Abstract
Endocrine disruptors, chemicals that disturb the actions of endogenous hormones, have been implicated in birth defects associated with hormone-dependent development. Phytoestrogens are a class of endocrine disruptors found in plants. In the current study we examined the effects of exposure at various perinatal time periods to genistein, a soy phytoestrogen, on reproductive development and learning in male rats. Dams were fed genistein-containing (5 mg/kg feed) food during both gestation and lactation, during gestation only, during lactation only, or during neither period. Measures of reproductive development and body mass were taken in the male offspring during postnatal development, and learning and memory performance was assessed in adulthood. Genistein exposure via the maternal diet decreased body mass in the male offspring of dams fed genistein during both gestation and lactation, during lactation only, but not during gestation only. Genistein decreased anogenital distance when exposure was during both gestation and lactation, but there was no effect when exposure was limited to one of these time periods. Similarly, spatial learning in the Morris water maze was impaired in male rats exposed to genistein during both gestation and lactation, but not in rats exposed during only one of these time periods. There was no effect of genistein on cued or contextual fear conditioning. In summary, the data indicate that exposure to genistein through the maternal diet significantly impacts growth in male offspring if exposure is during lactation. The effects of genistein on reproductive development and spatial learning required exposure throughout the pre- and postnatal periods.
Keywords: genistein, phytoestrogen, anogenital distance, water maze, spatial learning
The endocrine disruptor hypothesis proposes that exogenous compounds can interfere with endocrine function by altering the binding, release, or metabolism of endogenous hormones (Baskin et al., 2001; Colborn et al., 1993). Evidence in favor of this hypothesis has included rodent studies of plant-derived estrogenic compounds termed phytoestrogens. For example, studies of the soy-derived phytoestrogen genistein showed that exposure of pregnant females causes decreased birth weight, reduced anogenital distance (AGD), delayed puberty, altered mass of reproductive organs, and altered reproductive behavior in male offspring (Levy et al., 1995; Nagao et al., 2001; Santell et al., 1997; Wisniewski et al., 2003; Wisniewski et al., 2005).
Exposure of males to genistein not only impacts their reproductive development and function but also alters several sexually dimorphic behaviors including learning and memory. Sex-based differences in behavior are thought to be established by the organizational effects of gonadal hormones on brain development and morphology (Isgor and Sengelaub, 1998; Isgor and Sengelaub, 2003; Joseph et al., 1978; Leret et al., 1994; Lucion et al., 1996; Roof and Havens, 1992; Williams et al., 1990). Interestingly, studies have shown that exposure to estrogen or estrogen-like compounds (such as genistein) perinatally or in adulthood can diminish sex differences in behavior (Gupta et al., 2001; Lephart et al., 2002; Lephart et al., 2004; Leret et al., 1994; Lund and Lephart, 2001a; Lund et al., 2001; Pan et al., 2000). These data support the idea that sexually dimorphic behavioral traits are sensitive to endocrine disruption.
The sensitivity of sexually dimorphic traits to gonadal steroids is well-understood to depend on the developmental stage of the animal. In rodents, for example, gestational exposure to androgens masculinizes the external genitalia (Faber and Hughes, 1992; Grady et al., 1965) and pre- and postnatal exposure (up to postnatal day 10) masculinizes the CNS in rodents (Isgor and Sengelaub, 1998; Isgor and Sengelaub, 2003; Roof and Havens, 1992). With these findings in mind, we undertook the current study to examine whether the effects of genistein on reproductive development and sexually dimorphic behavior require exposure throughout gestation and lactation, as done by Wisniewski et al (Wisniewski et al., 2003; Wisniewski et al., 2005), or whether these effects can be seen when exposure is limited to either critical period.
Materials and Methods
Animals
Male and female Sprague-Dawley rats were obtained from the Drake University Breeding Colony. All animals had access to food and water ad lib. Animals were maintained on 12:12 light-dark cycle with lights on at 0600 h CST in a temperature-controlled vivarium. Adequate measures were taken to minimize pain or discomfort of the animals. Experiments were conducted in accordance with international standards on animal welfare and were in compliance with local and national regulations. All procedures were approved by the Drake University IACUC.
Diets
Genistein (4′,5,7-trihydroxyisoflavone) was purchased from Indofine Chemical Co., Inc. (Hillsborough, NJ) and mixed with a casein-based diet (5K96, Purina Mills, Richmond, IN) that contained less than 1 ppm phytoestrogens at a concentration of 5mg/kg of feed. The 5K96 diet is similar to the standard NIH-31 formula except that soy and alfalfa proteins are replaced with casein. 5K96 diet without genistein added served as the control diet in all experiments. All diets were sterilized by irradiation.
Procedure
For assessment of reproductive development and learning and memory of male offspring, dams were randomly assigned to one of four diet groups: control (C-C; litter n = 8), genistein (G-G; litter n = 9), gestation (G-C; litter n = 8), and lactation (C-G; litter n = 8). All dams were acclimated to their respective diets for 2 weeks prior to mating. Dams began mating at 70 days of age. Pregnancy was determined by presence of vaginal sperm plug or significant weight gain (20 g in one week). Day of birth was identified as postnatal day 1 (PND 1). Pups were exposed to genistein via gestating and/or lactating mothers consuming a 5 mg/kg dose of genistein in rat chow as described above. C-C dams were fed control diet during gestation (gestation day 1 (GD 1) through postnatal day 1 (PND 1) and during lactation (PND 2-PND 21). G-G dams were fed genistein diet during gestation and lactation. G-C dams were given genistein diet during gestation, and then switched to control diet from the day pups were born through PND 21. C-G dams were given control diet during gestation, and then switched to the genistein diet from the day that pups were born to PND 21. There was no significant group differences in the amount of food consumed during gestation and lactation (data not shown). At PND 21, pups were weaned and housed with same sex siblings and placed on control diet. On PND 70 1-4 males per litter were randomly selected for necropsy and analysis of reproductive organ weight. Remaining males (maximum of 2 per litter) were used for testing of learning and memory.
Measures of maternal behavior
For assessment of maternal behavior, observations were taken from a mixture of the litters described above and similarly treated litters that were used in the reproductive anatomy and learning and memory experiments. Litter n's for the assessment of maternal behavior were C-C, n = 14; G-G, n = 13; G-C, n = 7; C-G, n = 8. Observations were taken between 9:00am and 11:00am every day beginning on postnatal day 3 and ending in postnatal day 21. Measurements were obtained by observing the litters 3 times for 30 sec with an interval of 10 min between observations. This number of observations was chosen based on previous work by one of us (BJS) that more extensive observations do not provide new information on maternal behavior (Sanders and Gray, 1997). The specific behaviors recorded were nursing, licking of pups, and whether the dam was out of contact with the nest.
Measures of reproductive development and growth
Anogenital distance (AGD) was measured on PND 2, 7, 14, 21 using a caliper with a digital readout. Using an electronic balance, pups were weighed together and averaged on PND 2, but were weighed individually on all other days. At PND 21, pups were weaned and housed by sex with siblings. At PND 70, rats were weighed and then sacrificed with CO2 overdose and the wet weight of the testes, gonadal fat pad, epididymides, and seminal vesicles were obtained.
Assessment of spatial learning and memory in the Morris water maze
Testing of the performance of the rats in the Morris water maze followed standard methods as previously described (Kinney et al., 2003; Wrenn et al., 2004). Rats were approximately 5 mos old at the onset of testing. The maze consisted of a plastic, circular pool, with a diameter of 183 cm and a height of 76 cm. Water was added to a depth of 61 cm and rendered opaque by the addition of non-toxic white paint. Water temperature was maintained at approximately 22° C. Rats were tested by an investigator blind to treatment group on three components of the task: hidden platform training, probe trial testing, and visible platform training. In all components of the task, video tracking of the swim path was recorded and data collected by a personal computer equipped with commercially available water maze software (Actimetrics, Evanston, IL).
Hidden platform training took place on days 1-6 and days 8-9. Probe trials took place on day 7 and day 10, and there was a two-day break between day 4 and day 5; thus hidden platform testing spanned 12 days with the hidden protocol performed a total of 8 times. Each rat received four trials per day with each trial consisting of placing the rat, facing the wall of the pool, in a pseudorandomly selected start position. Rats were allowed a maximum of 60 sec to reach a hidden platform (circular, 15 cm in diameter) which was submerged 2 cm below the waterline in a fixed spatial location. Rats were removed from the platform after 30 sec and given a 30 sec rest period between consecutive trials. Performance measures recorded included latency to find the platform and swim speed.
In order to shed light on how the rats were reaching the platform, we supplemented our latency data by identifying the predominant swim strategy used on the first trial of each training day. To identify swim strategies we used the following decision algorithm which is modified from previously published methods (Brody and Holtzman, 2006; Graziano et al., 2003; Janus, 2004; Wolfer and Lipp, 2000):
If > than 50% of the swim path was within 10 cm of the edge of the pool and the latency to find the platform was > 15 sec, the strategy was identified as thigmotaxis.
If the swim path did not meet the criteria for thigmotaxis, was predominantly circular, and the latency to find the platform was > 15 sec, the strategy was identified as circling.
If the swim path did not meet the criteria for circling, entered all three quadrants after leaving the start quadrant, did not remain in any one quadrant for > 40% of the trial, and the latency to find the platform was > than 15 sec, the strategy was identified as scanning.
If the swim path did not meet the criteria for scanning, remained in a non-target quadrant for > 40% of the trial, and the latency to find the platform was > than 15 sec, the strategy was identified as incorrect probing.
If the swim path did not meet the criteria for incorrect probing, remained in the target quadrant for > 40% of the trial, and the latency to find the platform was > than 15 sec, the strategy was identified as correct probing.
If the swim path did not meet the criteria for correct probing, and consisted of at least one 180° turn, or one overlapping loop, or entered all quadrants, or was within 10 cm of the edge of the pool for > than 50% of the trial and the latency to find the platform was < 15 sec, the strategy was identified as indirect swimming.
If the swim path did not meet the criteria for indirect swimming and the latency to find the platform was < 15 sec, the strategy was identified as direct swimming.
Strategy identification was performed by independent observers who were uninformed of group identity. Since our decision algorithm relies on mostly objective criteria to define strategies, the two observers were found to be in agreement on > 98% of the tracings.
For the purpose of simplifying analysis, the swim strategies were categorized as thigmotaxis, non-spatially biased (circling and scanning), or spatially biased (incorrect probing, correct probing, indirect swimming, and direct swimming. Representative tracings of each strategy are shown in Figure 1.
Fig. 1.
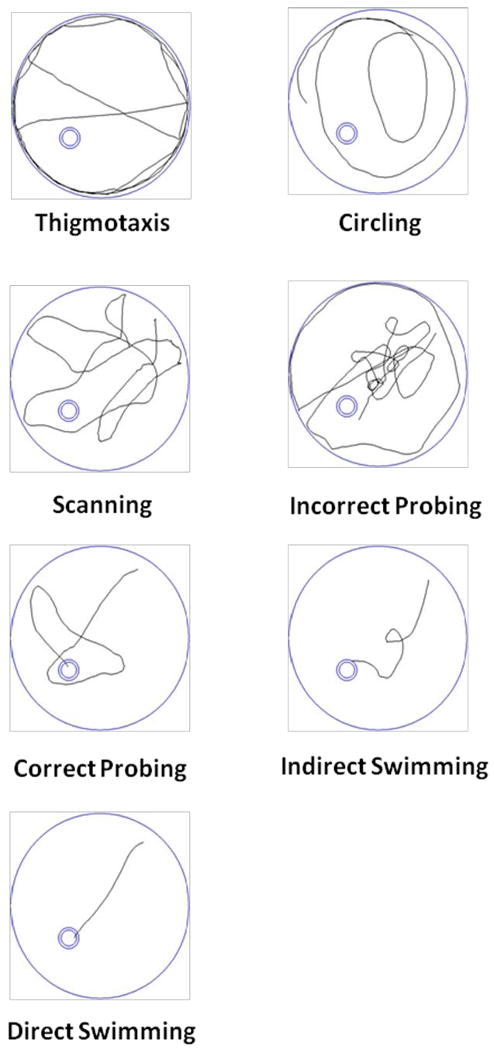
Water maze swim strategies. Representative examples of the various swim strategies used by the rats are shown as tracings of the swim path. In each tracing the large circle represents the edge of the pool and the small double circle represents the location of the hidden platform. Rats tended to use thigmotaxis as their initial strategy. As training progressed, rats tended to shift their strategy to a non-spatially biased swim path such as circling or scanning and then to a spatially biased path with the ultimate path being a direct swim to the platform.
Probe trials, in which the platform was not present, were performed on days 7 and 10. In the probe trials each rat was placed in the start position opposite the former platform location and allowed to swim for 60 sec. Performance measures in the probe trial included percent of time spent in each quadrant of the pool, swim speed, and thigmotaxis.
Visual platform training took place eleven days after the final probe test and was administered for two days. In the visible platform task, an upright marker (Sharpie Magnum) was placed on the platform and served as a conspicuous visible indicator of the location of the platform which varied from trial to trial. Four trials were administered per day in the same manner as hidden platform training. Performance measures in the visible task included latency to find the platform and swim speed.
Assessment of cued and contextual fear conditioning
Emotional learning was assessed using a cued and contextual conditioned fear paradigm as previously described (Bainbridge et al., 2008). Testing commenced approximately 25 days after the completion of Morris water maze protocol. All phases of conditioned fear training and testing were conducted in clear, plastic chambers measuring 32 cm × 25 cm × 23 cm (Med Associates, Georgia, VT). The floors of the chambers consisted of metal grids capable of delivering mild footshocks to the rats. The chambers were contained in wooden, sound attenuating cubicles measuring 64 cm × 76 cm × 42 cm. The cubicles contained small video cameras that captured video of rat behavior for automated data acquisition and analysis. The presence or absence of freezing behavior was recorded during all phases of the protocol by computer software interfaced with the video cameras. Freezing behavior, defined as the cessation of all movement except that associated with breathing, is a response exhibited by rats in the presence of threatening stimuli.
On the training day, rats were individually placed by an investigator blind to treatment group in the conditioning chamber and presented with two white noise (conditioned stimulus (CS), 90 dB) and footshock (unconditioned stimulus (US), 0.75 mA) pairings. Each pairing was preceded and followed by a 2 min exploration period. The CS-US pairings were comprised of 30 s of white noise and 1 s of footshock that overlapped and co-terminated with the CS. The chambers were cleaned with 70% ethanol between rats. Twenty-four and 48 hours after training, the rats were tested for contextual and cued fear conditioning, respectively.
For contextual conditioning, the rats were individually removed from their home cage and placed in the conditioning chamber under environmental conditions identical to those of the training day. No stimuli were presented to the rats during the contextual conditioning test. Freezing behavior was recorded for 5 min and served as a measure of the strength of association between the shock and the training environment.
In the cued fear conditioning test, rats were individually removed from their home cages and placed in the conditioning chamber. To isolate cued conditioning from contextual conditioning, the environmental context was altered by replacing the grid floor with a floor of different texture, and a plastic insert was placed along the walls in order to change their appearance and texture. Furthermore, the chambers were cleaned with an orange-scented Fantastik cleaning solution rather than 70% ethanol between subjects in order to alter the olfactory stimuli in the environment. Each rat was placed in the altered-context environment for 6 min. The first 3 min established the baseline exploration in the absence of any stimuli. During the last 3 min, the CS white noise was presented. The presence or absence of freezing behavior was recorded by computer software and served as the measure of the strength of the learned association between the auditory cue and the footshock.
Statistical Analyses
For the measures of AGD, postnatal body mass, reproductive organ wet mass, and adult body mass, litter means were calculated and the grand means were compared. Comparisons of anogenital distance and postnatal body weight were done by two-way repeated measures ANOVA using age and group as the factors. Comparisons of reproductive organ wet mass and adult body mass, were done by one-way ANOVA using group as the factor.
For maternal behavior, dependent measures of interest were collapsed across block of postnatal day (blocks were PND 3-6, 7-11, 12-16, and 17-21) and analyzed by two-way repeated measures ANOVA using block and group as the factors.
For the Morris water maze, subjects' platform latencies, percent thigmotactic, and swim speed from each daily set of four trials were averaged to obtain a mean per day, and the grand means of these values were compared by two-way repeated measures ANOVA using day of training and group as the factors. The last day of predominant thigmotactic swimming was determined for each C-C and G-G rat and compared by the Mann-Whitney test of ranks. In the probe trials, the percent time spent in the quadrant that had contained the platform was compared with percent time in the other quadrants by one-way repeated measures ANOVA.
For fear conditioning one way ANOVA was used to determine an effect of group on percent time freezing.
In all ANOVAs data were analyzed using SigmaStat (SyStat Software, Inc., San Jose, CA). The threshold for significance was p < 0.05. Post hoc pairwise comparisons were done when main effects were found to be significant.
Results
Maternal behavior
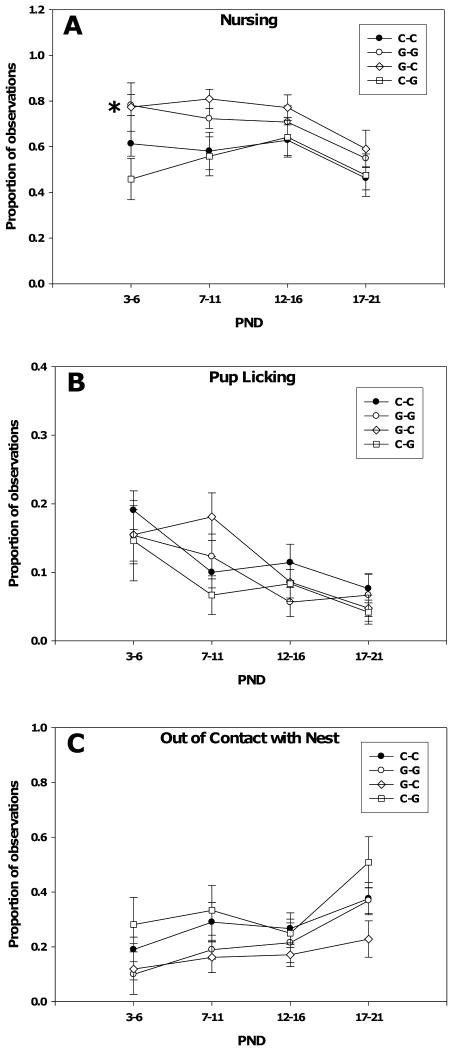
Measurements of maternal behavior are shown in Figure 2. For nursing behavior, two-way repeated measures ANOVA found significant main effects of PND block (F(3, 38) = 6.51, p < 0.001) and of group (F(3, 38) = 3.64, p = 0.02). There was not a significant interaction between block and group. The effect of group is due to the general decrease in nursing that occurs as the pups near weaning. Post hoc analysis determined that the effect of group was due to increased nursing in the G-G (p = .048) and G-C (p = 0.02) groups.
Fig. 2.
Maternal behavior of control and genistein-treated dams. (A) Dams that ate genistein throughout gestation and lactation (G-G) or only during gestation were observed to nurse significantly more than control dams (C-C) as indicated by the asterisk (p < 0.05, Fisher LSD) (B) Pup licking did not differ between the groups. (C) Dams' contact with the nest did not differ between the groups. Observations were performed everyday between PND 3 and 21. All data are pooled by blocks of postnatal days and expressed as the mean ± SEM.
For both licking of pups and contact with the nest, there was a significant main effect of PND block (licking: F(3, 38) = 8.38, p < 0.001; removal: F(3, 38) = 10.15, p < 0.001) but no effect of group or interaction between block and group. The effects of block were due to the decrease in licking and decrease in nest contact that occurs as weaning approaches.
Reproductive development and growth
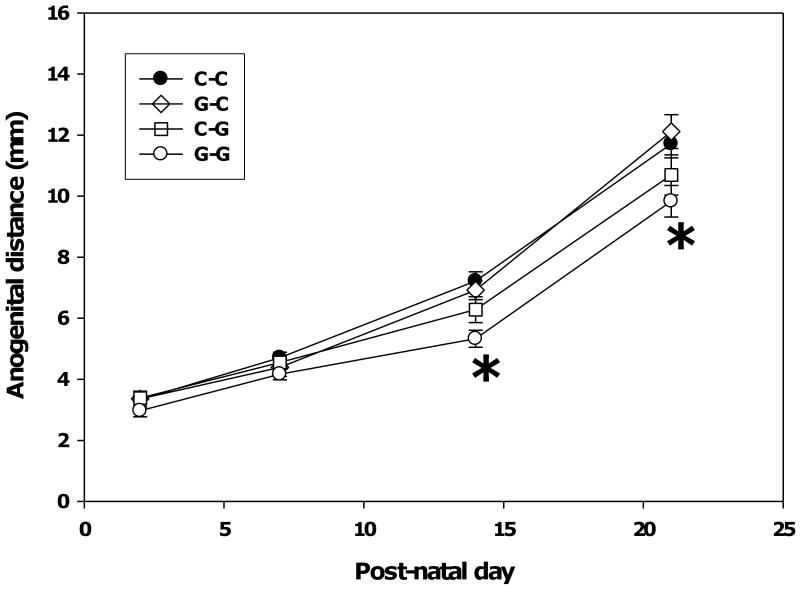
Two-way repeated measures ANOVA of AGD revealed significant main effects of age (F(3, 31) = 557.19, p < 0.001), genistein exposure group (F(3, 31) = 5.02, p 0.006), and a significant interaction between age and group (F(9, 93) = 2.59, p = 0.01). As illustrated in Figure 3, Tukey post hoc pairwise comparisons within age found that the G-G group had a significantly shorter mean AGD than the C-C group on PND 14 and PND 21 (p = 0.001). Anogenital distance was unaffected in the G-C and C-G groups (Figure 3) indicating that the impact of genistein on the development of the male external genitalia requires exposure throughout gestational and lactational development.
Fig. 3.
Effect of pre- and postnatal genistein on anogenital distance. Exposure to genistein (5 mg/kg of food) via the maternal diet during gestation and lactation (G-G) significantly reduced anogenital distance compared to exposure to phytoestrogen-free diet during gestation and lactation (C-C). Asterisks indicate p < 0.001 as determined by the Tukey post hoc test. Data are presented as the grand mean of litter means ± SEM; litter n's were 8-9 for each group.
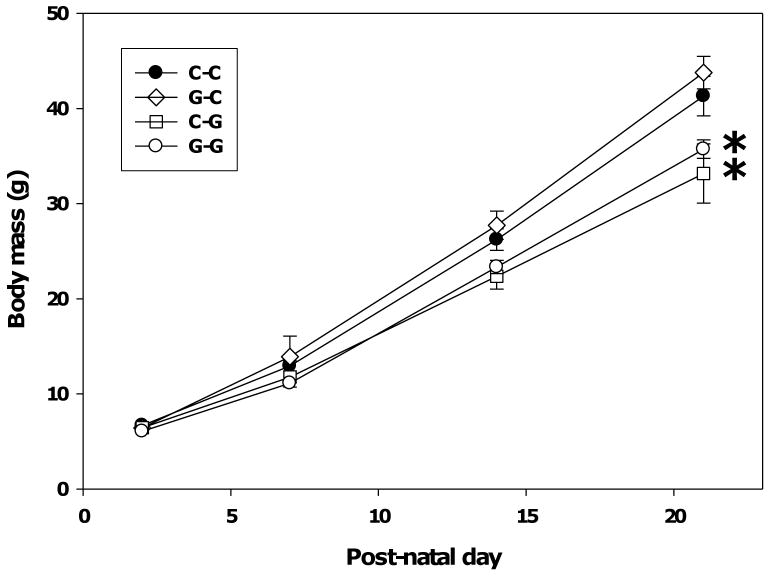
Two-way repeated measures ANOVA of postnatal growth (body mass) revealed significant main effects of age (F(3, 31) = 532.61, p < 0.001), genistein exposure group (F(3, 31) = 6.80, p = 0.001), and a significant interaction between age and group (F(9, 86) = 2.69, p =0.008). As illustrated in Figure 4, Tukey post hoc pairwise comparisons within age found that the G-G group had a significantly lower mean body mass than the C-C group on PND 21 (p = 0.02). As illustrated in Figure 4, post hoc analysis found that the C-G group had a significantly lower mean body mass than the C-C group on PND 21 (p < 0.001). The mean body mass of the G-C group was unaffected. These data indicate that lactational exposure to genistein reduces postnatal growth in male pups while gestational exposure alone does not impact growth.
Fig. 4.
Effect of pre- and postnatal genistein on postnatal growth. Exposure to genistein (5 mg/kg of food) via the maternal diet during gestation and lactation (G-G) and during lactation only (C-G) significantly reduced body mass on postnatal day 21 compared to exposure to phytoestrogen-free diet during gestation and lactation (C-C). Asterisks indicate p ≤ 0.01 as determined by the Tukey post hoc test. Data are presented as the grand mean of litter means ± SEM; litter n's were 8-9 for each group.
Reproductive measures at PND 70
Table 1 shows the wet weight of male reproductive organs and body mass on PND 70. Pre- and/or postnatal genistein exposure had no effect on any of these outcome measures as determined by one way ANOVA.
Table 1.
Adult Reproductive Measures at PND 70
| Dependent Measure | Control (C-C) | Genistein (G-G) | Gestation (G-C) | Lactation (C-G) |
|---|---|---|---|---|
| Reproductive Organs (wet weight, g) | ||||
| Testes | 3.72 ± 0.14 | 3.45 ± 0.06 | 3.68 ± 0.15 | 3.63 ± 0.06 |
| Gonadal Fat Pad | 10.61 ± 0.62 | 10.99 ± 0.45 | 11.18 ± 0.71 | 10.69 ± 0.48 |
| Epididymides | 1.13 ± 0.07 | 1.05 ± 0.05 | 1.21 ± 0.08 | 1.16 ± 0.06 |
| Seminal Vesicles | 0.36 ± 0.05 | 0.33 ± 0.05 | 0.37 ± 0.05 | 0.28 ± 0.03 |
| Body Mass (g)_ | 311 ± 12.87 | 315 ± 8.44 | 333 ± 12.26 | 318 ± 10.54 |
All data are presented as mean ± SEM using litter as the experimental unit.
Litter n's were 8-9 per group.
Spatial learning and memory in the Morris water maze
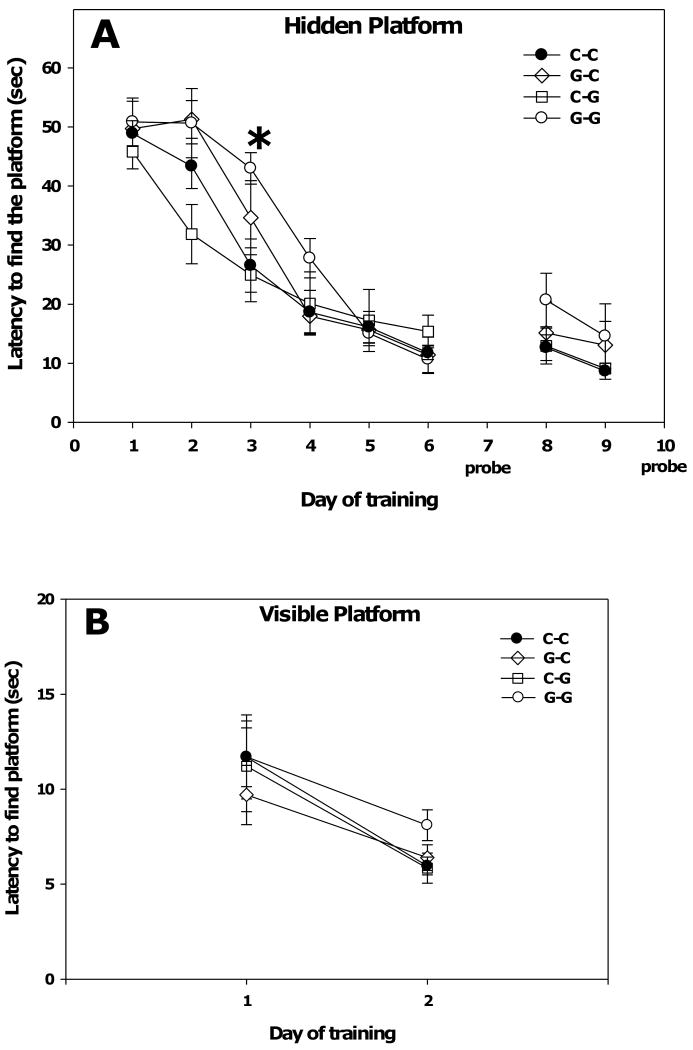
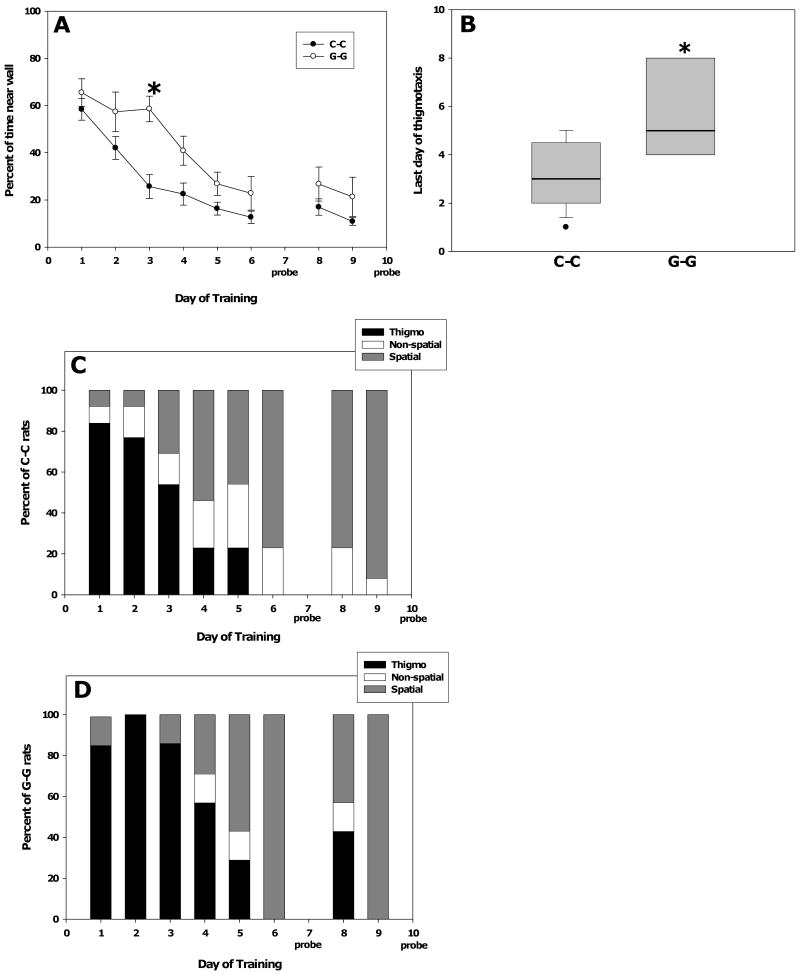
Two-way repeated measures ANOVA of latency of adult males to find the hidden platform in the water maze revealed a significant main effect of day (F(7,33) = 83.45, p < 0.001) in which latency decreased with training and a significant day × group interaction (F(21,231) = 1.77, P = 0.02). Post hoc Tukey analysis determined that there was a significant difference in latencies between the C-C group and the G-G group on day 3 (P=0.011, Figure 5A) which shifted the learning curve of the G-G group to the right. No significant differences were found between the C-C group and the G-C and C-G groups (Figure 5A). In order to further explore the difference in water maze performance between C-C and G-G rats, thigmotactic swimming, defined as swimming within 10 cm of the pool's edge, was analyzed. Two-way repeated measures ANOVA of the percent of swimming that was thigmotactic found a significant main effect of day (F(7, 33) = 93.83, p < 0.001) in which thigmotaxis decreased with training and a significant group × day interaction (F(21, 230) = 1.69, p = 0.03). Post hoc Tukey analysis determined that there was a significant difference in thigmotaxis between the C-C group and the G-G group on day 3 (P < 0.001, Figure 6A), and that on day 4 the difference in thigmotaxis between the C-C and G-G groups narrowly missed significance (p = 0.057).
Fig. 5.
Water maze performance in the hidden (A) and visible (B) platform tasks. (A) Exposure to genistein during gestation and lactation (G-G) significantly increased the latency to find the platform on day 3 of training compared to the unexposed group (C-C). Asterisk indicates p < 0.001 as determined by the Tukey post hoc test. (B) There was no effect of genistein exposure on the latency to find the visible platform. Data are presented as the mean ± SEM, and n's were C-C = 13, G-G = 7, G-C = 6, C-G = 11. Probe trials were performed on days 7 and 10.
Fig. 6.
Swim path analysis of hidden platform acquisition trials. (A) Exposure to genistein during both gestation and lactation (G-G) significantly increased thigmotaxis on day 3 of training relative to controls. Asterisk indicates p < 0.05 by the Tukey post hoc test. Data are the mean ± SEM. (B) Swim path identification showed that rats exposed to genistein through both gestation and lactation (G-G) had their last day of relying on thigmotactic swimming significantly later than C-C rats. Asterisk indicates p = 0.007 (Mann-Whitney test). The bold horizontal line in each box indicates the median. Boxes extend to the 25th and 75 percentiles. Whisker caps extend to the 10th and 90th percentiles. The data point is a subject that was below the 10th percentile. (C-D) Swim paths were identified and categorized for C-C (panel C) and G-G (panel D) rats as described in the Methods. The stacked bars in the graph show the percentage of rats that used thigmotaxis (black), non-spatial (white), or spatial (gray) strategies on the first trial of each training day. Note that on days 3 and 4 thigmotaxis is disappearing as a strategy to a greater extent in the C-C rats.
In terms of swim strategy, thigmotaxis is understood to be used by rodents early in training and to disappear as spatial learning occurs (Janus, 2004; Wolfer and Lipp, 2000). Hence, we determined the last day in which thigmotactic swimming was greater than 50% for each rat. Mann-Whitney analysis of these data found that the median last day of thigmotaxis was significantly greater for the G-G group than the C-C group (p = 0.007; Figure 6B).
To further analyze differences in water maze performance between the C-C and G-G rats, we identified swim strategy as described in the Methods. Figure 6C-D shows the proportion of rats of the C-C and G-G groups using various swim strategies. The data show that on days 3 and 4 thigmotaxis disappeared as a strategy to a greater extent in the C-C rats.
Swim speed of the rats during training in the hidden platform task was measured to rule out the potential confounding effect of genistein on the ability to swim (data not shown). Two repeated measures ANOVA found a significant main effect of day on swim speed (F(7, 34) = 9.25, p < 0.001) in which swim speed generally increased with training. There was no main effect of genistein exposure group and no significant interaction between day and group. These data rule out an effect of genistein on swim speed as an alternative explanation for the latency data described above.
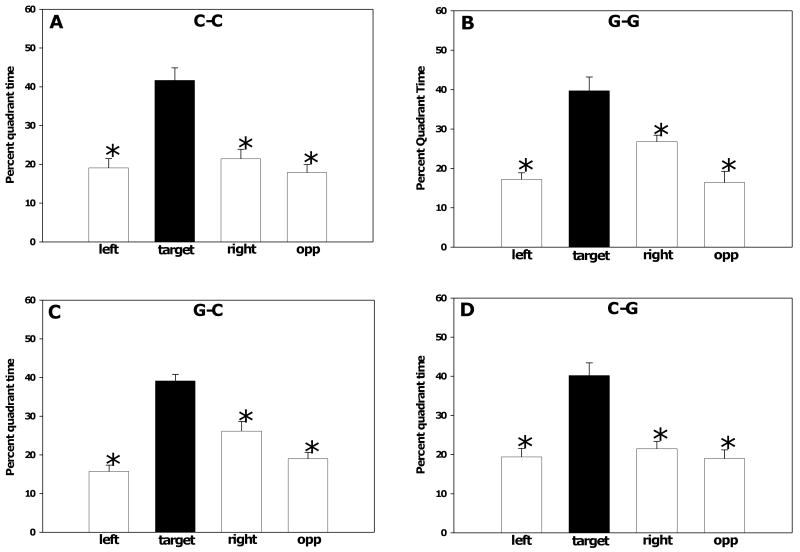
Probe trials, in which the platform was removed from the maze, were performed on days 7 (Figure 7) and 10 (data not shown) of the water maze experiment. During both probe trials, all experimental groups showed a significant preference for the quadrant that had contained the platform (target) over the opposite and adjacent quadrants. One way RM ANOVA within each group found that percent time spent in the target quadrant was significantly greater than time spent in the other quadrants (Bonferroni post hoc test, all p <0.001), demonstrating that learning had occurred in all groups by day 7 and was unchanged on day 10. These data indicate that despite the slower learning, the G-G rats were eventually able to encode the spatial location of the platform.
Fig. 7.
Water maze probe trial performance. All groups spent significantly more time in the quadrant that had contained the platform (target) during the acquisition of the hidden platform task than in the adjacent left, right, or opposite quadrants. (A) control group (C-C). (B) gestation and lactation group (G-G). (C) gestation only group (G-C). (D) lactation only group (C-G). Asterisks indicate p < 0.001. Data are presented as the mean ± SEM.
Finally, the rats were trained in a visible platform task in order to rule out the possible confound of an effect of perinatal genistein on vision (Figure 5B). Two way repeated measures ANOVA of latency to find the visible platform found a significant main effect of day (F(1, 33) = 20.61, p < 0.001), but no effect of group or group × day interaction. These data rule out impaired vision as an alternative explanation for the impaired spatial learning in the G-G group.
Cued and contextual fear conditioning
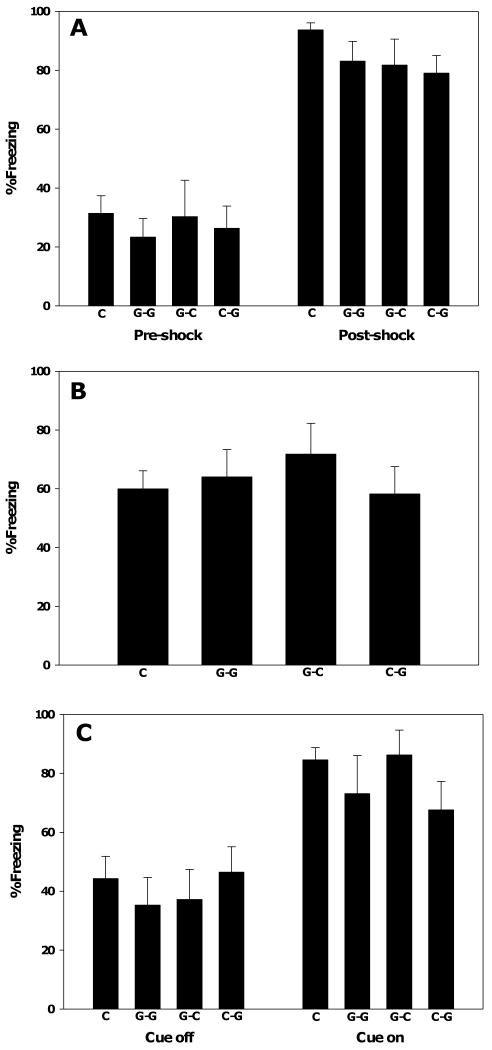
There was no significant effect of pre- and/or postnatal genistein exposure on freezing during the fear conditioning training day (Figure 8A). Similarly, genistein did not affect freezing during the test of contextual conditioning (Figure 8B) or the test of cued conditioning (Figure 8C). These data indicate that early exposure to genistein does not impact emotional learning in adulthood in male rats.
Fig. 8.
Cued and contextual fear conditioning. Pre- and postnatal genistein exposure through the maternal diet had no effect on freezing behavior during training (A), testing of conditioning to the training context (B), or testing of conditioning of the cue (C). Data are presented as the mean ± SEM; n's were C-C = 13, G-G = 7, G-C = 6, C-G = 10.
Discussion
The present report shows that genistein exposure through the maternal diet slows postnatal growth, decreases anogenital distance, and slows spatial learning in adulthood. The effects on anogenital distance and spatial learning required genistein exposure to span gestation and lactation. When exposure was limited to either period, no effect was seen on anogenital distance or spatial learning suggesting that deleterious actions of genistein during one period can be mitigated at another time in development. However, a critical period for the effect of genistein on postnatal growth was identified in which lactational, but not gestational, exposure produced an effect.
An important aspect of our study is that our data extend the literature on the parameters under which a phytoestrogen alters non-reproductive behaviors. Specifically, this study is the first to show that phytoestrogen exposure limited to development can disrupt hippocampal-dependent learning in adulthood. Such an approach has been recommended in the literature as a means of addressing the long-term health risks of endocrine disruption (Weiss, 2002). A previous study of the effect of dietary genistein on spatial memory showed that male rats were impaired by genistein exposure that had begun in gestation and had continued through adulthood when the cognitive testing was performed (Lund et al., 2001). The approach of using continuous exposure cannot discriminate between an organizational or activational mechanism of action. The present data suggest that genistein has an organizational effect on developmental processes in the hippocampus that results in impaired spatial learning in adulthood. Other studies have shown that manipulation of gonadal hormones during early development produce morphological changes in the hippocampus and that these changes correlate with spatial learning ability (Isgor and Sengelaub, 1998; Isgor and Sengelaub, 2003; Roof and Havens, 1992). Future studies in our lab will examine whether pre- and/or postnatal genistein treatment disrupts hippocampal morphology in a similar manner.
Our water maze data clearly show that the genistein exposed rats were impaired because of greater reliance on thigmotactic swimming. This observation is important because it raises the possibility that the organizational effects of genistein are anxiogenic rather than mnemonic. In “dry land” rodent behavioral paradigms such as the open field, thigmotaxis is a well-validated measure of anxiety-like behavior (Choleris et al., 2001; Cryan and Holmes, 2005; Martin, 1998). However, to our knowledge, whether thigmotaxis in the water maze is wholly reflective of an anxiety-like state or whether it is a default strategy relied upon in the absence of spatial encoding has not been rigorously addressed.
Although we cannot rule out a contribution of anxiety-like behavior to the water maze performance in the present study, we favor a mnemonic interpretation of the impairment in the G-G group for two reasons. Firstly, although dietary exposure to phytoestrogens during adulthood has produced anxiogenic effects (Hartley et al., 2003; Patisaul et al., 2005), developmental exposure has produced anxiolytic effects (Lephart et al., 2004; Lund and Lephart, 2001b). Secondly, we found no effect of genistein on baseline or post-training freezing in a fear conditioning task which argues against altered emotionality in adulthood after developmental genistein exposure. Further studies are needed to clarify under what conditions and in which direction phytoestrogens impact anxiety-like behavior and stress responses.
Our finding that early genistein affects the development of the male genitalia and postnatal growth corroborates previous studies (Wisniewski et al., 2003; Wisniewski et al., 2005). The present study did not find effects of early genistein on testis size or epididymis size, all of which have been reported previously (Atanassova et al., 2000; Roberts et al., 2000; Weber et al., 2001; Wisniewski et al., 2003). Interestingly, other published reports have found no effect of genistein on several reproductive development outcomes (Fritz et al., 2003; Masutomi et al., 2003). These seemingly conflicting studies have varied with respect to both species (rat vs. mouse) and strain (Long Evans rat vs. Sprague Dawley rat). Future studies should formally address the possibility that biological differences between animal models alter the effects of genistein on reproductive development.
Mechanistic explanations of the effects of genistein are open to speculation. Genistein binds both ERα and ERβ subtypes (with higher affinity for ERβ) and drives estrogen-dependent gene transcription in a transfection reporter gene assay (Kuiper et al., 1998) consistent with estrogen agonist activity. However, in some contexts, genistein acts as an estrogen antagonist (Patisaul et al., 2001). In the current study we show a disruption by genistein of anogenital distance, body mass, and spatial learning all of which are under strong influence from androgens (Isgor and Sengelaub, 2003; Keisler et al., 1991; Zegher et al., 1999). Thus, an androgenic mechanism for genistein cannot be ruled out especially in light of reports that genistein inhibits androgen metabolism and receptor expression (Fritz et al., 2002; Fritz et al., 2003; Weber et al., 1999).
One must consider the possibility that our behavioral effects are secondary to effects of genistein on maternal behavior. For example, exposure to the endocrine disruptor bisphenol-A during gestation in mice reduced subsequent nursing and increased the amount of time spent away from the nest (Palanza et al., 2002). Variation in maternal behavior can alter spatial memory (Barha et al., 2007) and hippocampal plasticity (Champagne et al., 2008). One study reported that dietary genistein has no effect on nursing behavior (Flynn et al., 2000). In the current study, we observed increased nursing behavior. We doubt that this increase is contributing to the spatial learning impairment reported herein because high levels of nursing enhance spatial learning (Liu et al., 2000), and nursing behavior was also increased in the G-C group which had unimpaired spatial learning.
In our lab we have observed that pregnant dams ingest approximately 0.45 mg/kg bw/day of genistein during gestation and 0.80 mg/kg bw/day of genistein during lactation (data not shown). This intake of genistein is comparable or higher than values reported in traditional Japanese (0.21-0.43 mg/kg bw/day) (Arai et al., 2000; Fukutake et al., 1996; Wakai et al., 1999) and Western vegetarians (0.14 mg/kg bw/day) diets (Kirk et al., 1999). It should be noted that in both of these diets total phytoestrogen intake is higher since soy-based foods contain other phytoestrogens in addition to genistein. Thus, genistein intake in the current study is relevant to human diet.
Our findings that genistein exposure through the maternal diet slows postnatal growth, decreases anogenital distance, and slows spatial learning in adulthood is significant because of two developing trends in Western societies. One is that congenital abnormalities of the male reproductive system are increasing in incidence in the U.S. and parts of Europe (Boisen et al., 2004; Nelson et al., 2005; Skakkebak et al., 2001). The other is that soy consumption is rising (United Soybean Board, 2003) due possibly to government recommendations (Food and Drug Administration, 1999). Interestingly, one study has reported that a maternal vegetarian diet is associated with hypospadias (North and Golding, 2000). The present data serve as a caution to pregnant woman who are considering a soy diet and to mothers who are considering soy infant formula for their sons.
Acknowledgments
This work was supported by Grant Number R15AT003586 from the National Center For Complementary & Alternative Medicine (NCCAM). The authors thank Liz Feauto for assistance with animal husbandry. The authors thank Stacy Knight, Michelle Gombas, Elizabeth Widlund, and Jeremy DeFoe for assistance with the mating and physiological monitoring of the subjects. The authors thank Bradley Dick for assistance with the analysis of the water maze data. The authors thank Rahul Parsa for helpful statistical conversations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10:127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: Evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141:3898–3907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- Bainbridge NK, Koselke LR, Jeon J, Bailey KR, Wess J, Crawley JN, Wrenn CC. Learning and memory impairments in a congenic C57BL/6 strain of mice that lacks the M2 muscarinic acetylcholine receptor subtype. Behav Brain Res. 2008;190:50–58. doi: 10.1016/j.bbr.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Pawluski JL, Galea LAM. Maternal care affects male and female offspring working memory and stress reactivity. Physiol Behav. 2007;92:939–950. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: Is there a connection? Environ Health Perspect. 2001;109:1175–1183. doi: 10.1289/ehp.011091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen K, Kaleva M, Main K, Virtanen H, Haavisto AM, Schmidt I, Chellakooty M, Damgaard I, Mau C, Reunanen M, Skakkebaek N, Toppari J. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. The Lancet. 2004;363:1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp Neurol. 2006;197:330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Colborn T, Saal FSv, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: Advances in modelling human depression and anxiety. Nature Rev Drug Disc. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Faber K, Hughes C., Jr Anogenital distance at birth as a predictor of volume of the sexually dimorphic nucleus of the preoptic area of the hypothalamus and pituitary responsiveness in castrated adult rats. Biol Reprod. 1992;46:101–104. doi: 10.1095/biolreprod46.1.101. [DOI] [PubMed] [Google Scholar]
- Flynn KM, Ferguson SA, Delclos KB, Newbold RR. Multigenerational exposure to dietary genistein has no severe effects on nursing behavior in rats. Neurotoxicol. 2000;21:997–1001. [PubMed] [Google Scholar]
- Food and Drug Administration. Food labeling: Health claims; soy protien and coronary heart disease. Food and Drug Adminstration, HHS. Final rule. Federal Register. 1999;64:57700–57733. [PubMed] [Google Scholar]
- Fritz WA, Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein downregulates androgen and estrogen receptor expression in the rat prostate. Mol Cell Endocrinol. 2002;186:89–99. doi: 10.1016/s0303-7207(01)00663-3. [DOI] [PubMed] [Google Scholar]
- Fritz WA, Cotroneo MS, Wang J, Eltoum IE, Lamartiniere CA. Dietary diethylstilbestrol but not genistein adversely affects rat testicular development. J Nutr. 2003;133:2287–2293. doi: 10.1093/jn/133.7.2287. [DOI] [PubMed] [Google Scholar]
- Fukutake M, Takahashi M, Ishida K, Kawamura H, Sugimura T, Wakabayashi K. Quantification of genistein and genistin in soybeans and soybean products. Food and Chem Toxicol. 1996;34:457–461. doi: 10.1016/0278-6915(96)87355-8. [DOI] [PubMed] [Google Scholar]
- Grady KL, Phoenix CH, Young WC. Role of the developing rat testis in differentiation of the neural tissues mediating mating behavior. J Comp Physiol Psychol. 1965;59:176–182. doi: 10.1037/h0021824. [DOI] [PubMed] [Google Scholar]
- Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods. 2003;130:33–44. doi: 10.1016/s0165-0270(03)00187-0. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (ltp) in rats. Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML, File SE. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology. 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55:179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R, Hess S, Birecree E. Effects of hormone manipulations and exploration on sex differences in maze learning. Behav Biol. 1978;24:364–377. doi: 10.1016/s0091-6773(79)90223-2. [DOI] [PubMed] [Google Scholar]
- Keisler L, Vom Saal F, Keisler D, Walker S. Hormonal manipulation of the prenatal environment alters reproductive morphology and increases longevity in autoimmune NZB/W mice. Biol Reprod. 1991;44:707–716. doi: 10.1095/biolreprod44.4.707. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Starosta G, Crawley J. Central galanin administration blocks consolidation of spatial learning. Neurobiol Learn Mem. 2003;80:42–54. doi: 10.1016/s1074-7427(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Kirk P, Patterson RE, Lampe J. Development of a soy food frequency questionnaire to estimate isoflavone consumption in US adults. J Am Diet Assoc. 1999;99:558–563. doi: 10.1016/S0002-8223(99)00139-X. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinol. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KDR, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Setchell KDR, Handa RJ, Lund TD. Behavioral effects of endocrine-disrupting substances: Phytoestrogens. ILAR J. 2004;45:443–454. doi: 10.1093/ilar.45.4.443. [DOI] [PubMed] [Google Scholar]
- Leret ML, Molina-Holgado F, Gonzalez MI. The effect of perinatal exposure to estrogens on the sexually dimorphic response to novelty. Physiol Behav. 1994;55:371–373. doi: 10.1016/0031-9384(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Levy JR, Faber KA, Ayyash L, Hughes CL., J The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proc Soc Exp Biol Med. 1995;208:60–66. doi: 10.3181/00379727-208-43832. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Lucion AB, Charchat H, Pereira GAM, Rasia-Filho AA. Influence of early postnatal gonadal hormones on anxiety in adult male rats. Physiol Behav. 1996;60:1419–1423. doi: 10.1016/s0031-9384(96)00246-6. [DOI] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci. 2001a;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Dietary soy phytoestogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001b;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KDR, Adlercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001;2:20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Animal models sensitive to anti-anxiety agents. Acta Psychiatr Scand. 1998;98:74–80. doi: 10.1111/j.1600-0447.1998.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–170. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol. 2001;15:399–411. doi: 10.1016/s0890-6238(01)00141-1. [DOI] [PubMed] [Google Scholar]
- Nelson CP, Park JM, Wan J, Bloom DA, Dunn RL, Wei JT. The increasing incidence of congenital penile anomalies in the united states. J Urol. 2005;174:1573–1576. doi: 10.1097/01.ju.0000179249.21944.7e. [DOI] [PubMed] [Google Scholar]
- North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. BJU International. 2000;85:107–113. doi: 10.1046/j.1464-410x.2000.00436.x. [DOI] [PubMed] [Google Scholar]
- Palanza P, Howdeshell KL, Parmigiana S, Saal FSv. Exposure to a low dose of bisphenol a during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110:415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Watson S, Clarkson TB. Soy phytoestrogens improve radial arm maze performance in ovariectomized retired breeder rats and do not attentuate benefits of 17β-estradiol treatment. Menopause. 2000:230–235. doi: 10.1097/00042192-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Dindo M, Whitten PL, Young LJ. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor alpha- and beta-dependent gene expression in the brain. Endocrinology. 2001;142:2946–2952. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Blum A, Luskin JR, Wilson ME. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus maze. Behav Neurosci. 2005;119:587–594. doi: 10.1037/0735-7044.119.2.587. [DOI] [PubMed] [Google Scholar]
- Roberts D, Veeramachaneni D, Schlaff W, Awoniyi C. Effects of chronic dietary exposure to genistein, a phytoestrogen, during various stages of development on reproductive hormones and spermatogenesis in rats. Endocrine. 2000;13:281–286. doi: 10.1385/ENDO:13:3:281. [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Gray MJ. Early environmental influences can attenuate the blood pressure response to acute stress in borderline hypertensive rats. Physiol Behav. 1997;61:749–754. doi: 10.1016/s0031-9384(96)00530-6. [DOI] [PubMed] [Google Scholar]
- Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr. 1997;127:263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- Skakkebak NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects: Opinion. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- United Soy Board, Consumer attitudes about nutrition, national report 2002-2003. http://www.soyfoods.org/press/FAQ_sales.htm.
- Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, Nakayama T, Wada M, Ohno Y. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33:139. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- Weber K, Setchell K, Stocco D, Lephart E. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5-alpha reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol. 2001;170:591–599. doi: 10.1677/joe.0.1700591. [DOI] [PubMed] [Google Scholar]
- Weber KS, Jacobson NA, Setchell KD, Lephart ED. Brain aromatase and 5-alpha reductase, regulatory behaviors and testosterone levels in adult rats on phytoestrogen diets. Proc Soc Exp Biol Med. 1999;221:131–135. doi: 10.1046/j.1525-1373.1999.d01-66.x. [DOI] [PubMed] [Google Scholar]
- Weiss B. Sexually dimorphic nonreproductive behaviors as indicators of endocrine disruption. Environ Health Perspect. 2002;110 3:387–391. doi: 10.1289/ehp.02110s3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol. 2003;169:1582–1586. doi: 10.1097/01.ju.0000046780.23389.e0. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Cernetich A, Gearhart JP, Klein SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav. 2005;84:327–334. doi: 10.1016/j.physbeh.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Wolfer D, Lipp H. Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Exp Physiol. 2000;85:627–634. [PubMed] [Google Scholar]
- Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, Starosta G, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Wenk GL, Crawley JN. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- Zegher Fd, Devlieger H, Eeckels R. Fetal growth: Boys before girls. Horm Res. 1999;51:258–259. doi: 10.1159/000023382. [DOI] [PubMed] [Google Scholar]