Abstract
Objective:
TNF-α is an inflammatory cytokine that plays a central role in promoting the cascade of events leading to an inflammatory response. Recent studies have suggested that TNF-α may play a key role in the formation and rupture of cerebral aneurysms, and that the underlying cerebral inflammatory response is a major determinate of outcome following subrarachnoid hemorrhage (SAH).
Methods:
We studied 14 comatose SAH patients who underwent multimodality neuromonitoring with intracranial pressure (ICP) and cerebral microdialysis as part of their clinical care. Continuous physiological variables were time-locked every 8 hours and recorded at the same point that brain interstitial fluid TNF-α was measured in brain microdialysis samples. Significant associations were determined using generalized estimation equations.
Results:
Each patient had a mean of 9 brain tissue TNF-α measurements obtained over an average of 72 hours of monitoring. TNF-α levels rose progressively over time. Predictors of elevated brain interstitial TNF-α included higher brain interstitial fluid glucose levels (β=0.066, P<0.02), intraventricular hemorrhage (β=0.085, P<0.021), and aneurysm size >6 mm (β=0.14, p<0.001). There was no relationship between TNF-α levels and the burden of cisternal SAH; concurrent measurements of serum glucose, or lactate-pyruvate ratio.
Interpretation:
Brain interstitial TNF-α levels are elevated after SAH, and are associated with large aneurysm size, the burden of intraventricular blood, and elevation brain interstitial glucose levels.
Keywords: tumor necrosis factor-α, intraventricular hemorrhage, cerebral inflammatory response, cerebral microdialysis, brain interstitial fluid
Introduction
The pathophysiology of aneurysmal subarachnoid hemorrhage (aSAH) has long remained elusive. The presence and subsequent lysis of red blood cells in the subarachnoid space and cerebral cisterns produces an inflammatory response mediated by pro-inflammatory cytokines (1). This response has been theorized to sequester nitric oxide, resulting in vasospasm and delayed cerebral ischemia or infarction, which is associated with poor outcomes (1, 2). However, strategies aimed solely at improving cerebral blood flow or mitigating vasospasm following aSAH have had limited success in improving overall morbidity or mortality (3-5). This may be explained in part by a perspective that incorporates cerebral vasospasm as but one sequela of a larger cerebral inflammatory response (CIR) occurring throughout the brain. In this view, interactions between neurons, vasculature, and microglia following aneurysm rupture might be altered by a diffuse tissue inflammatory response triggered by the initial bleeding event. (6).
TNF-α is a critical inflammatory cytokine that plays a central role in initiating and promoting the cascade of events leading to an inflammatory response. This includes the accumulation of oxygen radicals, the upregulation of expression of endothelial and leukocyte adhesion molecules, and the recruitment of macrophages and neutrophils (7). Recent studies have suggested that TNF-α levels are elevated in the CSF of SAH (8, 9).
Several studies have attempted to correlate levels of TNF- α in serum and cerebrospinal fluid (CSF) with poor outcome and have arrived at conflicting results (8-12). However, the blood-brain and blood-CSF barriers may be variably disrupted following aSAH, rendering these approaches unreliable in measuring the level of TNF-α directly associated with SAH pathology. By contrast, cerebral microdialysis allows reliable, continuous sampling of intracerebral interstitial fluid. We hypothesized that TNF-α measured within the brain interstitial fluid 4-6 days after aneurysm rupture (SAH day) is proportional to the cerebral inflammatory response. We studied a series of poor-grade aSAH patients to determine what variables were associated with the CIR as determined by TNF-α in brain interstitial fluid.
Subjects and Methods
Patients
We studied 14 SAH patients who were enrolled in the Columbia University SAH Outcomes Project (SHOP) between December 2007 and April 2009. SHOP is a prospective observational cohort study that involves archiving of the medical record and one-year follow-up of neurological, cognitive, and functional outcome. The present analysis was limited to patients who underwent brain multimodality neuromonitoring of intracranial pressure (ICP) and cerebral microdialysis as part of their clinical care as per institutional protocol, with a minimum of 24 hours of high-quality recoverable data. Microdialysis samples were processed hourly for lactate, pyruvate, and glucose in the ICU and used to guide clinical management. TNF-α levels were measured from effluent microdialysis samples stored at −70° C in a post-hoc fashion. SAH days 4-6 were chosen because there is evidence that TNF-α does not change in the serum of aSAH patients from ictus to SAH day 3, but it does increase in the cerebrospinal fluid from SAH days 4-10 (9, 11) This study was approved by the Columbia University Institutional Review Board (IRB).
Clinical Variables
Baseline clinical, demographic, and physiological parameters were recorded on admission to the Columbia University Medical Center neuro-ICU. Clinical status was evaluated by recording the patient's worst Hunt-Hess score during the course of hospitalization, and with Glasgow Coma Scale scores recorded every other hour by the ICU nursing staff.
Radiological Variables
Admission CT scans were independently evaluated by a study neurointensivist for the amount and location of subarachnoid blood (SAH sum score, scaled 0 = no blood, 30 = all cisterns and fissures completely filled), (13) intraventricular blood (IVH sum score, scaled 0 = no blood, 12 = all ventricles completely filled), (14) the presence and degree of hydrocephalus, (15) and the presence of global cerebral edema (2). Bicaudate index was defined as distance between the medial borders of the caudate nuclei divided by the distance between the skull temples (16). Aneurysm size was defined by admission CT angiogram or cerebral angiogram as largest cross-sectional diameter in millimeters.
Physiologic Variables
All physiological variables recorded at the bedside, including heart rate, mean arterial pressure, intracranial pressure (ICP), cerebral perfusion pressure (CPP), bladder temperature, and multimodality data were stored in an SQL database. The Solar 8000i utilizing the General Electric Medical Systems Information Technologies' Unity Network® is the patient physiologic monitor. A high resolution data acquisition system (BedmasterEX, Excel Medical Electronics, Jupiter, FL) uses the open architecture of the Unity Network® to automatically acquire vital signs, alarm, and waveform data from all the patient monitoring devices in the NICU. Digital data is acquired every 5 seconds and recorded in a SQL database. Waveform data is stored at a resolution of 240 Hz in binary files. Brain metabolism data are incorporated into the data acquisition system utilizing the communications (COM) port on the device and is plugged into a serial-to-TCP/IP interface device (Equinox ESP-8, Avocent, Sunrise, FL). All continuous physiological variables, including standard microdialysis variables, were time locked every 8 hours and measured at the same point that brain interstitial fluid TNF-α was measured.
Clinical Management
Patient care for SAH conformed to guidelines established by the American Heart Association (17). Hemodynamic and fluid management was targeted to maintain cerebral perfusion pressure (CPP) >70 mm Hg, unless a higher level was associated with favorable effects on the lactate:pyruvate ratio (goal <40) and intracranial pressure (goal <20 mm Hg). Hemoglobin levels below 7 mg/dL were used to trigger blood transfusion unless there was evidence of active cerebral or myocardial ischemia, in which case a trigger of 10 mg/dl was used. Fever was aggressively treated using surface cooling (Arctic Sun Cooling System, Medivance Inc, Louisville, CO), with shivering controlled using a stepwise institutional protocol (18).
Cerebral Microdialysis
A CMA 106 microdialysis perfusion pump (CMA Microdialysis®) was used to perfuse the interior of the catheter with sterile artificial cerebro-spinal fluid (Na+ 148 mmol/L, Ca2+ 1.2 mmol/L, Mg2+ 0.9 mmol/L, K+ 2.7 mmol/L, Cl− 155 mmol/L) at a rate of 0.3 μl/min. Samples were colle cted every 60 min into microvials, and immediately analyzed at the bedside for glucose, lactate and pyruvate (mmol/L) with the CMA 600 analyzer (CMA Microdialysis®). At least 1 hour passed after the insertion of the probe and the start of the sampling, to allow for normalization of changes due to probe insertion. The analyzer was automatically calibrated on initiation and every six hours using standard calibration solutions from the manufacturer. Quality controls at three different concentrations for each marker were performed daily.
Measurement of TNF-α
A detailed description of this procedure can be found elsewhere (19). In short, high capacity 96 well plates were coated with 100 μl/well of capture antibody (human TNF-α antibody) and left at 4°C overnight (eBioscience Inc, San Diego, CA, USA). 10μl of brain interstitial fluid, or microdialysate, were diluted into 90μl of Assay diluent, and incubated on the plate overnight at 4°C. 100 μl of biotin-tagged TNF-α antibody/well was then added and incubated for 1 hour at room temperature. Then 100 μl/well of Avidin HRP was added and incubated at room temperature for 30 minutes. Then substrate solution from eBioscience kit followed by stop solution were added. The plate was read on a plate reader at 450 nm. Purified human TNF-α was used to create a standard curve.
Calculation of TNF-α Recovery from Microdialysis
Purified human TNF-α was added to 10 ml of artificial cerebrospinal fluid to make four concentrations of TNF- α: 1000 pg/ml, 2000 pg/ml, 3000 pg/ml, and 4000 pg/ml. A CMA 106 microdialysis perfusion pump (CMA Microdialysis®) was used to perfuse the interior of the catheter with artificial cerebrospinal fluid at a rate of 0.3 μl/min with different concentrations of TNF- α. One sample was collected over 1 hour for each different TNF-α concentration and this was done in triplicate. TNF-α concentration was measured as described above. Fractional recovery was calculated by plotting actual TNF-α concentration in the Petri dish versus recovered TNF-α concentration in microdialysate, and found to be 4.5 %.
Statistical Analysis
Continuous variables were assessed for normality with skewness and kurtosis. Data that was not normally distributed was reported as medians with inter-quartile ranges. Data that was normally distributed was reported with means and standard deviations. Categorical variables were reported as count and proportions in each group. Grouping was based on clinical significance or median versus mean, depending on normality. To study associations between brain interstitial TNF-α and other variables, a generalized estimating equation (GEE) (20), accounting for between-subject and within-subject variation, was used. To study the association between brain interstitial fluid TNF-α and different demographic, physiological, and radiographic variables; we performed a univariate analysis using GEE with an AR1 (auto-regressor 1) function. For this analysis, the TNF-α response was studied as a continuous variable that was log transformed, because TNF-α was not normally distributed. All variables listed in tables 1 and 2 were tested for significance using a univariate GEE model. The β, Wald-Chi square, and p values are reported for all predictor variables. All statistical analyses were performed using SPSS 16 software (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Table 1.
Patient Characteristics (N=14)
| Demographic Variables: | ||
| Age (years) | 48 (34-59) | |
| Female | 10 (71) | |
| Caucasian | 6 (43) | |
| BMI (kg/m2) | 24 (23-28) | |
| Past Medical History of | ||
| Hypertension | 2 (14) | |
| Diabetes Mellitus | 1 (7) | |
| Clinical Variables: | ||
| Worst Hunt Hess Grade | ||
| 4 stupor/moderate hemiparesis | 1 (7) | |
| 5 coma/decerebrate posturing | 13 (93) | |
| APACHE II subscore* | 14 (11-17) | |
| GCS | 7 (5-13) | |
| Physiological Variables during monitoring: | ||
| GCS | 6 (4-8) | |
| Temperature (°C) | 37 (34.4-37.3) | |
| Systemic Glucose (mg/dl) | 138 (113-171) | |
| Cerebral Perfusion Pressure (mmHg) | 84 (74-103) | |
| Intracranial Pressure (cm H2O) | 10 (6-13) | |
| White Blood Cell Count (×103 cells/mm3) | 13 (10.8-17.1) | |
| Radiographic variables on Admission: | ||
| Bicaudate index16 | 0.17 (0.14-0.19) | |
| Occurrence of IVH | 12 (86) | |
| HIJDRA score13 | 18 (12-24) | |
| Global Cerebral Edema | 6 (43) | |
| Aneurysm size (mm) | 6 (5.2-8.3) | |
Data are given as N (%) or Median (25-75% IQR)
An ICU mortality prediction ranging from 0-125 that incorporates physiological derangements, age, and chronic health problems and excludes GCS
Table 2.
Microdialysis Characteristics
| Median | Normal range | IQR (25-75%) | |
|---|---|---|---|
| Microdialysis Variables: | |||
| Lactate (mmol/L) | 3.7 | (2.0-3.8) | (2.4-4.4) |
| Pyruvate (μmol/L) | 103 | (119-213) | (84-162) |
| Lactate:Pyruvate ratio (LPR) | 28.3 | (19-27) | (23.1-33.1) |
| Glucose (mmol/L) | 1.12 | (0.8-2.6) | (0.71-1.92) |
| Tumor Necrosis Factor-α (pg/ml) | 753.1 | (372.1-1100.5) |
N=91 measurements of all microdialysis values
Results
Study Cohort
Median age of these patients was 48 years old with the 25th percentile at 34 years old and 75th percentile at 59 years old, 43% (n=6) were Caucasian, and 71% (n=10) were female. 21% (n=3) of these patients were admitted with a good clinical grade (Hunt-Hess grade 1 to 3), however all these patients progressed to a Hunt-Hess grade 4 or 5 (stupor or coma) and required mechanical ventilation during their hospitalization (Table 1). The larger aneurysms (>6 mm) were found in the internal carotid (14%), the middle cerebral artery (14%), the anterior cerebral artery (14%), and the vertebral artery territories (7%). The smaller aneurysms were found in the internal carotid (14%), the middle cerebral artery (7%), and the anterior cerebral artery territories (28%).
Microdialysis Characteristics
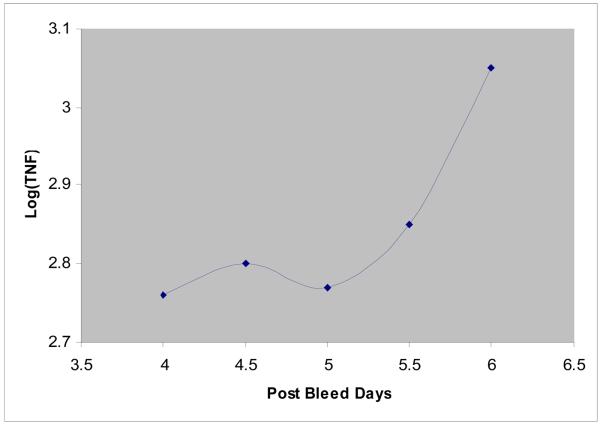
Median values for lactate, pyruvate, lactate:pyruvate ratio, glucose, and TNF-α are shown (Table 2). The microdialysis values were derived from 91 measurements of 14 patients over 3 days (SAH days 4-6). Four different concentrations were used to calculate the recovery of TNF-α in the microdialysate. From this, the actual concentration of TNF-α in the brain interstitial fluid was calculated. The brain interstitial TNF-α levels are averaged for all 14 patients at each of the 9 measured time points during the 72 hour monitoring period (Figure 1).
Figure 1.
mean of log TNF-α between SAH days 4-6
Predictors of TNF-α as a Linear Variable
There were no significant demographic predictors of TNF-α in this cohort of 14 patients. Of all the continuous physiological variables collected between SAH days 4-6, systemic glucose (β=0.005, p=0.063) approached significance, while brain interstitial fluid glucose (β=0.066, p=0.018) was significantly associated with TNF-α. All infusions were tested for significant associations with TNF-α, and none were significant, including insulin. The intraventricular sum score dichotomized patients into those with intraventricular hemorrhage and those without was a significant predictor of the TNF-α response (p=0.021, β=0.085). However, the most significant predictor of the TNF-α response on univariate analysis was aneurysm size (p<0.001), dichotomized at the median of 6 mm (Table 3). This is evident by the largest values for Wald-Chi square, 21.2 and β, 0.14.
Table 3.
Significant Predictors of TNF-α by GEE
| β | Wald Chi square |
p | |
|---|---|---|---|
| Demographic variables: | |||
| Age (>48years) | −0.069 | 2.18 | 0.14 |
| Female | −0.096 | 2.86 | 0.091 |
| Caucasian | 0.031 | 0.028 | 0.99 |
| BMI (kg/m2) | −0.003 | 0.95 | 0.33 |
| Past medical History of: | |||
| -Hypertension | 0.039 | 0.88 | 0.35 |
| -Diabetes Mellitus | 0.039 | 0.88 | 0.35 |
|
Admission Clinical Variables: |
|||
| Hunt Hess grade | 0.11 | 3.57 | 0.59 |
| Apache II subscore | 0.001 | 0.011 | 0.92 |
| GCS | −0.012 | 2.64 | 0.1 |
|
Continuous Variables during monitoring (q8): |
|||
| GCS | −0.012 | 1.33 | 0.25 |
| Temperature (°C) | 0.004 | 0.031 | 0.86 |
| Systemic glucose (mg/dl) | 0.005 | 3.46 | 0.063 |
| Cerebral Perfusion Pressure (mmHg) |
−0.005 | 0.685 | 0.41 |
| Intracranial Pressure (cm H2O) | 0.001 | 0.14 | 0.71 |
| White Blood Cell count (× 103cells/mm3) |
0.001 | 0.01 | 0.92 |
| MD Lactate (mmol/L) | −0.045 | 2.47 | 0.12 |
| MD Pyruvate (μmol/L) | 0.001 | 0.57 | 0.45 |
| MD LPR | −0.008 | 5.15 | 0.06 |
| MD Glucose (mmol/L) | 0.066 | 5.63 | 0.018 |
| Radiographic Variables: | |||
| Bicaudate Index | −0.43 | 0.14 | 0.71 |
| Existence of IVH | 0.085 | 5.33 | 0.021 |
| HIJDRA score | 0.006 | 0.71 | 0.93 |
| Global Cerebral Edema | 0.03 | 0.115 | 0.74 |
| Aneurysm Size (>6 mm) | 0.14 | 21.2 | <0.001 |
Discussion
In this study we found a progressive rise in brain interstitial TNF-α levels between SAH days 4 to 6. Large aneurysm size, the existence of intraventricular hemorrhage, and increased levels of glucose in the microdialysate were significantly associated with TNF-α elevation. The fact that TNF-α levels increase throughout SAH days 4-6 may provide an explanation for the cumulative risk of complications that occur after ictus. TNF-α levels may correlate with late complications of aSAH such as delayed cerebral infarction (DCI). There is growing evidence that DCI involves a progressive inflammatory burden that is independent of vasospasm. This is supported by the fact that clinical trials directed at ameliorating vasospasm have not resulted in improved outcomes (4, 5). Furthermore, current theories behind the evolution of DCI invoke impaired fibrinolytic activity, an inflammatory cascade, and endothelial dysfunction in the formation of microthrombi, all of which would likely involve TNF-α (21-24).
We found that large aneurysm size correlates with increasing TNF-α levels and there is both animal and human data that support this notion. When SAH is achieved by direct puncture of the ICA in rats, causing damage to vascular wall, blood and inflammatory mediators are released that result in a 40-50% mortality, similar to the vascular damage caused by an aneurysm (25, 26). In humans, non-aneurysmal SAH, like perimesencephalic and traumatic SAHs, are associated with DCI less frequently than aSAH (27-31). The fact that aneurysm size was shown to be associated with increasing TNF-α seems to be in support of our findings.
Even though the heme burden does not explain all the pathology seen in aSAH, it clearly is an important component to the pathology. Animal models of intraventricular hemorrhage (IVH) where blood is injected into the ventricles show a highly inflammatory pattern in the periventricular space (32, 33). In addition to this, the effect of IVH was studied in the aSAH population and shown to be an independent predictor of vasospasm and poor outcome (34, 35). Consistent with this, we found intraventricular hemorrhage to be associated with increased TNF-α.
Finally, we found brain interstitial fluid glucose to be significantly associated with increasing TNF-α in the brain. There is evidence to support hyperglycemia in the setting of an inflammatory response and that admission hyperglycemia is associated with poor outcome (36, 37). The correlation between brain interstitial fluid glucose and increasing TNF-α is preliminary, but is in accord with the increased insulin resistance seen in any inflammatory response. Furthermore, there is recent evidence that in addition to TNF-α being involved in growth and rupture of aneurysms (7, 8, 38, 39), the PPAR-γ (peroxisome proliferator-activated receptor) transduction pathway is similarly involved (40). When PPAR-γ agonists such as rosiglitazone were given to mice that spontaneously develop thoraco-abdominal aortic aneurysms, the incidence of aneurysm formation and rupture significantly decreased compared to controls. In addition, the expression of TNF-α, IL-6, and E-selectin were markedly reduced (40). As rosiglitazone is an insulin sensitizer, one would expect decreased serum glucose in this population too, even though this was not the case. Therefore, it is possible, that in the process of cerebral aneurysm formation and rupture, the PPAR-γ pathway is somehow inhibited, resulting in hyperglycemia and increased TNF-α levels. While this argument is derived from few data, it does provide a novel perspective of study of the cerebral inflammatory response after aSAH as it relates to the PPAR-γ pathway.
To our knowledge, this is the first study of the brain interstitial fluid TNF-α in patients after aneurysmal subarachnoid hemorrhage. With this in mind, there are several important limitations that can be addressed in future studies with a larger cohort of patients. Our patient pool was small and most patients were poor grade on admission. Second, defining the cerebral inflammatory response by TNF-α levels alone, is likely not a complete description of the inflammatory cascade occurring within the brain. TNF-α was chosen for two reasons. First, it is associated with almost all inflammatory cascades that have been described (41). Second, of all inflammatory mediators studied, TNF-α, had the most data in the aSAH population. In fact, SAH days 4-6 were chosen because there is evidence that TNF-α does not change in the serum of aSAH patients from ictus to SAH day 3, but it does increase in the cerebrospinal fluid from SAH days 4-10 (9, 11). We hope that this study, although preliminary in findings, will lead to further research of the cerebral inflammatory response in aneurysmal SAH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumont AS, D R, Chow MM, Lin CL, Calisaneller T, Ley KF, Kassell NF, Lee KS. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:133–5. doi: 10.1227/01.neu.0000068863.37133.9e. [DOI] [PubMed] [Google Scholar]
- 2.Claassen J, C J, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–32. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]
- 3.Haley EC, Jr, K N, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–74. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- 4.Pickard JD, M G, Illingworth R, Shaw MD, Teasdale GM, Foy PM, Humphrey PR, Lang DA, Nelson R, Richards P, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298:636–42. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vajkoczy P, M B, Weidauer S, Raabe A, Thome C, Ringel F, Breu V, Schmiedek P. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- 6.Cahill J, C J, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 7.Jayaraman T, P A, Shin YS, Li X, Mayer J, Chaudhry H, Niimi Y, Silane M, Berenstein A. TNF-alpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4:805–17. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki T, K H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. 2007;116:2830–40. doi: 10.1161/CIRCULATIONAHA.107.728303. [DOI] [PubMed] [Google Scholar]
- 9.Mathiesen T, E G, Ulfarsson E, Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. J Neurosurg. 1997;87:215–20. doi: 10.3171/jns.1997.87.2.0215. [DOI] [PubMed] [Google Scholar]
- 10.Fassbender K, H B, Rossol S, Bertsch T, Schmeck J, Schütt S, Fritzinger M, Horn P, Vajkoczy P, Kreisel S, Brunner J, Schmiedek P, Hennerici M. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001;70:534–7. doi: 10.1136/jnnp.70.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witkowska AM, B M, Socha K, Kochanowicz J, Mariak Z, Konopka M. TNF-alpha and sICAM-1 in intracranial aneurismal rupture. Arch Immunol Ther Exp (Warsz) 2009;57:137–40. doi: 10.1007/s00005-009-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber A, R K, Graninger W, Donner A, Illievich MU, Czech T. Ventricular cerebrospinal fluid and serum concentrations of sTNFR-I, IL-1ra, and IL-6 after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2000;12:297–306. doi: 10.1097/00008506-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Hijdra A, vG J, Nagelkerke NJ, Vermeulen M, van Crevel H. Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1250–6. doi: 10.1161/01.str.19.10.1250. [DOI] [PubMed] [Google Scholar]
- 14.Brouwers PJ, D D, Vermeulen M, Lindsay KW, Hasan D, vG J. Amount of blood on computed tomography as an independent predictor after aneurysm rupture. Stroke. 1993:809–14. doi: 10.1161/01.str.24.6.809. [DOI] [PubMed] [Google Scholar]
- 15.van Gijn J, H A, Wijdicks EF, Vermeulen M, van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985:355–62. doi: 10.3171/jns.1985.63.3.0355. [DOI] [PubMed] [Google Scholar]
- 16.Vermeij FH, H D, Vermeulen M, Tanghe HL, van Gijn J. Predictive factors for deterioration from hydrocephalus after subarachnoid hemorrhage. Neurology. 1994;44(10):1851–5. doi: 10.1212/wnl.44.10.1851. [DOI] [PubMed] [Google Scholar]
- 17.Bederson JB, CE J, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 18.Badjatia N, S E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, M S. Metabolic benefits of surface counter warming during therapeutic temperature modulation. Crit Care Med. 2009;37:1893–7. doi: 10.1097/CCM.0b013e31819fffd3. [DOI] [PubMed] [Google Scholar]
- 19.C. JR. ELISA. Theory and practice. Methods Mol Biol. 1995:1–218. doi: 10.1385/0-89603-279-5:1. [DOI] [PubMed] [Google Scholar]
- 20.Hilbe JHaJ. Generalized Estimation Equations. Chapman and Hall; Boca Raton, FL: 2003. [Google Scholar]
- 21.Frijns CJ, F R, Algra A, van Mourik JA, van Gijn J, Rinkel GJ. Early circulating levels of endothelial cell activation markers in aneurysmal subarachnoid haemorrhage: associations with cerebral ischaemic events and outcome. J Neurol Neurosurg Psychiatry. 2006:77–83. doi: 10.1136/jnnp.2005.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peltonen S, J S, Kaste M, Lassila R. Hemostasis and fibrinolysis activation after subarachnoid hemorrhage. J Neurosurg. 1997;87:207–14. doi: 10.3171/jns.1997.87.2.0207. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M, K A, Otawara Y, Hirashima Y, Takaku A, Ogawa A. Extrinsic pathway of blood coagulation and thrombin in the cerebrospinal fluid after subarachnoid hemorrhage. Neurosurgery. 1999;44:487–93. doi: 10.1097/00006123-199903000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Hirashima Y, N S, Endo S, Kuwayama N, Naruse Y, Takaku A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem Res. 1997;22:1249–55. doi: 10.1023/a:1021985030331. [DOI] [PubMed] [Google Scholar]
- 25.Adams HP, Jr, K N, Torner JC, Nibbelink DW, Sahs AL. Early management of aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1981;54:141–5. doi: 10.3171/jns.1981.54.2.0141. [DOI] [PubMed] [Google Scholar]
- 26.Bederson JB, G I, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–91. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 27.Cánovas D, G A, Jato M, Rubio F. Non-aneurysmal subarachnoid hemorrhage: 60 cases. Neurologia. 2006;21:704–9. [PubMed] [Google Scholar]
- 28.Fukuda T, H M, Ito H. Does traumatic subarachnoid hemorrhage caused by diffuse brain injury cause delayed ischemic brain damage? Comparison with subarachnoid hemorrhage caused by ruptured intracranial aneurysms. Neurosurgery. 1998;43:1040–9. doi: 10.1097/00006123-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Hui FK, T L, Tanaka T, Cawley CM, Zhang YJ. Clinical Differences Between Angiographically Negative, Diffuse Subarachnoid Hemorrhage and Perimesencephalic Subarachnoid Hemorrhage. Neurocrit Care. 2009;11:64–70. doi: 10.1007/s12028-009-9203-2. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, P J, Lee SH, Park SH, Kim YS, Hamm IS. Does non-perimesencephalic type nonaneurysmal subarachnoid hemorrhage have a benign prognosis? J Clin Neurosci. 2009;16:904–8. doi: 10.1016/j.jocn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Shigemori M, T T, Hirohata M, Maruiwa H, Kaku N, Kuramoto S. Clinical significance of traumatic subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 1990;30:396–400. doi: 10.2176/nmc.30.396. [DOI] [PubMed] [Google Scholar]
- 32.Pang D, S R, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1-3. Neurosurgery. 1986;19:540–73. doi: 10.1227/00006123-198610000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Xi G, K R, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371–83. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 34.Claassen J, B G, Kreiter K, Bates J, Du YE, Copeland D, Connolly ES, Mayer SA. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001;32:2012–20. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 35.Kramer AH, H M, Nathan B, Gress D, Dumont AS, Kassell NF, Bleck TP. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg. 2008;109:199–207. doi: 10.3171/JNS/2008/109/8/0199. [DOI] [PubMed] [Google Scholar]
- 36.Brierre S, K R, Deboisblanc BP. The endocrine system during sepsis. Am J Med Sci. 2004;328:238–47. doi: 10.1097/00000441-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Claassen J, V A, Kreiter KT, Kowalski RG, Du EY, Ostapkovich N, Fitzsimmons BF, Connolly ES, Mayer SA. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32:832–8. doi: 10.1097/01.ccm.0000114830.48833.8a. [DOI] [PubMed] [Google Scholar]
- 38.Fontanella M, R I, Gallone S, Rubino E, Fenoglio P, Valfrè W, Garbossa D, Carlino C, Ducati A, Pinessi L. Tumor necrosis factor-alpha gene and cerebral aneurysms. Neurosurgery. 2007:668–72. doi: 10.1227/01.NEU.0000255417.93678.49. [DOI] [PubMed] [Google Scholar]
- 39.Jayaraman T, B V, Li X, Mayer J, Silane M, Shin YS, Niimi Y, Kiliç T, Gunel M, Berenstein A. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery. 2005;57:558–64. doi: 10.1227/01.neu.0000170439.89041.d6. [DOI] [PubMed] [Google Scholar]
- 40.Jones A, D R, Torsney E, Howe F, Dunkley M, Gnaneswaran Y, Gaze D, Nasr H, Loftus IM, T M, Cockerill GW. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation. 2009;119(24):3125–32. doi: 10.1161/CIRCULATIONAHA.109.852467. [DOI] [PubMed] [Google Scholar]
- 41.C. M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]