Abstract
The Small Integrin-Binding LIgand, N-linked Glycoprotein (SIBLING) family is one category of non-collagenous proteins closely related to osteogenesis. In this study, the authors systematically evaluated the presence and distribution of four SIBLING family members, dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP) and osteopontin (OPN), in rat mandibular condylar cartilage using protein chemistry and immunohistochemistry. For protein chemistry, SIBLING proteins in the dissected condylar cartilage were extracted with 4 M guanidium-HCl, separated by ion-exchange chromatography, and analyzed by Western immunoblotting. Immunohistochemistry was employed to assess the distribution of these four SIBLING proteins in the condylar cartilage of 2-, 5- and 8-week-old rats. Results from both approaches showed that all four members are expressed in the condylar cartilage. DSPP, unlike that observed in dentin and bone, exists as a full-length form (uncleaved) in the condylar cartilage. The NH2-terminal fragment of DMP1 is mainly detected in the matrix of the cartilage while the COOH-terminal fragment is primarily localized in the nuclei of cells in the chondroblastic and hypertrophic layers. The data obtained in this investigation provide clues about the potential roles of these SIBLING proteins in chondrogenesis.
Keywords: condylar cartilage, temporomandibular joint, dentin sialophosphoprotein, dentin matrix protein 1, bone sialoprotein, osteopontin
Mandibular condylar cartilage is categorized as an articular cartilage, but is distinguished from the articular cartilages of the long bones in many aspects, such as embryonic origin, ontogenetic development, post-natal growth mode and histological structure33. As an important growth site for the mandible, the condylar cartilage is divided into five layers: articular, prechondroblastic, chondroblastic, hypertrophic and cartilage–bone interface14. In the condylar cartilage of rat, cell proliferation and extracellular matrix (ECM) production contribute to the growth of articular cartilage and subarticular layers. In addition to collagen and water, the ECM of the condylar cartilage contains a number of non-collagenous proteins (NCPs) and proteoglycans. The Small Integrin-Binding LIgand, N-linked Glycoprotein (SIBLING) family is one category of NCPs, which includes dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), osteopontin (OPN), and matrix extracellular phosphoglycoprotein (MEPE). Principally found in mineralized tissues, SIBLING family members are thought to play important roles in the formation of bone and dentin16.
DSPP, originally thought to be tooth-specific, is now found in the bone, cementum and some non-mineralized tissues at a remarkably lower level than in the dentin5, 8, 10, 19, 25. In the ECM of dentin, DSPP is present as proteolytically processed fragments, dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), which originate from the NH2-terminal and COOH-terminal regions of the DSPP amino acid sequence, respectively19. While DSP and DPP are abundant in the ECM of dentin26, 37, the intact, full-length form, representing the whole sequence of DSPP, has never been identified. Gene ablation experiments in mice have demonstrated that DSPP and/or its processed fragments (DSP and DPP) are critical for the mineralization of dentin35. The exact mechanisms by which DSPP functions in biomineralization are unknown.
DMP1 is an acidic phosphoprotein predominantly expressed in dentin, bone and cementum 13, 18. A lower level of expression for this protein has also been found in non-mineralized tissues such as the brain, kidney, pancreas, and salivary glands36. Like DSPP, DMP1 in the ECM of bone and dentin mainly occurs as proteolytically processed 37 kDa fragments and 57 kDa fragments, which originate from the NH2-terminal and COOH-terminal regions of the DMP1 amino acid sequence, respectively27. Recently, the full-length form of DMP1 has been identified in the ECM of rat dentin and bone, the concentration of which is considerably lower than its processed fragments in these two tissues15. The importance of DMP1 for dentin and bone mineralization has been demonstrated by gene ablation experiments in mice38, 39 and in human genetic mutation studies 9.
Bone sialoprotein is primarily found in bone, mineralizing cartilage, cementum, and reactionary dentin12, 23. The biological functions of BSP in mineralized tissues are largely unknown. Some data suggest that BSP acts as a nucleator for the formation of initial apatite crystals, and then, as this mineral grows on the collagen matrix, it acts as an inhibitor in directing the growth of the crystals12, 28.
OPN is present in relatively large quantities in non-mineralized tissues as well as mineralized tissues. In mineralized tissues, OPN is expressed in bone, cementum, predentin, and tertiary dentin 23, 28, 34. In vitro and in vivo studies show that OPN is an effective inhibitor of apatite formation and growth 3, 4, 34.
There is a large body of information about the biochemical properties and tissue expression of SIBLING family members in bone and dentin but little is known about these molecules, especially DSPP and DMP1, in the mandibular condylar cartilage. The main objectives of this investigation are to evaluate the presence or absence of the four SIBLING members and their relative expression level in the condylar cartilage of the rat mandible, and the difference in the expression pattern of these SIBLING family members and/or their processed fragments in association with the anatomical structure of the cartilage during postnatal growth. In this study, using protein chemistry and immunohistochemistry approaches the authors detected DSPP, DMP1, BSP and OPN in the rat condylar cartilage, which show remarkable variations between different layers of cartilage and at different ages. These findings provide novel information and clues about the potential roles of these molecules in the chondrogenesis of condylar cartilage and osteogenesis of the mandibular ramus.
Materials and Methods
Tissue acquisition/extraction of NCPs
Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) aged 2, 5, 8 and 12 weeks were used in this study. Mandibular condylar cartilage tissues from the 2-, 5- and 8-week-old rats were used for immunohistochemistry (IHC) staining while those from 12-week-old rats were used for protein chemistry analysis. The animal protocol was approved by the Animal Welfare Committee of Baylor College of Dentistry of the Texas A & M University System Health Science Center.
Extraction and separation of NCPs of the mandibular condylar cartilage
Twenty 12-week-old rats (40 mandibular condyles) were used for the extraction of NCPs. The condylar cartilage was carefully separated at the cartilage–bone interface under a dissecting microscope. The procedure for protein extraction and separation from the cartilage was similar to that routinely employed in the laboratory for extracting proteins from bone and dentin24. Briefly, the condylar cartilage was placed in 4 M guanidium-HCl (Gdm-HC; Acros Organics, Fairlawn, NJ, USA) solution (pH 7.2) containing proteinase inhibitors for 48 h. This procedure extracts NCPs (including the SIBLING family members) from the unmineralized phase (i.e. the condylar cartilage). NCPs in the mineralized phase cannot be extracted by Gdm-HCl without demineralization reagents. This protocol ensures that if there is a minor amount of contaminating mineralized tissue (mineralized cartilage or bone), the NCPs from the mineralized phase will not contaminate the samples from the non-mineralized cartilage. The Gdm-HCl extracts were subjected to Q-Sepharose (Amersham Biosciences, Uppsala, Sweden) ion-exchange chromatography with a gradient ranging from 0.1 to 0.8 M NaCl in a 6 M urea solution of pH 7.2. Acidic proteins from this extraction were eluted into sequential fractions, each in 1.0 ml of 6 M urea solution. Each separated chromatographic fraction in 6 M urea solution was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Stains-All staining and Western immunoblotting were carried out to evaluate the presence of DSPP, DMP1, BSP, OPN and their processed fragments.
SDS-PAGE and Western immunoblotting
For SDS-PAGE, 5–15% gradient gels were used in all the experiments. 60 μl of sample from each chromatographic fraction was loaded onto the gels. Stains-All staining was used for detecting the ECM proteins eluted from ion-exchange chromatography. For Western immunoblotting detection of DSPP/DSP, an anti-DSP polyclonal antibody5 (Table 1) was used at a dilution of 1:3000 in blocking buffer. For detection of DMP1, two types of anti-DMP1 antibodies were used. One was a monoclonal antibody generated using the NH2-terminal fragment of rat bone DMP1 as the antigen (designated as anti-DMP1-N-9B6.3, immunoreactive to the NH2-terminal region of DMP1)30 and was used at a dilution of 1:1000. The other was a polyclonal antibody referred to as anti-DMP1-C-857, which recognizes the COOH-terminal region of DMP115 and was used at a dilution of 1:2000. For detection of BSP, a monoclonal anti-BSP antibody known as anti-BSP-10D9.216 was used at a dilution of 1:2000. For detection of OPN, an anti-OPN polyclonal antibody24 was used at a dilution of 1:2000. Blots were washed three times in PBS containing 0.3% tween-20, and followed by incubation in secondary antibody with a 1:5000 dilution of alkaline phosphate-conjugated anti-mouse IgG or anti-rabbit IgG (Sigma-Aldrich, Louis, MO, USA). The blots were incubated in the chemiluminescent substrate CDP-star (Ambion, Austin, TX, USA) for 5 min and exposed to X-ray films.
Table 1. Antibodies used in this study.
| Antibody | Antibody type | Immunizing antigen | Immunoreactivity in Western blotting | Immunoreactivity in immunohistochemistry |
|---|---|---|---|---|
| Anti-DSP-2C12.3a | Monoclonal | Purified rat dentin DSP | No | Yes |
| Anti-DSPb | Polyclonal | Purified rat dentin DSP | Yes | Yes |
| Anti-DMP1-N-9B6.3c | Monoclonal | 37 kDa (N-terminal) | Yes | Very weak |
| Anti-DMP1-N-859d | polyclonal | Oligopeptide (residues 101-121) | Yes | Yes |
| Anti-DMP1-C-8G10.3e | Monoclonal | 57 kDa (C-terminal) | No | Yes |
| Anti-DMP1-C-857f | Polyclonal | Oligopeptide (residues 471-485) | Yes | Yes |
| Anti-BSP-10D9.2g | Monoclonal | Purified Rat bone BSP | Yes | Yes |
| Anti-OPNh | Polyclonal | Recombinant rat OPN | Yes | Yes |
This monoclonal antibody1 was used to detect DSP by immunohistochemistry analysis.
This polyclonal antibody5 was used to detect DSP by Western immunoblotting.
This monoclonal antibody30 was used to detect the NH2-terminal fragment of DMP1 by Western immunoblotting analysis.
This polyclonal antibody21 was used to detect the NH2-terminal fragment of DMP1 by immunohistochemistry and immunofluorescence analysis.
This monoclonal antibody2 was used to detect the COOH-terminal fragment of DMP1 by immunohistochemistry and immunofluorescence analysis.
This polyclonal antibody15 was used to detect the COOH-terminal fragment of DMP1 by Western immunoblotting analysis.
This monoclonal antibody16 was used to detect BSP by immunohistochemistry analysis and Western immunoblotting.
This polyclonal antibody24 was used to detect OPN by immunohistochemistry analysis and Western immunoblotting.
Immunohistochemistry (IHC)
IHC was performed to analyze the difference in the expression and distribution pattern of these SIBLING proteins in the mandibular condylar cartilage from 2-, 5- and 8-week-old rats. Under anesthesia, Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) at the above ages were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer. The mandibles were dissected and further fixed in the same fixative for 48 h, followed by decalcification in 8% EDTA (pH 7.4) at 4°C for approximately 4 weeks. Tissues were processed for paraffin embedding, and serial 5 μm sections were prepared. For immunohistochemical detection of DSPP/DSP: the anti-DSP monoclonal antibody designated as anti-DSP-2C12.31 (Table 1) was used at a dilution of 1:200. For DMP1, two types of antibodies were used at a dilution of 1:300: a monoclonal antibody referred to as anti-DMP1-C-8G10.32, which specifically recognizes the COOH-terminal fragment of DMP1 in IHC; the other was a polyclonal antibody known as anti-DMP1-N-85921, which recognizes the NH2-terminal fragment of DMP1. For BSP and OPN, antibodies were identical to those used in Western immunoblotting, and the dilution was 1:200. All IHC experiments were carried out using ABC kit and DAB kit (Vector Laboratories Inc, Burlingame, CA, USA), following the manufacturer's instructions.
Immunofluorescence analysis of DMP1 fragments
A double immunofluorescent staining approach was used to detect the location differences of DMP1 fragments in the condylar cartilage of rat mandible. The sections were first incubated with a mixture of the two primary antibodies, anti-DMP1-N-859 and anti-DMP1-C-8G10.3 (each diluted at 1:400), which recognize the NH2-terminal and COOH-terminal regions of DMP1, respectively. The sections were incubated with goat anti-rabbit F (ab')2 fragment conjugated with Alexa 488 (green) and goat anti-mouse F(ab')2 fragment conjugated with Alexa 546 (red, both from Invitrogen, Camarillo, CA, USA) at a dilution of 1:600. Nuclei were counterstained with TO-PRO-3 (Invitrogen, California, USA) at a dilution of 1:500 for 5 min to stain the nuclei (for blue color). After mounting with Slowfade, the images were captured and analyzed with a Leica TCS-SP2 confocal microscope (Heidelberg, Germany).
Table 1 is a summary of the antibodies used in this study. Detailed information for all of these antibodies can be found in the authors' previous publications1, 2, 5, 15, 16, 21, 24, 30.
Results
DSPP, DMP1, BSP and OPN in the Gdm-HCL extracts of the condylar cartilage
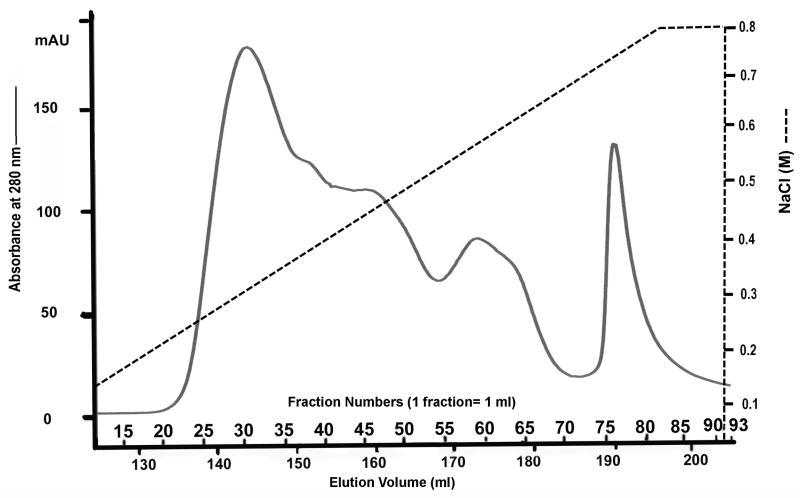
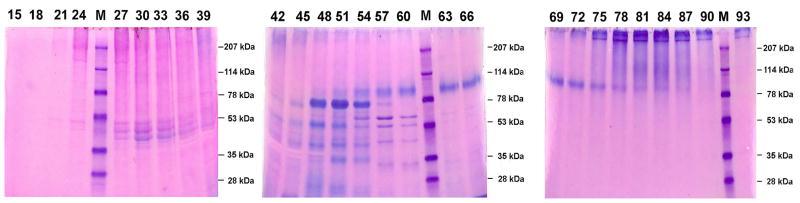
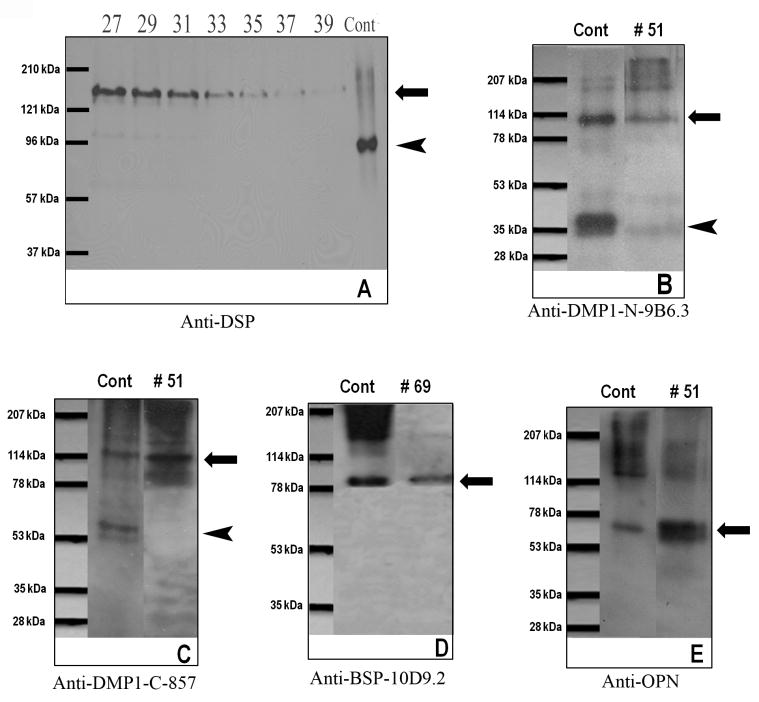
Q-Sepharose ion-exchange chromatography separated Gdm-HCl extracts of the cartilage into 120 fractions (Fig. 1). Each of the chromatographic fractions that might potentially contain any of the four SIBLING family members was assayed by Stains-All staining and Western immunoblotting. In this report, the Stains-All staining of fractions 15 through 93 is presented (Fig. 2) to illustrate the results. To show the identity of DSPP/DSP, Western immunoblotting of fractions 27 through 39 is shown while for that of the other three SIBLING family members, Western immunoblotting of one representative fraction is shown (Fig. 3).
Fig. 1.
Separation of NCPs extracted from the rat mandibular condylar cartilage. Q-Sepharose ion-exchange chromatography separated NCPs extracted from rat mandibular condylar cartilage into 120 fractions. Each fraction contained 1 ml of 6 M urea solution.
Fig. 2.
Stains-All staining for chromatographic fractions 15–93 of the Gdm-HCl extract from the rat mandibular condylar cartilage. Digits at the top of the figure represent fraction numbers. The blue protein bands in fractions 48–54 migrating between the 53 kDa and 78 kDa molecular weight markers represent OPN. The blue protein bands around 80 kDa (between the 78 kDa and 114 kDa markers) in fractions 54–81 represent BSP. The identification of these Stains-All positive protein bands as OPN and BSP was confirmed by Western immunoblotting (see Fig. 3).
Fig. 3.
Western immunoblotting for DSPP and its fragments, DMP1 and its fragments, BSP and OPN. (A) Western immunoblotting using anti-DSP polyclonal antibody for fractions 27–39 of Q-Sepharaose chromatography of NCPs extracted from rat condylar cartilage. Positive control (Cont): 0.3 μg of DSP isolated from rat incisor dentin; 27–39: 60 μl of sample from fractions 27-39 were treated with 2.5% of β-mercaptoethanol before loading. Long arrow indicates the migrating position of full-length DSPP; arrowhead indicates the migrating position of DSP. (B) Western immunoblotting using anti-DMP1-N-9B6.3 monoclonal antibody. Cont: 1 μg of the NH2-terminal (37 kDa) fragment of DMP1 and the full-length form of DMP1 isolated from rat long bone. 51: 60 μl of sample from fraction 51 of Q-Sepharaose chromatography of NCPs extracted from rat condylar cartilage. Both the 37 kDa fragment (arrowhead) and full-length form of DMP1 (long arrow) are detected in the extract from the rat condylar cartilage. In comparison with the DMP1 molecular species in the extract from rat long bone (Cont in Fig. 3B), the full-length form of DMP1 is more abundant in the extract from the cartilage. (C) Western immunoblotting using anti-DMP1-C-857 polyclonal antibody. Cont: 1 μg of the COOH-terminal (57 kDa) fragment and the full-length form of DMP1 isolated from rat long bone. 51: 60 μl of sample from fraction 51 of Q-Sepharaose chromatography of NCPs extracted from rat condylar cartilage. While the full-length form of DMP1 (long arrow) was visualized, the 57 kDa fragment (arrowhead) was hardly detectable in the extract from the rat condylar cartilage. (D) Western immunoblotting using anti-BSP-10D9.3 monoclonal antibody. Cont: 1 μg of BSP isolated from rat long bone. 69: fraction 69 of Q-Sepharaose chromatography of NCPs extracted from rat condylar cartilage. Arrow indicates the migrating position of BSP. (E) Western immunoblotting using the anti-OPN polyclonal antibody. Cont: 1 μg of OPN isolated from rat long bone. 51: fraction 51 of Q-Sepharaose chromatography of NCPs extracted from rat condylar cartilage. Arrow indicates the migrating position of OPN.
Stains-All staining failed to demonstrate any protein bands corresponding to the migration rates of DSPP, DSP or DPP on SDS-PAGE (Fig 2), whereas on Western immunoblotting, a clear band in fractions 27–37 corresponding to the migration rate of the full-length form of DSPP (180-190 kDa) was recognized by the anti-DSP antibody (Fig. 3A). This observation indicated that the amount of DSPP in the sample was not sufficient for disclosure by Stains-All staining, but could be clearly detected by Western immunoblotting. The migration rate of the protein bands in fractions 27–39 that were immunoreactive to the anti-DSP antibody were identical to the full-length form of mouse recombinant DSPP made in HEK-293 cells, which migrates between 180 and 190 kDa on 5–15% gradient gel (Sun and Qin, unpublished data). Before loading onto SDS-PAGE, the sample was treated with 2.5% β-mercaptoethanol to eliminate the disulfide-bond linked dimeric form of DSP; previous studies have shown that dentin ECM contain a dimeric form of DSP that migrates over 200 kDa on SDS-PAGE 16, 37. Taken together, the authors concluded that this 180-190 kDa protein band in fractions 27–39 (Fig. 3A) represented the full-length form of DSPP. DSP (the NH2-terminal fragment of DSPP) was hardly detectable in any of the chromatographic fractions (including fractions prior to 27). This observation clearly indicated that the majority of DSPP is not cleaved in the condylar cartilage of the rat mandible, which differs from the data obtained from dentin ECM, in which DSPP is hardly detectable whereas its cleaved products DSP and DPP are abundant.
Previous studies showed that during ion-exchange chromatography, the NH2-terminal (37 kDa) and COOH-terminal (57 kDa) fragments of DMP1 and the full-length form of DMP1 co-elute with OPN16. In this investigation, Western immunoblotting using the anti-DMP1-N-9B6.3 antibody showed the presence of the full-length form and the NH2-terminal (37 kDa) fragment of DMP1 (Fig. 3B) in the fractions, in which OPN eluted. The anti-DMP1-C-857 antibody (immunoreactive to the COOH-terminal region of DMP1) could hardly detect the 57 kDa fragment but clearly demonstrated the presence of the full-length form of DMP1 (Fig. 3C) in these fractions; a faint 57 kDa band could be observed only when a very large volume of sample was loaded or when the X-ray film was exposed for a prolonged period of time. The full-length form of DMP1 (long arrows in Figs. 3B and 3C) was detected by both the anti-DMP1-N-9B6.3 and anti-DMP1-C-857 antibodies in Western immunoblotting. In the condylar cartilage, the full-length form of DMP1 appeared more abundant than its processed fragments. The protein band representing BSP was clearly observed in fractions 57–81 by Stains-All staining (Fig. 2, the blue protein band migrating between the 78 and 114 kDa markers). The identification of this protein band as BSP was further confirmed by Western immunoblotting using the anti-BSP-10D9.2 antibody (Fig. 3D).
In the Q-Sepharose chromatography of Gdm-HCl extract from the rat condylar cartilage, OPN mainly eluted in fractions 48–54 and was clearly detected in these fractions by Stains-All staining (Fig. 2. the most remarkable blue protein band migrating just below the 78 kDa molecular weight marker in fractions 48–54). Western immunoblotting further confirmed the abundance of this protein in the condylar cartilage. A broad, dark protein band below the 78 kDa molecular marker was recognized by the anti-OPN antibody (Fig. 3E.).
Immunohistochemistry (IHC)
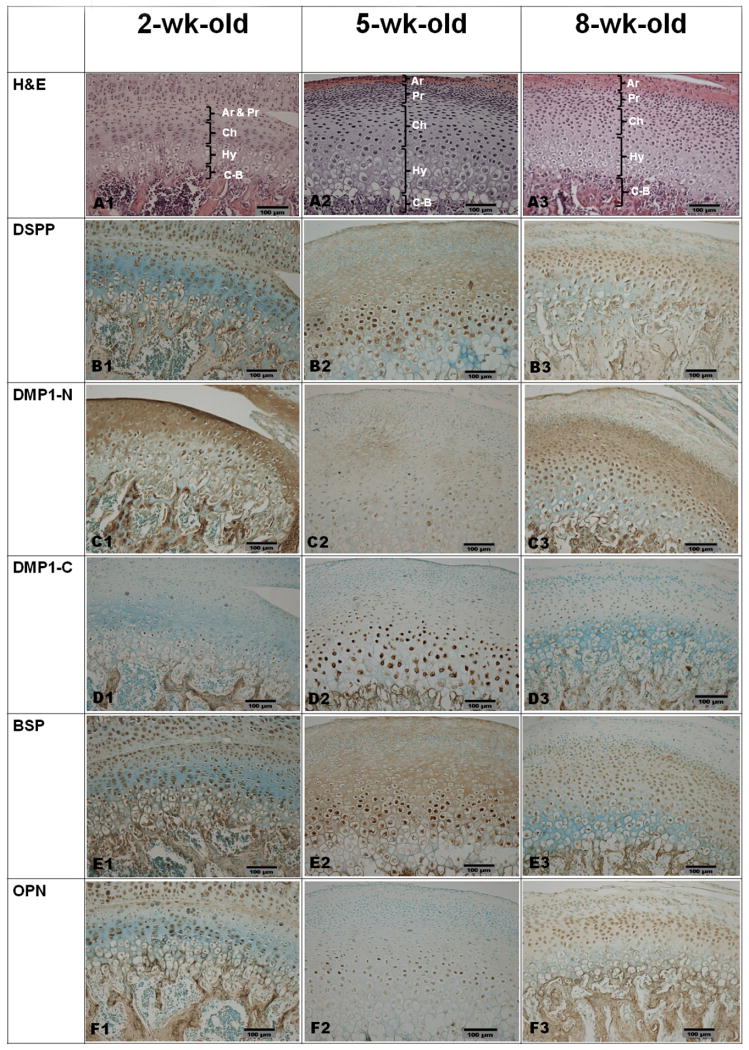
The condylar cartilage of the mandible can be divided into five layers based on histological appearance (Figs. 4A1, 4A2, 4A3): articular (Ar), prechondroblastic (Pr), chondroblastic (Ch), hypertrophic (Hy) and cartilage–bone interface (C-B). At 5 weeks after birth, the condylar cartilage is wider than at 2 or 8 weeks. Immunohistochemical staining showed that each of the four SIBLING family members had its own preference for distribution in specific layers, although all of them were detected in the cartilage. The distribution patterns also changed in relation to postnatal growth of the animals.
Fig. 4.
H&E and IHC staining for paraffin sections of the condylar cartilage from 2-, 5- and 8-week-old rats. Column 1, 2-week-old rat; column 2, 5-week-old rat; column 3, 8-week-old rat. Ar, articular layer; Pr, prechondroblastic layer; Ch, chondroblastic layer; Hy, hypertrophic layer; C-B, cartilage–bone interface. Row A, H&E staining; row B, IHC for DSPP; row C, IHC for the NH2-terminal fragment of DMP1; row D, IHC for the COOH-terminal fragment of DMP1; row E, IHC for BSP; row F, IHC for OPN. Bar equals 100 μm in all microphotographs. DSPP was observed in the cells of all five layers and in the ECM of the articular layer and prechondroblastic layer at 2 weeks (Fig. 4B1). At 5 and 8 weeks (Figs. 4B2 and 4B3), the signal for DSPP was detected in the cells of the chondroblastic and hypertrophic layers, as well as in the ECM of the articular, prechondroblastic and chondroblastic layers. The signal for the NH2-terminal fragment of DMP1 was mainly observed in the ECM of the articular layer and prechondroblastic layer at 2 weeks (Fig. 4C1). At 5 and 8 weeks (Figs. 4C2 and 4C3), the signal for the NH2-terminal fragment of DMP1 was mainly observed in the cells and the ECM of the chondroblastic and hypertrophic layers. Note that the IHC staining in the 5-week-old group is weaker than in the 8-week-old group. The signal for the COOH-terminal fragment of DMP1 (Figs. 4D1-4D3) was primarily observed in the nuclei of cells of the chondroblastic and upper part of the hypertrophic layers. BSP was observed in the cells of all five layers and in the ECM of the articular layer at 2 weeks (Fig. 4E1). At 5 weeks (Fig. 4E2), BSP signal was strong in the nuclei of cells in the prechondroblastic and chondroblastic layers and in the ECM of all five layers. At 8 weeks (Fig. 4E3), the signal for BSP was mainly observed in the prechondroblastic, chondroblastic and hypertrophic layers. OPN was observed in the cells of all five layers and in the ECM of the articular layer at 2 weeks (Fig. 4F1). At 5 and 8 weeks (Figs. 4F2 and 4F3), the signal for OPN was mainly observed in the nuclei of the chondroblastic layer and in the ECM of the prechondroblastic and chondroblastic layers.
DSPP was most pronounced within the cells in the articular layer and prechondroblastic layer at 2 weeks after birth, although the signal for DSPP could be observed across the whole area of the cartilage (Fig. 4B1). At 5 and 8 weeks (Figs. 4B2 and 4B3), DSPP was more prominent in the chondroblastic layer and the upper hypertrophic layer than in the superficial layer. DSPP was found in the ECM as well as within the cells.
The signal for the NH2-terminal fragment of DMP1 was very strong in the ECM of the articular and prechondroblastic layers but weaker in the chondroblastic and hypertrophic layers of the cartilage, 2 weeks after birth (Fig. 4 C1). At 5 weeks, the signal for the NH2-terminal fragment of DMP1 decreased across all layers of cartilage (Fig. 4C2), whereas at 8 weeks, the signal for the NH2-terminal fragment of DMP1 increased in the ECM of the prechondroblastic and chondroblastic layers (Fig. 4C3).
While the signal for the COOH-terminal fragment of DMP1 was weak across all layers of the condylar cartilage at 2 and 8 weeks after birth (Figs. 4D1 and 4D3), at 5 weeks a relatively strong immunoreactivity for this fragment was observed within the nuclei of cells in the chondroblastic layer and upper part of the hypertrophic layer adjacent to the chondroblasts (Fig. 4D2).
BSP was observed in significant amounts in all of the five layers of the condylar cartilage from all of the three age groups (Figs. 4E1, 4E2, 4E3). At 5 weeks, the signal for BSP was very strong in the ECM of the prechondroblastic and chondroblastic layers and within the cells of these two layers (Fig. 4E2).
At 2 weeks, OPN was prominent in the articular layer (Fig. F1), while at five weeks this protein was mainly detected in the cells of the chondroblastic layer (Fig. 4F2). The signal for OPN in the chondroblastic layer of the condylar cartilage from the 8-week-old rat (Fig. 4F3) appeared stronger than the level at 5 weeks. The IHC results are summarized in Table 2.
Table 2.
Summary of IHC results.
| 2 weeks | 5 weeks | 8 weeks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | Pr | Ch | Hy | C-B | Ar | Pr | Ch | Hy | C-B | Ar | Pr | Ch | Hy | C-B | |
| DSPP | ++ | ++ | + | + | + | + | + | ++ | ++ | + | + | + | ++ | ++ | + |
| DMP1-N | +++ | +++ | + | + | + | + | + | + | + | + | + | ++ | ++ | + | + |
| DMP1-C | + | + | + | + | + | + | + | ++ | ++ | + | + | + | + | + | + |
| BSP | ++ | ++ | ++ | ++ | ++ | ++ | +++ | +++ | ++ | ++ | + | ++ | ++ | ++ | ++ |
| OPN | ++ | ++ | ++ | + | ++ | - | + | + | - | + | + | + | ++ | + | ++ |
Ar, articular layer; Pr, prechondroblastic layer; Ch, chondroblastic layer; Hy, hypertrophic layer; C-B, cartilage–bone interface. +++ : strongly positive ; ++ : moderately positive; + : slightly positive; - : negative.
The distribution of the four SIBLING members showed the following general tendency. At 2 weeks after birth, relatively strong signals for these SIBLING proteins were observed in the articular and prechondroblastic layers, except for the COOH-terminal fragment of DMP1. As the animals grow (5 and 8 weeks after birth), the signals for these molecules either disappeared or became weaker in the articular layer, while their immunoreactivity in deeper layers (chondroblastic and hypertrophic layers) became stronger. In addition to their presence in the ECM, these four SIBLING members were also observed within the cells (fibroblasts, prechondroblasts, chondroblasts and hypertrophic chondrocytes).
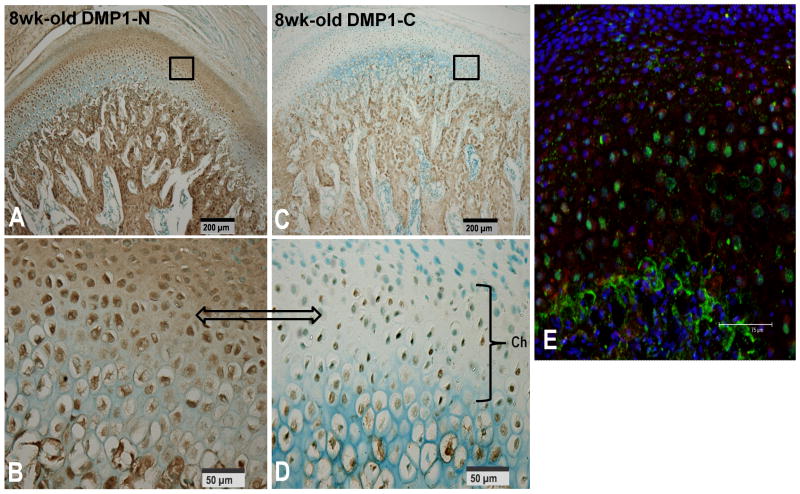
The NH2-terminal fragment of DMP1 showed a distribution profile different from that of the COOH-terminal fragment of DMP1 in the condylar cartilage of the mandible. To confirm these findings, the authors carried out double immunofluorescent staining experiments to investigate the localization of these two fragments on the condylar cartilage from 8-week-old rats. In this approach, the tissue section was initially incubated with a mixture of the two primary antibodies, anti-DMP1-N-859 and anti-DMP1-C-8G10.3, which react with the NH2-terminal and the COOH-terminal fragments of DMP1 in the same tissue section, respectively. The NH2-terminal fragment of DMP1 was mainly present in ECM and in the cytoplasm around the nuclei of cells in the prechondroblastic and chondroblastic layers (Fig. 5E), while the COOH-terminal fragment was primarily located in the nuclei of cells in the chondroblastic and hypertrophic layers. These findings are consistent with observations obtained from routine IHC analyses (see above).
Fig. 5.
Double staining immunofluorescence on the condylar cartilage from 8-week-old rats using a mixture of anti-DMP1-N-859 polyclonal and anti-DMP1-C-8G10.3 monoclonal antibodies. (A) Light microscopic IHC was performed with the anti-DMP1-N-859 polyclonal antibody. The NH2-terminal fragment of DMP1 was prominent in the chondroblastic and hypertrophic layers (boxed). (B) A higher magnification of the boxed area in A. (C) Light microscopic IHC was performed with the anti-DMP1-C-8G10.3 monoclonal antibody. The COOH terminal fragment was observed in the nuclei of cells in the chondroblastic and hypertrophic layers (boxed). (D) A higher magnification of the boxed area in C. (E) Double staining immunofluorescence analysis showed that the NH2-terminal fragment (red color) was mainly observed in the ECM of the chondroblastic and hypertrophic layers, or around the cell nuclei in the prechondroblastic, chondroblastic and hypertrophic layers, while the signal for the COOH terminal fragment (green color) was mainly found in the nuclei of cells in the chondroblastic and hypertrophic layers, and at the cartilage–bone interface.
Discussion
As both a site of growth and articulation, the condylar cartilage plays an important role in the development and function of the mandible. The protein-related macromolecules in the condylar cartilage of the mandible are classified into: collagens; proteoglycans; and non-collagenous proteins (NCPs). The collagen components of rat condylar cartilage include type I and type II collagens and differ from the articular cartilage of the long bone that contains only type II collagen22. The proteoglycans in the rat condylar cartilage are involved in regulating the activity and stability of proteins and signaling molecules within the matrix as well as in the matrix assembly. NCPs, in particular the SIBLING family members in the rat condylar cartilage, have not been given much attention, and their potential roles in chondrogenesis are largely unknown.
The SIBLING family members, DSPP, DMP1, BSP and OPN are known to play critical roles in osteogenesis, dentinogenesis and cementogenesis. There have been numerous studies on the localization, characterization and biological functions of these four SIBLING members in bone, dentin and cementum28. There are few reports of the distribution and characteristics of these NCPs in the condylar cartilage of the mandible17, 32, 39.
In this study, the authors systematically evaluated the presence and distribution of DSPP, DMP1, BSP and OPN in the condylar cartilage of the rat mandible, using protein chemistry and immunohistochemistry. For the protein chemistry analyses, condylar cartilage from 12-week-old rats was used; the bone and dentin tissues from rats at this age are routinely used by the authors for extraction of SIBLING proteins24 because rats at this age provide ample tissue mass while they are still young enough to maintain the momentum of growth. For the immunohistochemical experiments, condylar cartilage from rats aged 2, 5 and 8 weeks was used. At each of these ages, the condylar cartilage is a site of active growth, although more so at 2 weeks of age. Rats are typically weaned at 21 days of age, so this time interval spans an age (2 weeks) prior to the introduction of hard food to late adolescence 11 (8 weeks) in which articular function is significant and well-established. The mitotic cells of the condylar cartilage are located within the perichondrium (specifically the prechondroblastic layer), so this region is important for the growth that is so prominent at the condylar cartilage during the early part of the age span studied6, 32. With increasing age, the percentage of cells undergoing mitosis in the rat condylar cartilage declines steadily 31, while the articular function of the cartilage becomes more prominent and the articular layer is transformed from fibrous tissue to fibrocartilage in loaded areas7. In this study, immunoreactivity, especially for DSPP and the NH2 fragment of DMP1, changed from a primarily perichondrial localization at 2 weeks to more prominent expression in the chondroblastic and hypertrophic layers of the cartilage at 5 and 8 weeks, while becoming evident in the matrix. Viewed in this context, the shift of immunoreactivity for most SIBLING proteins from the superficial (perichondrial) layers to the deeper (cartilaginous) layers may reflect a shift from a role in growth regulation to a role in modulating ECM response to the increasing articular forces generated during the transition to hard food and the growth of the masticatory muscles6, 14, 32, 33. This shift of expression pattern suggests that these SIBLING family members may be actively involved in the development and function of the condylar cartilage: DSPP, DMP1, BSP and OPN along with other macromolecules such as collagens and proteoglycans may work collectively to ensure the proper formation and function of cartilage. DSPP in the ECM of dentin is primarily present as processed DSP and DPP fragments, originating from the NH2-terminal and COOH-terminal regions of the DSPP amino acid sequence, respectively4. The expression of DSPP has been observed in dentin17, bone28 and a number of tumor tissues10 but not in any type of cartilage. This investigation is the first to report the expression of DSPP in cartilaginous tissue. Although the level of DSPP expression in the rat condylar cartilage is lower than in dentin, it appears much higher in the cartilage than in the long bone, based on observations from both Western immunoblotting and immunohistochemistry analyses. Immunohistochemistry and protein chemistry experiments also revealed that the expression of DSPP in the growth plate cartilage of the long bone is higher than in the long bone (data not shown). The majority of DSPP in the condylar cartilage appears uncleaved (i.e. existing as the intact, full-length form); unlike in dentin, in which DSPP is primarily proteolitically processed into DSP and DPP. These findings suggest that the biological role of DSPP in the condylar cartilage may differ from that in dentin and bone. Further studies are warranted to analyze the roles of DSPP in the cartilage tissues.
Like DSPP, DMP1 in bone and dentin is primarily present as the processed NH2-terminal (37 kDa) and COOH-terminal (57 kDa) fragments, with only trace amounts of the full-length form of DMP115. In contrast to the observations in dentin and bone, the authors found that, compared with its processed fragments, there was more of the full-length form of DMP1 in the condylar cartilage. In addition to the features common to all SIBLING proteins, DMP1 and DSPP share several unique similarities2, 29. Both are proteolytically cleaved in the dentin and bone to yield NH2-terminal and COOH-terminal fragments. Their NH2-terminal fragments are highly glycosylated30, their COOH-terminal fragments are highly phosphorylated and are able to promote mineralization28, 29 and they show a similar localization in the tooth. The findings from this study added another similarity that in the condylar cartilage, both DSPP and DMP1 are primarily present as uncleaved forms. Futher studies are needed to determine why DSPP and DMP1 are not processed in the condylar cartilage, and to explore whether the biological roles of DSPP and DMP1 in the condylar cartilage differ from those in the dentin and bone.
The signal for the NH2-terminal fragment of DMP1 was localized differently from that of the COOH-terminal fragment in the condylar cartilage. The NH2-terminal fragment of DMP1 was mainly detected in the matrix or in the cytoplasm while the signal for the COOH-terminal fragment was mainly observed in the nuclei of cells in the chondroblastic and hypertrophic layers of the condylar cartilage. This was consistent with the findings that the localization of the NH2-terminal fragment of DMP1 differs from the COOH-terminal fragment in the rat dentin and long bone20. In the rat first molar, the NH2-terminal fragment was localized to predentin, whereas the COOH-terminal fragment was mainly restricted to mineralized dentin. In the growth plate of the long bone, the NH2-terminal fragment appeared in the proliferation and hypertrophic zones, while the COOH-terminal fragment was in the ossification zone. This localization difference in the cartilage strongly suggests that the two fragments of DMP1 may play different roles during chondrogenesis. It is also possible that the immunoreactivity revealed in IHC analyses by the antibodies against the NH2-terminal region of DMP1 represents the full-length form of DMP1, while the failure to detect the COOH-terminal portion of the full-length DMP1 by antibodies against the COOH-terminal region of the protein might be due to an unknown spatial structure that does not allow the antigenic epitopes of the COOH-terminal region to be exposed. The COOH-terminal portion of DMP1 might be wrapped inside the protein and could not be exposed. This could explain the abundance of the full-length form of DMP1 (found in Western immunoblotting) in the Gdm-Cl extract from the rat condylar cartilage. In addition to the different localization between the two fragments of DMP1, there were also sites in which they are co-localized. Co-localized signal can be found in the cytoplasm of prechondroblasts, chondroblasts and at the bone–cartilage interface.
BSP and OPN were clearly detected in the condylar cartilage. While DSPP and DMP1, or their processed fragments, could not be detected clearly by Stains-All staining, the Stains-All positive protein bands representing BSP and OPN were observed. IHC showed that at 2 weeks after birth, BSP and OPN were found in the ECM of the fast-growing superficial layer (articular layer and superficial part of prechondroblastic layer) while at later points (5 and 8 weeks), the main expression of these two proteins shifted into the deeper layers of the condylar cartilage.
In summary, with both protein chemistry and immunohistochemistry methods, the authors detected the four SIBLING family members, DSPP, DMP1, BSP and OPN in the condylar cartilage. The localization of the four SIBLING family members and/or their processed fragments varied in different layers of the cartilage. The findings from this investigation have increased the general body of knowledge about these four SIBLING proteins in different tissues and provided clues about the potential roles of these molecules in chondrogenesis.
Acknowledgments
The authors would like to thank Dr William T Butler and Dr Yongbo Lu for their valuable suggestions regarding the manuscript.
Funding: This work was supported by the National Institutes of Health Grant DE 005092 (C Qin).
Footnotes
Declarations
Competing Interests: None required
Ethical Approval: Not required
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004;112:163–170. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 2.Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004;23:371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 4.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 5.Butler WT, Bhown M, Brunn JC, D'Souza RN, Farach-Carson MC, Happonen RP, Schrohenloher RE, Seyer JM, Somerman MJ, Foster RA. Isolation, characterization and immunolocalization of a 53-kDal dentin sialoprotein (DSP) Matrix. 1992;12:343–351. doi: 10.1016/s0934-8832(11)80030-2. [DOI] [PubMed] [Google Scholar]
- 6.Copray JC, Dibbets JM, Kantomaa T. The role of condylar cartilage in the development of the temporomandibular joint. Angle orthod. 1988;58:369–380. doi: 10.1043/0003-3219(1988)058<0369:TROCCI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Copray JC, Liem RS. Ultrastructural changes associated with weaning in the mandibular condyle of the rat. Acta Anat. 1989;134:35–47. doi: 10.1159/000146731. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 9.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1230–1235. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56:619–628. doi: 10.1159/000126284. [DOI] [PubMed] [Google Scholar]
- 12.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 13.George A, Sabsay B, Simonian PAL, Veis A. Characterizationof a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 14.Hinton RJ, Carlson DS. Regulation of mandibular condylar cartilage growth. Sem Orthod. 2005;11:209–218. [Google Scholar]
- 15.Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, Bonewald L, Butler WT, Feng J, Qin C. Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int. 2008;82:401–410. doi: 10.1007/s00223-008-9140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, Wygant J, Butler WT, Qin C. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci. 2008;116:104–112. doi: 10.1111/j.1600-0722.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huq NL, Cross KJ, Ung M, Reynolds EC. A review of protein structure and gene organisation for proteins associated with mineralised tissue and calcium phosphate stabilisation encoded on human chromosome 4. Arch Oral Biol. 2005;50:599–609. doi: 10.1016/j.archoralbio.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 18.MacDougall M, Gu T, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- 19.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein arecleavage products expressed from a single transcript coded bya gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 20.Maciejewska I, Cowan C, Svoboda K, Butler WT, D'Souza R, Qin C. The NH2-terminal and COOH-terminal fragments of Dentin Matrix Protein 1 (DMP1) localize differently in the compartments of dentin and growth plate of bone. J Histochem Cytochem. 2009:57155–57166. doi: 10.1369/jhc.2008.952630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciejewska I, Qin D, Huang B, Sun Y, Mues G, Svoboda K, Bonewald L, Butler WT, Feng J, Qin C. Distinct Compartmentalization of Dentin Matrix Protein 1 Fragments in Mineralized Tissues and Cells. Cells Tissues Organs. 2009;189:186–191. doi: 10.1159/000151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizoguchi I, Takahashi I, Nakamura M, Sasano Y, Sato S, Kagayama M, Mitani H. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch Oral Biol. 1996;41:863–869. doi: 10.1016/s0003-9969(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 23.Moses K, Butler WT, Qin C. Immunohistochemical study of SIBLING proteins in reactionary dentin of rat molars at different ages. Eur J Oral Sci. 2006;114:216–222. doi: 10.1111/j.1600-0722.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 24.Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 25.Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H. The expression of dentin sialoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- 26.Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 2003;111:235–242. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 27.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 28.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 29.Qin C, Baba O, Brunn JC, McKee MD, Bonewald L, Butler WT. Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP) share unique properties including tissuelocalization, proteolytic processing, and high molecular weightforms. The 8th ICCBMT; 2005; pp. 174–177. [Google Scholar]
- 30.Qin C, Huang B, Wygant JN, McIntyre BW, McDonald CH, Cook RG, Butler WT. A chondroitin sulfate chain attached to the bone dentin matrix protein 1 NH2-terminal fragment. J Biol Chem. 2006;281:8034–8040. doi: 10.1074/jbc.M512964200. [DOI] [PubMed] [Google Scholar]
- 31.Sano K, Sekine J, Pe MB, Inokuchi T. Bromodeoxyuridine immunohistochemistry for evaluating age-related changes in the rat mandibular condyle decalcified by intravenous infusion. Biotech Histochem. 1992;67:297–302. doi: 10.3109/10520299209110038. [DOI] [PubMed] [Google Scholar]
- 32.Sasaguri K, Jiang H, Chen J. The effect of altered functional forces on the expression of bone-matrix proteins in developing mouse mandibular condyle. Arch Oral Biol. 1998;43:83–92. doi: 10.1016/s0003-9969(97)00075-7. [DOI] [PubMed] [Google Scholar]
- 33.Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage---a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- 34.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 35.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 36.Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 37.Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 38.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 39.Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, Kunieda T, Tsutsui TW, Boskey A, Bonewald LF, Feng J. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005;280:197–203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]