Abstract
Adult female rats with high levels of circulating estradiol are biased to use a place strategy to solve an ambiguous spatial navigation task and those with low levels are biased to use a response strategy. We examined the development of this hormonal modulation of strategy use by training juvenile female rats on an ambiguous navigation task and probing them for strategy use at postnatal day (PD) 16, 21, or 26, after administration of 17 β-estradiol or oil 48 and 24 hrs prior to testing. We found that rats could use either strategy successfully by PD21 but that estradiol did not bias rats to use a place strategy until PD26. In order to evaluate the stability of this effect over multiple navigation experiences, we retested oil-treated juveniles three times during adulthood. On the first adult navigation experience, rats were significantly more likely to use the same navigation strategy they used as juveniles, regardless of current estrous cycle phase. On the second and third adult tests, after rats had more experience with the task, previous navigation experience did not predict strategy use. Rats in proestrus were significantly more likely to use a place strategy while rats in estrus and diestrus did not appear to have a group bias to use either strategy. These results suggest that estradiol can modulate spatial navigation strategy use before puberty but that this effect interacts with previous navigation experience. This study sheds light on when and under what circumstances estradiol gains control over spatial navigation behavior in the female rat.
Keywords: estradiol, spatial navigation, development, place strategy, response strategy, hippocampus, striatum
Estrogens modulate learning and memory of spatial navigation tasks (see Daniel, 2006; Korol, 2004; Galea et al., 2008). These effects have been demonstrated by comparing females in a variety of hormonal conditions, including intact rats at different stages of their cycles, pregnant and/or lactating dams with virgins, and ovariectomized females with those replaced with estradiol (e.g., Galea et al., 2000; Luine et al., 2006; Sandstrom & Williams, 2004). A large body of evidence supports the view that females with higher levels of estrogens show enhanced hippocampus-dependent spatial learning and memory on both appetitively (e.g., Daniel et al., 1997; Liu et al., 2008; Daniel & Dohanich, 2001; Gibbs, 1999; Heikkinen et al., 2002) and aversively-motivated navigation tasks (e.g., Bimonte & Denenberg, 1999; Frye & Rhodes, 2002; Sandstrom & Williams, 2001, 2004; Markham et al., 2002; Frick & Berger-Sweeney, 2001), as well as on tasks like spontaneous alternation (Walf et al., 2009) and object recognition (Luine et al., 2003; Walf et al., 2009; Fernandez et al., 2008; Wallace et al., 2006), which do not require food deprivation, shock, or cool water for motivation. However, under certain conditions, estradiol has either no effect or impairs performance on many of these same tasks, including radial-arm maze (Ziegler & Gallagher, 2005; Galea et al., 2001; Luine & Rodriguez, 1994; Luine et al., 1998; Holmes et al., 2002), water maze (Berry et al., 1997; Chesler & Juraska, 2000; Fugger et al., 1998; Galea et al., 1995; Warren & Juraska, 1997; Frye, 1995), active avoidance (Diaz-Veliz et al., 1989; Daniel et al., 1999), and a 2-choice working memory water task (O’Neal et al., 1996). These varying effects of estradiol may be due to differences in the task, paradigm of hormone administration, dependent measures, age, or species used in the studies (for review, see Daniel, 2006). In addition to the effects of estrogens on hippocampal-dependent spatial navigation tasks, they have also been shown to impair learning on a number of tasks that rely on the dorsal striatum (e.g., White & McDonald, 2002), including win-stay, stimulus-response (Galea et al., 2001), cued (Daniel & Lee, 2004), and response navigation tasks (Korol & Kolo, 2002; Davis et al., 2005).
Several studies have directly examined the effects of estradiol on learning of spatial tasks that are almost identical but differ only in the strategy that is required to solve the task (Korol & Kolo, 2002; Davis et al., 2005; Zurkovsky et al., 2007). For example, on a plus-maze task that required rats to find a food reward using extra-maze cues (i.e., a place strategy), young adult ovariectomized rats administered 10 µg 17-β estradiol benzoate 48 and 24 hr prior to training learned more rapidly than their oil-treated counterparts (Korol & Kolo, 2002). However, when the task required rats to learn a motor response strategy (take a left or right turn) to locate the food and there were no extra-maze cues available, estradiol-treated rats had impaired learning compared to oil-treated controls. Estradiol administration has similar effects on place and response navigation when replacement is given chronically (e.g., a 0.5 mg 60-day release pellet) and when a more complex eight-arm radial maze is used to examine spatial learning (Davis et al., 2005). In addition, when rats are trained on a plus maze task in which either a place or response strategy may be used to find a food-baited arm, intact females in proestrus and estrus do not differ in their learning rate, but those in proestrus are more likely to use a place strategy and those in estrus are more likely to use a response strategy (Korol et al., 2004). These data have all been collected using young adult female rats as subjects, and to date, the development, and stability of estradiol effects on spatial navigation strategy choice and performance has never been examined.
Based on the findings described above indicating that the presence of high levels of estradiol increases the likelihood of using a place strategy and the absence of high levels of estradiol increases the probability of using a response strategy in adulthood, one might predict that prepubertal females would be biased towards using a response strategy until the rise in estradiol signaling the start of puberty activates neural networks to modulate place and response strategy use. Examination of the development of place and response strategies has shown that both male and female rats are able to locate a hidden platform in a water maze that is marked with a proximate cue by postnatal day (PD) 17, but they can not use extra-maze cues to find the hidden platform until PD20 (Akers & Hamilton, 2007; Rudy et al., 1987). Thus, both navigation strategies are available well before puberty, although it is unclear whether use of a response strategy is the “default” strategy for young rats when both strategies may be used and whether neural networks for navigation are sensitive to estradiol action prior to the onset of estrous cyclicity as are other neural and behavioral systems, such as female mating behaviors including lordosis and ear wiggling (Williams, 1987; Williams & Blaustein, 1988).
In addition to questions about the development of estradiol-modulated spatial navigation, studies in adult females have all used a between subject experimental design (Korol & Kolo, 2002; Davis et al., 2005; Quinlan et al., 2008), and therefore it is unclear whether navigation strategy use varies across the estrous cycle within individual rats. While there is no evidence that learning and working memory requiring place navigation varies across the estrous cycle in individual rats (Stackman et al., 1997), this issue has been examined very little. In addition, it is possible that experience using one navigation strategy may influence what strategy will be used upon subsequent experiences with the task. Thus estradiol may only modulate strategy use in inexperienced females, and experience may greatly influence the strategy used on subsequent spatial navigation tasks.
In order to avoid food deprivation in our juvenile subjects, we used a simplified water-based T-maze task in which the hidden escape platform could be found using either a place or response strategy to determine the development of estradiol-modulated navigation in rats from 16 to 26 days of age. Additionally, we retested females several times in adulthood at various stages of their estrous cycle to determine a) whether estradiol is able to maintain control over strategy selection over several experiences with the navigation task and b) the extent to which previous experience in the task interacts with the ability of estradiol to influence strategy use.
Materials and Methods
Subjects
Subjects were 80 female offspring from 10 timed-pregnant Sprague-Dawley CD rats purchased from Charles River Laboratories (Kingston, NY). Pregnant dams arrived in our colony at Duke University on their ninth day of gestation and were singly housed in individually-ventilated, transparent shoebox cages with corn cob bedding and ad libitum access to water and a standard diet (Rodent Diet 5001, PMI Nutrition International, Inc., Brentwood, MO). The temperature-controlled colony room was maintained on a 12:12 hr light:dark cycle with lights on at 7 a.m. daily. At birth, pups from all 10 litters were sexed and randomly assigned to foster mothers, and litters were culled to approximately 6 females and 4 males. Pups were weaned on postnatal day 26 (PD26) after the last age group of juveniles was tested. Females that were also behaviorally trained as adults were pair-housed at weaning, with the same food and living conditions described for dams above.
Treatments and experimental time points
Two females from each litter (n = 20 at each age) were behaviorally trained at PD16, PD21, or PD26. Forty-eight and 24 hours prior to the training day, one female from each litter was administered a subcutaneous (s.c.) injection of 5µg 17 β-estradiol (Sigma-Aldrich, St. Louis, MO) dissolved in 0.1ml sesame oil (Sigma-Aldrich, St. Louis, MO) in the nape of the neck, and the other pup from each litter was injected with the oil vehicle alone. Twice the amount of estradiol given on the same schedule to the adult female rat produces circulating estradiol just above those found in the intact rat in proestrus (75–90 pg/ml; Viau & Meaney, 1991), increases hippocampal CA1 spine density (Gould et al., 1990; Woolley & McEwen, 1993), and increases learning rate when a place strategy is needed to solve a hippocampal-dependent maze task (Korol & Kolo, 2002).
All oil-treated juveniles were raised to adulthood and tested again three times, at 3.5, 4.5, and 5.5 months of age in a repeated measures design. In order to determine estrous cycle status and confirm normal cycling, vaginal samples were taken daily at 09:30 for 3 weeks prior to each adult test. Cells were collected on a moistened cotton swab, rolled onto a glass slide, and examined at 10X magnification to determine estrous cycle status based on the proportion of leukocytes, epithelial, cornified, and nucleated cells (Matthews & Kenyon, 1984).
In addition to the 60 rats already described, twenty rats were ovariectomized (OVX) either at PD22 or PD44 (n = 10 each) and tested at 3.5 months of age. These ages were chosen because in our vivarium, offspring of timed pregnant Sprague Dawley rats reach puberty at PD34 as defined by vaginal opening; therefore, rats were ovariectomized either before or after puberty.
Ovariectomies
In order to remove the source of circulating gonadal hormones, rats were anesthetized for with a combination of 80 mg/kg ketamine plus 10 mg/kg xylazine and ovariectomized via bilateral dorsal incisions through the skin and muscle walls of the abdomen. The ovary and ovarian fat on each side of the body were exposed, tied off, and surgically removed with a scalpel. The site of removal was cauterized and carefully placed back into the abdominal cavity. The muscle wall and skin were sutured and antibiotic cream was applied to the wound site. Buprenorphine (0.5 mg/kg, i.p.) was administered at the end of all surgeries and again 12 hr later as analgesic. Rats were kept warm on heating pads until they woke up and then returned to their cagemates. Powdered food was offered in bowls on the cage floor for several days until rats were seen eating from the overhead food bin. All rats recovered without complications and were included in the study.
Apparatus and testing room
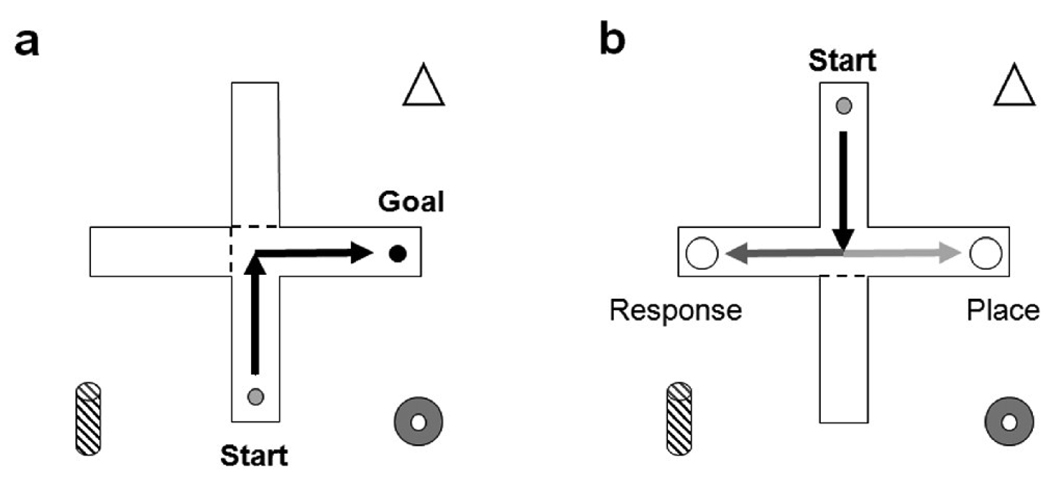
All behavioral training took place in a clear Plexiglas plus-maze placed inside a black plastic pool with a diameter of 1.8m. Each arm of the plus maze was 50.8 cm long and 15.2 cm wide, with a 15.2 cm x 15.2 cm center and 30 cm high walls. The maze was filled with approximately 15.5 cm of water mixed with black water-based paint, which was maintained at 24–25 °C. A platform with diameter 10.2 cm, hidden just below the water’s surface, was placed at the end of the goal arm. Rats could be prevented from entering arms of the maze by inserting a clear piece of Plexiglas at the base of the arm (see Figure 1).
Fig. 1.
Ambiguous water T-maze task. (a) Rats received 10 training trials on which they were always started from the south arm of the maze and had to navigate to the hidden platform at the end of the adjacent arm, with the other two maze arms blocked by clear plexiglass. The start and goal arms remained the same for all 10 training trials. (b) Immediately following 10 training trials, rats received a probe trial in which they were started from the north arm and only the opposite arm was blocked.
The maze was located in a 6.5 m × 3.8 m rectangular room (1.3 m from the North wall and 0.7 m from the East wall) that was rich with cues including a curtain, table with computer, cart with rat cages, counter, shelves, and walls with posters and objects with high-contrast patterns. We hung three additional cues of different shapes, colors, and contrast patterns 75 cm above the height of the water and outside the radius of the maze to provide additional extramaze cues in order to ensure that any failure of task learning in juveniles was not due to the rats’ poor visual acuity at these young ages. Hanging cues were quasi-randomly placed in four possible locations and counterbalanced across estradiol-treated rats within each juvenile age group, as well as within each adult test and across adult tests for each rat. No significant differences in latency or strategy were observed across cue configurations, therefore, all groups were combined for further data analysis. Oil-treated littermate controls were always trained in the same apparatus configuration as their estradiol-treated littermates.
Behavioral training and testing
Behavioral training and probe testing took place within a single session that consisted of 10 training trials and one probe trial during the lights-on portion of the day. Rats were trained in groups of four, which consisted of two estradiol-treated females from different litters and their two oil-treated littermates. Each rat was removed from its litter and placed in a clean holding cage with its littermate. Holding cages were then transported on a cart to the maze room, where the cart remained throughout behavioral training. During the 10 training trials, rats were always started from the arm closest to the experimenter (South arm) and the goal arm was either the West or East arm (counterbalanced across rats and tests). Clear plexiglass inserts blocked the other two arms, forming an “L” shaped route such that the rat could only enter the correct arm of the maze (see Figure 1). The apparatus remained in the same configuration for all 10 training trials.
To begin the first trial of the session, one rat was taken from its holding cage and placed in the South arm of the maze. A trial was considered complete when the rat climbed onto the escape platform at the end of the goal arm. If the rat did not find the platform within 60 s, it was guided to the platform by the experimenter. The rat was allowed to stay on the platform for 15 s and then placed on a dry towel in an opaque bucket. All walls of the maze were wiped with ethanol between trials to remove intramaze odor cues. Five consecutive trials were run in this fashion. The rat was then dried with a towel, and returned to its holding cage that was warmed by a space heater. The cue configuration and/or platform location were changed if necessary and the next rat completed 5 trials. After all 4 rats had completed 5 trials and were resting in holding cages, the first rat received 5 more training trials. Immediately following the last trial, the platform was removed and the Plexiglas inserts were moved so that only the South arm was blocked off, forming a T-shaped maze, in order to conduct a probe trial (see Figure 1b). Cues remained in the same configuration, and the rat was started from the North arm and allowed to swim for 60 s. During all training and probe trials, latencies to reach the end of any arms entered and latency to mount the platform were recorded by the experimenter and the path of the animal was recorded in real time using HVS Image software (Buckingham, WH, UK).
All rats were trained once as juveniles, at PD16, 21, or 26. Those administered oil as juveniles were raised to adulthood and tested again three times in adulthood as described above. All procedures were approved by the Institutional Animal Care and Use Committee of Duke University.
Time spent in start arm as a measure of strategy use
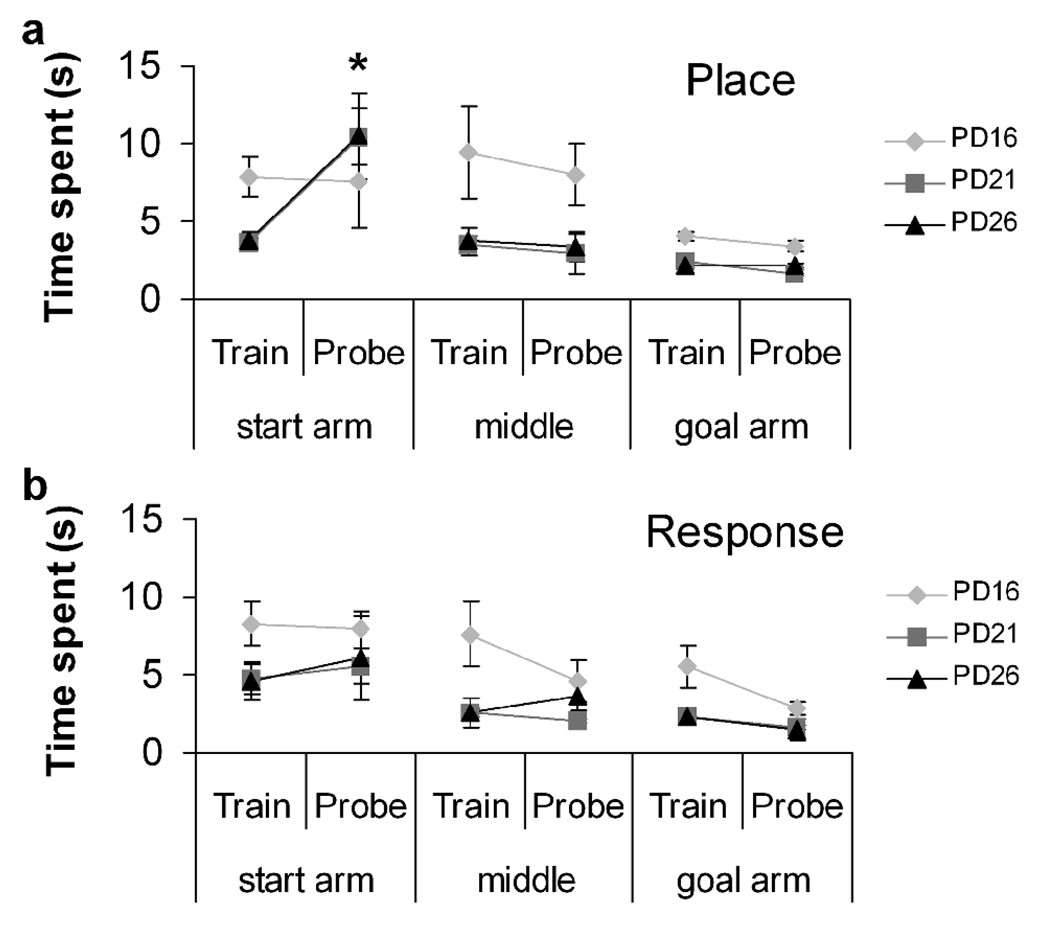
After 10 training trials, rats completed a probe trial which required them to start the maze from a novel arm 180° from the start arm used during training. Rats that returned to the same spatial location in the room that the platform had been located during training were considered to have used a “place” strategy, and rats that made the same turn (i.e., left or right) that they had taken on the training trials were categorized as using a “response” strategy. In order to confirm that rats were using different strategies to navigate rather than choosing an arm randomly, time spent in each segment of the maze (i.e., start arm, middle, goal arm) for each rat on each trial was examined. Rats trained at PD21 or PD26 that were categorized as place strategy users spent significantly more time in the start arm on the probe trial than during the last three training trials, when performance latency had reached asymptote (PD21: t(9) = 3.51, p = 0.007; PD26: t(14) = 2.32, p = 0.036), but rats at PD16 did not (p > 0.80; see Figure 2a). This increase in time spent occurred only in the start arm and occurred in a significant proportion of PD21 and PD26 place strategy users (PD21: χ2 = 11.15, p = 0.0008; PD26: χ2 = 8.89, p = 0.002), but was random in PD16 rats (χ2 = 0.09, p > 0.75). Once PD21 and PD26 place strategy rats reached the center of the maze, they spent no more time in these areas on the probe trial than they did on trials 8–10 (p > 0.10; Figure 2a). In contrast, rats that used a response strategy spent a similar amount of time in all parts of the maze during the probe trial as they did during their last 3 training trials indicating that they behaved similarly even though the start location had changed (p > 0.10, see Figure 2b). This analysis suggests that place strategy users have a representation of the environment to which they must reorient when they solve the task from the new location. In contrast, response strategy users simply employ the same motor response acquired during training (e.g., “take a left”) and therefore spend a similar amount of time in each segment of the maze on the probe as the last few training trials. Change in latency to reach the middle of the maze on a probe trial is a measure of strategy learning that is independent of arm choice. Therefore, this measure is a useful for assuring that rats have learned the task and are employing a strategy on the probe trial rather than choosing a goal arm at random or by mistake. This measure may be used in the future to confirm strategy use.
Fig. 2.
The difference between the time spent on the probe trial and training trials 8–10 for each segment of the maze. a) PD21 and PD26 rats that were categorized as using a place strategy spent significantly more time in the start arm during the probe than the last three training trials (PD21: t(18) = 3.618, p = 0.002; PD26: t(28) = 2.364, p = 0.025), while this was not the case for any other segment for PD21and PD26 rats or any segment for PD16 rats; b) All rats that used a response strategy spent a similar amount of time in each maze segment for the probe and training trials.
Statistical analysis
All analyses were calculated using an α value of 0.05. Because strategy choice was a binary dependent measure and group n’s were small, we used two-tailed exact binomial tests to determine whether strategy use of each group differed from the null hypothesis that there was no strategy bias (that is, 50% of the group used a place strategy and 50% used a response strategy). In addition, two-tailed Liebermeister’s quasi-exact test for small sample sizes, which is an appropriate Bayesian alternative to Fisher’s exact test when the frequency of one variable is not fixed (in this case, strategy used; see Seneta & Phipps, 2001), was used to determine differences in strategy use across groups in order to evaluate the effects of developmental age, estradiol treatment in adolescence, and estrous status in adulthood on strategy bias. Repeated measures ANOVAs were performed with latencies on the 10 training trials as the dependent measures and strategy (place or response) and estradiol status (estradiol- vs. oil-treated or estrous phase) as the independent measures to examine any differences in the rate of learning between groups. Because path length and latency measures were highly correlated, only latency measures are reported. T-tests were used to determine whether rats spent a significantly different amount of time in each part of the maze on the probe than on the last three training trials.
Results
Juvenile strategy use and learning
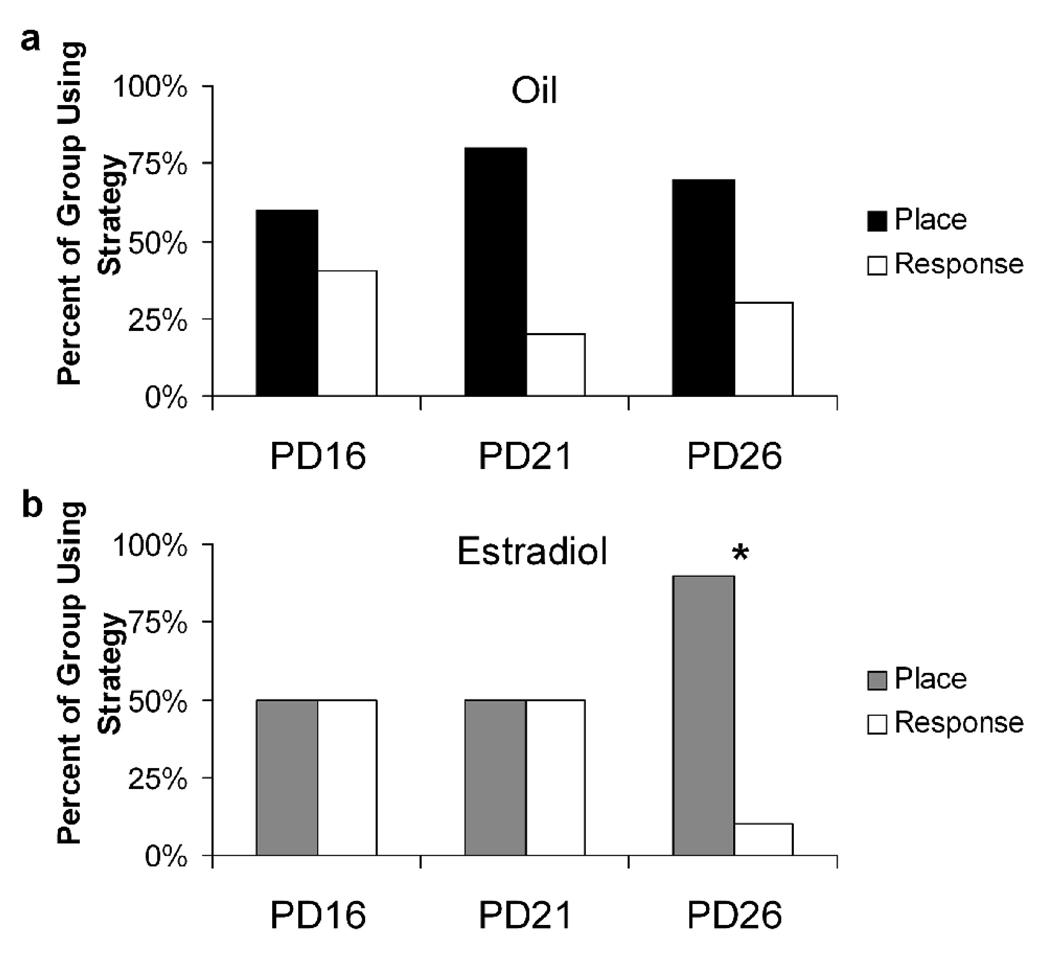
There were no significant differences in strategy use between estradiol- and oil-treated rats at any juvenile age (p > 0.15). However, 26-day-old estradiol-treated rats showed a significant bias toward using a place strategy when compared to chance (p = 0.021; Figure 3), while no other oil- or estradiol-treated groups showed a strategy bias (p > 0.15). In addition, PD26 rats administered estradiol were significantly more likely to use a place strategy than PD21 rats (p = 0.034), but there was no change in strategy use between PD16 and PD21 rats given estradiol or between any developmental age in oil-treated rats (p > 0.20). Together, these data suggest that estradiol was able to bias females to use a place strategy by PD26, at least a week before puberty.
Fig. 3.
The proportion of rats in each age group that used place and response strategies in control (a) and estradiol-treated (b) conditions shows that estradiol biased females to use a place strategy at PD26.
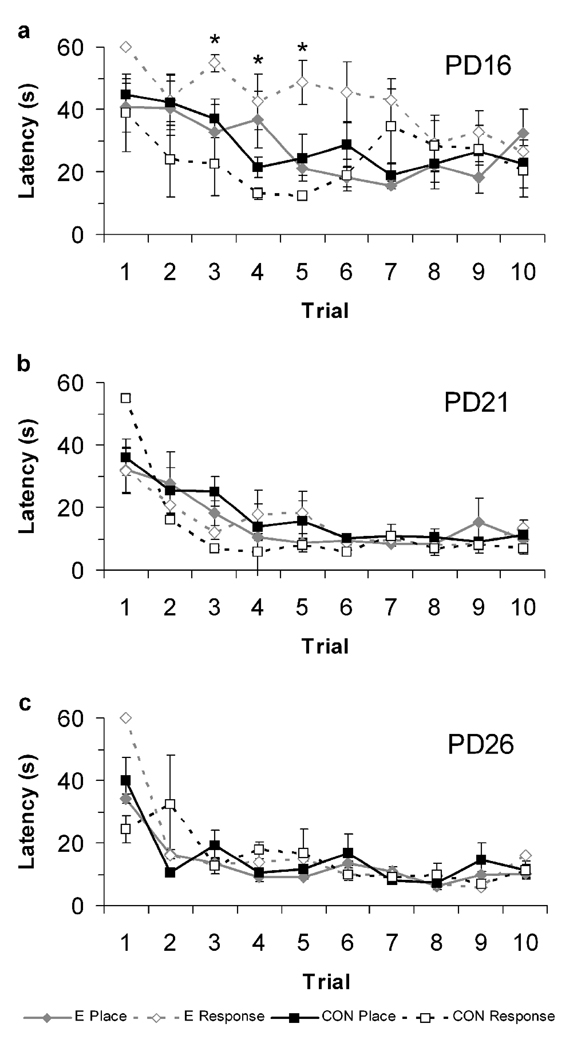
A repeated measures ANOVA with strategy and estradiol status as the independent variables and trial latency (trials 1–10) as the dependent variable were calculated for each age. The analysis for PD16 rats revealed a main effect of trial (F(9,144) = 4.87, p < 0.0001) and an estradiol status × strategy interaction (F(1,16) = 5.65, p = 0.030), but no other effects (p > 0.05; Figure 4a). Individual trials analyses revealed that PD16 rats given estradiol that were classified as response strategy users had significantly longer trial latencies than oil-treated rats using a response strategy on trials three (t(7) = 3.41, p = 0.011), four (t(7 )= 2.85, p = 0.025), and five (t(7) = 4.52, p = 0.003), but no other trials (p > 0.05). There were no differences in trial latency between oil and estradiol-treated place strategy users. ANOVAs for PD21 and PD26 revealed main effects of trial on PD21 (F(9,63) = 6.99, p < 0.0001) and PD26 (F(9,63) = 8.33, p < 0.0001) but no other effects (p > 0.10), indicating that all groups learned the task at similar rates (see Figure 4b and c).
Fig. 4.
Latencies on the first 10 trials of PD16 (a), PD21 (b), and PD26 (c) rats show that PD16 rats that were treated with estradiol and used a response strategy had significantly longer latencies than control rats that used a response strategy. * indicates p < 0.05.
Adult strategy use and learning
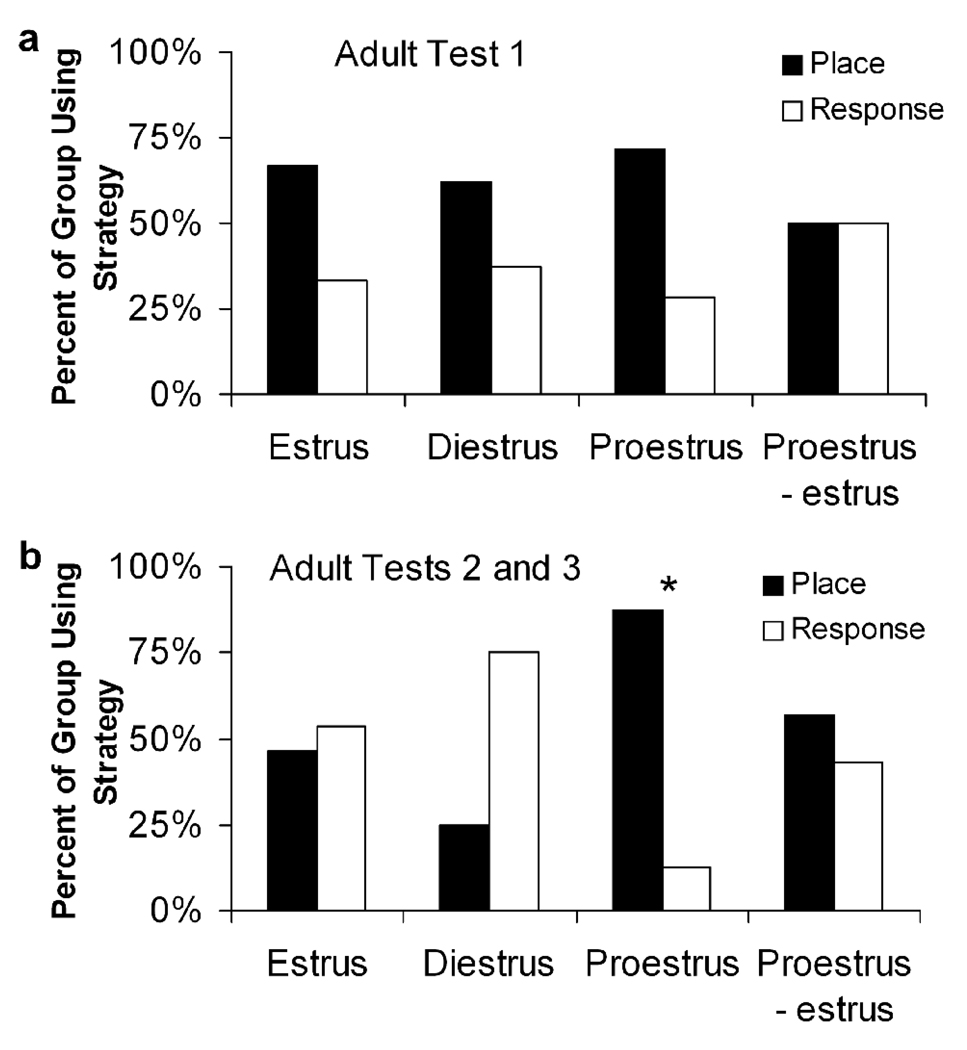
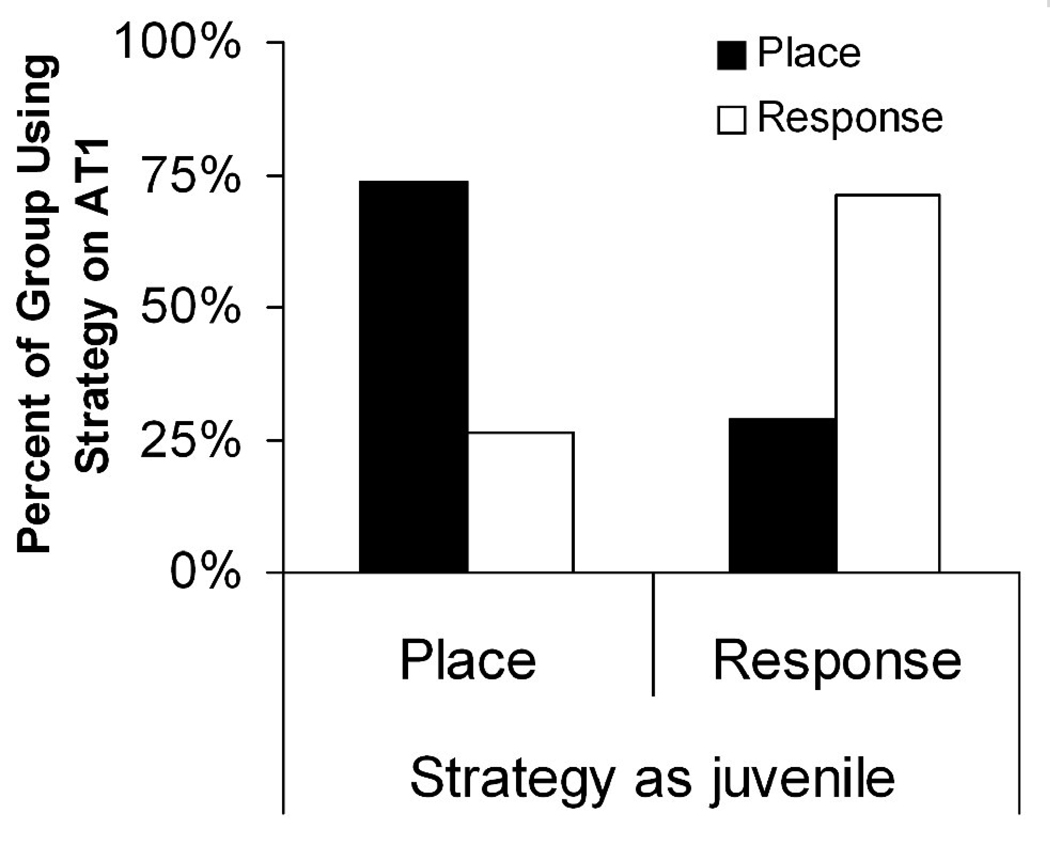
Once control rats grew to adulthood, they underwent the same training and testing paradigm three times in different estrous phases but with different cue configurations. On adult test 1 (AT1), 66% of rats in estrus, 63% in diestrus, 71% in proestrus, and 50% of rats transitioning from proestrus to estrus used a place strategy. None of these values was different from chance (p > 0.45; Figure 5a). Because we predicted that rats in proestrus would be more likely to use a place strategy than any other group, we directly compared the proestrus group to each other group and to all other groups combined, but none of these comparisons revealed any group differences (p > 0.25). To determine the effects of previous experience with the task, we evaluated the probability of using the same strategy on the first adult test that was used on the juvenile test. Regardless of estrous phase at AT1, rats were significantly likely to use the strategy on AT1 that they used as juveniles (p = 0.024; Figure 6). Together, these results suggest that previous experience, but not current estradiol status, strongly influenced choice behavior on AT1.
Fig. 5.
The proportion of rats on (a) adult test 1 and (b) adult tests 2 and 3 in each estrus cycle phase that used place and response strategies, showing that estrus phase did not affect strategy use until adult test 2, when females in proestrus were significantly more likely to use a place strategy than all other groups, χ2 = 12.583, p = 0.0003.
Fig. 6.
Comparison of strategy use across juvenile and adult tests revealed that rats were significantly likely to use the same strategy on adult test 1 as they did as juveniles, regardless of age at juvenile testing or current estrus phase. However, strategy use on the previous adult test had no impact on the strategy used on the current test for either adult test 2 or 3.
We also examined the effects of previous experience and estrous phase on the second (AT2) and third (AT3) adult tests using similar analyses. Neither strategy used as a juvenile nor strategy used on the previous test had a significant effect on strategy choice on either AT2 or AT3 (p > 0.25). When strategy selection was analyzed based on estrous phase, 60% of rats in estrus, 50% in diestrus, 90% in proestrus, and 50% transitioning from proestrus to estrus used a place strategy on AT2, and 40% in estrus, 0% in diestrus, 83% in proestrus, and 67% transitioning into estrus used a place strategy on AT3. Rats were biased to use a place strategy only during proestrus on AT2 compared to chance (p = 0.021), and proestrus rats were more likely to use a place strategy than rats in other estrous cycle phases (ps < 0.10) as well as to all other phases combined (p = 0.048). While there was no significant bias toward using a place strategy during any estrous phase on AT3 (p > 0.20), proestrus rats were more likely to use a place strategy than those in estrus (p = 0.060) and diestrus (p = 0.033). In addition, because no rats were tested in the same estrous phase twice, choice behavior from AT2 and AT3 were combined to confirm that rats in proestrus were significantly more likely to use a place strategy than chance (p = 0.004) but no strategy bias was present during any other estrous phase (p > 0.60; Figure 5b). These results suggest that high estradiol levels, but not previous experience, modulated strategy use on AT2 and AT3.
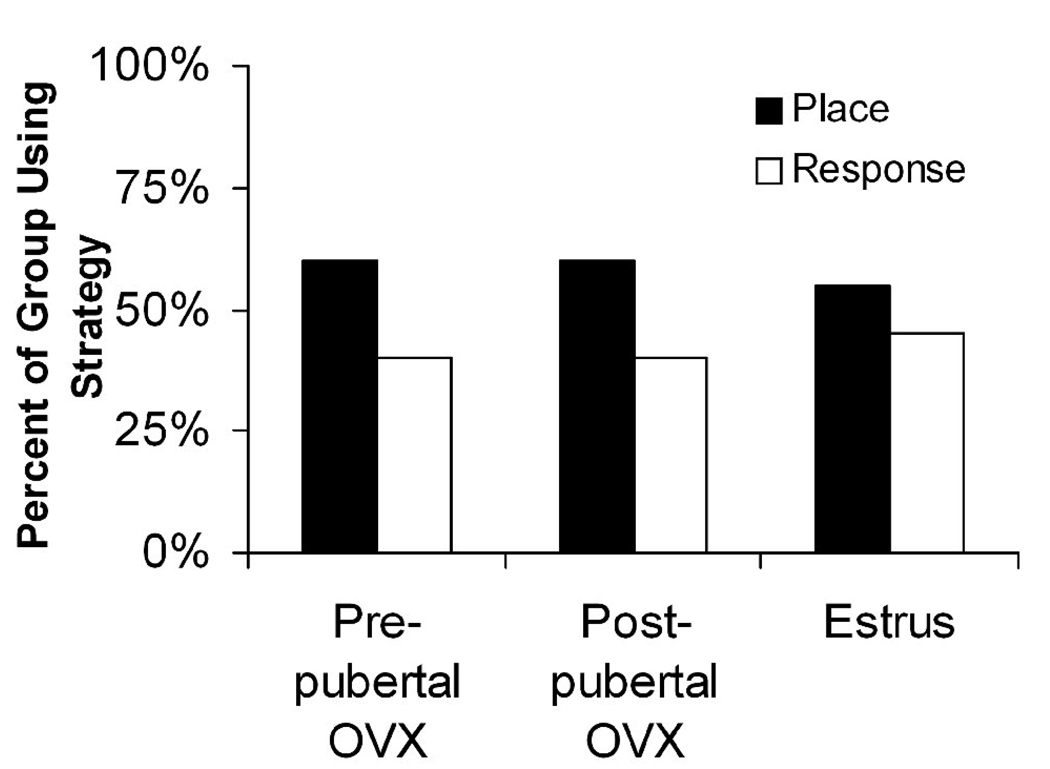
In addition to intact rats tested in adulthood, 60% of rats OVX at PD22 and 60% of rats OVX at PD44 used a place strategy to solve the task when they were tested once as young adults, revealing that there was no bias in either group (p > 0.75; Figure 7). These findings provide supporting evidence that rats with low or no circulating estradiol have no strategy bias in this task. Together, these results suggest that previous experience was the most influential factor on strategy choice at AT1, but high levels of estradiol biased rats to use a place strategy on AT2 and AT3.
Fig. 7.
All low/no estradiol groups in adulthood display no strategy bias.
Repeated measures ANOVAs were calculated for AT1, AT2, and AT3, with independent variables of strategy and estrous phase and dependent variables of latency on trials 1–10. On AT1, there was a main effect of trial, F(9,171) = 33.1, p = 0.000, and a trial × estrous phase × strategy interaction, F(27,171) = 1.93, p = 0.007, but no other effects, p > 0.10. Rats in proestrus that used a response strategy had significantly longer latencies on the first two training trials than those that used a place strategy (trial 1: t(5) = 3.58, p = 0.016; trial 2: t(5) = 3.71, p = 0.014), while response strategy users in estrus had significantly shorter latencies on the first training trial (t(4) = 6.52, p = 0.002). On AT2 and AT3, there were main effects of trial (AT2: F(9, 99) = 5.44, p < 0.0001; AT3: F(9, 144) = 16.68, p < 0.0001) but no other effects or interactions (p > 0.10).
Discussion
The results of the present study are consistent with previous findings that estradiol modulates spatial navigation strategy use in the adult female rat (see Korol, 2004) and extend these findings by showing that prepubertal females can use either place or response strategies by PD21 but their choice of strategy is not altered by estradiol administration until PD26. Our data provide no support for the hypothesis that prepubertal females, because of their low circulating estradiol levels, are more likely to use a response strategy in a spatial navigation task. We have also shown for the first time that the experience of using one spatial navigation strategy biases what strategy is used on the next spatial navigation task, irrespective of current estradiol status. However, after females have experience with multiple estrous cycles and spatial navigation, estradiol gains control of navigation strategy use and its influence can override the effects of prior experience with the task. Thus, we have found that both previous navigation strategy use and experience with cyclical estradiol influence the strategy used to navigate. Finally, we have shown that the normal hormonal transition of puberty is not required for the activational effects of estradiol on spatial navigation strategy use.
The development of estradiol’s modulation of navigation strategy use
We found that estradiol administration may be able to bias female rats to use a place strategy by PD26, as it does in young adult females (Korol et al., 2004; Quinlan et al., 2008). Thus, while both male and female rats have the ability to use extra-maze cues to perform place navigation by 20 days of age (Akers & Hamilton, 2007; Rudy et al., 1987), hippocampal network sensitivity to estradiol in females is not functional for several more days. Interestingly, these results parallel the precocious ability of estradiol to elicit other estrogen-sensitive behaviors such as lordosis and ear wiggling (Williams, 1987), as well as physiological changes such as the induction of progesterone receptors (Williams & Blaustein, 1988). And, it is not surprising that hippocampal and/or striatal sensitivity to estradiol occurs before puberty, as there is evidence that estrogen receptors (ERs) are expressed in both regions in the juvenile rat. Estrogen receptor α (ERα) is expressed at low levels in the hippocampus at PD0 and stabilizes at an adult-like baseline by PD15 after a transient increase that peaks between PD7 and PD10 (Perez et al., 2003; O’Keefe et al., 1995; Solum & Handa, 2001; Orikasa et al., 2000). Estrogen receptor β (ERβ) does not appear to be present in the hippocampus until at least PD14 (Perez et al., 2003), but it is present in the adult (e.g., Le Saux et al., 2006, but see also Orikasa et al., 2000). There is little evidence that ERs are present in the juvenile rat striatum (Toran-Allerand et al., 1992), but both receptor types have been detected in the mouse striatum throughout development at greater or equal levels than that of the adult (Kuppers & Beyer, 1999). Given the many complex mechanisms through which estradiol may influence navigation strategy use and the lack of detailed examination of ER expression during the developmental window that we studied, it remains to be explained why estradiol did not have a behavioral effect until PD26. It is possible that ER sensitivity and/or distribution of expression changes throughout the developmental window between PD21 and PD26 contribute to the development of estradiol modulation of spatial navigation strategy use.
Previous research has shown that low levels of estradiol (as during estrus) bias adult females to use a response strategy to solve an appetitively motivated navigation task (Korol et al., 2004; Quinlan et al., 2008). These results suggest that a response strategy is the “default” for females with no or low estradiol and predict that prepubertal females, who have very low circulating estradiol, are naturally response-biased. Surprisingly, the absence of estradiol did not bias rats at any age to use a response strategy in our water plus maze task. Juveniles, rats ovariectomized shortly before or after puberty and tested in adulthood, and adult females in estrus and diestrus were not, as a group, biased toward using a response strategy. We speculate that our task parameters, room cues, or use of an aversively-motivated water escape task favored place navigation over response navigation. For example, our testing room may be richer with salient geometric and landmark cues than those used by other labs examining this behavior or the use of aversive motivation may have increased rats’ attention to the cues in the environment, increasing the probability of using a place strategy in all rats. In addition, it is possible, although unlikely, that the handling needed for oil injections of prepubertal rats influenced strategy use in adulthood.
While estradiol administration did not influence strategy use in PD16 or PD21 females, rats were able to learn the task quickly and successfully at PD21 and their behavior and learning rates were similar to that of adult rats. In contrast, PD16 rats showed only slight decreases in trial latency over the 10 training trials, and those categorized as using a place strategy did not spend more time in the start arm on the probe than during training as PD21 and PD26 rats did, suggesting that they did not learn to employ a strategy but instead randomly chose a goal arm on the probe trial. However, estradiol disrupted the learning rates of only those PD16 rats categorized as using a response strategy, such that trial latencies were higher than those of all other PD16 groups on several trials. These data suggest that there was some qualitative difference between rats classified as place and response users at PD16. In addition, PD16 rats were likely to use the same strategy on the first test in adulthood as they used as juveniles, just as PD21 and PD26 rats did, suggesting that some rats may have learned a strategy at PD16. Thus, it is possible that PD16 rats were learning the task but became tired, which caused their trial latencies to stay high. Together, our results are consistent with previous research that navigation strategies develop between PD16 and PD21 (Akers & Hamilton, 2007; Rudy et al., 1987) and show for the first time that estradiol is able to modulate navigation strategy use by PD26, before the pubertal onset of naturally-cycling estradiol.
Previous experience and high estradiol levels modulate strategy use in adulthood
Previous studies examining estradiol effects on place and response strategy use have employed between-subjects designs in which subjects have had no previous experience with the task. Because females cycle for a great deal of their adult lives and likely have many experiences in which they must navigate through space, we examined the modulatory roles of previous navigation experience and estrous phase on strategy use. Because estradiol was able to bias females to use a place strategy on a first navigation experience as early as PD26 but in adulthood, the strategy used on a single previous navigation experience before puberty was a better predictor of the strategy used on the first test in adulthood than current estrous phase, it seems that a salient navigation experience in adolescence can overshadow the modulatory effects of estradiol. However, with increased experience with the task, a female is more likely to use a place strategy when in a high estradiol state, or estradiol is more able to prevent the gradual shift to use of a response strategy that has been observed in males (Packard & McGaugh, 1996; Chang & Gold, 2003). In our study rats received their initial navigation experience prior to puberty, though, such an experience may be extremely salient regardless of whether it occurs in adolescence or puberty. Our results suggest that the reason many previous studies have not found within-subjects effects of estrous cycle phase on place navigation (e.g., Stackman et al., 1997) is that the previous experience with the task was more influential on behavior than current estrous status.
Potential mechanisms of estrogen-modulated spatial navigation
There is evidence that estradiol has its effects on spatial navigation strategy use in the adult female rat via ERs within the hippocampus and striatum. Direct administration of estradiol to the striatum impairs response learning, and direct administration to the hippocampus enhances place learning (Zurkovsky et al., 2007), and local administration of ER antagonists to the hippocampus prevents the enhancing effects of systemic estradiol on place learning (Zurkovsky et al., 2006). In addition, estradiol has been shown to modulate hippocampal and striatal plasticity and behavior in a number of ways (for reviews, see Spencer et al., 2008 and Morissette et al., 2008) which may contribute to navigation learning and strategy use. Estradiol increases several aspects of hippocampal synaptic plasticity, such as NMDA and AMPA receptor activity to increase synaptic transmission (Foy et al., 1999; Teyler et al., 1980; Wong & Moss, 1991, 1992), the magnitude of hippocampal long-term potentiation in response to high frequency stimulation (Foy, 2001; Foy et al., 1999), dendritic spine density (Woolley & McEwen, 1993; Woolley et al., 1990), neurogenesis (Isgor & Watson, 2005; Mazzucco et al., 2006), spinogenesis (Mukai et al., 2007), and synaptic protein expression (Waters et al., 2009; Li et al., 2004).
Estradiol also appears to bias rats to use a hippocampal-dependent place strategy by directly modulating striatal dopaminergic activity, as administration of a dopamine receptor antagonist causes females with low levels of circulating estradiol (32 pg/ml) to switch from using a response strategy to a place strategy on an ambiguous navigation task, as females with high circulating estradiol (90 pg/ml) do (Quinlan et al., 2008). These data suggest that high levels of estradiol in the striatum may inhibit dopamine-mediated striatal function. However, estradiol has also been shown to increase striatal dopamine activity in a number of ways, suggesting that there are multiple mechanisms through which estradiol has its actions in the striatum. Estradiol potentiates dopamine release in the dorsolateral striatum both in vitro (Becker, 1990a; Xiao and Becker, 1998;Xiao et al., 2003) and in vivo (Becker, 1990b; Castner et al., 1993), upregulates striatal D2 receptors (Le Saux et al., 2006), increases dopamine receptor binding (Di Paolo et al., 1981; Levesque & Di Paolo, 1989), and enhances behavioral responses to dopaminergic drugs (Becker, 1990b; Jackson et al., 2006; Becker and Hu, 2008; Hu and Becker, 2008; Schultz et al., 2009). While complex, these data suggest that estradiol may act locally in the dorsolateral striatum to influence navigation strategy use.
The results of our study suggest that one or more of the mechanisms in the hippocampus and/or striatum involved in producing an estradiol-modulated bias to use a place strategy become functional around 26 days of age. Our data also point to a mechanism that that is modified by experience. These findings support the view that hormone-responsive circuits for cognitive function may be organized and respond to experience in a similar fashion to hormone sensitive neural circuits for reproductive behaviors like lordosis (Beach & Orndoff, 1974) and maternal behavior (Maestripieri & Zehr, 1998), in which increased experience and length of exposure to ovarian hormones increases hormonal control of behavior.
Acknowledgements
We would like to thank Lisa Myers for help with behavioral training and acknowledge support from the National Institute of Aging (AG09525).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers KG, Hamilton DA. Comparison of developmental trajectories for place and cued navigation in the Morris water task. Dev Psychobiol. 2007;49(6):553–564. doi: 10.1002/dev.20227. [DOI] [PubMed] [Google Scholar]
- Beach FA, Orndoff RK. Variation in the responsiveness of female rats to ovarian hormones as a function of preceding hormonal deprivation. Horm Behav. 1974;5(3):201–205. doi: 10.1016/0018-506x(74)90028-2. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5(2):157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118(2):169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects on performance of a hippocampal-dependent task. Behav Neurosci. 1997;111(2):267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24(2):161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610(1):127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav. 2000;38(4):234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18(10):787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32(3):217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82(2):142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66(1):11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84(2):132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Poyet P, Labrie F. Effect of chronic estradiol and haloperidol treatment on striatal dopamine receptors. Eur J Pharmacol. 1981;73(1):105–106. doi: 10.1016/0014-2999(81)90153-9. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Soto V, Dussaubat N, Mora S. Influence of the estrous cycle, ovariectomy and estradiol replacement upon the acquisition of conditioned avoidance responses in rats. Physiol Behav. 1989;46(3):397–401. doi: 10.1016/0031-9384(89)90010-3. [DOI] [PubMed] [Google Scholar]
- Dupon C, Kim MH. Peripheral plasma levels of testosterone, androstenedione, and oestradiol during the rat oestrous cycle. J Endocrinol. 1973;59(3):653–654. doi: 10.1677/joe.0.0590653. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28(35):8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR. 17beta-estradiol: effect on CA1 hippocampal synaptic plasticity. Neurobiol Learn Mem. 2001;76(3):239–252. doi: 10.1006/nlme.2001.4018. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81(2):925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115(1):229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57(1):5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956(2):285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERalpha on spatial learning. Horm Behav. 1998;34(2):163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP, Hampson E. Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Horm Behav. 1995;29(1):106–125. doi: 10.1006/hbeh.1995.1008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37(1):86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62(4):247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126(1–2):115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36(3):222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav. 2002;41(1):22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116(5):928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 2008;94(1–3):56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Watson SJ. Estrogen receptor alpha and beta mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134(3):847–856. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82(3):309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45(5):330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276(2):95–98. doi: 10.1016/s0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50(4):451–457. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Levesque D, Gagnon S, Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett. 1989;98(3):345–350. doi: 10.1016/0304-3940(89)90426-6. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126(1):183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62(3):230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144(7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34(2):149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Zehr JL. Maternal responsiveness increases during pregnancy and after estrogen treatment in macaques. Horm Behav. 1998;34(3):223–230. doi: 10.1006/hbeh.1998.1470. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42(3):284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Matthews MK, Jr, Kenyon R. Four- versus five-day estrous cycles in rats: vaginal cycling and pregnancy. Physiol Behav. 1984;33(1):65–67. doi: 10.1016/0031-9384(84)90014-3. [DOI] [PubMed] [Google Scholar]
- Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor alpha and estrogen receptor beta agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141(4):1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D'Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108(3–5):327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100(4):950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe JA, Li Y, Burgess LH, Handa RJ. Estrogen receptor mRNA alterations in the developing rat hippocampus. Brain Res Mol Brain Res. 1995;30(1):115–124. doi: 10.1016/0169-328x(94)00284-l. [DOI] [PubMed] [Google Scholar]
- O'Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21(1):51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Orikasa C, McEwen BS, Hayashi H, Sakuma Y, Hayashi S. Estrogen receptor alpha, but not beta, is expressed in the interneurons of the hippocampus in prepubertal rats: an in situ hybridization study. Brain Res Dev Brain Res. 2000;120(2):245–254. doi: 10.1016/s0165-3806(00)00016-x. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145(1):117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm Behav. 2008;53(1):185–191. doi: 10.1016/j.yhbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: dissociation of "proximal"- and "distal"-cue-based behaviors. Behav Neurosci. 1987;101(1):62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115(2):384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45(2):128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29(6):1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneta E, Phipps MC. On the comparison of two observed frequencies. Biometrical Journal. 2001;43(1):23–43. [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res Dev Brain Res. 2001;128(2):165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem. 1997;67(2):167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209(4460):1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Miranda RC, Hochberg RB, MacLusky NJ. Cellular variations in estrogen receptor mRNA translation in the developing brain: evidence from combined [125I]estrogen autoradiography and non-isotopic in situ hybridization histochemistry. Brain Res. 1992;576(1):25–41. doi: 10.1016/0006-8993(92)90606-a. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196(2):254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126(1):176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111(2):259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009 doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Williams CL. Estradiol benzoate facilitates lordosis and ear wiggling of 4- to 6-day-old rats. Behav Neurosci. 1987;101(5):718–723. doi: 10.1037//0735-7044.101.5.718. [DOI] [PubMed] [Google Scholar]
- Williams CL, Blaustein JD. Steroids induce hypothalamic progestin receptors and facilitate female sexual behavior in neonatal rats. Brain Res. 1988;449(1–2):403–407. doi: 10.1016/0006-8993(88)91064-5. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 beta-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543(1):148–152. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12(8):3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29(4):379–391. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jackson LR, Becker JB. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology. 2003;77(4):239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: assessment of estrogen replacement after ovariectomy. Brain Res. 2005;1052(2):163–173. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144(1):26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86(3):336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]