Abstract
Both attention biases to threat and a serotonin-transporter gene polymorphism (5-HTTLPR) have been linked to heightened neural activation to threat and the emergence of anxiety. The short allele of 5-HTTLPR may act via its effect on neurotransmitter availability, while attention biases shape broad patterns of cognitive processing. We examined individual differences in attention bias to emotion faces as a function of 5-HTTLPR genotype. Adolescents (N=117) were classified for presumed SLC6A4 expression based on 5-HTTLPR--low (SS, SLG, or LGLG), intermediate (SLA or LALG), or high (LALA). Participants completed the dot-probe task, measuring attention biases toward or away from angry and happy faces. Biases for angry faces increased with the genotype-predicted neurotransmission (low>intermediate>high). The reverse pattern was evident for happy faces. The data indicate a linear relation between 5-HTTLPR allelic status and attention biases to emotion, demonstrating a genetic mechanism for biased attention using ecologically valid stimuli that target socioemotional adaptation.
Keywords: anxiety, attention bias, serotonin transporter gene polymorphism, 5-HTTLPR, dot-probe task, intermediate phenotypes
Introduction
Anxiety is one of the most common and debilitating psychiatric disorders in adulthood. The large personal and societal costs have motivated researchers to find precursors of the disorder that could aid in early identification and intervention. The vast majority of adult cases of anxiety are first evident in childhood (Pine, Cohen, Gurley, Brook, & Ma, 1998), with a peak incidence in middle to late adolescence (Beesdo et al., 2007). As such, potential risk markers should be detectable early in life. The current paper examined the interrelation between two risk markers, namely attention bias to threat and the serotonin polymorphism (5-HTTLPR), in adolescence. These data may aid researchers in better understanding risk markers that may be at play in the emergence of anxiety.
There is extensive and consistent evidence linking anxiety to persistent biases to threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007), which may play a causal role in the disorder (Eldar, Ricon, & Bar-Haim, 2008; MacLeod, Campbell, Rutherford, & Wilson, 2004). A parallel literature has demonstrated that allelic variations in 5-HTTLPR also place individuals at increased risk (Lesch et al., 1996). The short allele (S) at 5-HTTLPR for the serotonin-reuptake transporter (SLC6A4) is associated with lower transcriptional efficiency compared to the long allele (L), reducing neurotransmitter availability by approximately 50%.
The data suggest that this reduction amplifies the individual’s response to threat, evident in hyper-reactive neuroendocrine (Jabbi et al., 2007; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009) and amygdala (Hariri et al., 2002; Kalin et al., 2008) responses in human and animal models (Munafo, Brown, & Hariri, 2008; Suomi, 2006). Attention biases to threat, when coupled with the S-allele, may act as a parallel cognitive conduit to increased stress reactivity.
Three adult studies have assessed the relations between attention biases and 5-HTT genotype employing threat words (Beevers, Gibb, McGeary, & Miller, 2007), threatening animals (Osinsky et al., 2008), and emotionally salient pictures (E. Fox, Ridgewell, & Ashwin, 2009) in psychiatric and healthy samples. Two (Beevers et al., 2007; Osinsky et al., 2008) found increased bias toward threat in individuals with at least one S-allele. The third (E. Fox et al., 2009) linked the L-allele to avoidance of threat and bias towards pictures of happy faces.
To date, one study has examined this potential relation in children (Gibb, Benas, Grassia, & McGeary, 2009). Children at high risk due to both maternal depression and the S-allele displayed a non-significant trend of attentional avoidance to sad, but not happy or angry, faces compared to children at low risk.
Clearly, many questions remain concerning the relation between 5-HTTLPR and attention bias toward threatening and appetitive stimuli. This study expands on this research in three ways. First, we focused on adolescents in light of evidence that attention biases may emerge early, before the gradual emergence of anxiety (Pine, Helfinstein, Bar-Haim, Nelson, & Fox, 2009). Second, our sample size allowed us to examine potential linear trends across three genotype groups (SS/SL/LL). Third, we used ecologically valid and socially relevant stimuli (emotion faces) that influence socioemotional adaptation (Ohman, 2002).
Methods and Materials
Participants
Participants (N=138, 66 male) were Caucasian adolescents (Agemean=15.05 years, SD=0.97, IQmean=115.8, SD=21.9) selected in infancy for a longitudinal study of socioemotional development. Twenty-six were excluded from the final analyses due to poor performance on the task (<63% correct, N=4), extreme bias scores (>2.5 SDs from mean, N=5), or missing genotype data (N=17). The final sample (N=112, 58 male, Agemean=14.97, SD=0.98, IQmean=117.8, SD=21.1) did not differ from the excluded participants on age, gender, or IQ (p’s>0.19). Adolescents were screened using the Schedule for Affective Disorders and Schizophrenia for School Aged Children–Present and Lifetime Version (Kaufman et al., 1997). The findings noted below remained intact after successive analyses controlling for all lifetime occurrences of any DSM-IV Axis 1 disorders (p’s<0.05).
The Institutional Review Board approved all procedures and families provided written assent/consent. Adolescents were compensated for their participation.
Genotyping
Genomic DNA was prepared from laboratory-collected saliva samples using Oragene•DNA kits (DNA genotek, Ottawa, Ontario, Canada).
SLC6A4 promoter (5HTTLPR) genotyping was performed in two stages using size discrimination for the S (103bp), L (146bp), LA (146bp), and LG (61bp) alleles. The 5HTTLPR region was amplified in a 20μl reaction: 1x Optimized Buffer A, 1x PCR enhancer, 0.25μM each primer [FAM-ATCGCTCCTGCATCCCCCATTAT (forward), GAGGTGCAGGGGGATGCTGGAA (reverse)], 0.125μM dNTP, 10ng DNA, 1.25u Platinum Taq polymerase (all Invitrogen Corp); 95°C (5 min), 40 cycles of 94°C (30sec), 52 °C (30sec), 68°C (1 min), and a final elongation, 68°C (10 min). S and L genotypes were discriminated directly from the PCR reaction products, rs25531 genotype was determined by digesting 10μl PCR mix with 50units HpaII (37°C, 1 hour, 1x restriction buffer). Samples were mixed with deionized formamide and GeneScan™-500 ROX Size Standard (Applied Biosystems), and genotypes resolved on a 3730 DNA Analyzer (Applied Biosystems).
Variation at the closely linked SNP rs25531 also modulates SLC6A4 efficiency with the relatively common LG allele having a similar profile to the S allele (Hu et al., 2006). Participants were therefore classified based on the presumed efficacy of serotonin neurotransmission: low (SS/SLG; N=26), intermediate (SLA/LALG; N=54), and high (LALA; N=37). Groups did not differ in age, gender, or IQ (p’s>0.15).
Dot Probe Task
Each trial (Figure 1a) began with a central fixation cross (500 ms). A face pair placed laterally on each side of the screen was then presented (either 500 or 1500 ms). Presentation times were varied in order to account for changes in bias linked to the chronometry of attention mechanisms (Mogg, Bradley, Miles, & Dixon, 2004). The pictures were each 11.1 cm × 8.9 cm, with 20.1 cm between their centers. Immediately following the face pairs, a white arrow appeared for 500 ms in the location just occupied by one of the pictures. Participants pressed one of two response keys to indicate arrow direction (up/down). Participants were seated 210 cm from the screen with stimuli at 5.2 degrees of visual angle. The inter-trial interval varied randomly at either 1900 or 2900 ms.
Figure 1.
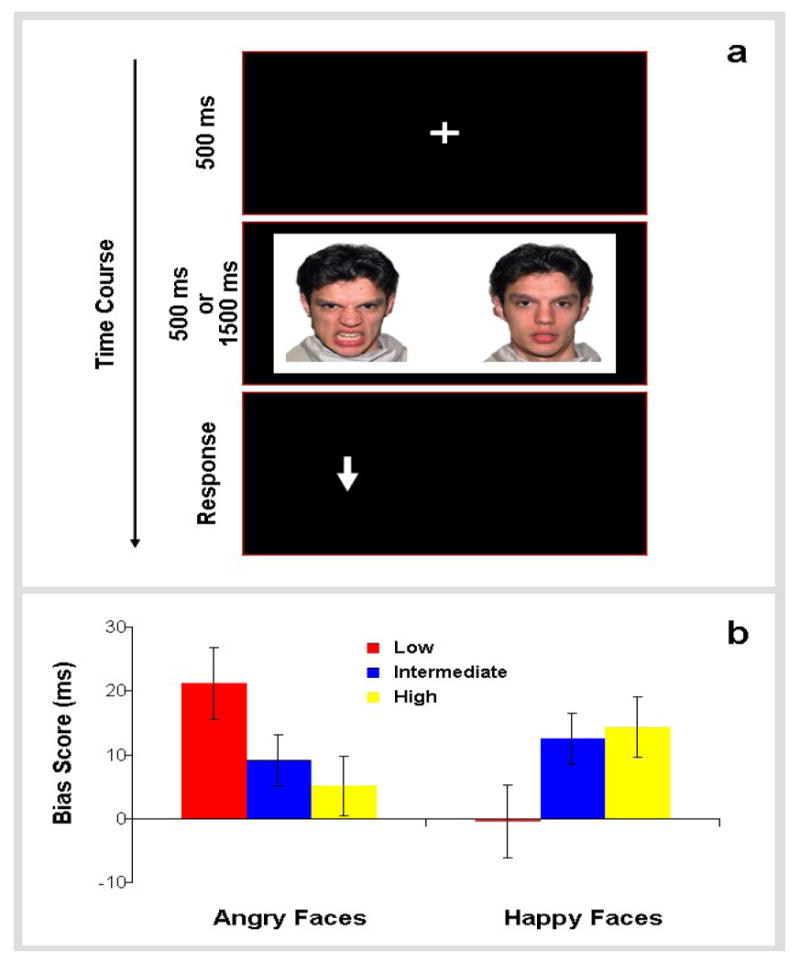
(a) Schematic of the attention bias task. (b) Attention bias pattern across the emotion faces for the polymorphism groups.
Three types of face pairs were presented: Angry/Neutral (64 trials), Happy/Neutral (64 trials), and Neutral/Neutral (32 trials). After 16 practice trials, the 160 experimental trials were presented in one of three pseudo-random orders. The faces (16 male; 16 female) were from the NimStim face stimulus set (Tottenham et al., 2009) and each was seen five times. Congruent trials had the arrow appear in the same location as the emotion face; incongruent trials had the arrow opposite the emotion face in the location of the neutral face.
Stimulus sex, congruency, presentation time (500/1500 ms), arrow direction (up/down), and arrow location (right/left) were counterbalanced. Stimuli were presented with a NANAO FlexScan 550i monitor controlled by the STIM stimulus presentation system from the James Long Company (Caroga Lake, NY).
Statistical Analysis
Trials with incorrect responses or reaction times (RTs) less than 200 ms were removed from analyses. The three groups did not differ on RTs across conditions (p’s>0.59). As in previous studies (Bar-Haim et al., 2007; Mogg et al., 2004), analyses focused on the attention bias patterns for the angry and happy faces. Bias scores were calculated by subtracting the mean RT for congruent trials from the mean RT for incongruent trials. Positive values indicate vigilance for the emotion stimuli; negative scores indicate avoidance.
A 2X2X3 repeated measures ANOVA was used to examine bias patterns as a function of face emotion (angry/happy), presentation time (500 ms/1500 ms), and genotype (low/intermediate/high). Presentation time did not interact with emotion-face or genotype (p’s>0.12). Therefore, mean bias scores were collapsed across presentation time for post-hoc t-tests, minimizing the number of analyses.
Results
One-sample t-tests against zero found significant vigilance to angry faces in adolescents with the low 5HTT neurotransmission genotype (M=21.16 ms, t(24)=3.97, p=0.001, d=1.62), which diminished as the presumed availability of serotonin increased across the intermediate (M=9.06 ms, t(49)=2.33, p=0.02, d=0.66), and high (M=5.12 ms, t(36)=1.03, p=0.31, d=0.34) 5HTT groups (Figure 1b). The opposite pattern was evident for the happy faces--the attention bias increased from the low (M=-0.49 ms, t(24)=-0.08, p=0.94, d=0.03), to intermediate (M=12.51 ms, t(49)=3.33, p=0.002, d=0.95), to high (M=14.31 ms, t(36)=3.02, p=0.005, d=1.01) 5HTT neurotransmission groups.
The emotion face-by-genotype interaction, F(2, 109)=4.54, p=0.01, f=0.29, revealed a significant linear contrast across groups. Adolescents with low 5HTT neurotransmission displayed more vigilance to threat than their counterparts with high neurotransmission, p=0.03 (with a trend versus the intermediate group, p=0.08). For happy faces, adolescents with low 5HTT neurotransmission showed less vigilance than the high neurotransmission group, p=0.05 (again, with a trend versus the intermediate group, p=0.07).
Discussion
Our findings revealed a linear relation between presumed levels of serotonin availability and the magnitude of attention biases towards both angry and happy faces. Importantly, gene-linked patterns of attention bias were already evident in adolescence--the developmental window during which anxiety often first emerges.
These findings echo recent research demonstrating that anxious adolescents show heightened amygdala activation to threat during a dot-probe task (Monk et al., 2008), and parallel work showing that healthy S-allele carriers exhibit an elevated amygdala response to threatening faces (Hariri et al., 2002). Empirical studies have also shown a causal link between attention bias to threat and anxiety in adults (MacLeod et al., 2004) and children (Eldar et al., 2008). The S-allele may prime both a neurobiological response to threat and information processing strategies that are biased to perceiving threat. The subsequently triggered biological and cognitive processes may then work in tandem to increase the vulnerability to anxiety.
Study limitations include (1) the small sample size for a gene-behavior study, which precluded systematic analyses of related factors, such as anxiety or personality (Bar-Haim et al., 2007), (2) no direct measure of serotonin levels despite the fact that the exact relation between allelic status and serotonin function is complex (Canli & Lesch, 2007), and (3) the reliance on a single measurement point, which precluded a developmental analysis of the main serotonin-attention relation.
Nevertheless, the current data are noteworthy for finding a systematic relation between presumed serotonin transmission and attention in a sample of adolescents. Future work should examine the role 5-HTTLPR variation may have in the attention bias-anxiety link across both positive and negative stimuli, perhaps moderating the ease by which the attention mechanism translates into anxiety during development.
Acknowledgments
We would like to thank Stacey Barton, Melissa Ghera, Dalit H. Marshall, and Kirsten VanMeenen for their assistance in data collection. Funding for the study was provided by grants to Nathan A. Fox from the John D. and Catherine T. MacArthur Foundation and NIH (MH074454; HD17899) and to Koraly Pérez-Edgar from the NIMH (MH073569) and NARSAD (Blowitz-Ridgeway Young Investigator Award).
Footnotes
Financial Disclosures The authors do not have any conflicts of interest, financial or otherwise, to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, van IJzendoorn M. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein M, Hofler M, Lieb R, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb B, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. Journal of Abnormal Psychology. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behaviour Research & Therapy. 2008;46:450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proceedings of the Royal Society B, Biological Sciences. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular Psychiatry. 2008;13(11):1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heilis A, Sabol SZ, G BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Campbell L, Rutherford EM, Wilson EJ. The causal status of anxiety-linked attentional and interpretive bias. In: Yiend J, editor. Cognition, emotion and psychopathology: Theoretical, empirical and clinical directions. New York, NY: Cambridge University Press; 2004. pp. 172–189. [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18(5):689–700. [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and Amygdala activation: A Meta-Analysis. Biological Psychiatry. 2008;65:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A. Automaticity and the amygdala: Nonconscious responses to emotional faces. Current Directions in Psychological Science. 2002;11:62–66. [Google Scholar]
- Osinsky R, Reuter M, Kupper Y, Schmitz A, Kozyra E, Alexander N, et al. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: Lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Annals of the New York Academy of Sciences. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon A, McCarry T, Nurse M, Hare T, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


