Abstract
Several studies have now documented that the serotonin transporter promoter region (5-HTTLPR) polymorphism predicts neural response to affective images in brain regions involved in the experience of emotion. However, the behavioral consequences of this genetic effect are less well known. The current study used eye-tracking methodology to examine how individuals genotyped for the 5-HTTLPR allocated their attention when simultaneously presented an array of positive and negative emotional scenes. Short 5-HTTLPR allele homozygotes displayed a bias to focus on positive images, particularly in the first half of the 30-second trial. In contrast, long 5-HTTLPR allele homozygotes viewed the stimuli in a more evenhanded fashion. Thus, short 5-HTTLPR allele homozygotes may be attempting to regulate greater reactivity to negative stimuli by purposefully turning their attention towards positive stimuli. Although this sensitivity may have benefits under benign conditions, it may also increase vulnerability to affective disorders when cognitive resources needed to turn attention away from negative stimuli are compromised.
Keywords: 5-HTTLPR, eye tracking, emotion, serotonin
Individuals with two copies of the short (SS) serotonin transporter gene-linked polymorphic region (5-HTTLPR) allele appear to be more sensitive to the social environment than their long (LL) 5-HTTLPR allele counterparts. For instance, short 5-HTTLPR allele homozygotes are almost twice as likely to become depressed when exposed to life stress than long allele homozygotes (e.g., Caspi et al., 2003; Kendler, Kuhn, Vittum, Prescott, & Riley, 2005). Similarly, assessments of the 5-HTTLPR genotype and life stress explained 31% of the variance in the severity of a major depressive episode (Zalsman et al., 2006), although it should also be noted that several studies have not found such a relationship (see Risch et al., 2009). Nevertheless, this line of work has generated a great deal of research aimed at understanding how the 5-HTTLPR affects sensitivity to emotional aspects of the environment.
Much of this research has explored the impact of the 5-HTTLPR genotype at a neural level (Hariri & Holmes, 2006). For instance, Hariri et al. (2002) first reported that carriers of the short 5-HTTLPR allele experienced greater amygdala reactivity, a brain region involved in the generation and expression of emotion, to angry and fearful facial expressions than individuals with two copies of the long 5-HTTLPR allele. This finding has been subsequently replicated in many other studies (for a meta-analysis, see Munafo, Brown, & Hariri, 2008). Surguladze et al. (2008) found greatest reactivity to fearful faces within the amygdala, fusiform gyrus, and ventral lateral PFC among short 5-HTTLPR allele homozygotes compared to individuals with one or two copies of the long 5-HTTLPR allele. Similarly, when viewing negative facial expressions, short 5-HTTLPR allele carriers had less functional connectivity between the amygdala and perigenual anterior cingulate cortex (pACC), a region believed to inhibit amygdala reactivity, than long 5-HTTLPR allele homozygotes (Pezawas et al., 2005). The “uncoupling” of this circuit may explain in part why the 5-HTTLPR genotype is associated with amygdala response to emotional stimuli (see also, Pacheco et al., 2009).
Other important research indicates that 5-HTTLPR polymorphism may influence neural responsiveness to a wider variety of stimuli than first thought. 5-HTTLPR genotype predicted differential activation in limbic (including the amygdala), striatal, and cortical brain regions in response to negative, positive, and neutral word stimuli (Canli et al., 2005). Further, greater amygdala reactivity was observed among short 5-HTTLPR allele carriers than long 5-HTTLPR allele homozygotes when contrasting fearful and happy facial expressions to fixation cross (Canli, Congdon, Todd Constable, & Lesch, 2008). Further, Heinz et al. (2005) reported that short 5-HTTLPR allele carriers had greater amygdala activation than long 5-HTTLPR allele homozygotes to aversive compared to neutral emotional scenes using images from the same collection as the current study. Thus, this heightened amygdala response in short 5-HTTLPR allele carriers appears to apply to a variety of emotional stimuli.
Heightened sensitivity to emotional information at a neural level presumably affects how short 5-HTTLPR allele homozygotes view their world—that is, what these individuals attend to in their environment (Bishop, 2008; Isaacowitz, 2006). Consistent with this possibility, Osinsky et al. (2008) found that short 5-HTTLPR allele carriers selectively attended to pictures of spiders presented for 2000ms than long 5-HTTLPR allele homozygotes. In an inpatient psychiatric sample, short 5-HTTLPR allele carriers displayed a stronger attentional bias for anxious word stimuli compared to long 5-HTTLPR allele homozygotes (Beevers, Gibb, McGeary, & Miller, 2007). Further, in a healthy adult sample, short 5-HTTLPR allele carriers had greater difficulty disengaging attention from happy and sad facial expressions than long allele homozygotes (Beevers, Wells, Ellis, & McGeary, in press, Study 1). In a replication study, individuals with two low expressing 5-HTTLPR (S or LG) alleles had greater difficulty disengaging their attention from happy, fearful, and sad facial expressions than high expressing 5-HTTLPR (LA) allele homozygotes. Individuals with one LA 5-HTTLPR allele did not differ from those with two LA alleles (Beevers et al., in press, Study 2). Taken together, these findings are generally consistent with an ERP study documenting short 5-HTTLPR allele carriers have stronger early posterior negativity potential (EPN) responses when passively viewing emotional scenes than long 5-HTTLPR allele homozygotes (Herrmann et al., 2007). Greater EPN reflects increased sensory encoding due to selective attention.
Although these studies provide important information about attentional biases for emotional stimuli associated with the 5-HTTLPR genotype, this assessment is incomplete. More specifically, these behavioral studies have primarily focused on processing of emotional stimuli presented for relatively brief durations. These studies typically obtain a “snapshot” of biased processing, as attentional bias is indirectly measured by reaction time immediately following stimulus offset. Use of multiple stimulus durations can assess the time course of sustained processing (e.g., Osinsky et al., 2008); however, assessing more than two or three stimulus durations often becomes difficult due to subject burden. Therefore, reaction time tasks may provide an excellent assessment of the early stages of selective attention, but yield a somewhat incomplete picture of how selective attention unfolds over time.
Hermans et al. (1999) suggested that tracking eye movements provides a relatively continuous index of information processing, which is ideal for assessing selective attention over relatively longer intervals. Under normal viewing conditions, individuals typically direct their line of gaze toward stimuli that attracts attention (Jonides, 1981). While attention can be moved without eye movements under certain laboratory conditions, the converse is not true; there is an obligatory shift in attention prior to every eye movement (Kowler, Anderson, Dosher, & Blaser, 1995). Shifts in gaze position thus closely follow and are guided by shifts in attentional focus (Kowler, 1995; Moray, Baddeley, & Weiskrantz, 1993). Further, measuring eye movements avoids problems of slow and variable motor responses. Mathews et al. (1996) suggested that differences in motor responding may potentially confound manual or vocal reaction time data. Visual attention is not confounded by such motor-slowing deficits. Thus, eye-tracking methodology may be particularly well suited to study associations between the 5-HTTLPR genotype and biased processing of emotional stimuli.
To examine the processing of emotional stimuli, we simultaneously presented positive and negative scenes for thirty seconds while recording the visual gaze of individuals genotyped for the 5-HTTLPR polymorphism. This approach is quite different from a typical imaging study of emotion processing, as single images or an array of similar images are typically presented. By presenting emotional stimuli that vary in content and measuring subsequent location of visual gaze, we can examine how attention is allocated when a variety of emotional stimuli are presented. Given greater neural responsiveness to emotional stimuli associated with the short 5-HTTLPR polymorphism, we hypothesized that the 5-HTTLPR genotype would influence selective attention for emotional stimuli.
Method
Participants
Participants were 45 undergraduate students (age: M = 18.89, SD = 0.92; 43% female; 68% White) with no current or past history of major depressive disorder, as determined by a SCID mood disorders module interview (First, Spitzer, Williams, & Gibbon, 1995). They participated in exchange for credit in their introductory psychology course. All participants had very low levels of self-reported depression (BDI-II M = 2.71, SD = 2.05). We were unable to genotype one participant for the 5-HTTLPR, so this person was excluded from analyses. Race, which was dichotomized into Caucasian versus non-Caucasian due to low numbers of minority participants, was not significantly associated with 5-HTTLPR allele frequency, χ2 (2, N = 44) = 2.60, p = .27, or time spent viewing images from each emotion category, Fs < 3.4, ps > .07. Similarly, there was no participant gender main effect or interaction with 5-HTTLPR allele status for predicting time spent viewing images (Fs < 1.8, ps > .12). Race and gender were therefore not included as a factor in analyses.
Assessments
Diagnostic assessment
Absence of major depressive disorder (MDD) was diagnosed with the Mood Disorders Module of the Structured Clinical Interview for the DSM-IV Diagnoses – Patient Version (SCID; First, Spitzer, Gibbon, & Williams, 1998). Two female assessors with at least a bachelor's degree in psychology conducted all interviews. Assessors participated in 15 hours of training, wherein they learned interview skills, reviewed diagnostic criteria for relevant Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; American Psychiatric Association, 1994), observed mock interviews, and role-played interviews. All SCID interviews conducted for this study were audio taped. Three advanced doctoral trainees acted as independent assessors and rated twenty percent of all interviews (n = 15). Diagnostic agreement was evaluated using the κ coefficient. Agreement between study interviewer and independent assessor diagnosis of MDD was very good (kappas [κ] = .85 – 1.0).
Self-report measures of depression
Participants completed the Beck Depression Inventory-II (BDI-II: Beck, Brown, & Steer, 1996), a widely used self-report measure of depression. The BDI-II consists of 21 items that measure the presence and severity of cognitive, affective, motivational, and physiological symptoms of depression. Past reports indicate adequate reliability and validity of these measures within college student samples (Beck, Steer, & Garbin, 1988).
Eye Tracking Paradigm
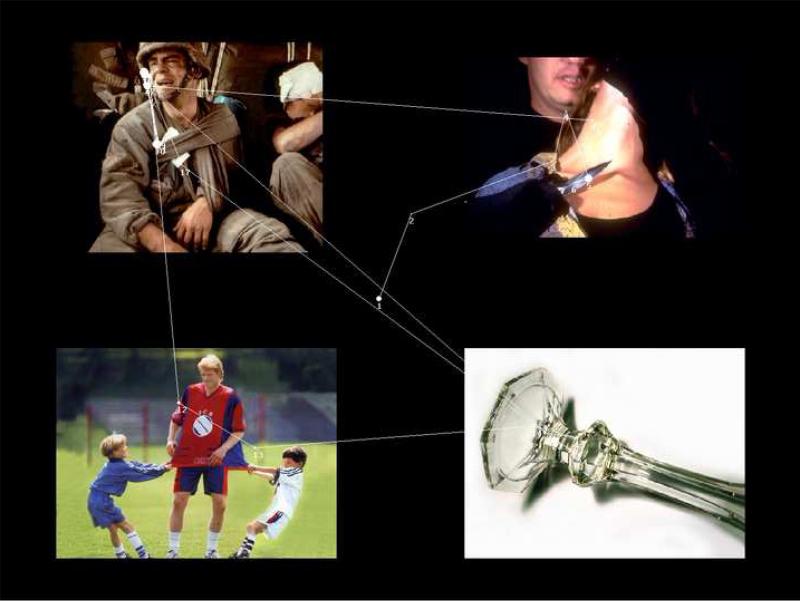
This task involved simultaneous presentation of four images selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). Images from this picture system are well standardized and have been used in studies of psychopathology. Each slide contained 4 images, one of 12 images selected from each of the following four categories: dysphoric, threat, neutral, and positive (see Figure 1).1
Figure 1.
An example of stimuli used in a trial with fixations superimposed on the image. Larger diameter of fixations indicates longer fixation duration. Numbers next to each fixation indicates sequence in which fixations occurred. Lines connecting fixations are intended to highlight the fixation sequence, but do not represent the actual scan path.
The IAPS images have been systematically rated for valence but not for specific emotion categories, such as dysphoric and threat. We conducted a pilot study to identify which emotion category best described the negative images. Ninety undergraduate students were asked to rate how “sad” and “threatening” each of the 24 negative images was on a 9-point scale (not at all = 1 to extremely = 9). Presentation order of the images was randomized across participants and presentation order for the rating categories were counterbalanced. As expected, the dysphoric images were rated as significantly more sad than threatening images (M = 6.73, SD = 1.89; M = 4.15, SD = 2.69, respectively), t(89) = 13.4, p < .001. Further, the threatening images were rated as significantly more threatening than dysphoric images (M = 7.38, SD = 1.86; M = 2.71, SD = 2.11, respectively), t(89) = −21.6, p < .001.
The IAPS stimuli have previously been rated on nine point scales for valence (unpleasant = 1 to pleasant = 9) and arousal (calm = 1 to excitement = 9). Valence ratings for the threatening and dysphoric images ranged from 2 to 4, whereas positive images were rated from 6 to 8. Neutral images had valence ratings of approximately 5. Comparisons revealed that the mean valence ratings for the positive (M = 7.3, SD = 0.4) and neutral (M = 5.1, SD = 0.2) images were significantly different from the other three categories (ts > 13.1, ps < .001), whereas the valence ratings for dysphoric (M = 2.4, SD = 0.4) and threatening (M = 2.6, SD = 0.6) images did not differ from each other (t = .58, ns). However, threatening (M = 6.7, SD = 0.6) images did have a significantly greater mean arousal rating than dysphoric (M = 4.9, SD = 0.5), positive (M = 4.6, SD = 0.7), or neutral (M = 2.8, SD = 0.3) images (ts > 7.8, ps < .001). In addition, neutral images had a significantly lower mean arousal rating than dysphoric, positive, or threatening images (ts > 7.9, ps < .001), whereas the dysphoric and positive images did not differ from each other in arousal (ts = 1.4, ns).
For each eye tracking trial, image location was randomly assigned to one of four screen quadrants, with the constraint that each stimulus category must occur in each of the four positions three times across 12 trials. Eight filler trials with four neutral images were presented to obscure the nature of the task. Participants completed a total of 20 trials (12 study + 8 filler). Stimuli location, stimuli selected for each trial, and the order in which stimuli were presented were all randomly determined for each participant by stimulus presentation software. Each trial began with a 1000 millisecond centrally presented fixation cross, followed by presentation of stimuli for 30 seconds.
Eye Tracking System
Line of visual gaze was assessed using a remote optics eye tracking system model R6 from Applied Science Laboratories (Cambridge, Massachusetts). Head location was fixed using a chin rest and forehead bar. The direction of gaze, measured with x and y coordinates, was sampled every 16.7 milliseconds (60Hz). Eye movements that were stable for more than 100ms within a 1° of visual angle were classified as a fixation. Areas of interest (AOIs) were also identified for each trial and corresponded with the total area for each of the four images. Thus, it was possible to determine total time spent viewing each of the four images presented during each trial. E-Prime software was used to present the stimuli and to automate the recording of eye location with the eye tracker software.
Genotyping
Genomic DNA were isolated from buccal cells using a modification of published methods (e.g., Freeman et al., 1997). The cheeks and gums are rubbed for 20 s with three sterile, cotton-tipped wooden swabs. The swabs are placed in a 50-ml capped polypropylene tube containing lysis buffer (500 μl of 1 M Tris-HCl; 200 mM disodium ethylene diaminetetracetic acid (EDTA), pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride). The subjects then rinse out the mouth vigorously with 10 ml of distilled water for 20 seconds and this was added to the 50-ml tube. The tubes were stored at 4°C until the DNA was extracted.
Serotonin transporter promoter region polymorphism (5-HTTLPR)
The 5-HTTLPR gene, which maps to 17q11.1-17q12, contains a 43 bp insertion/deletion in the 5’ regulatory region of the gene (Heils et al., 1996). This polymorphism in the promoter appears to be associated with variations in transcriptional activity: the long (L) variant (528 bp) has approximately three times the basal activity of the shorter (S) promoter (484 bp) with the deletion (Lesch et al., 1996). The assay is a modification of the method of Lesch and colleagues (Lesch et al., 1996). The primer sequences are: forward, 5’- GGCGTTGCCGCTCTGAATGC-3’ (fluorescently labeled), and reverse, 5’-GAGGGACTGAGCTGGACAACCAC-3’. These primer sequences yield products of 484 or 528 bp. Allele sizes are scored by two investigators independently and inconsistencies were reviewed and rerun when necessary. 5-HTTLPR allele frequency was SS = 12 (27.3%), SL = 24 (54.5%), LL = 9 (19.1%), which was within Hardy-Weinberg Equilibrium (p = .89).
Procedure
After signing an informed consent form, participants completed a demographics form, the BDI-II, and the Mood Disorders Module from the Structured Clinical Interview for DSM-IV (SCID; First et al., 1998). Participants were then escorted to the eye tracking room and seated in a height adjustable chair so their chin fit comfortably in the chin rest. The chin rest was positioned so that participants’ eyes were level with the middle of the 17-inch monitor on which the stimuli were presented. This ensured that all participants’ eyes were in the same location relative to the camera and the monitor. After adjusting the eye tracker to best capture each participant's right eye, a 9-point calibration was completed. Once calibration was complete the experimenter confirmed that the eye tracker was recording line of visual gaze within 1° of visual angle for each calibration point. Calibration was repeated until this criterion was met.
Once calibration was successful, the participant was informed that the task would begin shortly and all instructions would be presented on the computer screen. Participants were instructed to view the images naturally, as if they were watching television or viewing pictures in a photo album. The only constraint was that they were to view the images at all times during each trial. Further, they were also instructed to look at the fixation cross-hair prior to each trial in order to standardize the starting location of their gaze. The experimenter (located in an adjacent room) then monitored the stimulus presentation and eye tracking throughout each trial.
Results
A 4 (stimuli valence: dysphoric, threat, positive, neutral) × 3 (5-HTTLPR allele status: SS, SL, LL) mixed-plot analysis of variance examined whether 5-HTTLPR allele status was associated with percentage of time viewing stimuli. Analyses indicated significant effects for stimuli valence, F(3, 123) = 22.27, p < .001, partial η2 = .35, and stimuli valence × 5-HTTLPR allele status, F(6, 123) = 2.40, p = .03, partial χ2 = .11.
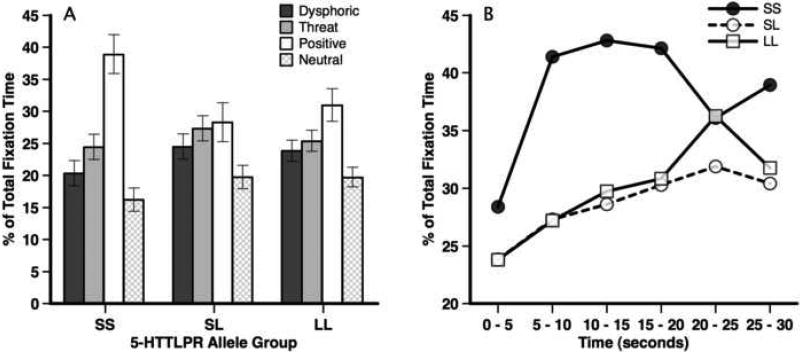
To decompose this interaction, we first compared 5-HTTLPR allele groups for percent fixation time for each stimulus valence. The effect of 5-HTTLPR allele group was significant for positive, F(2, 41) = 3.81, p = .03, partial η2 = .16, but not dysphoric, threat, or neutral stimuli (all Fs < 1.7, ps < .20, partial η2s < .08). For positive stimuli, the SS group spent significantly more time viewing positive stimuli than the SL and LL groups (effect size r = .39 and .32; ps < .05), whereas the SL and LL group did not differ from each other (effect size r = .08; p = .40; see Figure 2A).
Figure 2.
Percent of total fixation time viewing dysphoric, threat, positive, and neutral stimuli presented by 5-HTTLPR allele group (Panel A), and percent of total fixation time viewing positive stimuli across 5-second segments presented by 5-HTTLPR allele group (Panel B).
Within each 5-HTTLPR allele group, there were significant main effects for stimuli valence within the SS, F(3, 21) = 5.82, p = .005, partial χ2 = .45, SL, F(3, 69) = 12.87, p < .001, partial η2 = .35, and LL allele groups, F(3, 33) = 3.53, p = .03, partial η2 = .24; however, the valence effects were different across the groups. Within the SS group, LSD comparisons indicated significantly greater percent time spent looking at positive stimuli than dysphoric (effect size r = .61) and neutral (effect size r = .70, ps < .05) stimuli, but was marginally nonsignificant for threatening stimuli (effect size r = .47, p = .13). Within the SL group, a similar pattern emerged as the SS group, although effect sizes were generally smaller. Significantly more time was spent viewing positive than dysphoric (effect size r = .41) and neutral stimuli (effect size r = .65; ps < .05). No significant difference was observed between percent time viewing positive and threat stimuli (effect size r = .18, p = .30). However, in the LL group, time spent viewing positive stimuli only differed from neutral stimuli (effect size r = .57; p < .05). Time spent viewing positive, threat, and dysphoric stimuli did not differ (effect size r ranged from .21 to .38; ps > .35).
Finally, we examined percent time viewing positive stimuli in 5-second segments across the 30-second trial. The SS and SL groups differed for the 5-10 second segment, t(30) = −3.68, p = .001, effect size r = .56, 10-15 second segment, t(30) = −2.64, p = .02, effect size r = .43, and 15-20 second segment, t(30) = −2.06, p = .05, effect size r = .35. Similarly, the SS and LL groups differed at the 5-10, t(18) = −2.33, p = .03, effect size r = .48, and 10-15 second segments, t(18) = −1.82, p = .09, effect size r = .39. No other significant group differences were observed. As can be seen in Figure 2B, SL and LL groups gradually increased time spent viewing positive stimuli, whereas the SS group displayed a strong initial preference for viewing positive stimuli.
Discussion
When multiple sources of information compete for attention, an individual must direct attention to one source over another. Although many factors influence what a person ultimately selects to focus on, we hypothesized that the 5-HTTLPR genotype may affect how a person views emotional stimuli. When presented with an array of images depicting a variety of emotional scenes, short 5-HTTLPR allele homozygotes selectively directed their gaze toward positive stimuli. This tendency was particularly pronounced toward the beginning of the trial (e.g., first 5 to 15 seconds of a 30-second trial). Thus, when faced with a variety of positive and negative images, increased sensitivity to emotional stimuli conferred by the 5-HTTLPR genotype may lead short 5-HTTLPR allele homozygotes to direct their gaze towards positive stimuli.
There is accumulating evidence that the 5-HTTLPR genotype influences neural responses to negative and positive images in brain regions involved in the experience and generation of emotion (for a review, see Hariri & Holmes, 2006). Among short 5-HTTLPR allele homozygotes, heightened reactivity to negative images may be experienced as aversive and increase motivation to avoid negative images, whereas heightened reactivity to positive images may be experienced as pleasant and increase motivation to view positive images. This combined effect may serve to increase selective attention for positive stimuli among short 5-HTTLPR allele homozygotes. Long 5-HTTLPR allele homozygotes, in contrast, may be less reactive to emotional stimuli; therefore, they are less compelled to regulate their emotional responses by selectively attending to positive stimuli. Indeed, although long 5-HTTLPR allele homozygotes differentiated between neutral and emotional images, they did not selectively attend to a particular valence.
It is noteworthy that 5-HTTLPR genotype effects on selective attention were most apparent for short 5-HTTLPR homozygotes. Despite similar putative serotonin reuptake among short 5-HTTLPR allele homozygotes and heterozygotes (Lesch et al., 1996), several studies have found group differences between individuals with one or two copies of the short 5-HTTLPR allele in a variety of domains, including neural responses to emotional images (Surguladze et al., 2008), selective attention for emotional stimuli (Beevers et al., in press), and stress response (Gotlib, Joormann, Minor, & Hallmayer, 2008). This finding is also consistent with previous studies examining the interaction between life stress and depression, where risk for depression among people who have experienced life stress increases with number of short 5-HTTLPR alleles (Caspi et al., 2003; Kendler et al., 2005). Thus, some evidence suggests sensitivity to emotional aspects of the environment may be parametrically associated with number of short 5-HTTLPR alleles. Given this possibility, it seems important to examine 5-HTTLPR genotype groups separately whenever possible. Of course, larger sample sizes will be necessary to perform such analyses with adequate statistical power, which may prove to be more challenging for some areas of investigation, such as neuroimaging, than others.
Although there is ample evidence that the 5-HTTLPR genotype is associated with neural responses to emotional stimuli (Canli et al., 2005; Munafo et al., 2008), it will be important for future research to consider measuring neural and physiological responses to stimuli as eye movements are measured. This would help determine whether short 5-HTTLPR homozygotes are indeed experiencing greater reactivity to negative images and are selectively attending to positive stimuli in an effort to regulate this response. The integration of imaging, physiology, and eye tracking has produced intriguing results in other areas of inquiry (e.g., Dalton et al., 2005), so this may be a beneficial approach for studying associations between the 5-HTTLPR and emotion processing as well.
An important direction for future research will be to determine whether short 5-HTTLPR allele homozygotes are able to selectively attend to positive images when cognitive resources are limited. Consciously directing attention towards positive stimuli presumably requires cognitive effort, and a reduction in cognitive resources may hamper the ability of short 5-HTTLPR homozygotes to turn their attention away from negative stimuli. Indeed, information processing biases are often observed among depression vulnerable people after cognitive resources have been compromised in some fashion (e.g., Wenzlaff & Bates, 1998). Thus, short 5-HTTLPR allele carriers may be particularly prone to process negative information in the context of life stress or other times when cognitive resources are depleted (cf. Beevers, 2005).
Heightened reactivity to emotional stimuli may confer increased sensitivity to emotional aspects of the environment, which may have drawbacks (e.g., depression risk; Caspi et al., 2003; but also see, Risch et al., 2009), but it may also have some benefits. For instance, inheriting a short 5-HTTLPR allele is associated with better interpersonal sensitivity (Fiedorowicz et al., 2007), better work functioning and financial adjustment (Serretti, Mandelli, Lorenzi, & Smeraldi, 2005), and stronger associations between social support and mood state (Kaufman et al., 2004). Thus, short 5-HTTLPR allele homozygotes may be more sensitive to positive and negative aspects of the environment (e.g., social support versus social loss), whereas long 5-HTTLPR allele homozygotes may be less influenced by life circumstances.
Several limitations of this study should be noted. Small sample size is an important limitation, as effects observed in small samples are less likely to be replicated than effects initially observed in large samples (Maxwell, 2004). We interviewed subjects about current and past depression only, and not anxiety or other psychiatric disorders. Neutral images were typically inanimate objects, whereas the emotional images were typically animated. We only examined a single polymorphism, which predicted a relatively small amount of the variance in selective attention. Further, we did not genotype individuals for the single nucleotide polymorphism that occurs at the sixth nucleotide (adenine to guanine; A to G) in the long 5-HTTLPR allele (Wendland, Martin, Kruse, Lesch, & Murphy, 2006). Future work with larger samples sizes should consider examining the long 5-HTTLPR allele polymorphism and a variety of other genes (e.g., BDNF, DRD4) so that interactions among genes can be explored. As with any genetic association study, population stratification is a potential concern. This confound is unlikely as the vast majority of participants were Caucasian and ethnicity was unrelated to selective attention or allele status. Third variable explanations, such as the possibility that the 5-HTTLPR polymorphism is in linkage disequilibrium with another functional genetic marker, should also be considered as alternative explanations for the observed effects.
Despite these limitations, we believe this study makes an important and interesting contribution to how the 5-HTTLPR genotype contributes to biased processing of emotional stimuli. Individuals who inherit two copies of the short 5-HTTLPR allele appear to be more sensitive to emotional information in the environment. As a result, they selectively attend to positive stimuli. This form of selective attention may be possible under conditions of low stress; however, when stimuli are overwhelmingly negative or cognitive resources are depleted so that the ability to turn attention away from negative stimuli is hampered, this sensitivity to emotional stimuli may contribute to increased levels of distress.
Acknowledgments
Preparation of this article was facilitated by a grant (R01MH076897) from the National Institute of Mental Health to Christopher Beevers and shared equipment grants from the National Center for Research Resources (1S10RR023457-01A1) and the Department of Veteran Affairs to John McGeary. The authors thank Jennifer Kellough and the research staff from the Mood Disorders Laboratory at the University of Texas at Austin for their help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The following IAPS stimuli were used in the present study: Dysphoric – 2141, 2205, 2276, 2455, 2700, 2703, 2799, 2900, 3230, 9220, 9421, and 9530; Threat – 1120, 1300, 2811, 3500, 6260, 6312, 6313, 6350, 6510, 6560, 6562, and 6821; Positive – 1340, 2091, 2165, 2208, 2224, 2299, 2339, 2340, 2501, 4599, 4700, and 8461; Neutral – 2038, 2102, 2393, 2397, 2745, 2850, 5500, 5731, 7009, 7041, 7080, and 7185; Neutral filler – 2235, 2396, 2514, 2880, 5390, 5740, 7000, 7004, 7010, 7053, 7090, 7100, 7187, 7235, 7547, and 7950.
References
- Beevers CG. Cognitive vulnerability to depression: A dual process model. Clinical Psychology Review. 2005;25:975. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin Transporter Genetic Variation and Biased Attention for Emotional Word Stimuli Among Psychiatric Inpatients. Journal of Abnormal Psychology. 2007;116:208. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells T, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. doi: 10.1037/a0016198. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2008 doi: 10.1038/nn.2242. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Canli T, Congdon E, Todd Constable R, Lesch KP. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on neural correlates of affective processing. Biological Psychology. 2008;79:118–125. doi: 10.1016/j.biopsycho.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: A role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. PNAS. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Moser DJ, Hynes SM, Beglinger LJ, Schultz SK, Ellingrod VL. LA allelic heterozygosity of the 5HTTLPR polymorphism is associated with higher cognitive function and lower interpersonal sensitivity. Psychiatric Genetics. 2007;17:3–4. doi: 10.1097/YPG.0b013e328010f498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV--Patient version. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Graig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavioral Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of the human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2005;8:20. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Hermans D, Vansteenwegen D, Eelen P. Eye movement registration as a continuous index of attention deployment: Data from a group of spider anxious students. Cognition & Emotion. 1999;13:419. [Google Scholar]
- Herrmann MJ, Huter T, Muller F, Muhlberger A, Pauli P, Reif A, et al. Additive Effects of Serotonin Transporter and Tryptophan Hydroxylase-2 Gene Variation on Emotional Processing. Cerebral Cortex. 2007:1160–1163. doi: 10.1093/cercor/bhl026. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM. Motivated Gaze: The View From the Gazer. Current Directions in Psychological Science. 2006;15:68–72. [Google Scholar]
- Jonides J. Voluntary versus automatic control over the mind's eye movements. In: Long J, Baddeley AD, editors. Attention and performance IX. Guilford; Hillsdale: 1981. pp. 187–203. [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The Interaction of Stressful Life Events and a Serotonin Transporter Polymorphism in the Prediction of Episodes of Major Depression. Archives of General Psychiatry. 2005;62:529. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kowler E. Eye movements. In: Kosslyn SM, Osheron DM, editors. Visual cognition. Harvard University Press; Cambridge: 1995. pp. 215–265. [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Research. 1995;35:1897. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida; Gainsville, FL: 2008. [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behaviour Research & Therapy. 1996;34:695. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Maxwell SE. The Persistence of Underpowered Studies in Psychological Research: Causes, Consequences, and Remedies. Psychological Methods. 2004;9:147. doi: 10.1037/1082-989X.9.2.147. [DOI] [PubMed] [Google Scholar]
- Moray N, Baddeley AD, Weiskrantz L. Attention: Selection, awareness, and control: A tribute to Donald Broadbent. Clarendon Press/Oxford University Press; 1993. Designing for attention. p. 111. [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinsky R, Reuter M, Küpper Y, Schmitz A, Kozyra E, Alexander N, et al. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. Journal of Neuroscience. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, et al. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression: A Meta-analysis. JAMA: The Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Mandelli L, Lorenzi C, Smeraldi E. Social adjustment could be associated with the serotonin transporter gene in remitted patients with mood disorders and healthy subjects. Psychiatry Research. 2005;134:191–194. doi: 10.1016/j.psychres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Elkin A, Ecker C, Kalidindi S, Corsico A, Giampietro V, et al. Genetic variation in the serotonin transporter modulates neural system-wide response to fearful faces. Genes, Brain, and Behavior. 2008;7:543–551. doi: 10.1111/j.1601-183X.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Bates DE. Unmasking a cognitive vulnerability to depression:How lapses in mental control reveal depressive thinking. Journal of Personality and Social Psychology. 1998;75:1559–1571. doi: 10.1037//0022-3514.75.6.1559. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu X.-z., Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]