Abstract
The worldwide diversity of HIV-1 presents an unprecedented challenge for vaccine development 1-2. Antigens derived from natural HIV-1 sequences have elicited only limited breadth of cellular immune responses in nonhuman primate studies and clinical trials to date. Polyvalent “mosaic” antigens, in contrast, are designed to optimize cellular immunologic coverage of global HIV-1 sequence diversity 3. Here we show that mosaic HIV-1 Gag, Pol, and Env antigens expressed by recombinant, replication-incompetent adenovirus serotype 26 vectors markedly augmented both the breadth and depth without compromising the magnitude of antigen-specific T lymphocyte responses as compared with consensus or natural sequence HIV-1 antigens in rhesus monkeys. Polyvalent mosaic antigens therefore represent a promising strategy to expand cellular immunologic vaccine coverage for genetically diverse pathogens such as HIV-1.
The development of vaccine strategies that expand cellular immune breadth will be critical for achieving immunologic coverage of the enormous global genetic diversity of HIV-1 1-2. Moreover, the breadth of Gag-specific cellular immune responses has been shown to correlate with control of HIV-1 replication in humans 4 and with control of SIV challenges in vaccinated rhesus monkeys 5. Polyvalent “mosaic” proteins are assembled from natural sequences by in silico recombination and optimized to provide maximal coverage of potential T cell epitopes (PTEs) for a given valency 3. Mosaic antigens are full-length proteins that are designed to preserve natural antigen expression and processing. A 2-valent mosaic strategy consisting of two HIV-1 Gag, Pol, and Env antigens was utilized to balance the competing issues of theoretical coverage and practical utility. Here we report the breadth and magnitude of epitope-specific CD8+ and CD4+ T lymphocyte responses elicited by mosaic, consensus, and natural sequence HIV-1 antigens in rhesus monkeys.
We immunized 27 outbred rhesus monkeys with a single injection of recombinant adenovirus serotype 26 (rAd26) vectors 6 expressing the following antigens: (i) 2-valent mosaic (N=7), (ii) M consensus 7 (N=7), (iii) 2-valent combined clade B and clade C (N=7), or (iv) optimal natural clade C (N=6) HIV-1 Gag, Pol, and Env antigens. A total dose of 3×1010 viral particles of rAd26 vectors expressing these antigens was administered once i.m. to each animal. The optimal clade C antigens were the natural strain sequences selected to provide maximal PTE coverage of clade C sequences (see Methods). We assessed the breadth and magnitude of vaccine-elicited HIV-1-specific T lymphocyte responses by IFN-γ ELISPOT assays at week 4 following immunization utilizing pools and subpools of peptides that included all global PTEs found in at least 15% of HIV-1 M group sequences 8. All individual peptide responses were resolved, and cell-depleted IFN-γ ELISPOT assays were performed to determine if reactive peptides represented CD8+ or CD4+ T lymphocyte epitopes.
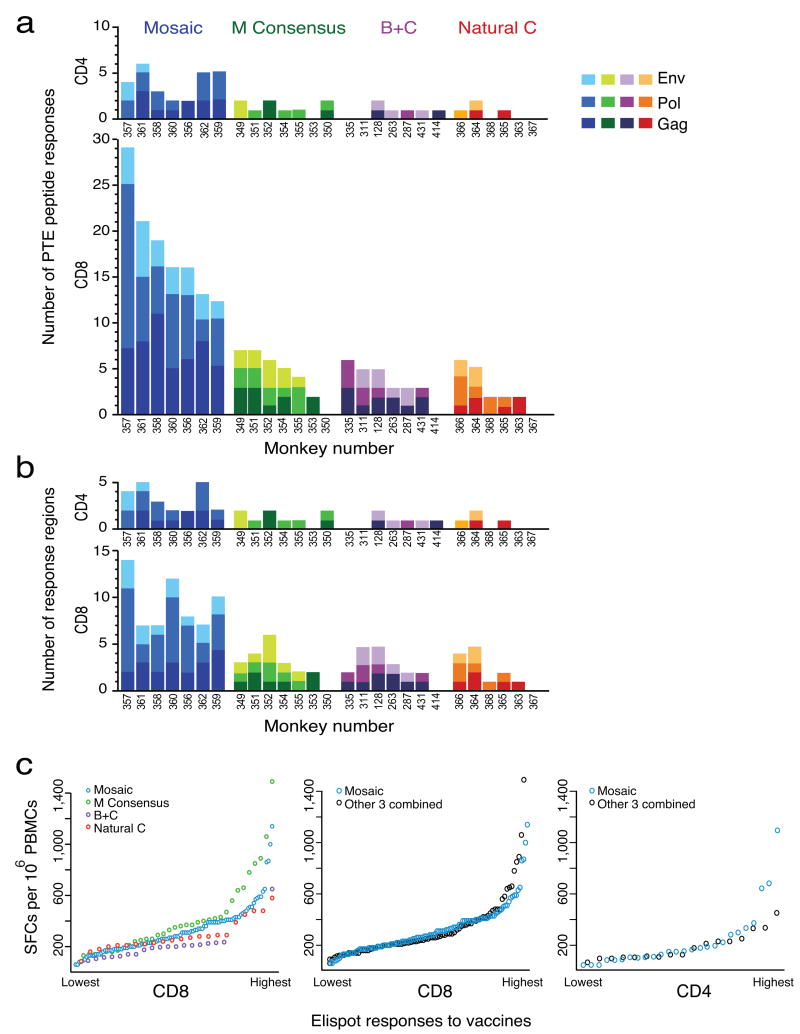
The total number of Gag-, Pol-, and Env-specific cellular immune responses to PTE peptides elicited by the mosaic antigens was 3.8-fold higher than the number of responses induced by the consensus or natural sequence antigens (Fig. 1a; P = 1 × 10-11, comparing the mosaic with the consensus antigens, the next highest group, based on a Poisson regression model 9-11). There were 4.4-fold more CD8+ than CD4+ T lymphocyte responses (P < 10-11) and fewer responses to Env than to Gag or Pol (P < 0.0007). The median number of CD8+ T lymphocyte responses was highest for the mosaic vaccine, followed by the consensus, the combined B+C, and the natural clade C vaccines (medians of 16, 5, 3, and 2 responses per animal in each group, respectively). Although there were fewer CD4+ T lymphocyte responses overall, the same relative pattern emerged with the highest number of CD4+ T lymphocyte responses to the mosaic vaccine, followed by the consensus, the combined B+C, and the natural clade C vaccines (medians of 4, 1, 1, and 0.5 responses per animal in each group, respectively). The best fitting Poisson regression model indicated similar relative degrees of augmentation for CD8+ and CD4+ T lymphocyte responses induced by the mosaic vaccine as compared with the other vaccines but greater absolute numbers of CD8+ T lymphocyte epitopes. T lymphocyte responses elicited by the consensus, the combined B+C, and the natural clade C vaccines were not statistically distinguishable.
Figure 1.
Breadth and magnitude of epitope-specific T lymphocyte responses to PTE peptides. (a) Numbers of epitope-specific CD4+ (top) and CD8+ (bottom) T lymphocyte responses to individual PTE peptides are shown following a single immunization of rAd26 vectors expressing mosaic (blue), M consensus (green), clade B + clade C (purple), or optimal natural clade C (red) HIV-1 Gag, Pol, and Env antigens. Individual monkeys are depicted on the x-axis. The different shades of each color reflect responses to the different antigens (Gag, Pol, Env) as indicated. (b) Numbers of CD4+ (top) and CD8+ (bottom) T lymphocyte response regions. (c) Magnitude of all Gag-, Pol-, and Env-specific CD8+ (left and middle panels) and CD4+ (right panel) T lymphocyte responses arranged from lowest to highest. Spot-forming cells (SFCs) per 106 PBMCs are shown for each epitope-specific response.
PTE peptides include multiple overlapping sequences that reflect naturally occurring HIV-1 polymorphisms 8, and thus PTE peptide responses encompass both the recognition of a particular epitope (breadth) and the cross-recognition of variants of that epitope (depth). We performed a conservative analysis of breadth by assessing the number of reactive epitopic regions per monkey in which all reactive PTE peptides that overlapped by 8 or more amino acids were counted as one event. In this conservative analysis, we observed that the mosaic antigens elicited 3.1-fold increased numbers of Gag-, Pol-, and Env-specific epitopic regions as compared with the consensus antigens or natural sequence antigens (Fig. 1b; P = 1.6 × 10-7, Poisson regression). The median number of CD8+ T lymphocyte epitopic regions was higher for the mosaic vaccine (median of 8 response regions, range 7-14) than the other vaccines (median of 2 response regions, range 0-6). Epitopes exhibited some clustering across animals as evidenced by regions of high epitope density (Supplementary Figs. 1 and 2). Complete alignments of all responses are also depicted (Supplementary Fig. 3).
These data show that the mosaic antigens substantially increased cellular immune breadth as compared with the M consensus or the natural clade C antigens. In addition to the evaluation of breadth, we assessed the magnitude of all individual CD8+ and CD4+ T lymphocyte responses. The magnitude of these responses proved comparable among all groups (Fig. 1c; P = 0.58 and P = 0.99, respectively, two-sided Kolmogorov-Smirnov tests), demonstrating that the mosaic antigens expanded cellular immune breadth without compromising the magnitude of individual epitope-specific responses.
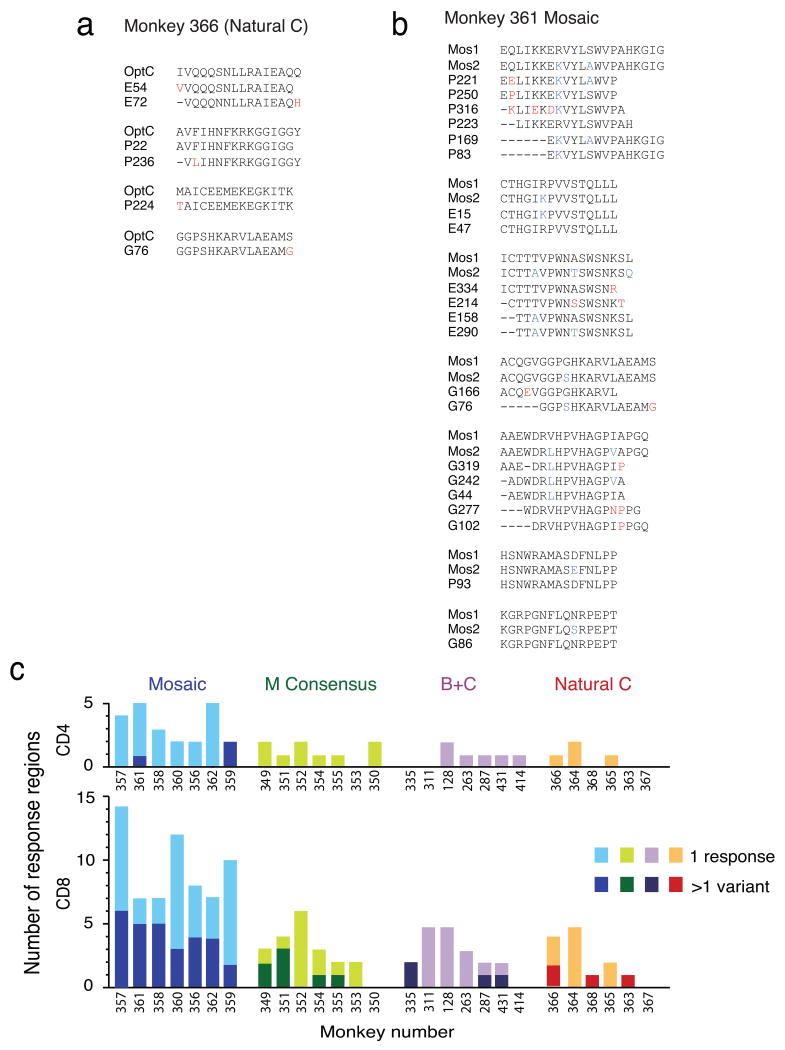
We next characterized the depth of the cellular immune responses elicited by the various vaccine regimens. We defined depth as the number of simultaneously elicited variant PTE peptides for a particular epitopic region. Inducing responses to multiple epitope variants may increase immunologic coverage of infecting virus sequences and may also block common escape routes in vivo or force the virus down suboptimal escape routes that incur high fitness costs 3. The consensus and natural sequence antigens elicited responses that were characterized by a high degree of sequence identity between the vaccine sequences and the reactive PTE peptides, as exemplified by the responses in monkey 366 (Fig. 2a; see also Supplementary Fig. 3). In contrast, the mosaic antigens elicited responses that were characterized by multiple reactive PTE peptides in particular epitopic regions. These peptides represented common variants and typically reflected the polymorphisms contained in the mosaic vaccine sequences, as exemplified by the responses in monkey 361 (Fig. 2b; see also Supplementary Fig. 3). A summary analysis demonstrates that the mosaic antigens elicited a greater frequency of cellular immune responses to peptides with two or more targeted variants as compared with the consensus or natural sequence antigens (Fig. 2c; P = 0.001, Wilcoxon rank-sum test comparing the mosaic with the other groups).
Figure 2.
Depth of epitope-specific T lymphocyte responses to PTE peptides. (a) Example of mapped T lymphocyte responses in monkey 366 that received the optimal natural clade C antigens. (b) Example of mapped T lymphocyte responses in monkey 361 that received the 2-valent mosaic antigens. In (a) and (b), vaccine sequences are shown on the top (OptC, Mos1, Mos2), and reactive PTE peptides are shown beneath the vaccine sequences denoted by the antigen (G, Gag; P, Pol; E, Env) and the PTE peptide number. The minimal overlap region is shown in bold. Sequence polymorphisms between the two mosaic antigens are shown in blue. Differences between the vaccine sequences and the reactive PTE peptides are shown in red. Complete alignments of all positive peptides organized by response regions are shown in Supplementary Fig. 3. (c) Depth of CD4+ (top) and CD8+ (bottom) T lymphocyte responses following immunization with rAd26 vectors expressing mosaic, M consensus, clade B + clade C, or optimal natural clade C antigens. Individual monkeys are depicted on the x-axis. One response variant (light shade) or >1 response variants (dark shade) are shown for each epitopic region.
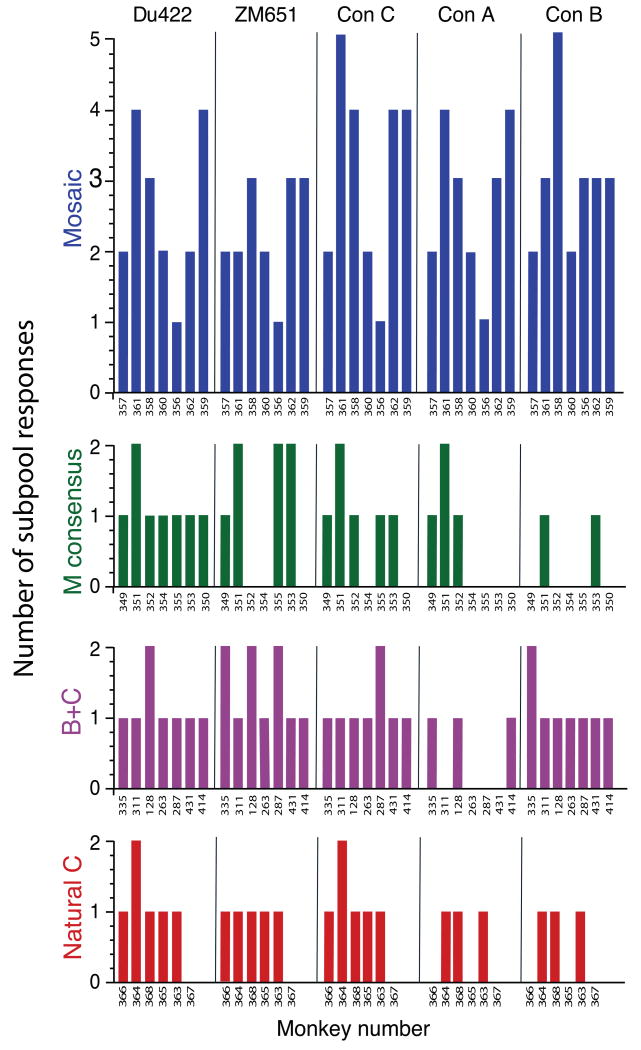
To complement the analysis with PTE peptides, we also assessed the breadth of cellular immune responses in the vaccinated monkeys utilizing overlapping peptides covering 5 different HIV-1 Gag sequences: clade C DU422, clade C ZM651, consensus C, consensus A, and consensus B. Cellular immune breadth was determined by assessing reactivity to subpools of 10 overlapping peptides spanning each Gag sequence. The mosaic antigens elicited greater breadth of T lymphocyte responses as compared with the consensus or natural sequence antigens against all five Gag sequences (Fig. 3; P = 1 × 10-7, binomial regression). In particular, the mosaic antigens proved superior to the optimal natural clade C antigens for inducing responses against clade C Gag. The mosaic antigens also elicited comparable responses to Gag from multiple clades, whereas the natural clade C antigens exhibited diminished responses to clade A Gag and clade B Gag (Fig. 3). The breadth of Gag-specific responses elicited by the consensus, the combined B+C, and the natural clade C vaccines were not statistically distinguishable, consistent with the results utilizing PTE peptides (Fig. 1).
Figure 3.
Breadth of epitope-specific T lymphocyte responses to five HIV-1 Gag sequences from clades A, B, and C. Breadth of cellular immune responses was assessed utilizing subpools of overlapping peptides spanning the following strains of HIV-1 Gag: clade C DU422, clade C ZM651, consensus C, consensus A, and consensus B. Numbers of positive subpools are shown following a single immunization of rAd26 vectors expressing mosaic (blue), M consensus (green), clade B + clade C (purple), or optimal natural clade C (red) HIV-1 Gag, Pol, and Env antigens. Individual monkeys are depicted on the x-axis.
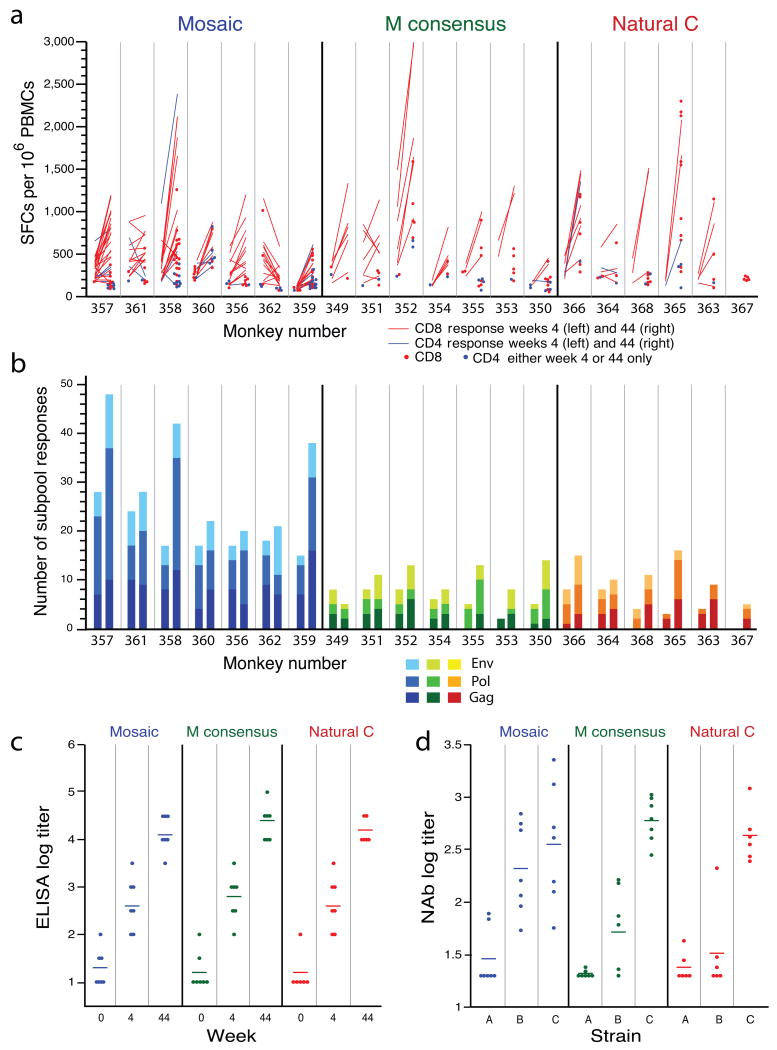
To assess the durability of these observations, we boosted the monkeys that received the mosaic, consensus, and optimal natural clade C antigens at week 40 with a total dose of 3×1010 viral particles of the heterologous vector rAd5HVR48 12 expressing HIV-1 Gag, Pol, and Env antigens that matched the initial immunization sequences. Cellular immune breadth was determined by assessing reactivity to subpools of 10 PTE peptides at week 4 (post-prime) and at week 44 (post-boost). The majority of CD8+ and CD4+ T lymphocyte responses that were observed after the priming immunization expanded following the boost (Fig. 4a, red and blue lines), although new responses were also detected (Fig. 4a, red and blue dots). At week 44, the frequency of high magnitude, epitope-specific cellular immune responses was comparable among groups (Fig. 4a), but the number of subpool responses elicited by the mosaic antigens (median 27 responses per animal) remained substantially higher than the number induced by the consensus antigens (median 11 responses per animal) or the optimal natural clade C antigens (median 10 responses per animal) (Fig. 4b; P < 0.001, Wilcoxon rank-sum tests for all pairwise comparisons).
Figure 4.
Cellular and humoral immune responses following the boost immunization. (a) Magnitude and (b) breadth of T lymphocyte subpool responses at week 4 post-prime (left side of each panel) and at week 44 post-boost (right side of each panel) for each monkey. Monkeys were primed at week 0 with rAd26 vectors and boosted at week 40 with rAd5HVR48 vectors expressing mosaic, M consensus, or optimal natural clade C HIV-1 Gag, Pol, and Env antigens. Individual monkeys are depicted on the x-axis. In (a), red denotes epitope-specific CD8+ T lymphocyte responses, blue denotes epitope-specific CD4+ T lymphocyte responses, lines depict responses observed at both timepoints, and dots depict responses observed at only one timepoint. In (b), the different shades of each color reflect responses to the different antigens (Gag, Pol, Env) as indicated. (c) Env-specific ELISA endpoint titers are shown at weeks 0, 4, and 44. (d) Neutralizing antibody (NAb) titers to the tier 1 clade A (DJ263.8), clade B (SF162.LS), and clade C (MW965.26) viruses are shown at week 44. NAb titers to murine leukemia virus as a negative control were <20 for all samples (data not shown).
We also measured Env-specific humoral immune responses following the boost immunization by ELISAs (Fig. 4c) and luciferase-based pseudovirus neutralization assays 13 (Fig. 4d). All groups exhibited comparable ELISA titers to clade C gp140 and comparable neutralizing antibody (NAb) responses to the tier 1 clade C virus MW965.26. The mosaic antigens elicited slightly higher NAb responses to the tier 1 clade B virus SF162.LS as compared with the consensus or natural clade C antigens (P = 0.02, Wilcoxon rank-sum test). NAb responses to the tier 1 clade A virus DJ263.8 were low in all groups, and no NAb responses to tier 2 viruses were detected (data not shown). Thus, the mosaic Env immunogens elicited noninferior antibody responses as compared with the consensus or natural sequence Env antigens.
Our data demonstrate that mosaic HIV-1 Gag, Pol, and Env antigens augmented both the breadth and depth of epitope-specific cellular immune responses as compared with consensus or natural sequence antigens in rhesus monkeys, in agreement with theoretical predictions (Supplementary Fig. 4). In particular, a 2-valent set of mosaic antigens proved superior to a 2-valent set of clade B and clade C antigens, indicating that the augmented cellular immune breadth and depth was dependent on the mosaic antigen design 3,14, which maximized inclusion of common epitope variants and excluded rare epitope variants that are unlikely to be useful in a vaccine. The results with the mosaic antigens in this study likely reflected the efficiency of rAd26 vectors in eliciting CD8+ T lymphocyte responses 5-6 together with the capacity of the mosaic antigens to augment CD8+ T lymphocyte breadth. The mosaic antigens also significantly enhanced CD4+ T lymphocyte breadth, but these effects were less marked and were characterized by lower total numbers of CD4+ T lymphocyte responses.
The importance of expanding cellular immune breadth has been demonstrated by recent studies showing that the breadth of Gag-specific T lymphocyte responses is correlated with control of HIV-1 replication in chronically infected humans 4 and with control of SIV replication following challenge of vaccinated rhesus monkeys 5. In the phase 2b STEP study, the rAd5-based HIV-1 vaccine candidate expressing natural clade B Gag, Pol, and Nef antigens elicited only a limited breadth of cellular immune responses, and no vaccine benefit was observed 15-16. This narrow breadth of cellular immune responses likely provided insufficient immunologic coverage of the diversity of infecting viruses. Moreover, viral escape from CD8+ T lymphocytes has been reported to occur during acute HIV-1 infection 17, and cellular immune responses against variant epitopes have been shown to block SIV mutational evolution in rhesus monkeys in vivo 18, thus suggesting the importance of expanding cellular immune depth. We were unable to assess the protective efficacy of mosaic HIV-1 vaccines in the present study as a result of the lack of an appropriate preclinical HIV-1 challenge model. Modeling the protective efficacy of mosaic vaccines against SIV challenges in nonhuman primates also has intrinsic limitations, since the observed diversity of SIV in natural hosts and HIV-1 in humans differs substantially and is influenced by different underlying biology 19-20.
In summary, this study demonstrates that 2-valent mosaic HIV-1 Gag, Pol, and Env antigens expanded cellular immune breadth and depth in rhesus monkeys as compared with consensus or natural sequence antigens. These findings have major implications for HIV-1 vaccine development, since global virus diversity and viral escape from cellular immune responses represent critical hurdles in the development of a T cell-based HIV-1 vaccine. A 2-valent cocktail of mosaic antigens is also practical and potentially feasible for clinical development. Moreover, the mosaic antigen strategy is generalizable and should be applicable for other genetically diverse pathogens in addition to HIV-1.
Methods
Antigen design
2-valent mosaic Gag, Pol, and Env antigens were constructed to provide optimal coverage of HIV-1 M group sequences in the Los Alamos HIV-1 Sequence Database essentially as described 3,21, using the coverage of epitope length peptides in the global database as the selection criterion. The global sequence data set was utilized for each antigen, restricted to include only full length proteins and one sequence per individual. This set of proteins was then fractured into all possible fragments of 9 contiguous amino acids (9-mers). The 9-mer frequencies were tallied, and we used a genetic algorithm to design and select in silico recombinant sequences that resembled real proteins and that maximized the number of 9-mer sequence matches between the vaccine candidates and the global database. The optimal clade C antigens were selected using a similar selection strategy, but we utilized the clade C dataset as the input sequences. We selected the single clade C Gag, Pol, and Env natural sequences that provided optimal coverage of all 9-mers in the global clade C dataset (C.IN.-.70177 Gag, C.ZA.04.04ZASK208B1 Pol, C.SN.90.90SE_364 Env). The clade B antigens were previously selected to be near-consensus or consensus sequences with good coverage of the clade B dataset (B.CAM-1 Gag, B.IIIB Pol, B.Con Env) 22 and were used to complement the optimal clade C antigens for the 2-valent clade B + C vaccine approach. Pol antigens contained RT and IN without PR and included point mutations to eliminate catalytic activity as described 22. Env gp140 antigens contained point mutations to eliminate cleavage and fusion activity. Vaccine sequences are depicted in Supplementary Fig. 4.
Vector production
Recombinant, replication-incompetent, E1/E3-deleted adenovirus serotype 26 (rAd26) and hexon-chimeric rAd5HVR48 vectors expressing these antigens were produced in PER.55K cells and purified essentially as described 6,12. The HIV-1 antigens were expressed in the adenovirus E1 region under control of a human cytomegalovirus promoter.
Animals and immunizations
27 outbred rhesus monkeys that did not express the MHC class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17 were housed at New England Primate Research Center (NEPRC), Southborough, MA. Immunizations involved 3 × 1010 viral particles rAd26 or rAd5HVR48 vectors expressing mosaic, M consensus, clade B + clade C, or optimal natural clade C HIV-1 Gag, Pol, and Env antigens delivered as 1 ml injections i.m. in both quadriceps muscles at weeks 0 and 40. All animal studies were approved by our Institutional Animal Care and Use Committees.
IFN-γ ELISPOT assays
HIV-1-specific cellular immune responses in vaccinated monkeys were assessed by interferon-γ (IFN-γ) ELISPOT assays essentially as described 5,12. HIV-1 Gag, Pol, and Env potential T cell epitope (PTE) peptides that included all PTEs found in at least 15% of HIV-1 M group sequences 8 as well as HIV-1 Gag peptides from clade C DU422, clade C ZM651, consensus C, consensus A, and consensus B strains were obtained from the NIH AIDS Research and Reference Reagent Program. Spot-forming cells (SFC) per 106 PBMC were calculated. Media backgrounds were typically < 15 SFC per 106 PBMC. Positive responses were defined as > 55 SFC per 106 PBMC and > 4-fold background.
Epitope mapping
Comprehensive CD8+ and CD4+ T lymphocyte epitope mapping was performed utilizing Gag, Pol, and Env PTE peptides (NIH AIDS Research and Reference Reagent Program). IFN-γ ELISPOT assays were conducted at week 4 following immunization initially with complete peptide pools as well as with subpools containing 10 PTE peptides that were pooled sequentially by peptide number. All peptide subpools with positive responses were then deconvoluted, and epitopes were confirmed with individual 15 amino acid PTE peptides. Cell-depleted IFN-γ ELISPOT assays were performed to determine if reactive peptides represented CD8+ or CD4+ T lymphocyte epitopes. Cell depletions were performed by negative bead selection and were >98% efficient. Partial epitope mapping utilizing PTE subpools was also performed 4 weeks following the boost immunization at week 44. All borderline responses were retested and only considered positive if confirmed. Partial epitope mapping utilizing subpools containing 10 overlapping Gag peptides was also performed to assess breadth to HIV-1 Gag from various clades.
Humoral immune assays
Env-specific humoral immune responses were evaluated by direct ELISAs utilizing HIV-1 clade C Env gp140 and luciferase-based pseudovirus neutralization assays essentially as described 13.
Statistical analyses
All statistical analyses were done using the package R 11 (http://www.R-project.org). To analyze the breadth of cellular immune responses to mapped PTE peptides (Fig. 1a), we fit Poisson regression models that predicted the number of reactive peptides as a function of vaccine group, antigen (Gag, Pol, Env), and lymphocyte subpopulation (CD4, CD8). Our models included random effects to accommodate animal-to-animal variation and were fit with the lme4 library 9-10 (http://cran.r-project.org/web/packages/lme4) of the package R. The data fit the models well (dispersion parameter 1.0), and there were no significant interactions among the three explanatory factors. For example, the 3.8-fold enhancement in the number of PTE peptides recognized by monkeys that received the mosaic antigens as compared to those that received the consensus or natural sequence antigens (Fig. 1a) applied equally to PTEs from Gag, Pol, and Env and held for responses by CD8+ as well as CD4+ T lymphocytes. The analysis of the number of reactive epitopic regions (Fig. 1b) also included Poisson regression models with random effects and again fit well (dispersion parameter 0.87) without any significant interactions. Comparisons of the magnitude of CD8+ and CD4+ T lymphocyte responses (Fig. 1c) were performed utilizing 2-sided Kolmogorov-Smirnov tests. Non-parametric tests to compare the breadth and depth of responses per monkey between different vaccines were also performed (Figs. 1a, 2c). We initially employed Kruskal-Wallis tests to determine if there was a difference among the 4 vaccine groups. In each case this was highly significant, and we then assessed all pairwise comparisons between the 4 vaccine groups using Wilcoxon rank-sum tests. In each of these comparisons, the mosaic vaccine elicited significantly more responses per monkey than the other 3 vaccines. To analyze the breadth of responses to HIV-1 Gag from various sequences (Fig. 3), we fit the data to binomial regression models. These models used the vaccine group as an explanatory variable and included random effects to account for animal-to-animal and strain-to-strain variation.
Supplementary Material
Acknowledgments
We thank B. Haynes, N. Letvin, P. Swanson, F. Stephens, B. Hahn, J. McElrath, J. Goudsmit, M. Pau, N. Michael, M. Marovich, and M. Pensiero for generous advice and assistance. Peptides were obtained from the NIH AIDS Research and Reference Reagent Program. We acknowledge support from NIH grants AI058727 (D.H.B.), AI066305 (D.H.B.), AI066924 (D.H.B.), AI078526 (D.H.B.), AI084794 (D.H.B.), RR000168 (NEPRC), AI067854 (B.K.), AI061734 (B.K.), and directed research funding from Los Alamos National Laboratory (B.K.).
Footnotes
Author Contributions: W.F., B.K., and D.H.B. designed the antigens. S.L.K., P.A., L.F.M., Y.H.S., and D.H.B. generated the vaccine vectors. K.L.O., N.L.S., A.L.P., A.M.R., D.M.L., S.L.C., and D.H.B. designed and conducted the cellular immunologic assays. A.L.P., K.B., J.R.P., and M.S.S. designed and conducted the humoral immunologic assays. A.C. and K.G.M. led the animal work. M.M. and B.K. led the data analysis, and J.S. wrote the response mapping software. D.H.B. and B.K. designed the study, and D.H.B. led the study. All co-authors contributed to writing the manuscript.
Competing Interests Statement: The authors report no financial conflicts of interest.
References
- 1.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korber BT, Letvin NL, Haynes BF. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. Journal of virology. 2009;83:8300–8314. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer W, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 4.Kiepiela P, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbink P, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santra S, et al. A centralized gene-based HIV-1 vaccine elicits broad cross-clade cellular immune responses in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10489–10494. doi: 10.1073/pnas.0803352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24:6893–6904. doi: 10.1016/j.vaccine.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Pinheiro JC, Bates DM. Mixed-effects models in S and S-plus. Springer; New York: 2000. [Google Scholar]
- 10.Bates D, Maechler M. The lme4 Package. 2009. http://cran.r-project.org/web/packages/lme4.
- 11.Team RDC. R Foundation for Statisical Computing; Vienna, Austria: 2009. R: A language and environment for statisical computing. http://www.R-project.org. [Google Scholar]
- 12.Roberts DM, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 13.Montefiori D. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays Current Protocols in Immunology. John Wiley & Sons; 2004. [DOI] [PubMed] [Google Scholar]
- 14.Kong WP, et al. Expanded breadth of the T-cell response to mosaic human immunodeficiency virus type 1 envelope DNA vaccination. Journal of virology. 2009;83:2201–2215. doi: 10.1128/JVI.02256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElrath MJ, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goonetilleke N, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. The Journal of experimental medicine. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barouch DH, et al. Dynamic immune responses maintain cytotoxic T lymphocyte epitope mutations in transmitted simian immunodeficiency virus variants. Nat Immunol. 2005;6:247–252. doi: 10.1038/ni1167. [DOI] [PubMed] [Google Scholar]
- 19.Barry AP, et al. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. Journal of immunology (Baltimore, Md. 2007;178:8002–8012. doi: 10.4049/jimmunol.178.12.8002. [DOI] [PubMed] [Google Scholar]
- 20.Sodora DL, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nature medicine. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurmond J, et al. Web-based design and evaluation of T-cell vaccine candidates. Bioinformatics. 2008;24:1639–1640. doi: 10.1093/bioinformatics/btn251. [DOI] [PubMed] [Google Scholar]
- 22.Priddy FH, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clinical infectious diseases. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.