II NKT cells with a sulfatide compound reduced protection, and could even counteract the protection offered by α-GalCer treatment (124). The most parsimonious explanation for these observations is that IFNγ production by type I NKT cells improves the CTL and NK cell responses, while IL-13 production by type II NKT cells inhibits the immune response (Fig. 2). As T, NK, and NKT cells are unresponsive to IL-13, the results also suggest that the effects of NKT cells depend on an intermediate cell, such as DCs (125). It should be noted however that studies using other tumor injection models found that improved protection of CD1d-deficient mice was not related to IL-13 (126, 127). Protection through adoptive transfer of NKT cells is also dependent on whether the NKT cells are CD4+ or CD4−, and upon the tissue used to isolate NKT cells, which relates at least partially to production of IL-4 (128). In humans, peripheral blood CD4− NKT cells expressed only IFNγ and TNFα, whereas CD4+ NKT cells produced both TH1 and TH2 type cytokines following stimulation (129, 130). A possible role for cytotoxic effector functions by NKT cells can also not be ruled out. Song and colleagues found that human NKT cells can directly kill monocytes pulsed with lysate from human neuroblastoma cell lines (131). Using immunocompromised mice, the authors found that human NKT cells reduced the number of monocytes in the tumors and inhibited growth of the xenograft, indicating that NKT cells also inhibit pro-tumor immunity by killing tumor-associated macrophages. Tissue localization and tumor type may therefore greatly affect cytokine expression by NKT cells and determine whether they promote or inhibit the anti-tumor response.
CONCLUSIONS
Although first described over 20 years ago (132), the complexity by which the immune system directs a TH1 or TH2 response is only now being appreciated. The concept of polarized populations of immune cells has now been expanded to include CD8+ CTLs, macrophages, and NKT cells. Whether these populations can be defined by genetic programs remains to be determined, but the effects of this polarization on the anti-tumor response demonstrates the importance for further research. Inhibiting immune suppression by blocking the activity of regulatory immune cells, or blocking self-suppression of the CTL response by inhibitory molecules such as PD-1 and CTLA-4, holds great potential for improving anti-tumor responses. These approaches will likely be enhanced by therapies that also dampen the effect of pro-tumor immune molecules released by other leukocytes that enhance angiogenesis, tissue remodeling and cell survival pathways, and in combination, may increase clinical efficacy of adoptive transfer therapies to engender durable anti-tumor immunity. Early clinical results have shown success in some of these areas, although the ability to inhibit peripheral tolerance and improve the anti-tumor response appears directly related to the severity of autoimmunity that is also induced. Being able to release the full potential of the immune response, while also being able to appropriate direct and control that response, is key to the future of immunotherapy.
Acknowledgments
The authors would like to acknowledge all of the scientists who made contributions to the areas of research reviewed here that were not cited due to space constraints. The authors acknowledge support from Postdoctoral Awards from the Department of Defense Breast Cancer Research Program (BR), the American Cancer Society (DGN), and the American Association for Cancer Research (NIA), as well as grants from the National Institutes of Health, the Dr. Susan Love Research Foundation, and a Department of Defense Era of Hope Scholar Award (LMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 2.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 3.De Palma M, Coussens LM. Immune cells and inflammatory mediators as regulators of tumor angiogenesis. In: Figg WD, Folkman J, editors. Angiogenesis: An integrative approach from science to medicine. New York: Springer; 2008. pp. 225–238. [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 11.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cell Mol Immunol. 2008;5:401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 15.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 16.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 17.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 18.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 19.Daniel D, Meyer-Morse N, Bergsland EK, Dehne K, Coussens LM, Hanahan D. Immune enhancement of skin carcinogenesis by CD4+ T cells. J Exp Med. 2003;197:1017–1028. doi: 10.1084/jem.20021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 21.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2:373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 22.Herrero JI, Lorenzo M, Quiroga J, et al. De Novo neoplasia after liver transplantation: an analysis of risk factors and influence on survival. Liver Transpl. 2005;11:89–97. doi: 10.1002/lt.20319. [DOI] [PubMed] [Google Scholar]
- 23.Fung JJ, Jain A, Kwak EJ, Kusne S, Dvorchik I, Eghtesad B. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 2001;7:S109–S118. doi: 10.1053/jlts.2001.28645. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis. 2001;60:433–441. doi: 10.1136/ard.60.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bei R, Masuelli L, Palumbo C, Modesti M, Modesti A. A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: Inflammation in their induction and impact on tumor growth. Cancer Lett. 2009;281:8–23. doi: 10.1016/j.canlet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 27.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 30.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 31.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 36.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto S, Greene M, Sehon AH. Immunosuppressor T cells in tumor bearing host. Immunol Commun. 1975;4:201–217. doi: 10.3109/08820137409055774. [DOI] [PubMed] [Google Scholar]
- 39.Berendt MJ, North RJ. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980;151:69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awwad M, North RJ. Immunologically mediated regression of a murine lymphoma after treatment with anti-L3T4 antibody. A consequence of removing L3T4+ suppressor T cells from a host generating predominantly Lyt-2+ T cell-mediated immunity. J Exp Med. 1988;168:2193–2206. doi: 10.1084/jem.168.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 42.Betts G, Twohig J, Van den Broek M, Sierro S, Godkin A, Gallimore A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br J Cancer. 2007;96:1849–1854. doi: 10.1038/sj.bjc.6603824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 44.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 45.Jordanova ES, Gorter A, Ayachi O, et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 46.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 47.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 48.Merlo A, Casalini P, Carcangiu ML, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–1752. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 49.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Adv Drug Deliv Rev. 2006;58:948–961. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 51.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerena-Lewis M, Crawford J, Bonomi P, et al. A Phase II Trial of Denileukin Diftitox in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer. Am J Clin Oncol. 2009 doi: 10.1097/COC.0b013e318187dd40. [DOI] [PubMed] [Google Scholar]
- 53.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 54.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 55.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B, Rong G, Wei H, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 62.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 63.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 64.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 65.Nam JS, Terabe M, Kang MJ, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69:2010–2017. doi: 10.1158/0008-5472.CAN-08-3479. [DOI] [PubMed] [Google Scholar]
- 67.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 69.Kortylewski M, Yu H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol. 2008;20:228–233. doi: 10.1016/j.coi.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z, Laurence A, Kanno Y, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 74.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 75.Girardi M, Oppenheim D, Glusac EJ, et al. Characterizing the protective component of the alphabeta T cell response to transplantable squamous cell carcinoma. J Invest Dermatol. 2004;122:699–706. doi: 10.1111/j.0022-202X.2004.22342.x. [DOI] [PubMed] [Google Scholar]
- 76.Daniel D, Chiu C, Giraud E, et al. CD4+ T Cell-mediated antigen-specific immunotherapy in a mouse model fo cervical cancer. Cancer Res. 2005;65:2018–2025. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- 77.DeNardo DG, Baretto JB, Andreu P, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:19–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 81.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 82.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002;196:129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 86.Noguchi Y, Jungbluth A, Richards EC, Old LJ. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1996;93:11798–11801. doi: 10.1073/pnas.93.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 88.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 89.Gao Y, Yang W, Pan M, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kacha AK, Fallarino F, Markiewicz MA, Gajewski TF. Cutting edge: spontaneous rejection of poorly immunogenic P1.HTR tumors by Stat6-deficient mice. J Immunol. 2000;165:6024–6028. doi: 10.4049/jimmunol.165.11.6024. [DOI] [PubMed] [Google Scholar]
- 91.Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol. 2000;165:6015–6019. doi: 10.4049/jimmunol.165.11.6015. [DOI] [PubMed] [Google Scholar]
- 92.Fallarino F, Gajewski TF. Cutting edge: differentiation of antitumor CTL in vivo requires host expression of Stat1. J Immunol. 1999;163:4109–4113. [PubMed] [Google Scholar]
- 93.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol. 2006;176:905–913. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 94.Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167:6497–6502. doi: 10.4049/jimmunol.167.11.6497. [DOI] [PubMed] [Google Scholar]
- 95.Dobrzanski MJ, Reome JB, Dutton RW. Therapeutic effects of tumor-reactive type 1 and type 2 CD8+ T cell subpopulations in established pulmonary metastases. J Immunol. 1999;162:6671–6680. [PubMed] [Google Scholar]
- 96.Dobrzanski MJ, Reome JB, Hollenbaugh JA, Dutton RW. Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses. J Immunol. 2004;172:1380–1390. doi: 10.4049/jimmunol.172.3.1380. [DOI] [PubMed] [Google Scholar]
- 97.Olver S, Apte S, Baz A, Kienzle N. The duplicitous effects of interleukin 4 on tumour immunity: how can the same cytokine improve or impair control of tumour growth? Tissue Antigens. 2007;69:293–298. doi: 10.1111/j.1399-0039.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- 98.Mattes J, Hulett M, Xie W, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eguchi J, Kuwashima N, Hatano M, et al. IL-4-transfected tumor cell vaccines activate tumor-infiltrating dendritic cells and promote type-1 immunity. J Immunol. 2005;174:7194–7201. doi: 10.4049/jimmunol.174.11.7194. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi M, Kobayashi H, Pollard RB, Suzuki F. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J Immunol. 1998;160:5869–5873. [PubMed] [Google Scholar]
- 101.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 102.Lebel-Binay S, Laguerre B, Quintin-Colonna F, et al. Experimental gene therapy of cancer using tumor cells engineered to secrete interleukin-13. Eur J Immunol. 1995;25:2340–2348. doi: 10.1002/eji.1830250833. [DOI] [PubMed] [Google Scholar]
- 103.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 104.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 106.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volpert OV, Fong T, Koch AE, et al. Inhibition of angiogenesis by interleukin 4. J Exp Med. 1998;188:1039–1046. doi: 10.1084/jem.188.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schuler T, Kornig S, Blankenstein T. Tumor rejection by modulation of tumor stromal fibroblasts. J Exp Med. 2003;198:1487–1493. doi: 10.1084/jem.20030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aspord C, Pedroza-Gonzalez A, Gallegos M, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 112.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 113.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 114.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smyth MJ, Crowe NY, Pellicci DG, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 116.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J Immunol. 1999;163:2387–2391. [PubMed] [Google Scholar]
- 119.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 120.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 121.Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–645. doi: 10.1111/j.1349-7006.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berzofsky JA, Terabe M. KNT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 123.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ambrosino E, Terabe M, Halder RC, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 125.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 127.Terabe M, Khanna C, Bose S, et al. CD1d-restricted natural killer T cells can down-regulate tumor immunosurveillance independent of interleukin-4 receptor-signal transducer and activator of transcription 6 or transforming growth factor-beta. Cancer Res. 2006;66:3869–3875. doi: 10.1158/0008-5472.CAN-05-3421. [DOI] [PubMed] [Google Scholar]
- 128.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song L, Asgharzadeh S, Salo J, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]


