Abstract
Brain-derived neurotrophic factor (BDNF) is closely linked with neuronal survival and plasticity in psychiatric disorders. In this work, we engineered degradable, injectable alginate microspheres and non-degradable, implantable poly(ethylene vinyl acetate) matrices to continuously deliver BDNF to the dorsal hippocampus of rats for two days or more than a week, respectively. The antidepressant-like behavioral effects of BDNF delivery were examined in the Porsolt forced swim test. Rats were sacrificed 10 days after surgery and tissue samples were analyzed by western blot. A small dose of BDNF delivered in a single infusion, or from a two-day sustained release alginate implant, produced antidepressant-like behavior, whereas the same dose delivered over a longer period of time to a larger tissue region did not produce antidepressant-like effects. Prolonged delivery of BDNF resulted in a dysregulation of plasticity-related functions: increased dose and duration of BDNF delivery produced increased levels of TrkB, ERK, CREB, and phosphorylated ERK, while also producing decreased phosphorylated CREB. It is evident from this work that both duration and magnitude of BDNF dosing are of critical importance in achieving functional outcome.
Keywords: neurotrophin, BDNF, hippocampus, depression, neuroplasticity, polymeric drug delivery
1 Introduction
Major depression is a complex disorder characterized by profound behavioral and biochemical changes that severely impact quality of life. Depression is associated with decreases in structural plasticity, altered cellular resilience and neuronal atrophy in both experimental and clinical settings (Manji et al., 2003, Duman et al., 2000, Duman, 2004, Ongur et al., 1998). Since direct administration of neurotrophic factors in animals is known to increase sprouting and growth of neurons, it has been suggested that the learning and adaptive deficiencies observed in depressive phenotypes are the consequence of decreased neuroplasticity due to loss of neurotrophic support in the brain (Korte et al., 1995, Garcia, 2002).
Of all the neurotrophins, brain-derived neurotrophic factor (BDNF) has received the most attention for its role in the loss of central nervous system plasticity that may underlie major depression. Clinical and experimental models of depression are associated with decreased expression of BDNF (Smith et al., 1995b, Duman and Monteggia, 2006), whereas antidepressant treatment is associated with increased expression of BDNF (Duman, 2005). Furthermore, direct administration of exogeneous BDNF produced antidepressant-like effects in the forced swim and learned helplessness paradigms in rats (Siuciak et al., 1997, Shirayama et al., 2002, Hoshaw et al., 2005). The causal role of BDNF in the etiology of depression is complex; however, it is clear that BDNF or the signaling pathways related to its function are potential targets for the development of new therapies.
The delivery of proteins, small molecules and other active agents to the brain is a challenge to testing the local action of pharmaceuticals. Since many drugs do not normally cross the blood brain barrier, they must be directly infused into the brain region of interest by implantation of a permanent guide cannula affixed to the skull. Repeated infusions cause significant tissue damage, which limits the treatment duration of animal studies. Furthermore, the biological activity and concentration of drugs after periodic administration is not always known. It would therefore be advantageous to deliver sustained levels of drug directly to local brain regions without the use of a permanently implanted guide cannula.
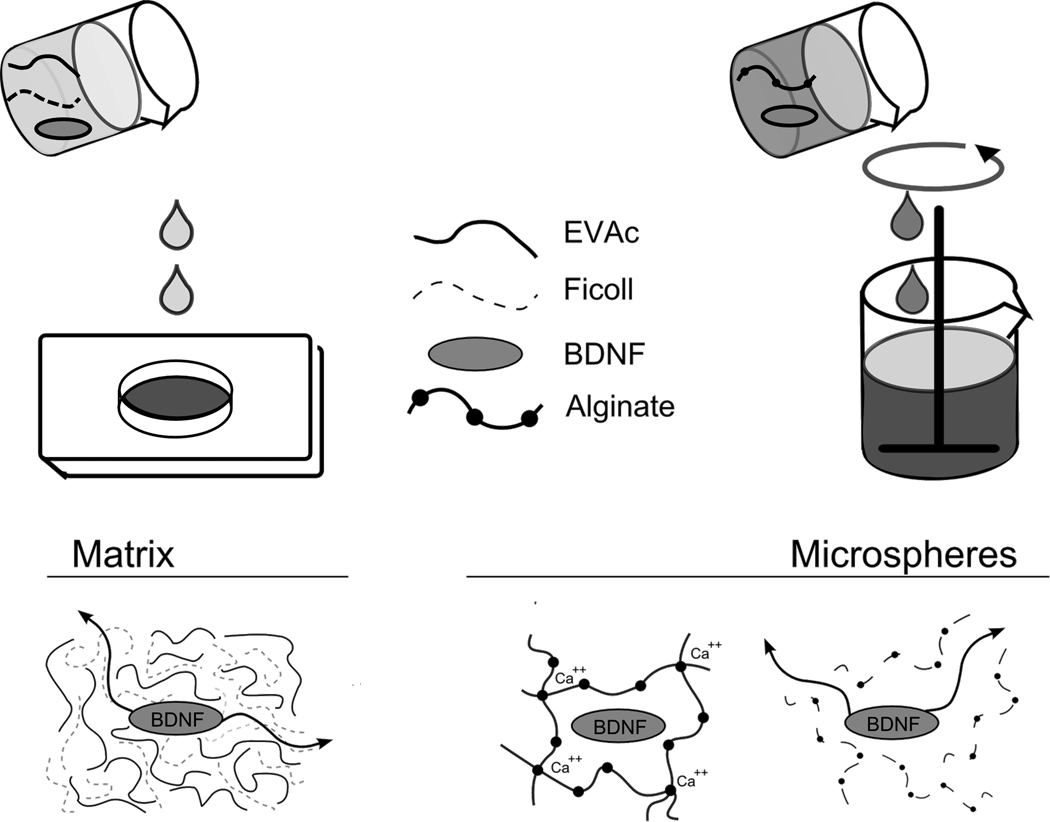
In this work, we developed implantable, sustained-release polymers to deliver BDNF to local brain regions (figure 1). Poly(ethylene vinyl acetate) (EVAc) is an inert, biocompatible, nondegradable polymer that has been used for FDA-approved applications in humans. To create solid matrices of polymer containing drug, the drug, polymer, and an inert codispersant are dissolved in an organic solvent, which is evaporated to yield a solid drug-polymer matrix. After implantation, the codispersant dissolves and drug diffuses through an interconnected network of pores to the surrounding tissue site. Biomaterials constructed from EVAc can be designed to deliver active agents over the course of weeks or months (Smith et al., 1995a, Saltzman et al., 1999, Gruber, 2006). Alginate is a biodegradable polymer that has been used extensively for the delivery of growth factors in vivo. An injectable form of alginate is constructed by cross-linking droplets of alginate-drug solution with Ca2+, or other bivalent cations, to form stable polymer microspheres containing drug. After implantation, Ca2+ ions diffuse out of the polymer network, allowing alginate to dissolve and liberating trapped drug. Microspheres composed of alginate can be designed to release drug over durations of days or weeks (Cho et al., 1998, Elcin et al., 2001, Ueng et al., 2004).
Figure 1. Schematic describing EVAc and alginate biomaterial construction.
Porous EVAc matrices were constructed by dissolving polymer with drug and an inert dispersant in organic solvent. This mixture was poured onto a chilled aluminum mold and the solvent was subsequently evaporated. Biodegradable alginate microspheres were created by adding cross-linker to an emulsified alginate-drug solution.
We applied BDNF-releasing biomaterials with varying release kinetics in a rat model of antidepressant efficacy in order to explore how delivery rate affects biological outcomes relevant to depression. Our goals were two-fold: first, to examine behavioral and biochemical effects of BDNF delivery to the hippocampus, and, second, to develop drug delivery devices which could be used for future exploration of the biological effects of compounds that do not normally cross the blood brain barrier. To our knowledge, these experiments are the first to report the application of polymeric protein delivery devices in the study of psychiatric illness.
2 Results
2.1 Biomaterial Characterization
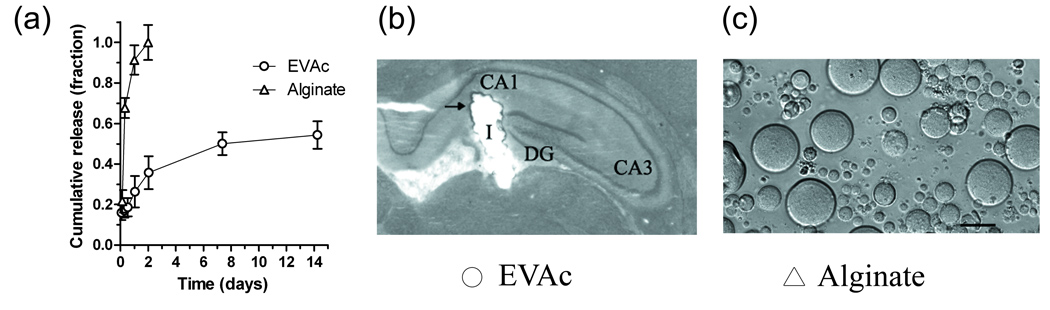
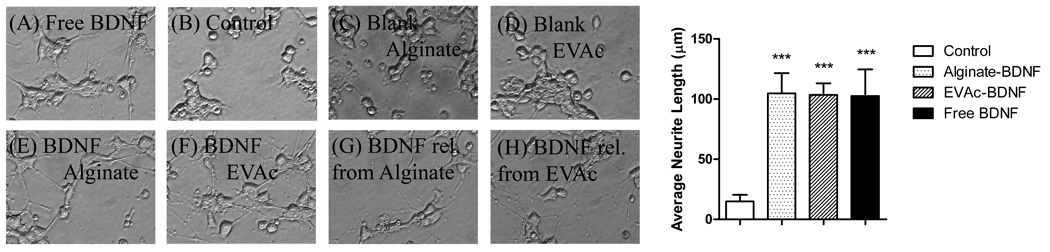
To determine the rate of release of BDNF from polymer material, samples of BDNF-EVAc and BDNF-alginate were incubated in buffer solution at 37° C (figure 2). Alginate microspheres were observed to release the majority of BDNF within 2 days, whereas EVAc implants released BDNF for more than 7 days (not all of the BDNF was released from EVAc implants during the time-frame studied here). To determine whether the released BDNF was bioactive, free BDNF, BDNF obtained from controlled release supernatant, and implants containing BDNF were applied to PC12-TrkB cell cultures. A neuronal phenotype, evident by process outgrowth, was observed in all cells that were treated with BDNF, regardless of the source of BDNF (figure 3). Minimal neurite extension was observed in non-treated or material-only control cells. EVAc and alginate materials were therefore effective vehicles to deliver bioactive BDNF to the brain.
Figure 2. Polymeric biomaterials were designed to deliver a sustained dose of BDNF to the hippocampus.
(a) Controlled release measurements of BDNF from alginate and EVAc biomaterials. The majority of BDNF was released from alginate materials over a period of 1–2 days; EVAc materials continued releasing BDNF for 7 days. Release is expressed as a fraction of total protein released for alginate and as fraction total theoretical loading for EVAc. (b) Image of an EVAc disc implanted into the rat hippocampus. Rats received solid EVAc matrices and were sacrificed 10 days later. The EVAc implant (I) reached the target location of the DG/CA1 regions of the dorsal hippocampus. Nissl stain revealed only minimal tissue damage surrounding the implant location (arrowhead) (c) Light microscope image of alginate microspheres. Microspheres were of round, uniform morphology, with an average diameter of 38 µm. Scale bar = 50µm.
Figure 3. PC12-TrkB response to BDNF.
(a) Serum deprived PC12-TrkB cells extended neurites in response to free BDNF. (b–d) However, no neurite extension was observed for non-treated or blank-material controls. Neurite extension was observed in the presence of polymer-encapsulated and polymer-released BDNF (50ng/ml).. (i) Neurite length was significantly greater in BDNF treated cells when compared to non-treated cells, regardless of the source of BDNF (error bars represent standard deviation).
Nissl staining was used to verify BDNF delivery coordinates. To further explore the accuracy of implant placement, a series of pilot experiments were performed in which rats received BDNF-EVAc implants and were sacrificed after behavioral testing for Nissl staining. Quantitative measures of EVAc-BDNF implant placement (e.g., implant length) were compared to behavioral scores in the forced swim test. No correlation between implant placement and behavioral score were identified (data not shown). For n=40 animals, an independent observer who was familiar with stereotactic surgery methods judged that the EVAc implants reached the target tissue region of CA1/DG (sample image provided in figure 2). In general, the implants were well tolerated and there was minimal damage to the tissue region surrounding the implant. We are therefore confident that the surgical method used to place BDNF-EVAc biomaterials was effective and accurate.
2.2 Behavioral Effects of BDNF Infusions
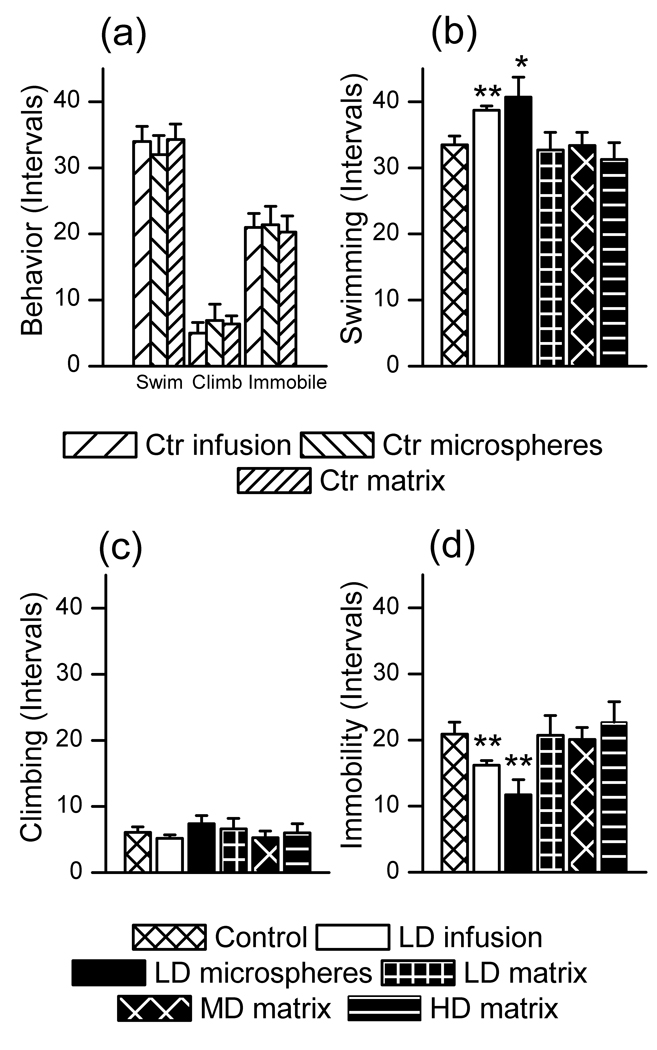
To determine how varying modes of delivery affected the antidepressant-like action of BDNF, we examined rat behavior in a modified version of the Porsolt forced swim test. The EVAc implant was smaller than a typical cannula used for infusions and Nissl staining confirmed that the polymer was well-tolerated, so we did not expect that the presence of the implant would influence baseline behavior. No differences in swimming, climbing or immobility measures were observed for rats that received saline infusions, blank microsphere or blank EVAc implants (figure 4a, one-way ANOVA p = 0.95), indicating that the vehicle does not alter baseline behavior. Therefore, the three material controls were pooled into a single group for statistical analysis of behavior. Single, bilateral infusions of BDNF in saline or implantation of BDNF-releasing microspheres (doses of 0.25µg per hemisphere) produced significantly decreased immobility compared to control (figure 4b). However, BDNF-releasing EVAc implants did not produce antidepressant-like behavior, regardless of the total dose administered (0.25, 3.8 or 11 µg per hemisphere, figure 4b). Increased swimming behavior was observed in the BDNF-infusion and BDNF-alginate groups, although climbing behavior was not different than control (figure 4c–d). These results demonstrate that varying modes of BDNF delivery produced varying behavioral effects.
Figure 4. Effect of BDNF on behavioral outcomes in the forced swim test for varying delivery methods.
(a) No differences in swimming, climbing or immobility behavior were observed for control groups that received saline infusions, blank microsphere or blank EVAc implants (one-way ANOVA, p=0.95), indicating that the vehicle itself does not alter behavior. (b) A single, LD infusion of BDNF and LD microsphere implants produced antidepressant-like effects, as evident by a decrease in immobility compared to pooled control (p=0.0023 and 0.0022, respectively). However, rats that received LD, MD or HD matrix implants did not behave differently than control rats (p=0.95, 0.73 and 0.58, respectively). (c,d) LD infusions of BDNF and LD microsphere implants produced increased swimming behavior compared to control rats (p=0.0012 and 0.039, respectively). However, rats that received LD, MD or HD matrices did not behave differently than control rats (p=0.79, 0.74 and 0.44, respectively). No differences were observed in climbing behavior. Statistical comparisons were performed for each dosing group compared to pooled, no-dose controls.
To determine the effect of BDNF on activity, rats were tested in the open field paradigm prior to their first forced swim session. While the open field test was conducted under standardized conditions that have been previously demonstrated to be sensitive to the effects of benzodiazepine anxiolytics, the present study did not observe any effect of BDNF on this psychological realm. No differences in total movement, number of center entries, or duration of center activity were observed for any of the groups (data not shown). We conclude that under the conditions tested here, BDNF delivery did not affect activity levels.
2.3 Biochemical Effects of BDNF
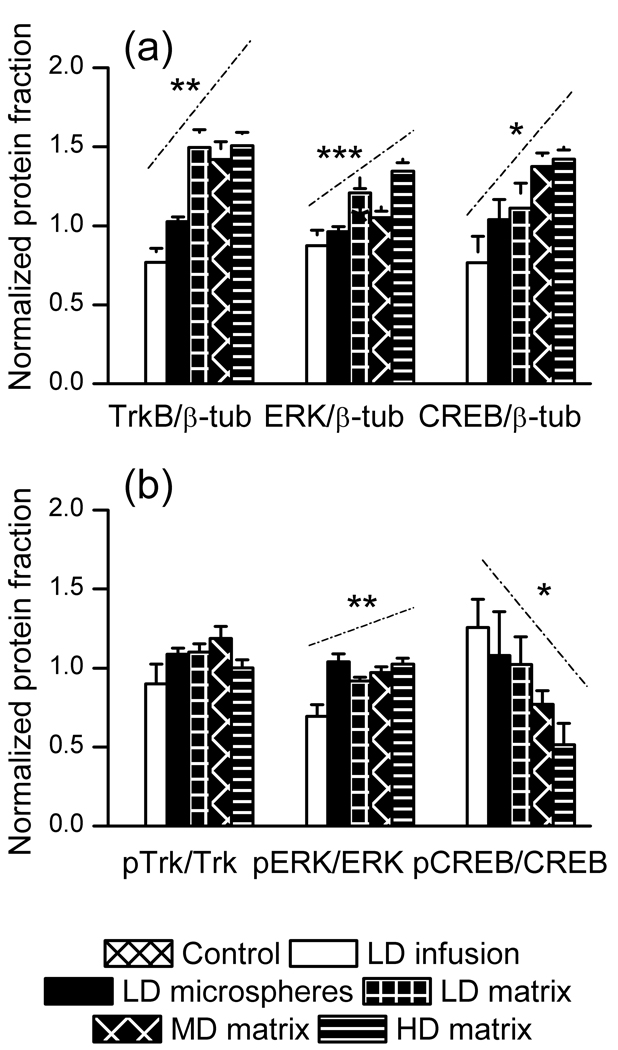
To determine how varying rates of BDNF delivery affected biochemical pathways regulated by BDNF, rats that received behavioral testing were sacrificed two days after the final forced swim session (10 days after surgery) and western blot was performed on hippocampal tissue samples obtained from the CA1/DG regions. In order to better visualize how dose and delivery duration affect BDNF function, we plotted all dosing groups on a single x-axis, where the dosing groups were ranked by increasing dose and duration of BDNF exposure (HD matrix > MD matrix > LD matrix > LD alginate > LD infusion) and the material control has a value of 1. Increasing dose and duration of BDNF exposure produced an increase in total TrkB, ERK and CREB; phosphorylated TrkB was unaffected, phosphorylated ERK was increased, and phosphorylated CREB was decreased (figure 5). These results demonstrate that varying modes of BDNF delivery produced varying effects on biochemical pathways that are regulated by BDNF.
Figure 5. Activation of the TrkB-ERK-CREB signaling pathway as a function of BDNF delivery.
LD infusion, LD alginate, LD EVAc, MD EVAc, and HD EVAc are ranked in order of increasing dose and duration of BDNF exposure, with material controls represented by a value of 1. Total levels of TrkB, ERK, and CREB increased for higher dose and duration of BDNF exposure (p = 0.0036, 0.00030, and 0.022, respectively). Phosphorylated ERK increased and phosphorylated CREB decreased for higher dose and duration of BDNF exposure (p = 0.023 and 0.028, respectively), whereas phosphorylated TrkB was unchanged (p = 0.33). The slope of each signficant relationship is depicted by a dashed line appearing above the data.
3 Discussion
Sustained, local drug delivery from polymer-based biomaterials is often less invasive and more effective than conventional therapy (Saltzman, 2001). The development of controlled release biomaterials for application in the brain could allow for the delivery of psychotropic agents that would not otherwise be tested. Polymeric drug delivery devices have been applied in both the central and peripheral nervous systems (Menei et al., 1994, Aebischer et al., 1996, Valtonen et al., 1997, Xudong and Shoichet, 1999, Terenghi, 1999, Bloch et al., 2001, Stokols et al., 2006), and for the controlled delivery of BDNF (Mittal et al., 1994, Sakane and Pardridge, 1997, Vejsada et al., 1998, Penschuck et al., 1999, Loh et al., 2001, Aszmann et al., 2002, Patist et al., 2004, Winter et al., 2008, Shi et al., 2008). However, to our knowledge, this is the first study to utilize controlled release biomaterials in a model of rodent behavior.
We observed that implantable biomaterials were effective vehicles to deliver BDNF to the hippocampus of rats. Biodegradable, alginate microspheres and nondegradable, EVAc implants were designed to release sustained levels of neurotrophin for several days or more than a week, respectively. BDNF released from these devices remained bioactive. The surgical method resulted in accurate implant placement in the CA1 and DG regions of the dorsal hippocampus, and the polymers were well-tolerated. No obvious cell death was observed in culture, and minimal tissue damage was observed in whole-brain. We observed that EVAc and alginate materials were effective vehicles that could be used to deliver varying rates and doses of bioactive BDNF to a local tissue region.
EVAc and alginate materials may be of interest for sustained-release application in the central nervous system beyond what was examined in this series of experiments. Alginate implants are an advantageous delivery method for several reasons. The formulation conditions utilized for the manufacture of alginate microspheres allows for high loading of drug in polymer with the use of an aqueous solvent (the use of an organic solvent in EVAc formulations could decrease the efficacy of some active agents). The high loading of alginate microspheres becomes particularly advantageous when compared to the loading of BDNF that was achieved for other polymeric microsphere formulations (1:1000 for poly(lactide-co-glycolide), data not shown). Second, microsphere doses can be administered by the same infusion protocols used for fluid infusions and thus pose only minimal change to established delivery methods. EVAc implants pose a separate set of advantages. Since solid matrices are formulated by a solvent-cast method, no drug is lost in the formulation steps and total loading of drug in polymer is known exactly. Furthermore, the geometry of the EVAc implants can be altered in order to target larger tissue regions, and EVAc materials can also be designed to deliver sustained doses of drug for longer periods of time than alginate materials. The best choice of drug-delivery material will therefore depend on a combination of drug delivery requirements, which include the desired material platform, dose and release kinetics.
In these studies, EVAc matrices released BDNF throughout the period of time studied, whereas alginate microspheres released the majority of encapsulated BDNF over 1–2 days. The half-life of BDNF in the brain is unknown, however, the half-life of nerve growth factor (NGF), a neurotrophin of similar size and charge, is reported to be less than one hour (Krewson and Saltzman, 1996). Since BDNF and NGF both activate the Trk family of receptors, it is likely that the half-life of BDNF is also on the order of hours. The BDNF delivery methods utilized here can therefore be ranked in order of magnitude and temporal/spatial duration of BDNF exposure: HD matrices > MD matrices > LD matrices > LD microspheres > LD infusion.
In this work, distinct behavioral differences in the forced swim test were observed between rats that received different formulations of BDNF. As previously reported (Shirayama et al., 2002), a one-time, bilateral infusion of 0.25µg of BDNF produced antidepressant-like effects in the forced swim test. Delivery of BDNF from alginate microspheres was also antidepressant-like, whereas delivery of BDNF from EVAc matrices was not antidepressant-like, even when the total dose was high (11µg). The loss of antidepressant-like activity of BDNF was not due to lower effective concentration. Since delivery of BDNF as a single infusion or from alginate microspheres produced an increase in swimming behavior with no change in climbing behavior, it is possible that BDNF function was mediated via serotonergic action (Cryan, et al., 2005). Although these experiments were not designed to study the serotonin system, selectively enhanced swimming behavior is consistent with other published reports demonstrating enhanced serotonin function and the sprouting and survival of serotonergic neurons after direct delivery of BDNF (Siuciak et al., 1996, Mamounas et al., 1995). Our results suggest that sustained exposure to BDNF may interfere with its previously reported antidepressant-like effects.
Behavioral effects after infusion of BDNF have been examined for several modes of neurotrophin delivery (summarized in table 2). BDNF produced antidepressant-like effects when applied as a continuous, high-dose infusion to the midbrain (Siuciak et al., 1997), as a one-time, low-dose infusion into the hippocampus (Shirayama et al., 2002), and as a one-time, low-dose infusion to the ventricles (Hoshaw et al., 2005). In contrast, BDNF produced prodepressive-like behavior when administered as a continuous, low-dose infusion to the Ventral-Tegmental Area (VTA) of the Nucleus Accumbens (NAc) (Eisch et al., 2003).
Table 2.
| Mode of BDNF Delivery |
|||||
|---|---|---|---|---|---|
| Study | Location | Method | Delay | Dose | Outcome |
| Siuciak, et al., 1997 | Midbrain | Continuous | 7 days | 12 or 24 µg/day |
Decreased immobility in the forced swim test; decreased latency to escape and increased number of escapes in learned helplessness |
| Shirayama, et al., 2002 | Hippocampus | Single Dose | 3, 7 or 10 days |
0.05,0.25, 1 µg |
Decreased immobility in the forced swim test; decreased latency to escape and increased number of escapes in learned helplessness; behavioral effects blocked by infusion of MEK inhibitor |
| Eisch, et al., 2003 | Ventral- Tegmental Area |
Continuous | 7 days | 2.5 µg/day | Decreased latency to immobility in the forced swim test |
| Hoshaw, et al., 2005 | Ventricles | Single Dose | 3, 6 or 12 days |
0.1, 1µg | Decreased immobility in the forced swim test at 3 or 6 but not 12 day following the infusion 1 but not 0.1 µg doses of BDNF |
| Current Study 2009 | Hippocampus | Sustained Release Polymers |
7 days | 0.25-11µg | Dose and duration dependent effects in the forced swim test |
Published studies describe antidepressant-like and prodepressive-like effects of BDNF when BDNF was delivered to different locations with different dosing schedules
The different behavioral outcomes of BDNF administration observed in prior studies were attributed to region-specific functionality of the neurotrophin. Since there are drugs which are known to exert one type of action in one brain region and exert an alternate (sometimes opposite) action in other brain regions (Jussofie, 1993, Reuss et al., 2000, Wortwein et al., 2006), it would not be surprising to observe region-specific functionality for BDNF action. The fact that the BDNF receptor, TrkB, undergoes precise regional and developmental regulation emphasizes this point: it is likely that the local function of BDNF is strongly influenced by the underlying distribution of its receptor (Anderson et al., 1995). However, there are also known instances of drugs whose activity depends on the duration or periodicity of administration protocol (Koopmans et al., 1996, Laursen et al., 2001, Samaha et al., 2008), raising the possibility that the biological effect of increased levels BDNF could depend on the duration over which it is delivered.
The temporal pattern of delivery is particularly important to consider for direct delivery of BDNF or for therapies that are designed to increase levels of BDNF in a sustained, non-activity-dependent manner. It is known that sustained levels of BDNF can result in down-regulation of TrkB and activation of cell death pathways, and prior exposure of cells to BDNF regulates sensitivity of cells to BDNF in vitro. For example, primary hippocampal cultures that were primed with a single exposure to BDNF on day 0 could not be stimulated by BDNF to produce c-fos on day 6 (Frank, 1996). The sustained presence of BDNF can also be harmful to cells. Neuronal necrosis was observed after 48 hours of continuous exposure of BDNF in primary cortical cultures (Kim et al., 2003), and sustained application of BDNF to the spinal cord was neurotoxic above doses of 1 µ g per day (Boyd and Gordon, 2002).
Sustained delivery of BDNF is known to produce a variety of complex effects in vivo; certain delivery paradigms produced down-regulation of plasticity-related cellular processes. Although continuous infusion of high doses of BDNF to the midbrain produced antidepressant-like effects in the forced swim test (Siuciak et al., 1997), the same delivery protocol produced compensatory deactivation of TrkB, with total levels of TrkB protein reduced by 70% after 6 days of continuous BDNF delivery (Frank et al., 1997). In other studies, BDNF was used to increase the excitability of neurons in order to induce seizures in rats (Xu et al., 2004). It was observed that serial bolus infusions of BDNF in the hippocampus produced seizures, whereas the same dose delivered continuously did not. Levels of phosphorylated TrkB were down-regulated after continuous delivery when compared to bolus delivery, yet, at sufficiently high, continuously delivered doses, BDNF regained kindling activity. Since TrkB is unlikely be entirely deactivated (i.e., there will always be some receptors present), it appeared that continuous exposure to BDNF produced down-regulation of receptor activity that was overcome by increasing the dose of BDNF (Xu et al., 2004). In our experiments, we observed that animals which exhibited antidepressant-like behavior had lower levels of TrkB than animals that did not exhibit antidepressant-like behavior. Prior experiments designed to examine the role of BDNF in neuroplasticity have used a variety of methodologies, with different delivery locations, durations, and doses, and with behavioral and biochemical metrics that are measured at different times. Direct comparison of each of these studies with our work is not possible, due to methodological differences, however, taken collectively, two key features of BDNF function are evident: (1), the relationship between neuroplasticity-related behavior and neuroplasticity-related biochemistry is complex, and (2), the precise effect of BDNF individual components of neuroplasticity-related processes depends on how it is delivered.
It is possible to speculate on the association of the observed behavioral with these biochemical measurements, and it is particularly interesting to consider how delivery duration may affect the typical direction of association of members of the pathway. It was expected that TrkB, ERK and CREB would be regulated in the same direction (Hu et al., 2000, Grewal et al., 1999). Instead, we observed biochemical changes for different doses and duration of delivery that occurred in distinct directions for different members of the pathway. Since CREB regulates gene transcription activity through phosphorylation dependent and independent mechanisms, increased levels of CREB as well as pCREB have been implicated in the action of some antidepressant drugs (Blendy, 2006). Yet, in these experiments, antidepressant-like effects were not observed in animals that exhibited increased levels of CREB. These results suggest adaptation of the CREB pathway to sustained BDNF exposure that has not been observed in prior acute studies.
It was also expected that up- or down-regulation of Trk, ERK and CREB would reflect up- or down-regulation of protein levels associated with neuronal plasticity. In these experiments, levels of pCREB/CREB were observed to decrease in the same animals where TrkB, ERK and CREB tended to increase; the animals with low levels of pCREB/CREB also did not demonstrate reduced immobility after BDNF exposure. Phosphorylation of CREB is known to regulate gene transcription (Chrivia et al., 1993, Bito et al., 1996, Shaywitz and Greenberg, 1999), and this regulation of gene transcription by phosphorylated CREB produces complex downstream effects in gene translation that are believed to be necessary for the induction of late-phase long term potentiation (L-LTP). L-LTP is the mechanism by which alterations in neuronal plasticity occur, and neuronal plasticity is one of the mechanisms by which neurotrophins are believed to exert therapeutic effect. Therefore, the observed decrease in pCREB/CREB in animals that did not have a behavioral response to treatment suggests that sustained delivery of BDNF produced deficits in neuronal plasticity-related biochemical pathways, as ultimately measured by behavior in the forced swim paradigm. In future studies, it would be interesting to characterize the full dose-response relationship for BDNF function, in order to determine the precise dose and duration-dependent threshold over which BDNF produces dysregulation of CREB function.
In these studies, BDNF was delivered to the rat hippocampus via three vehicles: saline infusion, alginate microspheres, and EVAc implants. The rate and duration of BDNF exposure varies for each vehicle, as does the material that comes into contact with the hippocampal cells. An important question follows: is the lack of behavioral effect observed with BDNF-EVAc due to material effect, (for example, an increase in hippocampal damage)? We feel confident in concluding that our observations are not solely due to material effect for several reasons. First, EVAc is known to be biocompatible in the brain (During et al., 2004), and neuronal regeneration has been observed in the presence of EVAc in other systems (Tornqvist, et al., 2000, and Barras, et al., 2002). There is no expected mechanism by which the presence of EVAc, a solid, non-degradable polymer could halt part (e.g., phosphorylation of CREB) but not all (e.g., phosphorylation of ERK) of neuroplasticity-related processes. Second, BDNF was reported to produce neuroplasticity-related behavioral effects in the presence of significant hippocampal damage produced by a permanently implanted guide cannula (Cirulli and Chiarotti, 2004). The EVAc implant produces less hippocampal damage than a permanently implanted guide cannula, due to the lower volume and stationary nature of the implant. Guide cannulae commonly produce significant amounts of brain damage, yet we are not aware of any study that has demonstrated a lack of behavioral effect due solely to the presence of the cannula. Third, there were no differences in baseline behavior between vehicle controls. Fourth, in all cases, regardless of delivery vehicle, BDNF was bioactive and produced dose- and duration-dependent biochemical effects. It is therefore not likely that the lack of behavioral effect observed in these studies is due to differences in delivery material alone. We conclude that particular durations of delivery could interfere with the neuroplasticity-related antidepressant-like function of BDNF. The dose- and duration-dependent effects of BDNF are therefore important considerations in the development of therapies that seek to manipulate BDNF function in the brain.
EVAc and alginate-based biomaterials both effectively delivered bioactive BDNF to the rat hippocampus for varying experimental durations. We observed antidepressant-like behavioral effects when BDNF was delivered over a short period of time to a confined target region; these effects were not observed when BDNF was delivered over a longer period of time to a larger tissue site. A significant increase in total protein levels was observed for TrkB, ERK and CREB for greater dose and duration of BDNF delivery. Levels of pTrkB/TrkB were unchanged and levels of pERk/ERK were increased whereas levels of pCREB/CREB tended to decrease for greater dose and duration of BDNF exposure. Our behavioral and biochemical results are in line with reports of BDNF-induced desensitization of plasticity-related function observed both in vitro and in vivo; these results support a growing body of evidence that suggests that the biological effects of BDNF can depend on the precise delivery paradigm. The observed decrease in pCREB/CREB suggests that sustained delivery of BDNF produced a downregulation in gene transcription activities, which may have been responsible for functional deficits in neuronal plasticity on a behavioral level. In sum, dysregulation of BDNF-associated, plasticity-related pathways was observed after sustained delivery of BDNF in the dorsal hippocampus of the rat. Our results highlight an important consideration for the development of therapies to target BDNF-related pathways: the manner in which an autoregulatory pathways is perturbed can influence its biological outcome.
4 Experimental Procedure
4.1 Biomaterial Construction
BDNF-containing microspheres were fabricated by dissolving 4% w/v of alginic acid (viscosity 200.000–400.000 cps, Sigma-Aldrich, St. Louis, MO) with BDNF in molecular grade water containing 0.2% HPMC (Sigma). The BDNF solution was dialyzed through a 10kDa dialysis membrane (Thermo Scientific, Rockland, IL) to remove excessive buffer salts. To prevent endotoxin contamination, reagent solutions were filtered and all equipment was heated to 250°C for two hours prior to use. The alginate-BDNF solution was passed through a 0.22µm PES membrane (Millipore, Kankakee, IL) and stored at 4°C prior to use. Three ml of alginate-BDNF solution and 1 ml of 30% Tween 80 were added dropwise to a mixture of 13.5 ml isooctane and 5% Span 80 undergoing homogenization at 17,500 RPMS. Cross-linking solution (2.5 ml of 8% filtered calcium chloride) was added dropwise to the beaker at a rate of 4 ml/minute. The cross-linked mixture was homogenized for 3 minutes. Isopropanol (13.5 ml) was added to the beaker over 30 seconds and homogenized for 3 minutes. The resulting solution was centrifuged and washed twice with isopropanol. After the final wash, the supernatant was gently poured off, and the remaining isopropanol was evaporated off with an air stream. The CaCl2-crosslinked alginate particles were resuspended in water, frozen and lyophilized for 48 hours to yield a free flowing powder.
EVAc matrices were fabricated to contain 1, 7.6 and 22 µg BDNF / mg EVAc. The desired quantity of BDNF was first dissolved in water with an inert sugar, Ficoll (400kD, Sigma). The amount of added Ficoll was calculated to account for buffer salts present in the BDNF solution (0.92 mg salt / mg BDNF) to bring the total loading of non-polymer to 50% of implant weight. The mixture was frozen, lyophilized and ground to a fine powder. One hundred mg of EVAc were dissolved in 2ml of methylene chloride (Sigma). The protein-sugar powder (100mg) was added to the polymer solution and vortexed briefly; the resulting suspension was poured into a chilled aluminum mold (−80°C) and allowed to freeze for several minutes. The polymer disks were stored at −20°C for 48 hours and then lyophilized for 48 hours to remove all traces of solvent. The disks were stored at −20°C until use.
4.2 Biomaterial Characterization
Controlled release experiments were conducted at 37°C; alginate particles and EVAc containing BDNF were incubated in 1.1ml of PBS. Controlled release samples (1ml) were removed and replaced at regular intervals: 2hrs, 7 hrs, 12hrs, 1, 2, 7, and 14 days for EVAc implants; 3hrs, 8hrs, 1 day and 2 days for alginate microspheres. Protein released from alginate microspheres was not detectable in media after 2 days. BDNF was quantified by BCA assay (Pierce, Rockland, IL). Microsphere diameter was measured with a light microscope at 60× magnification.
Polymer encapsulated BDNF was subjected to several processing steps, and so it was necessary to confirm that the encapsulated BDNF was bioactive. PC12-TrkB cells were cultured in DMEM media (Invitrogen, Carslbad, CA) containing 10% FBS (Invitrogen), 5% Horse Serum (Invitrogen) and Penicillin Streptomycin (Invitrogen) on collagen-coated (10 µg/well) 6-well plates to 50% confluency. Medium was replaced with serum-free DMEM containing BDNF (in free form, with polymer carrier, or obtained from controlled release samples) at a concentration of 50ng/ml. Each material treatment was applied to three wells. Control cells were exposed to serum-free DMEM or serum-free DMEM with blank polymer. After 4 days of BDNF exposure, the cells were examined under light microscope at 20× magnification. Neurite extension was quantified using ImageJ (NIH, Bethesda, MD); values were averaged for 3 samples for each dosing group, with error reported as standard deviation.
4.3 Stereotactic Surgery
The stereotactic procedure followed the design of a prior study that observed antidepressant-like effects of BDNF delivery to the hippocampus (Shirayama et al., 2002). Stereotactic coordinates were chosen to target the CA1 and DG regions of the dorsal hippocampus. A total of 72 male Sprague-Dawley rats (275–325g; Charles River Laboratory) were housed in pairs, maintained on a 12-hour light-dark cycle and given ad libitum access to food and water. Surgical dates were counterbalanced among 8 experimental groups (doses are described in table 1). The night prior to surgery, food was withheld. On day 1, rats were anesthetized with an intraperitoneal injection of pentobarbitol (55 mg/kg). Burr holes were drilled bilaterally (AP = −3.8mm, ML = ±2.0mm). Alginate microsphere solutions (6.25 µg polymer per µl saline) were prepared no more than two hours prior to surgery. For saline and free BDNF infusions and implantation of blank alginate and alginate-BDNF microspheres, a Hamilton syringe was lowered to DV = −3.8 in order to pierce the membrane surrounding the hippocampus, raised to DV = −3.4, and 1µl of fluid was infused over 10 minutes, followed by a 5 minute waiting period to prevent backflow. Implants were cut to size (0.25 or 0.5mg) on the day of surgery. For blank EVAc and EVAc-BDNF implants, a 22-gage guide cannula containing the polymer implant was lowered to DV = −3.8, raised to DV = −3.4mm, and the implant was pushed out with a dummy cannula. All infusions and implantations were bilateral. Incisions were closed using wound clips and the rats were treated with post-operation, interpertoneal injections of analgesia (carprofen, 5mg/kg/day) for 3 days.
Table 1.
| Dose | Method | Vehicle | BDNF(µg) |
|---|---|---|---|
| Control (Ctr) | Infusion | Saline | 0 |
| Low (LD) | Infusion | Saline | 0.25 |
| Control (Ctr) | Microspheres | Alginate | 0 |
| Low (LD) | Microspheres | Alginate | 0.25 |
| Control (Ctr) | Implant | EVAc | 0 |
| Low (LD) | Implant) | EVAc | 0.25 |
| Medium (MD) | Implant | EVAc | 3.8 |
| High (HD) | Implant | EVAc | 11 |
BDNF dosing scheme for animal surgeries
4.4 Behavioral Tests
Open field activity sessions were conducted in a quiet, dimly-lit room between 9am and 12pm on day 7 following surgery. Rats were placed in a 60×60cm white plastic arena and allowed to explore the arena for 15 minutes. The arena was cleaned with water prior to each test and behaviors were taped for later blind scoring. A 3×3 grid was overlayed on the viewing screen and total number and duration of center-field entries (counted when all four limbs entered the center square) were recorded.
Initial forced swim sessions occurred between 1 and 6pm in a dark room on day 7 following surgery. Rats were placed in a Plexiglass cylinder containing water (24°C, 18 inches deep), allowed to swim freely for 15 minutes, and then dried with paper towels and placed on a heating pad before returning to home cage. A 5 minute re-test occurred between 1 and 6pm on day 8. Swim sessions were taped for later scoring. Climbing was counted as activity during which both front paws reached above the waterline onto the plexiglass surface for the duration of the five second interval. Swimming was counted for all active, non-climbing intervals. Immobility was counted during intervals during which the rat made only the minimum effort necessary to stay afloat. Climbing, swimming and immobility behaviors were recorded in 5 second intervals by a rater blind to the experimental manipulations.
In the original paradigm described by Porsolt (Porsolt et al., 1977), rats were were allowed to swim for 15 minutes on day 1, and pharmacological treatment was provided immediately after this first session. Rats were re-tested, with behavioral scoring, in a second, 5-minute swim session on day 2. Antidepressant treatment is known to produce a decrease in the time spent immobile during the day 2 session. In our experiments, rats were allowed to recover from surgery for 7 days prior to behavioral testing, and so they received pharmacological treatment prior to the first swim session. This modified paradigm is similar to other studies that have infused drugs without the use of permanent cannulae (Shirayama et al., 2002).
4.5 Sacrifice, Tissue Collection and Western Blots
Rats were sacrificed by rapid decapitation on day 12. The brains were quickly removed, placed on dry ice, and stored at −80°C until use. On the day of processing, brains were brought to −20° C for several minutes, placed on a dry-ice chilled aluminum mold (ASI Instruments, Avon Lake, OH), and sliced through the coronal plane into 2mm sections. Hippocampal tissue was obtained with two 1mm-diameter tissue punches (Fine Science Tools, Foster City, California) from the CA1 and DG regions immediately surrounding the infusion or implant location in each hemisphere. Tissue samples were dissociated with two 10 second sonication bursts in 200µl of ice-cold sonication buffer (137mM NaCl, 20mM Tris-HCl, 1% igepal, 10% glycerol) with 1:100 protease and phosphotase inhibitor coctails 1 and 2 (Sigma). A BCA protein assay (Pierce, Rockland IL) was used to determine total protein levels for each sample.
For Western Blots, five microliters of Laemmili buffer (20% glycerol, 2% sodium dodecyl sulfate) were added to 15µl of sample containing 20µg of tissue and boiled at 100°C for 10 minutes. The denatured proteins were loaded onto gradient Tris-glycine gels (Invitrogen), separated by SDS-polyacrylamide gel electrophoresis for 2–3 hours at 100V, and transferred to a 0.2 µm nitrocellulose membrane (Bio-Rad, Hercules, California) overnight at 20V. All immunoblotting proceeded as follows, with four 15-minute washes in excess 0.1% Tween-20 PBS between each step: two hour block in 5% milk, one hour incubation with primary antibody, and one hour incubation with secondary antibody. The following antibodies were used: ERK Rb (1:2000, Cell Signaling), pERK Ms (1:1000, Cell Signaling), TrkB Ms (1:500, BD Biosciences), pTrk Rb (1:250, Cell Signaling), CREB Rb (1:1000, Sigma), pCREB Ser133 Rb (1:1000, Sigma), β-tubulin Rb (1:5000, ABCam).
To verify infusion location, fresh-frozen tissue was sliced to a thickness of 15–30µm on a freezing cryostat (Leica, Belair Instrument Co., Springfield, NJ) and inverted onto warm, poly-L-lysine-coated glass slides(American Master Tech Scientific, Inc., Lodi, CA). Nissl staining was performed with 0.1% w/v Cresyl Violet (Sigma).
4.6 Statistical Analysis
The infusion group had 6 dosed animals with 7 controls, the microsphere group had 9 dosed animals with 9 controls, and the matrix groups had 8–10 animals for each dose with 9 control animals. Except where stated, error bars represent standard error of the mean. Statistical analysis was performed using GraphPad Prism statistical software (v5.02, GraphPad Software, La Jolla, CA). ANOVA was first used to check for statistical differences between group means with α=0.05. Post hoc testing of behavioral data utilized a two-tailed Welch’s t test. Post hoc testing of biochemical data utilized a regression analysis (the method is described elsewhere, (Sheskin, 2007)).
Acknowledgments
The authors gratefully acknowledge funding provided by the National Institutes of Health (NS45236, PHS DA011717, MH066172 and MH025642), as well as support from the Interdisciplinary Research Consortium on Stress, Self-control and Addiction (UL1-DE19586), the NIH Roadmap for Medial Research/Common Fund (AA017537), and the CT Department of Mental Health and Addiction Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aebischer P, Schluep M, Deglon N, Joseph JM, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC, Regli F, Baetge EE. Intrathecal delivery of cntf using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat. Med. 1996;2:696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Alderson RF, Altar CA, DiStefano PS, Corcoran TL, Lindsay RM, Wiegand SG. Differential distribution of exogenous BDNF, NGF, and NT-3 in the brain corresponds to the relative abundance and distribution of high-affinity and low-affinity neurotrophin receptors. J. Comp. Neurol. 1995;357:296–317. doi: 10.1002/cne.903570209. [DOI] [PubMed] [Google Scholar]
- Aszmann OC, Korak KF, Kropf N, Fine E, Aebischer P, Frey M. Simultaneous GDNF and BDNF application leads to increased motoneuron survival and improved functional outcome in an experimental model for obstetric brachial plexus lesions. Plas. Reconstr. Surg. 2002;110:1066–1072. doi: 10.1097/01.PRS.0000020990.82332.43. [DOI] [PubMed] [Google Scholar]
- Barras FM, Pasche P, Bouche N, Aebischer P, Zurn AD. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J. Neurosci. Res. 2002;70:746–755. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol. Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bloch J, Fine EG, Bouche N, Zurn AD, Aebischer P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp. Neurol. 2001;172:425–432. doi: 10.1006/exnr.2001.7778. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur. J. Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Cho NH, Seong SY, Chun KH, Kim YH, Chan KI, Ahn BY, Jeong SY. Novel mucosal immunization with polysaccharide-protein conjugates entrapped in alginate microspheres. J. Control. Release. 1998;53:215–224. doi: 10.1016/s0168-3659(97)00255-1. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RPS, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cirulli B, Chiarotti A. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris Water Maze and performance in the Elevated Plus-Maze. Hippocampus. 2004;14:802–807. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: A case of neuronal life and death? Biol. Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duman RS. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol. Aging. 2005;26:88–93. doi: 10.1016/j.neurobiolaging.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol. Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- During MJ, Freese A, Sabel BA, Saltzman WM, Deutch A, Roth RH, Langer R. Controlled release of dopamine from a polymeric brain implant: In vivo characterization. Ann. Neurol. 2004;25:351–356. doi: 10.1002/ana.410250406. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, De Wit J, Simonak RS, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol. Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: Implications for tissue engineering and wound healing. Artif. Organs. 2001;25:558–565. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- Frank L. BDNF down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur. J. Neurosci. 1996;8:1220–1230. doi: 10.1111/j.1460-9568.1996.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Frank L, Wiegand SJ, Siuciak JA, Lindsay RM, Rudge JS. Effects of BDNF infusion on the regulation of TrkB protein and message in adult rat brain. Exp. Neurol. 1997;145:62–70. doi: 10.1006/exnr.1997.6440. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, metaplasticity, and antidepressants. Curr. Mol. Med. 2002;2:629–638. doi: 10.2174/1566524023362023. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJS. Extracellular-signal-regulated kinase signalling in neurons. Curr. Opin. Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Gruber CJ. The combined contraceptive vaginal ring (nuvaring): Evaluation of the clinical and pharmacological evidence. Women’s Health. 2006;2:351–356. doi: 10.2217/17455057.2.3.351. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Hu BR, Liu CL, Park DJ. Alteration of map kinase pathways after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2000;20:1089–1095. doi: 10.1097/00004647-200007000-00008. [DOI] [PubMed] [Google Scholar]
- Jussofie A. Brain region-specific effects of neuroactive steroids on the affinity and density of the GABA-binding site. Biol. Chem. Hoppe-Seyler. 1993;374:265–270. doi: 10.1515/bchm3.1993.374.1-6.265. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hwang JJ, Behrens MM, Snider BJ, Choi DW, Koh JY. TrkB mediates BDNF-induced potentiation of neuronal necrosis in cortical culture. Neurobiol. Dis. 2003;14:110–119. doi: 10.1016/s0969-9961(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Koopmans SJ, Sips HCM, Krans HMJ, Radder JK. Pulsatile intravenous insulin replacement in streptozotocin-diabetic rats is more efficient than continuous delivery: Effects on glycaemic control, insulin-mediated glucose metabolism and lipolysis. Diabetologia. 1996;39:391–400. doi: 10.1007/BF00400670. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewson CE, Saltzman WM. Transport and elimination of recombinant human NGF during long-term delivery to the brain. Brain Rese. 1996;727:169–181. doi: 10.1016/0006-8993(96)00378-2. [DOI] [PubMed] [Google Scholar]
- Laursen T, Gravholt CH, Heickendorff L, Drustrup J, Kappelgaard AM, Jørgensen JOL, Christiansen JS. Long-term effects of continuous subcutaneous infusion versus daily subcutaneous injections of growth hormone (GH) on the insulin-like growth factor system, insulin sensitivity, body composition, and bone and lipoprotein metabolism in GH-deficient adults. J. Clin. Endocrinol. Metab. 2001;86:1222–1228. doi: 10.1210/jcem.86.3.7323. [DOI] [PubMed] [Google Scholar]
- Loh NK, Woerly S, Bunt SM, Wilton SD, Harvey AT. The regrowth of axons within tissue defects in the CNS is promoted by implanted hydrogel matrices that contain BDNF and CNTF producing fibroblasts. Exp. Neurol. 2001;170:72–84. doi: 10.1006/exnr.2001.7692. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J. Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray NA, Zarate CA, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol. Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Menei P, Benoit JP, Boisdron-Celle M, Fournier D, Mercier P, Guy G, Vick NA, Brem H. Drug targeting into the central nervous system by stereotactic implantation of biodegradable microspheres. Neurosurgery. 1994;34:1058–1064. doi: 10.1227/00006123-199406000-00016. [DOI] [PubMed] [Google Scholar]
- Mittal S, Cohen A, Maysinger D. In vitro effects of brain derived neurotrophic factor released from microspheres. NeuroReport. 1994;5:2577–2582. doi: 10.1097/00001756-199412000-00043. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(d,l-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25:1569–1582. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Giorgetta O, Fritschy JM. Neuronal activity influences the growth of barrels in developing rat primary somatosensory cortex without affecting the expression pattern of four major GABA(a) receptor subunits. Brain Res. Dev. Brain Res. 1999;112:117–127. doi: 10.1016/s0165-3806(98)00171-0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Reuss B, Hertel M, Werner S, Unsicker K. Fibroblast growth factors-5 and -9 distinctly regulate expression and function of the gap junction protein connexin43 in cultured astroglial cells from different brain regions. Glia. 2000;30:231–241. [PubMed] [Google Scholar]
- Sakane T, Pardridge WM. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res. 1997;14,:1085–1091. doi: 10.1023/a:1012117815460. [DOI] [PubMed] [Google Scholar]
- Saltzman WM. Drug Delivery: Engineering Principles for Drug Therapy. New York City: Oxford University Press; 2001. [Google Scholar]
- Saltzman WM, Mak MW, Mahoney MJ, Duenas ET, Cleland JL. Intracranial delivery of recombinant nerve growth factor: release kinetics and protein distribution for three deliveery systems. Pharm. Res. 1999;16:232–240. doi: 10.1023/a:1018824324275. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Reckless GE, Seeman P, Diwan M, Nobrega JN, Kapur S. Less is more: Antipsychotic drug effects are greater with transient rather than continuous delivery. Biol. Psychiatry. 2008;64:145–152. doi: 10.1016/j.biopsych.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of Parametric and Non-Parametric Statistical Procedures. Fourth Edition. Florida, USA: CRC Press; 2007. [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shi GD, Jia LS, Yuan W, Shi JG, Tan JM, Chu C. Preparation of injectable sustained-release nanoparticles carrying brain-derived neurotrophic factor and evaluation of their drug releasing characteristics. Academic Journal of Second Military Medical University. 2008;29:538–542. [Google Scholar]
- Shirayama Y, Chen ACH, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacology Pharmacol. Biochem. Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Smith AL, Cordery PM, Thompson ID. Manufacture and release characteristics of Elvax polymers containing glutamate receptor antagonists. J. Neurosci. Methods. 1995a;60:211–217. doi: 10.1016/0165-0270(95)00014-l. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995b;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 2006;12:2777–2787. doi: 10.1089/ten.2006.12.2777. [DOI] [PubMed] [Google Scholar]
- Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist N, Bjorklund L, Almqvist P, Wahlberg L, Stromberg I. Implantation of bioactive growth factor-secreting rods enhances fetal dopaminergic graft survival, outgrowth density, and functional recovery in a rat model of Parkinson’s disease. Exp. Neurol. 2000;164:130–138. doi: 10.1006/exnr.2000.7411. [DOI] [PubMed] [Google Scholar]
- Ueng SWN, Yuan LJ, Lee N, Lin SS, Chan EC, Weng JH. In vivo study of biodegradable alginate antibiotic beads in rabbits. J. Orthop. Res. 2004;22:592–599. doi: 10.1016/j.orthres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high- grade gliomas: A randomized double-blind study. Neurosurgery. 1997;41:44–49. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Vejsada R, Tseng JL, Lindsay RM, Acheson A, Aebischer P, Kato AC. Synergistic but transient rescue effects of BDNF and GDNF on axotomized neonatal motoneurons. Neurosci. 1998;84:129–139. doi: 10.1016/s0306-4522(97)00497-1. [DOI] [PubMed] [Google Scholar]
- Winter JO, Gokhale M, Jensen RJ, Cogan SF, Rizzo JF. Tissue engineering applied to the retinal prosthesis: Neurotrophin-eluting polymeric hydrogel coatings. Mater. Sci. Eng. Biomim. Mater. Sens. Syst. 2008;28:448–453. doi: 10.1016/j.msec.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortwein G, Husum H, Andersson W, Bolwig TG, Mathe AA. Effects of maternal separation on neuropetide Y and calcitonin gene-related peptide in "depressed" flinders sensitive line rats: A study of gene-environment interactions. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:684–693. doi: 10.1016/j.pnpbp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Xu B, Michalski B, Racine RJ, Fahnestock M. The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, Trk expression and seizure-related morphological changes. Neurosci. 2004;126:521–531. doi: 10.1016/j.neuroscience.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Xudong C, Shoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339. doi: 10.1016/s0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]