Abstract
The limited availability of fresh osteochondral allograft tissues necessitates the use of banking for long-term storage. A vitrification solution containing a 55% cryoprotectant formulation, VS55, previously studied using rabbit articular cartilage, was evaluated using porcine articular cartilage. Specimens ranging from 2–6mm in thickness were obtained from 6mm distal femoral cartilage cores and cryopreserved by vitrification or freezing. The results of post-rewarming viability assessments employing alamar-Blue demonstrated a large decrease (p<0.001) in viability in all 3 sizes of cartilage specimen vitrified with VS55. This is in marked contrast with prior experience with full thickness, 0.6mm rabbit cartilage. Microscopic examination following cryosubstitution confirmed ice formation in the chondrocytes of porcine cartilage vitrified using VS55. Experiments using a more concentrated vitrification formulation (83%), VS83, showed a significant treatment benefit for larger segments of articular cartilage. Differences between the VS55 and the VS83 treatment groups were significant at p < 0.001 for 2mm and 4mm plugs, and at p < 0.01 for full thickness, 6mm plugs. The percentage viability in fresh controls, compared to VS55 and VS83, was 24.7% and 80.7% in the 2mm size group, 18.2% and 55.5% in the 4mm size group, and 5.2% and 43.6% in the 6mm group, respectively. The results of this study continue to indicate that vitrification is superior to conventional cryopreservation with low concentrations of dimethyl sulfoxide by freezing for cartilage. The vitrification technology presented here may, with further process development, enable the long-term storage and transportation of living cartilage for repair of human articular surfaces.
Keywords: Cartilage, cryopreservation, vitrification, osteochondral grafts
Introduction
Advances in low temperature biology have produced high viability preservation methods for cells and tissues [33]. However, in general the development of preservation methods is not straightforward and methods that work for many cells in suspension and connective tissues do not work for certain cell types and tissues, including chondrocytes in intact articular cartilage [reviewed, 30]. Process development requires the optimization of chemical and thermal treatments to achieve maximal survival and stability. In a recent editorial 11] the need for ice-free cryopreservation methods was emphasized. The consensus opinion was that viable tissues such as blood vessels, corneas and cartilage that have proven refractory to cryopreservation by conventional freezing methods, despite decades of intense research by many investigators, can only be successfully preserved if steps are taken to prevent or control the ice that forms during cooling and warming. Mathematical modeling may ultimately improve our ability to optimize freezing procedures for tissues [13], but has not yet contributed to significant advances. Ice free tissue cryopreservation using vitrification have been shown to be effective in cardiovascular tissues [31;3;26;27]. Vitrification, an amorphous solidification of a supercooled liquid, can be achieved by adjusting the solute composition, the cooling rate and warming rate such that nucleation and growth of ice crystals is essentially prevented [30].
More recently we have extended our vitrification studies to musculoskeletal tissues employing a rabbit articular cartilage model and obtained excellent in vitro and in vivo results [25;28]. Articular cartilage is generally considered to be an immunologically privileged tissue and must contain living cells at the time of transplantation. Although, fresh osteochondral allografts have proven to be effective and functional for transplantation, the limited availability of fresh allograft tissues necessitates the use of osteoarticular allograft banking with long-term storage [1;16;17;21]. Conventional cryopreservation by means of freezing employing 1-2M Me2SO results in death of 80–100% of the chondrocytes in articular cartilage plus extracellular matrix damage due to ice formation [25;28;4]. These detrimental effects are major obstacles preventing successful clinical utilization of osteochondral allografts [21;29;32] and for future tissue engineered cartilage products in development. However, human articular cartilage is at least six times thicker than the rabbit cartilage we have previously used (~0.6mm), therefore porcine femoral condyle articular cartilage was employed in this study because it approximates the thickness of human knee joint cartilage.
The tissue vitrification technology described here employs 55% or 83% weight/volume cryoprotective agents. The original formulation (VS55, a mixture of dimethylsulfoxide [Me2SO], formamide, and propylene glycol) and method of use was licensed from the American Red Cross, where it was intended for organ preservation [6;7;18]. The tissue vitrification methods were then further developed [14;15] and correlations with matrix preservation investigated [24;4]. In this report the vitrification solution, VS55, and method that resulted in ~80% chondrocyte preservation in our previous studies employing full thickness rabbit cartilage [25;28] and VS83 were evaluated using porcine articular cartilage specimens of varying thickness as a model of human articular cartilage.
Materials and Methods
Femoral cartilage was obtained aseptically from the femoral weight bearing condyles of sexually mature domestic Yorkshire cross pigs weighing between 25 and 30 Kg. These pigs were skeletally immature, maturity is achieved at weights >200 Kg and two years of age. No animals were sacrificed for these studies. Bona fide excess tissues from other approved studies at the Medical University of South Carolina were employed. In this study, three different thicknesses of porcine cartilage plug (Table 1), 6mm osteochondral cores obtained using a biopsy punch, were harvested post-mortem, randomized to avoid bias and vitrified or frozen as described below. The bone was trimmed away resulting in full thickness articular cartilage cores of ~6mm.
Table 1.
Pig Cartilage Sample Size
| Cartilage Size | Tissue Dimension |
|---|---|
| Thin Size Plug | 6mm Diam. × 2mm Depth |
| Thick Size Plug | 6mm Diam. × 4mm Depth |
| Full Size Plug | 6mm Diam. × 6mm Depth |
Conventional Cryopreservation
Cartilage specimens were cryopreserved in polyethylene vials using slow rate (−1°C /min) cooling with 1.4M ME2SO in DMEM culture medium plus 10% fetal bovine serum from −4°C to −80°C [2]. The cryopreserved tissue specimens were then stored at −160°C in vapor phase nitrogen for a minimum of 24 hours. Thawing was accomplished in two steps, whereupon the containers were transferred to an ice-bath for elution of the cryoprotectant. This was achieved in one step in which the tissue samples were transferred to DMEM.
Vitrification Protocol
The cartilage specimens were gradually infiltrated with precooled vitrification formulations of ME2SO, formamide and 1,2-propanediol in EuroCollins solution at 4°C in six steps consisting of 0, 12.5, 25, 50, 75 and 100% of each formulation to achieve final cryoprotectant concentrations of either 55 or 83%. The final cryoprotectant concentrations were 3.10 or 4.6M ME2SO, 3.10 or 4.6M formamide, and 2.21 or 3.3M propylene glycol, respectively. Finally, the cartilage specimens were placed in glass scintillation vials (Dia. × H, 25mm × 60mm) containing 2 ml of pre-cooled vitrification solution. The top of the vitrification solution was then covered with 0.7 ml of 2-methylbutane (isopentane, freezing point: −160° C, density: 0.62) at 4° C to prevent direct air contact. A thermocouple was inserted into a separate dummy sample of the same vitrification solution and its output monitored via a digital thermometer throughout the cooling process. Samples were cooled rapidly (43°C/min) to −100°C by placing the samples in a precooled bath containing isopentane in a - 135°C mechanical storage freezer. Upon achieving −100°C the specimens were removed from the bath and stored at −135°C in the mechanical storage freezer, which resulted in slow cooling (3 C/min) to − 135°C. The samples were held at −135°C for a minimum of 24 hours. Vitrified cartilage was rewarmed in two stages, first, slow warming to −100°C (~30°C/min) at the top of the mechanical storage freezer and then rapidly warmed to melting (~225°C/min) in a 30% ME2SO in water bath at room temperature. After rewarming, the vitrification solution was removed in seven sequential 15 minute steps at 4°C into DMEM culture medium as previously described [27;25;28].
Viability Assessment
Viability assessments were initiated within an hour of completion of the rewarming and cryoprotectant elution protocol. Cartilage specimens were incubated under cell culture conditions in media containing alamarBlue. The alamarBlue™ assay utilizes a water soluble fluorometric viability indicator based on the detection of metabolic activity, specifically, an oxidation-reduction (REDOX) indicator which both fluoresces and changes color in response to chemical reduction of the growth medium caused by cell metabolism. Samples were read on a spectrofluorometer at 590 nm. The data is expressed as relative fluorescent units/mg dry weight of tissue.
Histology Methods
Cryosubstitution was utilized to visualize the presence of ice in cryopreserved specimens [25;28]. Cryosubstitution was performed on thin thickness, 2mm cartilage specimens using chilled (−90°C) 1% osmium tetroxide in 100% methanol in high-density polyethylene scintillation vials containing cryopreserved specimens at −90°C. The tissues were dehydrated with cryosubstitution medium over a period of several days at −90°C. The vials were then placed in a −20°C storage freezer overnight, followed by 4°C for 1 hour, and then finally brought to room temperature. Finally, the tissues were transferred to 100% acetone, infiltrated with Araldite® resin (Electron Microscopy Sciences, Fort Washington, PA), polymerized, sectioned and stained with Toluidine blue for viewing by light microscopy. Selected blocks were then thin sectioned (75 nm) for transmission electron microscopy. The sections were double-stained with uranyl acetate followed by lead citrate and viewed in a JEM-1210 transmission electron microscope (JEOL USA, Inc., Peabody, MA) at an accelerating voltage of 80 kV.
Statistical Methods
One-way ANOVA was employed to compare experimental groups with post hoc testing using Tukey's method. P-values < 0.05 were considered significantly different.
Results
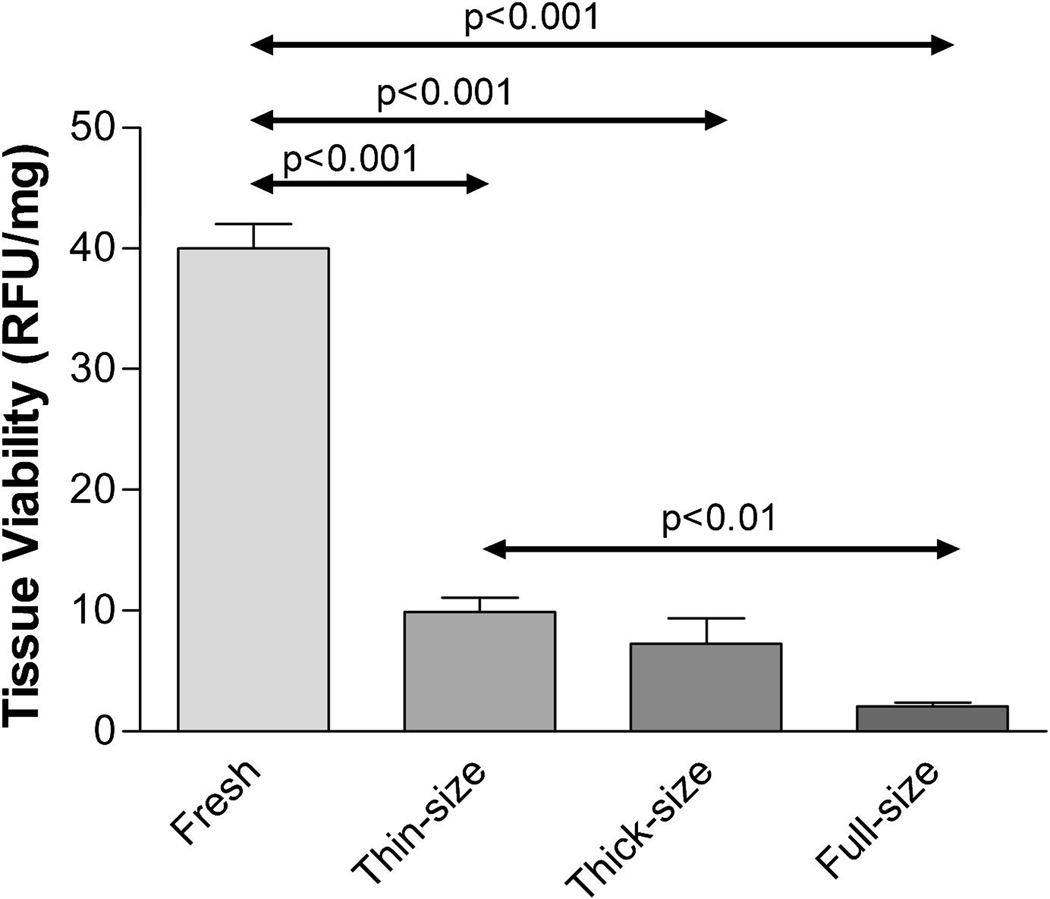
The vitrification solution, VS55, previously tested on rabbit articular cartilage, was evaluated using porcine articular cartilage. Three different sizes of cartilage were obtained from pigs (Table 1) and vitrified using our standard protocol with VS55. Comparisons between size groups demonstrated that there was a significant difference for thin versus full sized plugs (p<0.01), but not for thin versus thick and thick versus full VS55 treated groups (Fig. 1).
Figure 1.
Pig cartilage viability using alamarBlue. Cartilage in different sizes vitrified using VS55 and compared with fresh controls. Data presented as the mean ±1 standard error, n≥6.
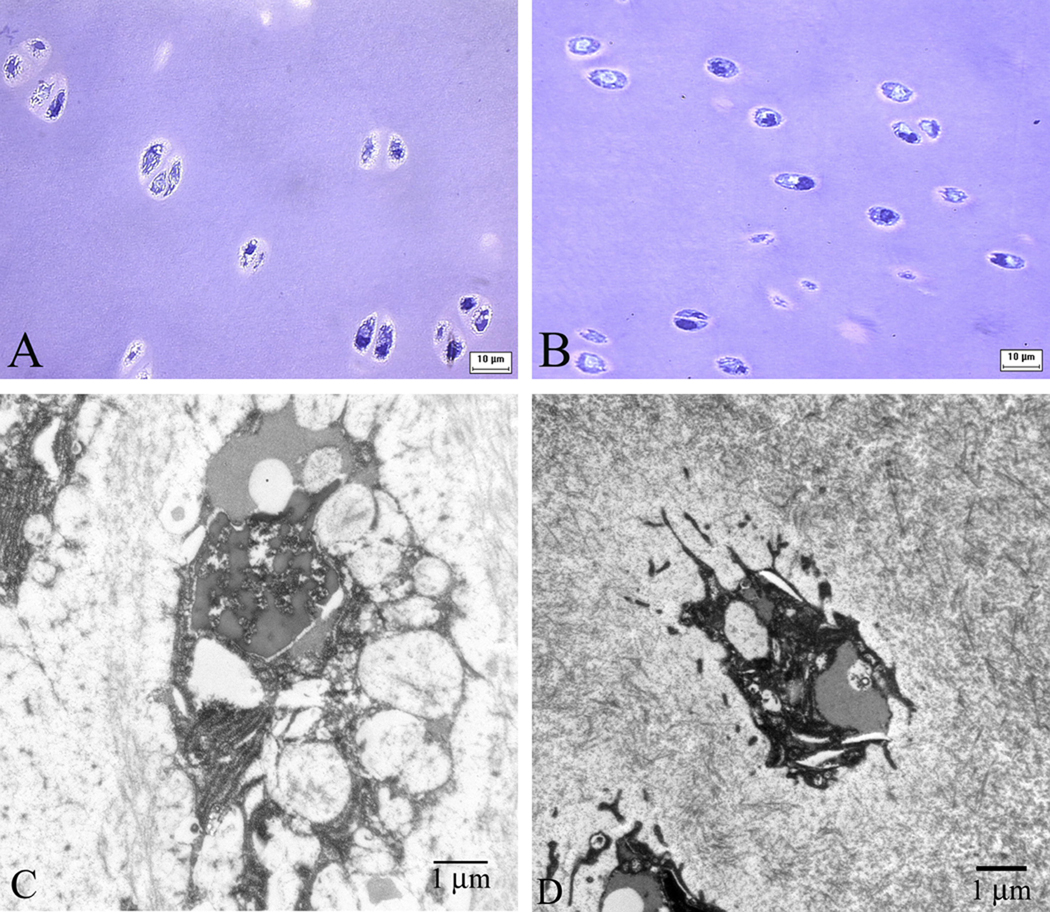
Microscopic examination following cryosubstitution confirmed ice formation in the chondrocytes of porcine cartilage vitrified using VS55 verified the presence of ice in these specimens (Fig. 2). Light microscopy of vitrified and cryosubstituted pig cartilage (thin size) showed the irregular shape of chondrocytes with considerable cytoplasmic disruption (Fig 2, A). Electron microscopy demonstrated chondrocytes with nuclear disruption and large secretory vacuoles. Cytoplasmic projections have a “spiked” appearance that may be due to ice formation (Fig 2, C). Light microscopy of thin sized pieces of cartilage vitrified with VS83 appeared to be free of ice (Fig. 2, B), however small structures were observed that we interpret as due to ice crystals using electron microscopy (Fig. 2,D) similar to our previous observations employing VS55 in rabbit cartilage [28].
Figure 2.
Cryosubstitution of thin size cartilage vitrified using VS55 (A & C) and VS83 (B & D). Magnification: A & B=X100; C=X12000; D=X10000.
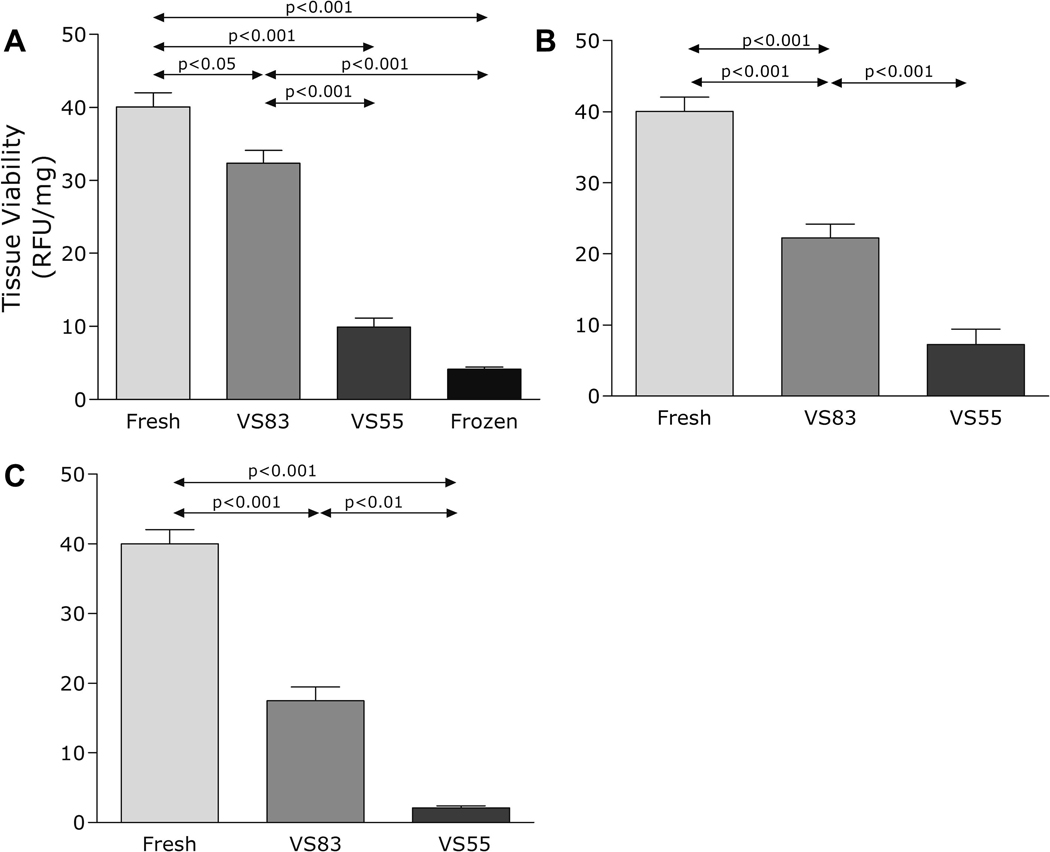
We then conducted a series of experiments using a higher concentration of 83% vitrification solution, VS83, a concentrated version of VS55. One-way ANOVA showed a significant treatment benefit of VS83 on cartilage tissues (Fig. 3A–C). Post hoc tests using Tukey's method determined that the difference between the VS55 and the VS83 treatment was significant at p < 0.001 in thin and thick size plugs, and at p < 0.01 in full size plugs. The percentage viability in fresh controls, compared to VS55 and VS83, was 24.7% and 80.7% in the thin size group, 18.2% and 55.5% in the thick size group, and 5.2% and 43.6% in the full size group, respectively. There was also a significant difference between the thin versus thick (p<0.01) and thin versus full thickness (p<0.001) VS83 treated groups, but not the thick versus full thickness group (not shown on figure).
Figure 3.
Pig cartilage plugs vitrified using VS55 and VS83; viability assayed using alamarBlue and compared with fresh and frozen controls. A - Thin size, B - Thick size, C - Full size. Data presented as the mean ±1 standard error, n≥6.
Discussion
Isolated chondrocytes are relatively easy to cryopreserve in suspensions [22]. However, in contrast, most studies using a variety of animal articular cartilage models [17;21;28;34] and human cartilage biopsies [29] have revealed no more than 20% chondrocyte viability following conventional cryopreservation by freezing procedures employing low concentrations of either ME2SO or glycerol as cryoprotectants. Ohlendorf et al [21] used a bovine articular cartilage, osteochondral plug model to develop a clinical cryopreservation protocol. This protocol employed slow rate cooling and 8% ME2SO as the cryoprotectant. They observed loss of viability in all chondrocytes except those in the most superficial layer at the articular surface. In marked contrast, Muldrew et al [19] previously investigated chondrocyte survival in a similar sheep model. These researchers observed ~50% cell survival post-cryopreservation, predominantly close to the articular surface and deep at the bone/cartilage interface. The middle layer was devoid of viable cells. More recently, Muldrew et al. demonstrated improved results using a step-cooling cryopreservation protocol, achieving ~62% chondrocytes recovery, but cell survival post-transplantation was poor and again there was significant loss of cells in the mid-portion of the graft [20]. The reason for lack of cell survival deeper than the superficial layers of articular cartilage is most likely multifactorial [12]. Surface cells freeze and thaw more rapidly than cells located deep within the matrix. This phenomenon could result in a greater opportunity for ice to form, both within cells and in the extracellular matrix, deeper within the articular cartilage. We observed ice deeper in the tissue in our prior studies of rabbit articular cartilage cryopreservation [28]. Furthermore, typically employed concentrations of ME2SO (8–20%) may not penetrate adequately to limit intracellular ice formation. Recent data from Jomha et al. [10] demonstrated that increasing ME2SO concentrations to 6M can result in higher overall cell survival (40%) after cryopreservation. Whether or not these investigators were achieving vitrification or partial vitrification within the tissues is not clear.
In agreement with the majority of the literature, we have found that cryopreservation by freezing results in very poor chondrocyte preservation [25;28]. The results of the present study continue to indicate that vitrification strategies are superior to conventional cryopreservation by freezing. However, VS55 produced much lower levels of chondrocyte viability in 2 to 6mm thick porcine cartilage samples (Fig. 2) than in our previous studies with ~0.6 mm thick rabbit cartilage where ~80% viability was observed [28]. This observation of very low viability with VS55 using full thickness large animal cartilage is in agreement with Johma et al. [9]. Employing VS83 we obtained ~80% chondrocyte preservation in 2mm cartilage specimens (~80% viability) and about 55% viability in 4mm specimens indicating that this vitrification approach should be excellent for small cartilage specimens for chondrocyte culture or cartilage biopsies (Fig. 3). Cryosubstitution studies revealed that ice formation occurred in porcine cartilage preserved with VS55 (Fig. 2). A more concentrated vitrification solution (VS83) resulted in significantly better preservation of the chondrocytes in porcine articular cartilage than VS55 (Fig. 2), although ultrastructural ice was still observed. However, when VS83 was employed on full thickness cartilage (~6mm) only ~43% chondrocyte viability was observed. We believe that this low outcome was due to poor cryoprotectant permeation resulting in small destructive ice crystals (Fig. 2D) and not cytotoxicity because much higher levels of cell viability were observed in thinner cartilage specimens which were exposed to the cryoprotectants for the same length of time (Fig. 3A). Alternative vitrification protocols that may improve survival of chondrocytes in full thickness grafts are being developed employing longer incubation times and lower sub-zero incubation temperatures for the final steps in addition and removal of cryoprotectants to minimize the risks of cryoprotectant cytotoxicity with longer incubation times. Alternative formulations, such as VS442 [35] and 40% ethylene glycol with 0.6M sucrose [8], which are less cytotoxic and that do not require the cumbersome multistep addition and removal procedures employed with VS55 and VS83 are also needed. Further support for a vitrification approach to preservation of articular cartilage was recently reported by Pegg et al. [23] in which cryoprotectants were added in a step-wise manner during cryopreservation employing a 'liquidus-tracking' method that completely avoids the crystallization of ice and does not require rapid warming.Very thin cartilage specimens (~1mm) preserved in this way incorporated sulphate (35S) into newly synthesized glycosaminoglycans and approached 70% of that of fresh control cartilage. The author indicated that this process is far from ideal [23] and application to thicker cartilage specimens is required to effectively compare this method with the literature. Despite the warming rate advantage this method would be difficult to perform routinely as an aseptic process for human cartilage preservation due to the necessity of continuous addition of cryoprotectants during the cooling process [23], although further research may prove this opinion wrong.
In contrast, the preservation technology presented here can be performed aseptically in a manner similar to frozen products such as heart valves. The major technical limitations of this vitrification strategy being the rapid warming rates and high cryoprotectant concentrations required to prevent ice growth during re-warming. The duration of post-rewarming cryoprotectant elution may also be stressful for orthopedic surgeons employing vitrified cartilage for transplantation. Strategies to overcome these limitations are being developed. Concern has previously been expressed regarding one of the vitrification formulation components, formamide, being a potential mutagen [5]. This issue may require that vitrified tissue product labeling excludes implantation in pregnant women if formamide is employed. Studies of the collagen matrix have demonstrated better preservation in vitrified than in frozen cryopreserved porcine articular cartilage [4]. Biomechanics studies of vitrified cartilage still need to be performed.
Conclusions
A more concentrated vitrification formulation was required for preservation of relatively porcine articular cartilage compared with our earlier experience with rabbit articular cartilage. Further process development employing the new VS83 formulation may enable the long-term storage and transportation of full thickness living cartilage for surgical repair of human articular surfaces. This cartilage may be in the form of either osteochondral allografts or, prospectively, tissue-engineered cartilage constructs.
Acknowledgments
This study was supported by a U.S. Public Health Grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant # R44 AR472731.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bakay A, Csonge L, Papp G, et al. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22(277):281. doi: 10.1007/s002640050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brockbank KGM. Method for Cryopreserving Musculoskeletal Tissues. 5,131,850. US patent. 1992
- 3.Brockbank KGM, Lightfoot FG, Song YC, Taylor MJ. Interstitial ice formation in cryopreserved homografts: A possible cause of tissue deterioration and calcification in vivo. Journal of Heart Valve Disease. 2000;9(2):200–206. [PubMed] [Google Scholar]
- 4.Brockbank KGM, MacLellan WR, Xie J, Hamm-Alvarez SF, Chen ZZ, Schenke-Layland K. Quantitative Second Harmonic Generation Imaging of Cartilage Damage. Cell and Tissue Banking. 2008;9:299–308. doi: 10.1007/s10561-008-9070-7. [DOI] [PubMed] [Google Scholar]
- 5.Brockbank KGM, Walsh JR, Song YC, Taylor MJ. Encyclopedia of Biomaterials and Biomedical Engineering. Vol. 24. New York: Marcel Dekker; 2003. Vitrification: Preservation of Cellular Implants; pp. 1–26. [Google Scholar]
- 6.Fahy GM. Vitrification. In: McGrath JJ, Diller KR, editors. Low Temperature Biotechnology: Emerging Applications and Engineering Contributions. New York: American Society of Mechanical Engineers; 1988. pp. 113–146. [Google Scholar]
- 7.Fahy GM, Saur J, Williams RJ. Physical problems with vitrification of large systems. Cryobiology. 1990;27:492–510. doi: 10.1016/0011-2240(90)90038-6. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi M, Tsuchiya H, Otoi T, Agung B, Yamamoto N, Tomita K. Influence of freezing with liquid nitrogen on whole-knee joint grafts and protection of cartilage from cryoinjury in rabbits. Cryobiology. 2009;59:28–35. doi: 10.1016/j.cryobiol.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Johma NM, Anoop PC, Bagnall K, McGann LE. Comparison of high cryoprotectant concentrations for cryopreservation of porcine articular cartilage. Cell Preservation Technology. 2003;1(3):201–206. [Google Scholar]
- 10.Jomha NM, Anoop PC, Bagnall K, McGann LE. Effects of Increasing Concentrations of Dimethyl Sulfoxide During Cryopreservation of Porcine Articular Cartilage. Cell Preservation Technology. 2002;1(2):111. [Google Scholar]
- 11.Kaiser J. New Prospects of Putting Organs on Ice. Science. 2002;295(5557):1015. doi: 10.1126/science.295.5557.1015. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson JOM, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1994;17:243–256. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson JOM. Cryopreservation: Freezing and Vitrification. Science. 2002;296:655–656. doi: 10.1126/science.296.5568.655d. [DOI] [PubMed] [Google Scholar]
- 14.Khirabadi BS, Song YC, Brockbank KGM. Method of cryopreservation of tissues by vitrification. #6,740,484. United States Patent. 2004
- 15.Khirabadi BS, Song YC, Brockbank KGM. Method of cryopreservation of tissues by vitrification. #7,157,222. United States Patent. 2007
- 16.Malinin TI, Martinez OV, Brown MD. Banking of massive osteoarticular and intercalary bone allografts-12 years' experience. Clin Orthop. 1985;197:44–57. [PubMed] [Google Scholar]
- 17.Marco F, Leon C, Lopez-Oliva F, et al. Intact articular cartilage cryopreservation. In Vivo evaluation. Clin Orthop. 1992;283:11–20. [PubMed] [Google Scholar]
- 18.Mehl PM. Nucleation and crystal growth in a vitrification solution tested for organ cryopreservation by vitrification. Cryobiology. 1993;30:509–518. doi: 10.1006/cryo.1993.1051. [DOI] [PubMed] [Google Scholar]
- 19.Muldrew K, Hurtig M, Schachar N, McGann LE. Localization of freezing injury in articular cartilage. Cryobiology. 1994;31:31–38. doi: 10.1006/cryo.1994.1004. [DOI] [PubMed] [Google Scholar]
- 20.Muldrew K, Novak K, Studholme C, Wohl G, Zernicke R, Schachar N, McGann LE. Transplantation of articular cartilage following a step-cooling cryopreservation protocol. Cryobiology. 2001;43:260–267. doi: 10.1006/cryo.2001.2349. [DOI] [PubMed] [Google Scholar]
- 21.Ohlendorf C, Tomford WW, Mankin HJ. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res. 1996;14:413–416. doi: 10.1002/jor.1100140311. [DOI] [PubMed] [Google Scholar]
- 22.Pegg DE, Wusteman MC, Wang L. Cryopreservation of articular cartilage. Part 1: conventional cryopreservation methods. Cryobiology. 2006;52(3):335–346. doi: 10.1016/j.cryobiol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Pegg DE, Wang L, Vaughan D. Cryopreservation of articular cartilage. Part 3: the liquidus-tracking method. Cryobiology. 2006;52(3):360–368. doi: 10.1016/j.cryobiol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Schenke-Layland K, Xie J, Haydarkhan-Hagvall S, Hamm-Alvarez SF, Stock UA, Brockbank KGM, MacLellan WR. Optimized preservation of extracellular matrix damage in cardiac tissues: Implications for long-term graft durability. Annals of Thoracic Surgery. 2007;83:1641–1650. doi: 10.1016/j.athoracsur.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Song YC, An YH, Kang QK, Li C, Boggs JM, Chen ZZ, Taylor MJ, Brockbank KGM. Vitreous preservation of articular cartilage grafts. Journal of Investigative Surgery. 2004;17:65–70. doi: 10.1080/08941930490422438. [DOI] [PubMed] [Google Scholar]
- 26.Song YC, Hagen PO, Lightfoot FG, Taylor MJ, Smith AC, Brockbank KGM. In vivo evaluation of the effects of a new ice-free cryopreservation process on autologous vascular grafts. Journal of Investigative Surgery. 2000;13(5):279–288. doi: 10.1080/08941930050206300. [DOI] [PubMed] [Google Scholar]
- 27.Song YC, Khirabadi BS, Lightfoot FG, Brockbank KGM, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nature Biotechnology. 2000;18:296–299. doi: 10.1038/73737. [DOI] [PubMed] [Google Scholar]
- 28.Song YC, Lightfoot FG, Chen Z, Taylor MJ, Brockbank KGM. Vitreous preservation of rabbit articular cartilage. Cell Preservation Technology. 2004;2(1):67–74. [Google Scholar]
- 29.Stone BB, Defranzo BE, Dicesare C, et al. Cryopreservation of human articular cartilage for autologous chondrocyte transplantation. Cryobiology. 1998;37:445–446. (abstract) [Google Scholar]
- 30.Taylor MJ, Song YC, Brockbank KGM. Vitrification in Tissue Preservation: New Developments. In: Benson E, Fuller B, Lane N, editors. Life in the Frozen State. vol. 22. London: Taylor and Francis Books; 2004. pp. 603–641. [Google Scholar]
- 31.Taylor MJ, Song YC, Kheirabadi BS, Lightfoot FG, Brockbank KGM. Vitrification fulfills its promise as an approach to reducing freeze-induced injury in a multicellular tissue. Advances in Heat and Mass Transfer in Biotechnology, Volume number HTD-Vol 363/BED-Vol 44. 1999:93–102. [Google Scholar]
- 32.Tomford WW, Fredericks GR, Mankin HJ. Studies on cryopreservation of articular cartilage chondrocytes. J Bone Joint Surg Am. 1984;66:253–259. [PubMed] [Google Scholar]
- 33.Walsh JR, Taylor MJ, Brockbank KGM. Storage and Transport Issues for Tissue Engineered Medical Products. In: Picciolo GL, Schutte E, editors. Tissue Engineered Medical Products (TEMPs), ASTM STP 1452. West Conshohocken, PA: ASTM International; 2003. [Google Scholar]
- 34.Wu FJ, Davisson TH, Pegg DE. Preservation of tissue-engineered articular cartilage. Cryobiology. 1998;37:410. [Google Scholar]
- 35.Yin H, Cui L, Liu G, Cen L, Cao Y. Vitreous cryopreservation of tissue engineered bone composed of bone marrow Mesenchymal stem cells and partially demineralized bone matrix. Cryobiology. 2009;59:180–187. doi: 10.1016/j.cryobiol.2009.06.011. [DOI] [PubMed] [Google Scholar]