Abstract
Herpesviruses commandeer distinct cellular pathways to enter target cells. The mechanism by which herpes simplex virus (HSV) selects a pH-dependent, endocytic route or a pH-independent route remains to be elucidated. We investigated the role of the non-glycosylated viral envelope protein UL45 in HSV entry via endocytosis. UL45 plays a role in mediating cell-cell fusion and has been proposed to functionally interact with gB to regulate membrane fusion. Thus, we also probed the impact of UL45 on the structure and function of gB present in virions. A UL45 deletion virus successfully entered cells via low pH, endocytic pathway with wild type kinetics. In the absence or presence of UL45, the antigenic conformation of virion gB appeared unaltered. Antibodies to gB neutralized infection of the UL45-deletion virus and wild type virus to a similar extent, regardless of whether the target cells supported low pH endocytic or non-endocytic entry routes. Lastly, HSV virions were inactivated by low pH regardless of the presence of UL45. The results, together with previous studies, suggest that UL45 plays distinct roles in cell-cell fusion and virus-cell fusion during acid-dependent entry.
Keywords: herpes simplex virus, viral endocytosis, UL45, glycoprotein B
Herpes simplex virions can use pH-dependent endocytosis to initiate infection of host cells (Nicola et al., 2003). Other human herpesviruses have also been shown to utilize low pH, endocytic pathways for entry (Akula et al., 2003; Finnen et al., 2006; Ryckman et al., 2006). HSV may use an acid-dependent pathway to enter mucosal epithelial cells at the portal of entry in the human host (Nicola et al., 2005). Infection of neurons isthought to occur via a pH-independent mechanism (Lycke et al., 1988; Nicola et al., 2005; Stiles et al., 2008). Direct, acid-independent penetration at the cell surface is the entry pathway in Vero cells (Wittels and Spear, 1991), which are a commonly used cell type to study HSV biology. The utilization of multiple cellular pathways to enter different physiologically relevant cell types is an emerging theme among herpesviruses (Frampton et al., 2007; Miller and Hutt-Fletcher, 1992; Nicola et al., 2005; Nicola et al., 2003; Raghu et al., 2009; Ryckman et al., 2006; Van de Walle et al., 2008).
How HSV entry via a particular cell pathway is determined is not well-understood. Both virus determinants (Delboy et al., 2006; Roller et al., 2008) and host factors such as cell receptors (Arii et al., 2009; Delboy et al., 2006; Gianni et al., 2004; Milne et al., 2005; Roller et al., 2008; Stiles et al., 2008) influence the route taken. Entry by an endocytic mechanism may also be distinguished by the involvement of host kinases (Cheshenko et al., 2003; Cheshenko et al., 2005; Clement et al., 2006; Hoppe et al., 2006; Nicola et al., 2005; Nicola and Straus, 2004; Petermann et al., 2009). For the human herpesviruses EBV (Wang et al., 1998) and HCMV (Wang and Shenk, 2005), distinct viral envelope protein complexes determine target cell tropism and entry pathway. For HSV, glycoproteins gB, gD, and gH-gL are required for entry via pH-independent (Spear, 1993) and pH-dependent (Nicola and Straus, 2004) pathways. We theorize that one or more specific HSV envelope proteins direct the virus to the low pH endosomal route. Several HSV envelope proteins are non-essential for replication on Vero cells, which support pH-independent penetration of HSV at the cell surface (Wittels and Spear, 1991). With the exception of gC (Nicola and Straus, 2004), these proteins have not been evaluated for potential involvement in entry via endocytosis.
The HSV UL45 membrane protein lacks consensus sites for addition of N-linked carbohydrates. The UL45 gene is expressed late and encodes a 172 residue,~ 18 kilodalton type II membrane protein that is dispensable for growth in Vero cells (Cockrell and Muggeridge, 1998; Visalli and Brandt, 1991; Visalli and Brandt, 1993). The UL45 protein is required for cell-cell fusion induced by a syncytial variant of HSV. Specifically, syncytium-formation due to a Y854K mutation in the cytoplasmic tail of gB requires wild type UL45 (Haanes et al., 1994). Thus, UL45 may mediate fusion events during HSV infection through a functional interaction with gB. gB is conserved among all herpesviruses and plays an essential role in HSV fusion and entry (Heldwein and Krummenacher, 2008). Recently, pH-dependent conformational changes were detected in HSV gB (Dollery et al., 2009). We investigated a potential role for UL45 in HSV entry by pH-dependent endocytosis and examined its influence on the structure and function of gB in virions.
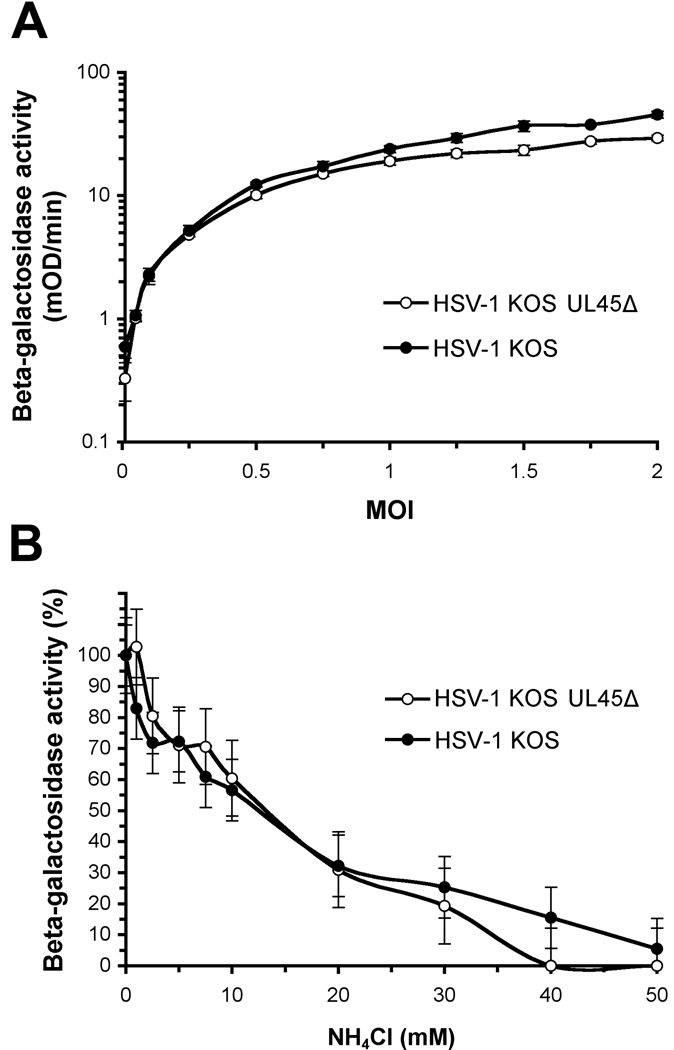
CHO cells that express the cellular receptor nectin-1 are a model system to study low pH, endocytic entry of HSV. We measured the ability of a UL45-null mutant, HSV-1 KOS UL45Δ (Visalli and Brandt, 1991)(provided by C. Brandt) to enter these cells. HSV-induced expression of beta-galactosidase is frequently used as a reporter of successful viral entry. CHO-nectin-1 cells are CHO-K1 cells stably transformed to express nectin-1 and contain the Escherichia coli lacZ gene under the control of the HSV ICP4 promoter (Geraghty et al., 1998) (provided by G. Cohen and R. Eisenberg). The level of entry of HSV-1 UL45Δ into CHO nectin-1 cells was very similar to wild type KOS at a range of MOIs (Fig. 1A), suggesting that UL45 is non-essential for efficient entry into these cells.
Fig. 1.
(A) Entry of HSV-1 KOS UL45Δ into CHO-nectin-1 cells. HSV-1 KOS or KOS UL45Δ was added to CHO-nectin-1 cells at MOIs ranging from 0 to 2. Beta-galactosidase activity of cell lysates was quantified at 6 hr post-infection as an indication of viral entry. Entry of the two viruses was not statistically different (p = 0.40, Student’s t-test). (B) Effect of ammonium chloride on the cell entry of HSV-1 KOS UL45Δ. CHO-nectin-1 cells were treated with indicated concentration of ammonium chloride for 30 min. HSV-1 KOS or KOS UL45Δ (MOI of 1) was added to cells for 6 hr in the continued presence of agent. Entry was measured as the percent of beta-galactosidase activity of cell lysates relative to that obtained in the absence of lysosomotropic agent. Data are means of quadruplicate determinations with standard deviation. Entry was not statistically different in the presence of NH4Cl (p = 0.94, Student’s t-test).
Different strains of HSV can travel distinct entry routes in the same cell type (Delboy et al., 2006; Roller et al., 2008). For example, the ANG path strain of HSV enters CHO-nectin-2 cells via a pH-independent pathway that is distinct from the pH-dependent entry of the rid1 strain (Delboy et al., 2006). To assess the contribution of UL45 to the selection of entry route, we tested the effect of a lysosomotropic agent on entry into CHO-nectin-1 cells. Ammonium chloride is a weak base that buffers the pH of acidic cellular compartments and characteristically inhibits entry of viruses that require a low pH for entry, such as HSV (Nicola et al., 2003). Entry of HSV-1 UL45Δ was inhibited by ammonium chloride in a concentration-dependent manner (Fig. 1B). All together, the data suggest that the absence of UL45 bestows no apparent defect in the ability of HSV to engage the low pH, endosomal entry route.
To address whether the absence of UL45 alters the conformation of gB, we characterized the reactivity of UL45Δ virions with mouse monoclonal antibodies (MAbs) to gB. Two-fold dilutions of ~ 5 × 105 PFU wild type or UL45Δ virions were blotted directly to nitrocellulose membranes. Blots were probed with MAbs designated SS10, SS106 and SS144 (Bender et al., 2007; Bender et al., 2005; Heldwein et al., 2006) (provided by R. Eisenberg and G. Cohen) and H126, H1817, and H1359 (Virusys). All six antibodies tested displayed equivalent reactivity with gB from both viruses (Table 1). The ectodomain of gB is an elongated, rod-like structure comprised of several folded domains (Heldwein et al., 2006). The MAbs evaluated detect distinct epitopes across the gB molecule (Table 1) (Bender et al., 2007), suggesting that in the absence of UL45, gB is not globally altered. This contrasts with virions containing highly fusogenic forms of gB. Virion gB from such fusion-from-without strains of HSV has a pronounced reduction in reactivity with MAbs to Domain I, the fusion domain of gB (Roller et al., 2008).
Table 1.
Effect of deletion of UL45 from HSV-1 KOS on the reactivity and neutralization activity of gB-specific antibodies
| Reactivitya | Neutralizationb | ||||||
|---|---|---|---|---|---|---|---|
| Vero | HaCaT | ||||||
| MAb | Notes | wt | UL45Δ | wt | UL45Δ | wt | UL45Δ |
| H126 | Domain I | + | + | + | + | + | + |
| H1359 | Domain III | + | + | − | − | − | − |
| H1817 | Domain VI | + | + | nd | nd | nd | nd |
| SS10 | Domain IV | + | + | + | + | + | + |
| SS106 | Domain V | + | + | + | + | + | + |
| SS144 | Domain V | + | + | + | + | + | + |
| αUL45 | Polyclonal to UL45pc |
+ | − | nd | nd | nd | nd |
measured by dot blot or Western blot
+, > 50% plaque reduction at MAb dilution < 0.002; −, < 50% plaque reduction
(Visalli and Brandt, 1993), provided by C. Brandt.
wt, wild type; nd, not determined
Glycoprotein-specific antibodies that neutralize virus infection in the absence of complement target glycoprotein function. To investigate the influence of UL45 on the function of gB in virus entry, MAbs specific for gB were tested for their ability to neutralize UL45-null HSV. Since CHO-nectin-1 cells do not support plaque formation, Veroand HaCaT cells were employed. Table 1 shows that HSV-1 UL45Δ was sensitive to neutralization by MAbs H126, SS10, SS106 and SS144. In contrast, neutralization by H1359 was not detected. Each antibody tested neutralized wild type KOS to a similar extent. Thus, by these measures, the absence of UL45 does not appear to alter the conformation or function of gB.
Vero cells support HSV entry by pH-independent fusion with the plasma membrane, and HaCaT cells support a pH-dependent, endocytic mechanism (Nicola et al., 2005). All of the MAbs to gB tested neutralized UL45Δ to a similar extent whether assayed on Vero or HaCaT cells (Table 1). Within the limits of these experiments, no difference in the role of UL45 in these two entry pathways was detected. Although UL45 influences cell-cell fusion (syncytium formation) determined by gB, the results in Table 1 provide no evidence for direct association between the two molecules in the virion envelope. Additional studies will address whether there is indeed a physical interaction between UL45 protein and gB.
Inactivation of virions by acid pretreatment is a hallmark of viruses that can utilize a pH-activated entry pathway. When viruses such as Semliki Forest virus are treated with low pH buffers, their fusion glycoproteins are prematurely activated, so when the acid-treated virus is added to cells, it is incompetent for entry (Bron et al., 1993). Entry function of isolated herpes simplex virions is inactivated by low pH pretreatment in an irreversible and temperature-dependent manner (Nicola et al., 2003). The target of inactivation in the virion is likely one or more of the HSV glycoproteins that involved in membrane fusion.
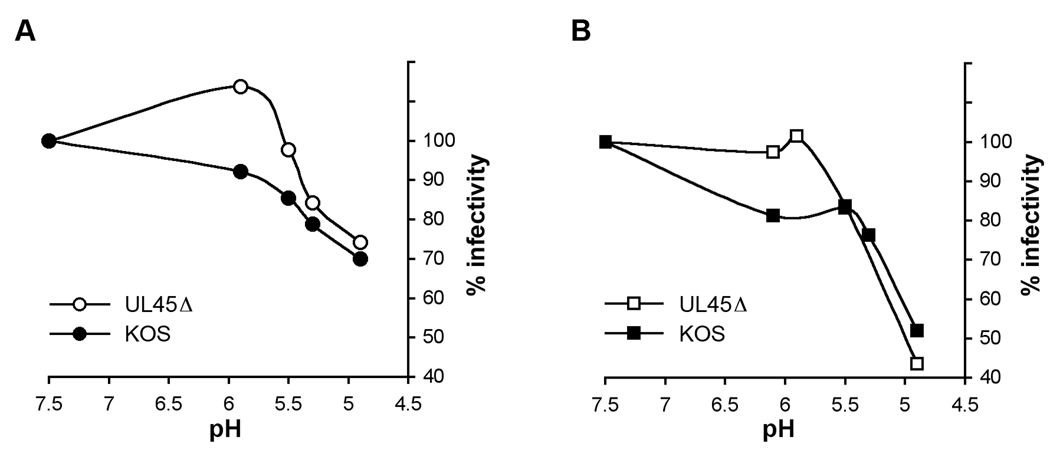
To address whether UL45 determines sensitivity to low pH inactivation of virions, we analyzed the effect of pH on the infectivity of HSV-1 UL45Δ particles. HSV was diluted in sodium bicarbonate-free, serum-free DMEM buffered withmM HEPES (Life Technologies), mM 2-(N-morpholino) ethanesulfonic acid (MES; Sigma), and 5 mM succinate (Sigma) containing 0.2% BSA to achieve final pHs ranging from 7.5 to 4.9 (Nicola et al., 2003). Samples were neutralized to pH 7.6 with pretitrated amounts of 0.05 N NaOH and diluted in sodium bicarbonate buffered DMEM (pH 7.6) with 10% fetal bovine serum. Virions were assayed for successful infection by plaque formation on HaCaT or Vero cells (Fig. 2A and B). Infectivity of UL45-null particles was impaired by a similar range of pHs to wild type. Thus, the UL45 envelope protein does not confer sensitivity of HSV to acid inactivation. Interestingly, treatment of UL45Δ with pH 6.0 caused an apparent increase in infectivity of HaCaT cells. This may indicate a small difference between the cell types tested. A pH < 5.0 is required to reduce wild type plaque formation (Fig. 2 (Tuyama et al., 2006; Whitbeck et al., 2006). However, a pH < 6.0 reduces HSV particle entry as measured by beta-galactosidase reporter assay (Nicola et al., 2003). This discrepancy may reflect assay differences or different roles of pH in HSV infection when measured at early vs. later times post-infection.
Fig. 2.
Low pH inactivation of HSV-1 UL45Δ. Samples of HSV-1 strain KOS or KOS UL45Δ were adjusted to a range of pHs as shown, incubated at 37°C for 10 min, and then neutralized to pH 7.6. Treated virions were incubated with (A) HaCaT or (B) Vero cells, and plaque formation was measured as an indication of virus entry and infection. The infectivity of samples that were treated with pH 7.5 was defined as 100%. Inactivation of HSV-1 UL45Δ was not statistically different from wild type (p = 0.34 for HaCat and p = 0.91 for Vero, Student’s t-test).
Inhibition of infectivity was observed whether pH-treated virions were assessed on cells that support pH-dependent (HaCaT) or pH-independent (Vero) pathways (Fig. 2A and B). Interestingly, infectivity measured on Vero cells appeared more sensitive to inactivation by pH 4.9. However, the non-physiological pH of 3.0 completely inactivated virions regardless of which cells were used. These data further suggest that UL45 plays a dispensable role in HSV entry regardless of entry route. Although studies with lysosomotropic agents demonstrate that exposure to low intracellular pH is not required for infection of Vero cells, acid pretreatment does impair HSV infectivity for Vero cells. Low pH may disrupt one or more element of the glycoprotein machinery that is common to both pathways.
Virus-cell fusion during entry and cell-cell fusion or syncytium formation are distinct processes but share several features in common (Spear, 1993). NormalUL45 expression is required to allow cell-cell fusion induced by gB syn mutants (Haanes et al., 1994). This led to the notion that UL45 protein may play an important role as a mediator of fusion events during HSV infection. UL45 may impact gB in the infected cell, but the conformation of virion gB appears to be unaffected by UL45 protein. Together, the results suggest that despite the function of UL45 in regulating cell-cell fusion, UL45 has little impact on virus-cell fusion or viral entry by an acid-dependent mechanism.
Acknowledgments
This investigation was supported by Public Health Service grants AI-60702 and AI-083850 (A.V.N) and by training grant AI-07617 from the National Institute of Allergy and Infectious Diseases and a grant from the Japan Health Sciences Foundation (SAA4832).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol. 2003;77(14):7978–7990. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arii J, Uema M, Morimoto T, Sagara H, Akashi H, Ono E, Arase H, Kawaguchi Y. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J Virol. 2009;83(9):4520–4527. doi: 10.1128/JVI.02601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J Virol. 2007;81(8):3827–3841. doi: 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender FC, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J Virol. 2005;79(18):11588–11597. doi: 10.1128/JVI.79.18.11588-11597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R, Wahlberg JM, Garoff H, Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. Embo J. 1993;12(2):693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Del Rosario B, Woda C, Marcellino D, Satlin LM, Herold BC. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol. 2003;163(2):283–293. doi: 10.1083/jcb.200301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Liu W, Satlin LM, Herold BC. Focal adhesion kinase plays a pivotal role in herpes simplex virus entry. J Biol Chem. 2005;280(35):31116–31125. doi: 10.1074/jbc.M503518200. [DOI] [PubMed] [Google Scholar]
- Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174(7):1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell AS, Muggeridge MI. Herpes simplex virus 2 UL45 is a type II membrane protein. J Virol. 1998;72(5):4430–4433. doi: 10.1128/jvi.72.5.4430-4433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delboy MG, Patterson JL, Hollander AM, Nicola AV. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol J. 2006;3(1):105. doi: 10.1186/1743-422X-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollery SD, Delboy MG, Nicola AV. Low pH-induced conformational change in herpes simplex virus glycoprotein B. J Virol. 2009;84 doi: 10.1128/JVI.02573-09. ((in revision)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnen RL, Mizokami KR, Banfield BW, Cai GY, Simpson SA, Pizer LI, Levin MJ. Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells. J Virol. 2006;80(21):10325–10334. doi: 10.1128/JVI.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton AR, Jr, Stolz DB, Uchida H, Goins WF, Cohen JB, Glorioso JC. Equine herpesvirus 1 enters cells by two different pathways, and infection requires the activation of the cellular kinase ROCK1. J Virol. 2007;81(20):10879–10889. doi: 10.1128/JVI.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280(5369):1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J Virol. 2004;78(22):12268–12276. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanes EJ, Nelson CM, Soule CL, Goodman JL. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J Virol. 1994;68(9):5825–5834. doi: 10.1128/jvi.68.9.5825-5834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cell Mol Biosci. 2008;65 doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- Hoppe S, Schelhaas M, Jaeger V, Liebig T, Petermann P, Knebel-Morsdorf D. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J Gen Virol. 2006;87(Pt 12):3483–3494. doi: 10.1099/vir.0.82231-0. [DOI] [PubMed] [Google Scholar]
- Lycke E, Hamark B, Johansson M, Krotochwil A, Lycke J, Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch Virol. 1988;101(1–2):87–104. doi: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- Miller N, Hutt-Fletcher LM. Epstein-Barr virus enters B cells and epithelial cells by different routes. J Virol. 1992;66(6):3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79(11):6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79(12):7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77(9):5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78(14):7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann P, Haase I, Knebel-Morsdorf D. Impact of Rac1 and Cdc42 signaling during early herpes simplex virus type 1 infection of keratinocytes. J Virol. 2009;83(19):9759–9772. doi: 10.1128/JVI.00835-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J Virol. 2009;83(10):4895–4911. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller DG, Dollery SJ, Doyle JL, Nicola AV. Structure-function analysis of herpes simplex virus glycoprotein B with fusion-from-without activity. Virology. 2008;382(2):207–216. doi: 10.1016/j.virol.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80(2):710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, FL: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- Stiles KM, Milne RS, Cohen GH, Eisenberg RJ, Krummenacher C. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology. 2008;373(1):98–111. doi: 10.1016/j.virol.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyama AC, Cheshenko N, Carlucci MJ, Li JH, Goldberg CL, Waller DP, Anderson RA, Profy AT, Klotman ME, Keller MJ, Herold BC. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: optimal candidate for microbicide combinations. J Infect Dis. 2006;194(6):795–803. doi: 10.1086/506948. [DOI] [PubMed] [Google Scholar]
- Van de Walle GR, Peters ST, VanderVen BC, O'Callaghan DJ, Osterrieder N. Equine herpesvirus 1 entry via endocytosis is facilitated by alphaV integrins and an RSD motif in glycoprotein D. J Virol. 2008;82(23):11859–11868. doi: 10.1128/JVI.00868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli RJ, Brandt CR. The HSV-1 UL45 gene is not required for growth in Vero cells. Virology. 1991;185:419–423. doi: 10.1016/0042-6822(91)90790-i. [DOI] [PubMed] [Google Scholar]
- Visalli RJ, Brandt CR. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 1993;29(2):167–178. doi: 10.1016/0168-1702(93)90057-t. [DOI] [PubMed] [Google Scholar]
- Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102(50):18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kenyon WJ, Li Q, Mullberg J, Hutt-Fletcher LM. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72(7):5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbeck JC, Zuo Y, Milne RS, Cohen GH, Eisenberg RJ. Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM. J Virol. 2006;80(8):3773–3780. doi: 10.1128/JVI.80.8.3773-3780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittels M, Spear PG. Penetration of cells by herpes simplex virus does not require a low pH- dependent endocytic pathway. Virus Res. 1991;18(2–3):271–290. doi: 10.1016/0168-1702(91)90024-p. [DOI] [PubMed] [Google Scholar]