Abstract
Morphogenesis during eye development requires retinoic acid (RA) receptors plus RA-synthesizing enzymes, and loss of RA signaling leads to ocular disorders associated with loss of Pitx2 expression in perioptic mesenchyme. Several Wnt signaling components are expressed in ocular tissues during eye development including Dkk2, encoding an inhibitor of Wnt/β-catenin signaling, which was previously shown to be induced by Pitx2 in the perioptic mesenchyme. Here, we investigated potential cross-talk between RA and Wnt signaling during ocular development. Genetic studies using Raldh1/Raldh3 double null mice deficient for ocular RA synthesis demonstrated that Pitx2 and Dkk2 were both down-regulated in perioptic mesenchyme. Chromatin immunoprecipitation and gel mobility shift studies demonstrated the existence of a DR5 RA response element upstream of Pitx2 that binds all three RA receptors in embryonic eye. Axin2, an endogenous readout of Wnt/β-catenin signaling, was up-regulated in cornea and perioptic mesenchyme of RA deficient embryos. Also, expression of Wnt5a was expanded in perioptic mesenchyme of RA deficient eyes. Our findings demonstrate excessive activation of Wnt signaling in the perioptic mesenchyme of RA deficient mice which may be responsible for abnormal development leading to defective optic cup, cornea, and eyelid morphogenesis.
Keywords: Retinoic acid, Wnt signaling, Perioptic mesenchyme, Cornea, Eyelid, Raldh1, Raldh3, Pitx2, Dkk2, Axin2, Wnt5a
Introduction
Retinoic acid (RA) is a vitamin A derivative essential for embryonic development. Vitamin A deficiency is known to cause various ocular malformations including retinal defects, microphthalmia, coloboma and vision impairment. Ocular morphogenesis during embryonic development is a multi-step process involving induction, proliferation, differentiation and migration of cells. The vertebrate eye is formed from neural ectoderm, surface ectoderm and perioptic mesenchyme, which is derived from neural crest cells. An essential function of perioptic mesenchyme is to contribute multiple mature cell lineages that are required for the normal ocular anterior segment and eyelid development (Le Liévre and Le Douarin, 1975). In humans, defects in survival, migration and differentiation of perioptic mesenchymal cells can lead to anterior segment dysgenesis and increased risk of glaucoma (Gould et al., 2004). Also, mutation of human PITX2 leads to Axenfeld-Rieger syndrome characterized by ocular anterior segment defects (Semina et al., 1996; Mears et al., 1998; Kozlowski and Walter, 2000; Vieira et al., 2006; Weisschuh et al., 2006).
A role for RA has been well established in various stages of eye development based upon the overlapping expression patterns of RA-synthesizing enzymes (retinaldehyde dehydrogenases; RALDHs) and retinoic acid receptors (RARs) in ocular tissues, combined with corresponding loss-of-function studies in mouse (Lohnes et al., 1994; Ghyselinck et al., 1997; Wagner et al., 2000; Mic et al., 2004; Matt et al., 2005; Molotkov et al., 2006; Matt et al., 2008). RA synthesis is carried out by three RALDH enzymes (encoded by Raldh1, Raldh2, and Raldh3) with ocular Raldh1 expression limited to the dorsal retina and ocular Raldh3 expression limited to the ventral retina (Duester, 2008). RA serves as a ligand for nuclear RA receptors (RARα, RARβ, and RARγ) which bind to DNA as heterodimers with retinoid X receptors (RXRα, RXRβ, and RXRγ) (Mark et al., 2006). RA signaling is transduced when RA binds to the RAR component of RAR/RXR heterodimers that are bound to RA response elements (RAREs) of specific target genes (Mic et al., 2003). Loss of ocular RA synthesis in mouse embryos carrying mutations of both Raldh1 and Raldh3 does not disrupt eye dorsoventral patterning as originally proposed, but it does disrupt anterior segment morphogenesis leading to excessive perioptic mesenchyme growth associated with dysgenesis of cornea and eyelid and rotation of the optic cup along the dorsoventral axis (Matt et al., 2005; Molotkov et al., 2006). As Raldh1 and Raldh3 are expressed in the retina but not the perioptic mesenchyme, RA signaling for eye morphogenesis occurs in a paracrine fashion (Molotkov et al., 2006).
Besides RA signaling, many components of the Wnt signaling pathway are also expressed in ocular tissues. A role for Wnt signaling during embryonic eye development has been established in regulation of distinct processes including eye field specification, morphogenetic movements, proliferation, differentiation and apoptosis (Fuhrmann, 2008). In short, canonical Wnt/β-catenin signaling is activated by binding of Wnt ligands to frizzled and LRP5/6 co-receptors followed by stabilization and translocation of β-catenin to the nucleus where it regulates transcription of target genes by interacting with TCF/LEF transcription factors (Van de Wetering et al., 1997; Logan and Nusse, 2004; Angers and Moon, 2009). Among the several Wnt ligands and signaling components expressed in the developing eye, Dkk2 (an antagonist of canonical Wnt/β-catenin signaling) is highly expressed in the perioptic mesenchyme, and recently it has been shown that Dkk2 is a critical downstream target of Pitx2 in neural crest derived perioptic mesenchyme (Gage et al., 2008). Pitx2 encodes a homeodomain transcription factor which plays an essential role during ocular anterior segment patterning and development (Gage et al., 1999; Hjalt et al., 2000; Gage et al., 2005). Heterozygous mutations in human PITX2 results in Axenfeld-Rieger syndrome characterized by anterior segment dysgenesis and high risk of developing glaucoma (Semina et al., 1996; Mears et al., 1998; Kozlowski and Walter, 2000; Vieira et al., 2006; Weisschuh et al., 2006). Global and neural crest specific Pitx2 knockout mice exhibit a similar eye phenotype including abnormal anterior segment differentiation, vasculogenesis, eyelid defects and coloboma (Gage et al., 1999; Kitamura et al., 1999; Lu et al., 1999; Evans and Gage, 2005). The eye defect of Pitx2 knockout mice is quite similar to that reported for Raldh1/Raldh3 and RARb/g double knockout mice (Matt et al., 2005; Molotkov et al., 2006; Matt et al., 2008). Previous studies in embryos lacking either ocular RA synthesis or RA receptors demonstrate that Pitx2 is down-regulated in the perioptic mesenchyme (Matt et al., 2005; Matt et al., 2008; See and Clagett-Dame, 2009). The observation that Pitx2 functions as an inducer of Dkk2 in perioptic mesenchyme provides a new paradigm for inhibition of canonical Wnt signaling through a critical role of Pitx2 during eye development (Gage et al., 2008).
Here, we investigate potential cross-talk between RA and Wnt signaling during eye development. We report that the Pitx2 gene contains a DR5 RARE located 4.3 kb upstream. We also demonstrate that RA activity in the perioptic mesenchyme is required for expression of not only Pitx2 but also Dkk2 which affects Wnt/β-catenin signaling, and for repression of Wnt5a. RA inhibition of Wnt signaling in the perioptic mesenchyme provides a mechanism for proper anterior segment formation, eyelid development, and optic cup orientation along the dorsoventral axis.
Materials and methods
Generation of Raldh1;Raldh3 Double Null Mutant Embryos
Raldh1-/-;Raldh3-/- double homozygous embryos were generated by crossing the two single mutant lines as previously described (Molotkov et al., 2006). Following mating, noon on the day of vaginal plug detection was considered embryonic day 0.5 (E0.5). Embryos were genotyped by PCR analysis of yolk sac DNA. All mouse studies conformed to the regulatory standards adopted by the Animal Research Committee at the Burnham Institute for Medical Research.
Chromatin Immunoprecipitation
Chromatin Immunoprecipitation (ChIP) was performed according to the manufacturer’s ChIP protocol (Active Motif, Carlsbad, CA). In separate experiments, ten E8.5 wild-type mouse embryos or eighteen E12.5 wild-type mouse eyes were dissected, pooled, and cross-linked with 1% formaldehyde at room temperature for 15 min; eye tissue included the whole eye and surrounding perioptic mesenchyme. Isolated nuclei (in 650 μl of shearing buffer) from E8.5 mouse embryos were sonicated for twelve pulses of 10 sec each (Duty cycle-6, Output 30%) using a Branson Sonifier 450 (or in the case of nuclei from E12.5 eye samples for twenty pulses of 10 sec each at 40% power output) using a microtip probe from Misonix Digital Sonicator 4000 (Cole-Parmer Instrument Company, Vernon Hills, IL, USA). Samples were sonicated on ice to shear DNA to an average size of 500 bp followed by centrifugation at 13,000 rpm for 10 min. At this point, a small portion of supernatant was stored as input control. For immunoprecipitation, 150 μl of sheared chromatin mixed with 3 μg of either anti-RARα (sc-551, Santa Cruz Biotechnology), anti-RARβ (Affinity Bioreagents), anti-RARγ (sc-550 Santa Cruz Biotechnology), or control IgG antibodies was used for each ChIP reaction incubated with 25 ml pre-blocked protein G-coated magnetic beads (Active Motifs, Carlsbad, CA) for 4 h at 4°C. Beads were washed and eluted DNA-protein complexes were reverse cross-linked and purified. The immunoprecipitated DNA was analyzed by PCR. ChIP analysis was performed at least in three independent experiments. PCR products were separated by 3% agarose gel electrophoresis and visualized using ethidium bromide staining. RARE specific and non-specific primer sequences for mouse Pitx2, RARβ, and Raldh1 (Aldh1a1) genes used in this study were:
Pitx2 DR5a-F: 5’-CAAGATACTGGTCTGTTACCTTCC-3’
Pitx2 DR5a-R: 5’-GTTTCCGAATTACCTATCTGAGAGG-3’
Pitx2 DR5b-F: 5’-CATTTTAAGTCCCTCTCTGACAACC-3’
Pitx2 DR5b-R: 5’-GTGCAAGAGCCTGGTAATCCCT-3’
Pitx2 NS-F: 5’-GAAATTTGTTCCACTCTGGAGAACC-3’
Pitx2 NS-R: 5’-GGTAATGATGGGAAGGGGCTAATC-3’
RARb DR5-F: 5’-TGGCATTGTTTGCACGCTGA-3’
RARb DR5-R: 5’-CCCCCCTTTGGCAAAGAATAGA-3’
RARb NS-F: 5’-AGTACAGACCTTCCAAGAGTGCCT-3’
RARb NS-R: 5’-GTCATGGGAAAGAGAGGTTGAGC-3’
Raldh1 DR5-F: 5’- TGCACACACACCCTTAGCACAG -3’
Raldh1 DR5-R: 5’- CAGGTGACAGGCTCAGCAAATTG -3’
Electrophoretic Gel Mobility Shift Assay (EMSA)
Sixteen wild-type E12.5 embryo eyes including surrounding perioptic mesenchyme tissue were dissected out and nuclear protein extracts were prepared as described (Dignam et al., 1983). Biotin-labeled double-stranded oligonucleotide probes containing RARE sequences (DR5a and DR5b) were bound to nuclear extracts. Binding reactions were performed using the LightShift Chemiluminescent EMSA Kit (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. Reaction mixtures were incubated for 20 min at room temperature. Binding reactions were subjected to EMSA on 6% non-denaturing polyacrylamide gels in 0.5 Tris-Borate-EDTA buffer, and detection was performed using a LightShift Chemiluminescent EMSA Kit (Pierce) according to the manufacturer’s instructions. For supershift analysis, nuclear extracts were incubated with a 3 μg of anti-RARα (sc-551, Santa Cruz Biotechnology), anti-RARβ (Affinity Bioreagents), or anti-RARγ antibodies (sc-550 Santa Cruz Biotechnology) for 20 min on ice before adding probe. Sequences of wild-type and mutated oligonucleotides (underlined bases indicate mutations introduced) containing the DR5 RA response elements used in gel shift reactions are as follows (the complementary strand was also synthesized and annealed to the strand shown here which was biotin-labeled):
Pitx2 RARE DR5a WT: 5’-TTAGGTAATTCATTAGAAAGTCAATACAGAC-3’
Pitx2 RARE DR5a Mut: 5’-TTAGGTGATAAATTAGAGAGAAAATACAGAC-3’
Pitx2 RARE DR5b WT: 5’-TTTAAATCAGATCATCGAAGAGTCACCAGAAA-3’
Pitx2 RARE DR5b Mut: 5’-TTTAAATCGGAAAATCGAACAGAAACCAGAAA-3’
In situ Hybridization
To prepare cryosections for in situ hybridization, whole heads of E12.5 mouse embryos were dissected out, washed twice with 1xPBS (phosphate-buffered saline) and fixed in 4% paraformaldehyde (Sigma) for 2 h at 4°C. Embryo heads were then cryo-protected in 10% sucrose (Sigma) for 3 h followed by 30% sucrose overnight at 4°C. Heads were embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA), oriented, frozen and stored at -80°C. Heads were cryo-sectioned at 12 μm and processed for in situ hybridization as described (Wilkinson and Nieto, 1993) with the following digoxigenin-labeled antisense riboprobes: Pitx2, Vax2, Dkk2, Axin2 and Wnt5a. Riboprobes were prepared as described previously (Wilkinson and Nieto, 1993).
TOPgal lacZ Detection
Mice carrying the TOPgal Wnt/β-catenin reporter transgene which places lacZ (encoding β-galactosidase) under the transcriptional control of LEF/TCF response elements (DasGupta and Fuchs, 1999), was mated to our wild-type and Raldh1-/-;Raldh3-/- double mutant mice to detect Wnt/β-catenin activity in RA-deficient embryos. β-galactosidase activity was detected by staining with X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) for 6 h at 37°C.
Results
Pitx2 expression is impaired in perioptic mesenchyme of Raldh1;Raldh3 double null mutant
Raldh1-/- ;Raldh3-/- double null mutants exhibit dysgenesis of the ocular anterior segment caused by excessive invasion of perioptic mesenchyme, which leads to abnormal thickening of corneal stroma and premature closure of the eyelid folds (Matt et al., 2005; Molotkov et al., 2006). Pitx2 is one of the key genes expressed in the neural crest derived perioptic mesenchyme surrounding the optic cup and it plays an important role in anterior segment formation (Evans and Gage, 2005). Previous studies reported that Pitx2 expression was reduced or absent in perioptic mesenchyme of RA deficient mice (Matt et al., 2005; Matt et al., 2008; See and Clagett-Dame, 2009). At E12.5, we show that Pitx2 expression is down-regulated in the perioptic mesenchyme of our Raldh1-/-;Raldh3-/-double null mutants (Fig. 1A-B; n=6). This finding confirms that Pitx2 expression is dependent on paracrine RA signals from Raldh1 and Raldh3 expressed in the retina. The loss of Pitx2 expression in Raldh1-/-;Raldh3-/- double null mutants suggests Pitx2 may be a RA target gene.
Fig. 1.
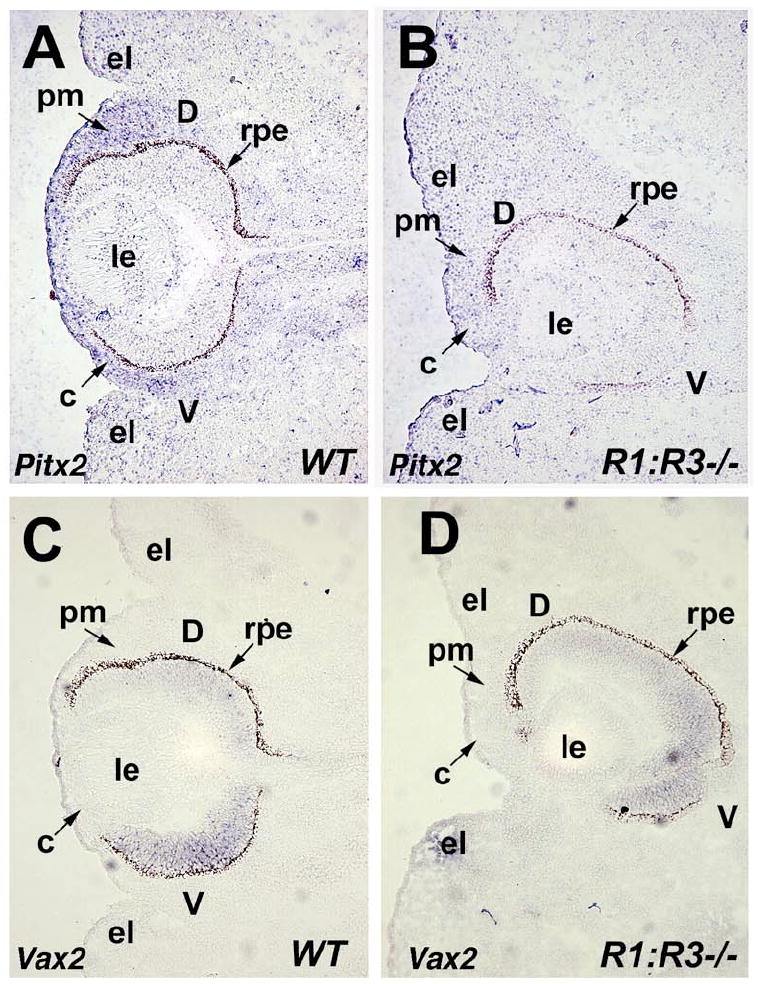
Ablation of Raldh1 and Raldh3 impairs Pitx2 expression in perioptic mesenchyme. (A-B) In situ hybridization with digoxigenin-labeled Pitx2 antisense riboprobe on frontal sections through the eye of E12.5 wild-type (WT) and double null (Raldh1-/-;Raldh3-/-) embryos. Note that Pitx2 expression in the perioptic mesenchyme of R1;R3-/- embryo is severely reduced compared to WT control indicated by arrow. (C-D) Vax2 shows normal expression pattern in the ventral retina of wild-type (WT) compared to double null (Raldh1-/-;Raldh3-/-) eyes, however note the dorsoventral rotation of the optic cup in the R1;R3-/- double mutant. c, cornea; d, dorsal retina; el, eyelid fold; le, lens; pm, perioptic mesenchyme; rpe, retinal pigment epithelium; v, ventral retina.
As a control, Vax2 expression is shown in wild-type and Raldh1-/- ;Raldh3-/- double null mutants (Fig. 1 C-D). Vax2 is expressed in the ventral retina (Barbieri et al., 2002; Mui et al., 2002), and loss of RA synthesis does not alter its expression although the optic cup exhibits rotation along the dorsoventral axis which places the ventral retina deeper inside the head (Fig. 1D). Sections of the double mutant eye also show corneal mesenchyme thickening and collapse of the dorsal eyelid fold, while the ventral eyelid fold is still easily observed (Fig. 1B, D).
RA directly regulates Pitx2 in vivo
We investigated whether Pitx2 regulation by RA is direct or indirect. In silico analysis of the mouse Pitx2 upstream regulatory region was performed to identify retinoic acid response elements (RAREs). Two putative DR5-type RAREs (direct repeat with 5 bp between) were located in the Pitx2 upstream regulatory region at -4.3 kb (DR5a) and -3.6 kb (DR5b) relative to the ATG start codon (Fig. 2A). These DR5 elements are conserved and display a high degree of sequence homology between mouse, rat and human (Fig. 2B). To test if Pitx2 expression is directly regulated by RAR binding to these DR5 RAREs in vivo, we performed chromatin immunoprecipitation (ChIP) using antibodies specific for RARα, RARβ, and RARγ on cross-linked DNA-protein complexes from either E8.5 whole embryos (Fig. 2C) or E12.5 eye tissues (Fig. 2D). ChIP analysis of both whole embryo and eye tissue reveals that all three RARs bind specifically with DR5a element located at -4.3 kb in Pitx2 regulatory region, but no binding was observed using IgG control and non-specific primers for a region several kb away that does not contain a RARE. Interestingly, the DR5b element was not able to recruit any of the RA receptors in either embryo or eye indicating that only the DR5a element is functionally active. As a control, we also show that the RARβ gene, which is transcriptionally activated by RA via a well characterized DR5 RARE (Mendelsohn et al., 1991), recruited all three RARs in both embryo and eye ChIP (Fig. 2C-D). Also, the Raldh1 (Aldh1a1) gene, which contains a DR5 RARE (Balmer and Blomhoff, 2005), recruited all three RARs in eye ChIP (Fig. 2D). Collectively, ChIP data provide evidence that Pitx2 is a direct target of RA action in the developing eye.
Fig. 2.
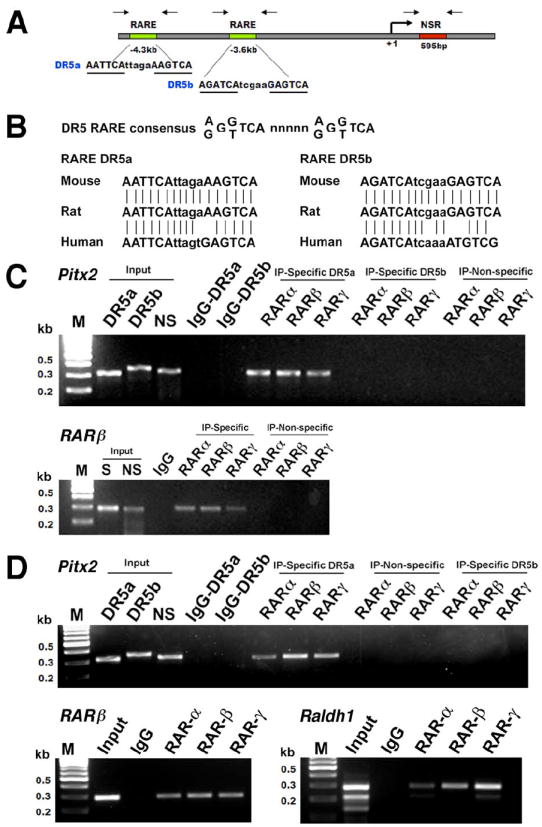
Recruitment of retinoic acid receptors to the mouse Pitx2 promoter in vivo. (A) Schematic representation of the mouse Pitx2 upstream region containing two putative DR5 retinoic acid response elements (RARE) located 3.6 and 4.3 kb upstream of transcription start site. Arrows indicate the location of primer pairs used to amplify the RARE-containing regions and a non-specific negative control region (NSR) located 595 bp downstream for chromatin immunoprecipitation (ChIP) assay. (B) Nucleotide sequence alignment of conserved DR5a and DR5b RAREs in mouse, rat, and human Pitx2 genes. ChIP assay was performed using (C) E8.5 mouse embryos and (D) E12.5 eyes. The cross-linked protein-DNA complexes were immunoprecipitated with anti-RAR-α, RAR-β, and RAR-γ antibodies. Mouse IgG was used as a negative control. Input material and immunoprecipitates were analyzed by PCR with specific primers flanking the RARE and non-specific primers located several kb away for the mouse Pitx2, RARβ, and Raldh1 promoters. S: RARE specific primers, NS: Non-specific primers.
We used an electrophoretic gel mobility shift assay (EMSA) to further examine the mouse Pitx2 RAREs. Using a nuclear extract from wild-type E12.5 embryonic eyes, we found that a double-stranded 31-mer oligonucleotide probe containing the DR5a RARE located at -4.3 kb was shifted to a more slowly migrating species (Fig. 3, lane 2); a probe containing the DR5b RARE located at -3.6 kb was shifted much less efficiently than the DR5a probe (Fig. 3, lane 7). DR5a and DR5b probes with mutations introduced into the direct repeats exhibited no detectable shifted species (Fig. 3, lanes 3 and 8). Specificity of the DR5a shifted species for binding to RARs was demonstrated by inclusion of RAR antibodies in the gel shift reaction which resulted in supershifted species for RARα, RARβ, and RARγ antibodies (Fig. 3, lanes 4-6). Combined with the ChIP results demonstrating binding of DR5a but not DR5b to RARs in vivo (Fig. 2D), our findings support DR5a as a functional RARE for the Pitx2 gene in embryonic eye and suggest that DR5b is ineffective as a RARE.
Fig. 3.
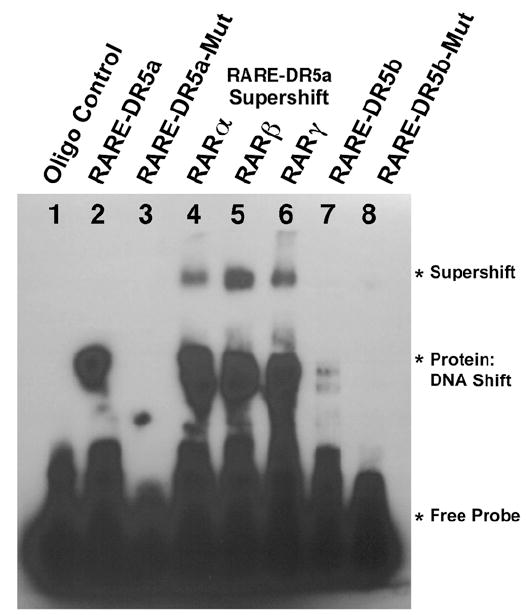
Electrophoretic gel mobility shift assays on the mouse Pitx2 RAREs. (Lane 1) DR5a 31-mer probe incubated without nuclear extract added. (Lanes 2-3) DR5a and DR5a-mutant probes, respectively, incubated with E12.5 eye nuclear extract. (Lanes 4-6) DR5a probe supershifted by adding RAR antibodies along with the E12.5 eye nuclear extract. (Lanes 7-8) DR5b and DR5b-mutant probes, respectively, incubated with E12.5 eye nuclear extract.
Retinoic acid interferes with Dkk2 expression in perioptic mesenchyme
Dkk2 is a member of the dickkopf family of secreted proteins that acts as an antagonist of canonical Wnt signaling (Mao and Niehrs, 2003; He et al., 2004). During eye development, onset of Dkk2 expression is first detected in the anterior segment perioptic mesenchyme at E11.5 which then extends to mesenchyme surrounding the optic cup and the developing cornea (Monaghan et al., 1999; Gage et al., 2008). Recently, Dkk2 was identified as a direct target of Pitx2 during anterior segment morphogenesis and evidence was presented for a role in negative regulation of canonical Wnt signaling activity which is essential for normal ocular morphogenesis (Gage et al., 2008). We examined E12.5 Raldh1-/-;Raldh3-/- double null mutants and wild-type littermate controls for expression of Dkk2 in ocular tissues to ascertain the role of canonical Wnt signaling in perioptic mesenchyme. In the Raldh1-/-; Raldh3-/- double null mutant, Dkk2 expression was consistently reduced in perioptic mesenchyme and cornea (Fig. 4A-B, n=6). As Raldh1-/-;Raldh3-/- double null mutant eyes completely lack RA signaling at E11.5 (Molotkov et al., 2006), the residual Dkk2 expression we observe at E12.5 is likely due to an RA-independent mechanism. A reduction of Dkk2 expression in RA deficient mice eyes suggests that increased Wnt signaling may contribute to the ocular defects observed in these mice.
Fig. 4.
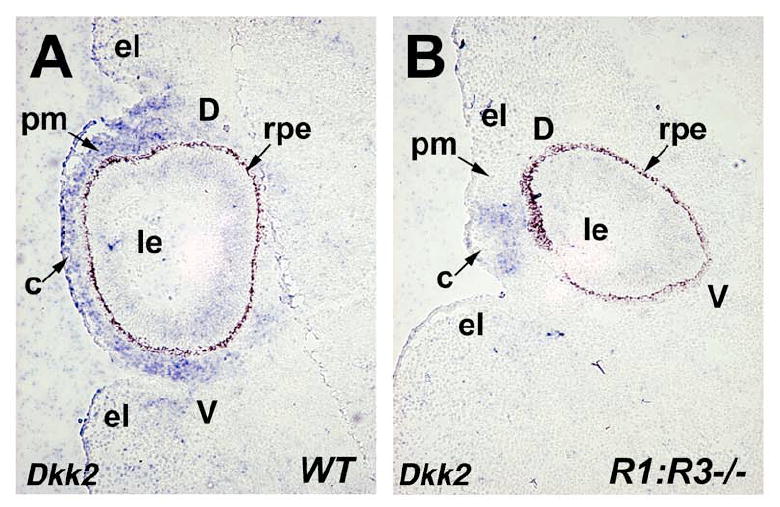
Loss of Raldh1 and Raldh3 interferes with Dkk2 expression in perioptic mesenchyme. (A-B) In situ hybridization with Dkk2 antisense riboprobe on frontal sections through the eye of E12.5 wild-type (WT) and double null (Raldh1-/-;Raldh3-/-) embryos. Note that Dkk2 expression in the perioptic mesenchyme of R1;R3-/- embryo (B) is severely reduced compared to WT control (A) indicated by arrow. c, cornea; d, dorsal retina; el, eyelid fold; le, lens; pm, perioptic mesenchyme; rpe, retinal pigment epithelium; v, ventral retina.
Activated Wnt signaling in the developing eyes of RA deficient mice
Our Dkk2 mRNA expression data suggests that activation of Wnt signaling may occur in the perioptic mesenchyme of RA deficient mice. To begin assessing active Wnt signaling in the eye, we used Raldh1; Raldh3 null mice carrying the TOPgal transgene, a reporter for the canonical Wnt signaling pathway (DasGupta and Fuchs, 1999). TOPgal does not reflect all Wnt/β-catenin signaling present in embryos (see Axin2 data below), but it is a very useful marker for examining the eyelid front. Staining of E12.5 whole-mount embryos shows that TOPgal expression in wild-type eyes exists along a thin line around the entire optic cup that corresponds with the migrating front of the eyelid (Fig. 5A; n=6). However, the Raldh1-/-;Raldh3-/- double null mutant eye appears to be missing the dorsal eyelid fold, although a portion of the ventral eyelid fold may be present (Fig. 5B; n=6). Sagittal sections of these same eyes also show a loss of the eyelid front in the double mutant, and further demonstrate that TOPgal expression is up-regulated along the edge of the dorsal retina in the double mutant (Fig. 5C-D; n=6). Frontal sections of these eyes region reveal that wild-type eyes normally lack TOPgal expression in the perioptic mesenchyme (except for weak activity in the eyelid fold domains) and have very little expression in the dorsal retina, whereas in the double mutant TOPgal expression was positive in the perioptic mesenchyme near where the cornea should form and is much stronger in the dorsal retina (Fig. 5E-F, n=6). Previous studies reported that TOPgal expression is transiently active in the developing mouse retina, but such expression is independent of Wnt/β-catenin signaling (Fuhrmann, 2008). However, TOPgal expression in the perioptic mesenchyme of the double mutant may reflect an increase in Wnt/β-catenin signaling when RA signaling is lost (see Axin2 data below).
Fig. 5.
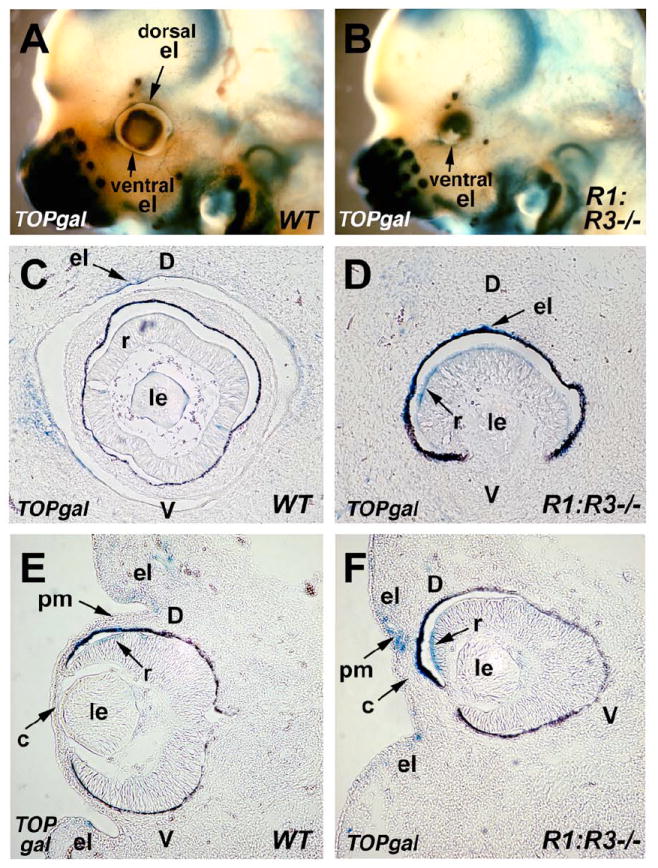
Activation of the Wnt reporter TOPgal in wild-type and RA deficient mouse eyes. (A-B) Staining of whole-mount heads of E12.5 wild-type (WT) and double null (Raldh1-/-;Raldh3-/-) embryos carrying the TOPgal reporter. Arrows indicate TOPgal expression in both the dorsal and ventral eyelid folds of WT, but only in the ventral eyelid fold of the mutant. (C-D) Sagittal sections through the eyes of TOPgal stained E12.5 wild-type (WT) and double null (Raldh1-/-;Raldh3-/-) embryos showing the loss of the normal eyelid folds in the mutant, and ectopic TOPgal expression in the retina. (E-F) Frontal sections showing that the mutant lacks the dorsal eyelid domain, but retains the ventral eyelid domain, and also showing that the mutant has increased TOPgal expression in a central domain of perioptic mesenchyme located where the cornea would normally develop and nearby in the retina. c, cornea; d, dorsal retina; el, eyelid fold; le, lens; pm, perioptic mesenchyme; r, retina; v, ventral retina.
As a more reliable marker of Wnt/β-catenin signaling in perioptic mesenchyme, we examined mRNA expression of Axin2, an gene that is induced by the Wnt/β-catenin pathway (Jho et al., 2002). Axin2 mRNA was detected in the eyelid folds of E12.5 wild type eyes, but was very low in the perioptic mesenchyme and cornea directly adjacent to the optic cup (Fig. 6A; n=6). For the Raldh1-/-;Raldh3-/- double null mutant, Axin2 expression was significantly increased in the perioptic mesenchyme of the cornea and the collapsed dorsal eyelid fold, and expression was increased in the perioptic mesenchyme of the ventral eyelid fold (Fig. 6B; n=6).
Fig. 6.
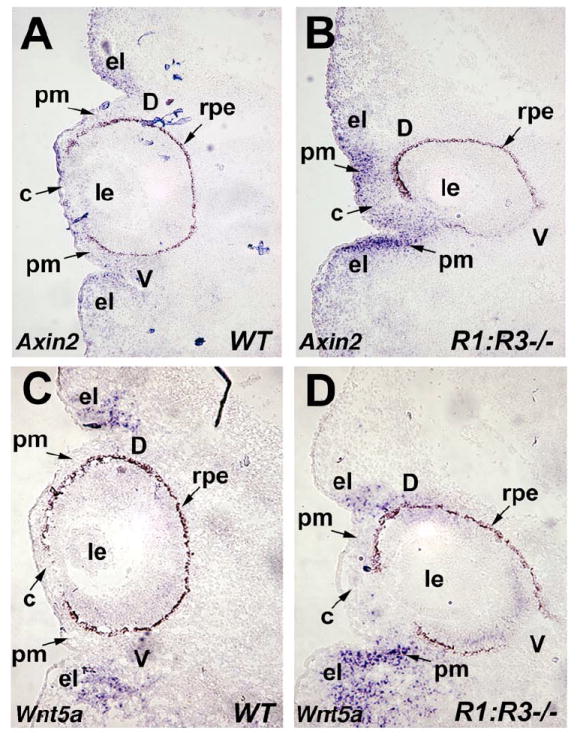
Enhanced expression of Wnt markers in mouse eyes deficient for RA synthesis. (A-B) In situ hybridization with Axin2 antisense riboprobe on frontal sections through the eye of E12.5 wild-type (WT) and double null (Raldh1-/-;Raldh3-/-) embryos. Note that Axin2 expression in the underlying perioptic mesenchyme, cornea, and ventral eyelid fold of R1;R3-/- embryo is greatly increased compared to WT control indicated by arrow. (C-D) Wnt5a mRNA expression in frontal sections of E12.5 wild-type (WT) and double null (Raldh1-/-;Raldh3-/-) eyes. The Wnt5a expression domain in the ventral eyelid fold and perioptic mesenchyme of R1;R3-/- embryo is increased significantly compared to the wild-type control. c, cornea; d, dorsal retina; el, eyelid fold; le, lens; pm, perioptic mesenchyme; rpe, retinal pigment epithelium; v, ventral retina.
Wnt5a encodes a Wnt ligand whose expression is detected in the eyelid folds of the developing mouse eye (Liu et al., 2003). We also observed expression of Wnt5a in the eyelid folds of E12.5 wild-type eyes, and we found that Raldh1-/-;Raldh3-/- double mutant eyes exhibit an expansion of Wnt5a expression in the ventral eyelid mesenchyme, but not in the remnant of the dorsal eyelid fold (Fig. 6C-D; n=6). As the dorsal eyelid fold is still observable in this section of the double mutant, it appears that the Wnt5a-positive dorsal eyelid domain has moved much further ventrally than normal (Fig. 6D). In addition to functioning in the non-canonical Wnt pathway (Angers and Moon, 2009), Wnt5a may also activate or inhibit Wnt/β-catenin signaling depending on the cellular context (Mikels and Nusse, 2006). Thus, our observation of increased expression of both Wnt5a and Axin2 expression in the mesenchyme around the ventral eyelid fold of RA deficient eyes (compare Figs. 6B and 6D) suggests a possible role for Wnt5a in stimulating the observed increase in ocular Wnt/β-catenin signaling.
Discussion
Previous genetic studies on the role of RA in eye development using Raldh gene knockout or RA receptor mutants demonstrated the individual contribution of RA synthesizing enzymes (Fan et al., 2003; Matt et al., 2005; Molotkov et al., 2006) and RA receptors (Lohnes et al., 1994; Ghyselinck et al., 1997; Matt et al., 2008) crucial for ocular morphogenesis. During embryonic eye development, RA signaling controlled by RARβ and RARγ expressed in the perioptic mesenchyme is required for normal morphogenetic movements necessary for invagination of the optic cup, closure of the choroid fissure and mesenchymal growth to correctly generate the cornea and eyelids. RA produced in the neural retina, the retinal pigmented epithelium and the corneal ectoderm by Raldh1 and Raldh3 is secreted and then acts in a paracrine fashion on the perioptic mesenchyme to regulate cell survival during anterior eye formation. Absence of RA signaling results in down-regulation of Pitx2, Foxc1 and Eya2 encoding transcription factors within perioptic mesenchyme required for proper anterior segment morphogenesis. Previous studies on RA deficient models (including Raldh or RAR knockout mice as well as vitamin A deficient rats) suggest Pitx2 is a key effector of RA signaling in the neural crest derived perioptic mesenchyme (Matt et al., 2005; Matt et al., 2008; See and Clagett-Dame, 2009). Global and neural crest specific Pitx2 mutant mice exhibit corneal and eyelid dysgenesis, iris stroma, sclera, thickening of mesenchyme between the lens and cornea, coloboma, and defects in the optic nerve (Evans and Gage, 2005). Importantly, mutations in human PITX2 lead to Axenfeld-Rieger syndrome, a congenital disorder characterized by ocular anterior segment dysgenesis and increased risk of glaucoma (Semina et al., 1996; Kozlowski and Walter, 2000; Weisschuh et al., 2006). In this study, we present evidence that Pitx2 in the perioptic mesenchyme is transcriptionally regulated directly by RA via a conserved DR5 RARE located 4.3 kb upstream of the Pitx2 promoter. Gel-shift and chromatin immunoprecipitation (ChIP) studies in embryonic eye tissue demonstrate that all three RARs are able to recruit to this RARE in vivo, identifying Pitx2 as a direct RA-inducible gene. An earlier study on F9 embryonal carcinoma (EC) cells did not identify Pitx2 as a RA responsive gene in their microarray experiments (Eifert et al., 2006). Also, Pitx2 expression in lateral plate mesoderm of early embryos does not require RA signaling (Niederreither et al., 2001). However, taking into account both expression studies in RA-deficient embryos as well as our RAR ChIP findings in perioptic mesenchyme, it is clear that ocular Pitx2 expression is dependent upon RA signaling for induction and most likely a direct target of RA signaling.
In the present study, we investigated the integrated role of RA and Wnt signaling during embryonic eye development. Several RA and Wnt signaling (canonical and non-canonical) components including enzymes, ligands and receptors are expressed in developing ocular tissues and their mutual interaction in various stages of eye development cannot be neglected. A recent study on the role of Wnt signaling in eye development identified Dkk2 as an important downstream effector of Pitx2 function in perioptic mesenchyme and suggested a Pitx2-Dkk2 auto-regulatory feedback mechanism for proper ocular anterior segment development (Gage et al., 2008). In our genetic study using Raldh1-/-;Raldh3-/- double mutants, Dkk2 expression was severely reduced in the perioptic mesenchyme and developing cornea, suggesting up-regulation of Wnt/β-catenin signaling may have occurred. We also demonstrated an increase in Axin2 expression in the perioptic mesenchyme, cornea, and ventral eyelid fold of Raldh1-/-;Raldh3-/- double mutants. As Axin2 expression is a well-established endogenous readout of the Wnt/β-catenin signaling pathway (Jho et al., 2002), our findings provide strong evidence that a loss of RA signaling in Raldh1-/-;Raldh3-/- double mutants results in an up-regulation of Wnt/β-catenin signaling in mesenchymal ocular tissues. Our findings suggest a model in which cross-talk between RA and Wnt signaling is required for eye morphogenesis with Pitx2 being the intermediary (Fig. 7).
Fig. 7.
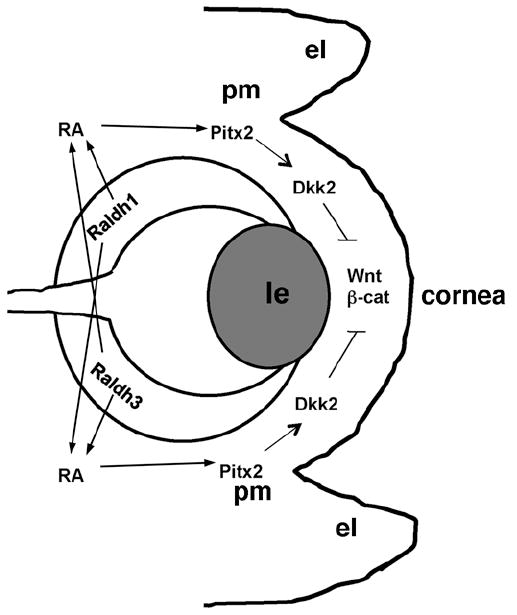
Model for cross-talk between RA and Wnt signaling during eye morphogenesis. Our findings demonstrate that RA synthesized by the dorsal (Raldh1) or ventral (Raldh3) retina is secreted and acts directly with nuclear RA receptors to induce Pitx2 in the perioptic mesenchyme; elimination of both enzymes is necessary to observe ocular defects as RA generated by either Raldh1 or Raldh3 can provide RA to both dorsal and ventral perioptic mesenchyme (Molotkov et al., 2006). Our model further suggests that a major downstream target of RA-induced Pitx2 is Dkk2 whose induction in the perioptic mesenchyme antagonizes Wnt/β-catenin signaling to allow proper morphogenesis of the perioptic mesenchyme, eyelids, and cornea. el, eyelid fold; le, lens; pm, perioptic mesenchyme; RA, retinoic acid.
Earlier studies on the role of Wnts in mouse eye development showed that Wnt5a is expressed in the eyelid fold perioptic mesenchyme (Liu et al., 2003). We observed that Wnt5a expression was up-regulated in RA deficient eyes at E12.5. Although Wnt5a has mostly been associated with non-canonical Wnt signaling (Slusarski et al., 1997), Wnt5a may also activate or inhibit Wnt/β-catenin signaling depending on the context (Mikels and Nusse, 2006). As we observe an increase of both Wnt/β-catenin signaling (Axin2 expression) and Wnt5a expression in the ventral eyelid and surrounding perioptic mesenchyme in RA deficient eyes, we suggest that Wnt5a may activate Wnt/β-catenin signaling in the eye. However, as Wnt5a may down-regulate Wnt/β-catenin signaling under some circumstances (Torres et al., 1996), our observation of increased Wnt5a expression in the same ventral eyelid region where increased Axin2 expression is observed may indicate a response by the RA-deficient eye to dampen the increased Wnt/β-catenin signaling seen in RA-deficient eyes. Our studies have not ruled out that excessive Wnt5a expression may result in excesses in both canonical and non-canonical Wnt signaling, especially as abnormal dorsoventral rotation of the optic cup may be the result of aberrant non-canonical Wnt signaling which is known to effect morphogenetic movements (Angers and Moon, 2009).
Interaction between RA and Wnt signaling pathways have previously been reported during embryogenesis. A recent study demonstrated that loss of RA in Raldh2 null embryos increases Wnt/β-catenin signaling during body axis extension by de-repressing Wnt8a and Wnt3a (Zhao and Duester, 2009). This finding supports earlier studies in vitamin A deficient quail embryos (Olivera-Martinez and Storey, 2007), suggesting RA/Wnt interaction during trunk development is conserved. Another relevant example is the recent demonstration of deregulated RA signaling in the early developing eyes of Wnt co-receptor Lrp6 mutant embryos that exhibit complete loss of Raldh1 expression and ectopic expression of Raldh3 (Zhou et al., 2008). Several putative TCF/LEF binding sites were identified in the Raldh1 and Raldh3 promoter regions indicating the possibility of direct Wnt-mediated regulation of RA synthesis during early eye development (Zhou et al., 2008).
In conclusion, our studies here demonstrate that RA signaling is initially required to activate Pitx2 expression within the perioptic mesenchyme around the optic cup which in turn induces Dkk2 to locally suppress Wnt signaling in this tissue. Such cross-talk between ocular RA and Wnt signaling may be required for proper formation of the anterior segment as well as normal optic cup orientation and eyelid development.
Acknowledgments
We thank the following for mouse cDNAs used to prepare in situ hybridization probes: F. Costantini (Axin2), P. Gage (Dkk2), G. Lemke (Vax2), A. McMahon (Wnt5a), M. Mercola (Pitx2). We also thank Y. Yamaguchi for providing TOPgal mice. This work was funded by National Institutes of Health grant EY013969 (G.D.).
References
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature Rev Mol Cell Biol. 2009;10:468–77. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J Steroid Biochem Mol Biol. 2005;96:347–354. doi: 10.1016/j.jsbmb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Barbieri AM, Broccoli V, Bovolenta P, Alfano G, Marchitiello A, Mocchetti C, Crippa L, Bulfone A, Marigo V, Ballabio A, Banfi S. Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development. 2002;129:805–813. doi: 10.1242/dev.129.3.805. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–31. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifert C, Sangster-Guity N, Yu LM, Chittur SV, Perez AV, Tine JA, McCormick PJ. Global gene expression profiles associated with retinoic acid-induced differentiation of embryonal carcinoma cells. Mol Reprod Dev. 2006;73:796–824. doi: 10.1002/mrd.20444. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S-I, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, Rosenberg KI. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317:310–24. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Dupé V, Dierich A, Messaddeq N, Garnier J-M, Rochette-Egly C, Chambon P, Mark M. Role of the retinoic acid receptor beta (RARb) during mouse development. Int J Dev Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- Gould DB, Smith RS, John SW. Anterior segment development relevant to glaucoma. Int J Dev Biol. 2004;48:1015–1029. doi: 10.1387/ijdb.041865dg. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zheng X. LDL receptor-related proteins 5 and 6 in Wnt/b-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV, Amendt BA, Murray JC. The Pitx2 Protein in Mouse Development. Dev Dyn. 2000;218:195–200. doi: 10.1002/(SICI)1097-0177(200005)218:1<195::AID-DVDY17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Jho E, Zhang T, Domon C, Joo C-K, Freund J-N, Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Kozlowski K, Walter MA. Variation in residual PITX2 activity underlies the phenotypic spectrum of anterior segment developmental disorders. Hum Mol Genet. 2000;9:2131–2139. doi: 10.1093/hmg/9.14.2131. [DOI] [PubMed] [Google Scholar]
- Le Liévre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest, analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Liu H, Mohamed OA, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annual Review of Pharmacology & Toxicology. 2006;46:451–80. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupé V, Garnier J-M, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140–8. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, Ritch R, Koop B, Kuo WL, Collins C, Marshall J, Gould DB, Pearce W, Carlsson P, Enerbäck S, Morissette J, Bhattacharya S, Hogan B, Raymond V, Walter MA. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld Rieger anomaly. Am J Hum Genet. 1998;63:1316–1328. doi: 10.1086/302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Developmental analysis of the retinoic acid-inducible RAR-β2 promoter in transgenic animals. Development. 1991;113:723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270–277. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Mikels A, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLos Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- Mui SH, Hindges R, O’Leary DDM, Lemke G, Bertuzzi S. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development. 2002;129:797–804. doi: 10.1242/dev.129.3.797. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Storey KG. Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development. 2007;134:2125–35. doi: 10.1242/dev.000216. [DOI] [PubMed] [Google Scholar]
- See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325:94–105. doi: 10.1016/j.ydbio.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel Bartelt J, Bierke Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Vieira V, David G, Roche O, de la Houssaye G, Boutboul S, Arbogast L, Kobetz A, Orssaud C, Camand O, Schorderet DF, Munier F, Rossi A, Delezoide AL, Marsac C, Ricquier D, Dufier JL, Menasche M, Abitbol M. Identification of four new PITX2 gene mutations in patients with Axenfeld-Rieger syndrome. Mol Vis. 2006;1:1448–1460. [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Dräger UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Weisschuh N, Dressler P, Schuettauf F, Wolf C, Wissinger B, Gramer E. Novel mutations of FOXC1 and PITX2 in patients with Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci. 2006;47:3846–3852. doi: 10.1167/iovs.06-0343. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods in Enzymology. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Molotkov A, Song L, Li Y, Pleasure DE, Pleasure SJ, Wang YZ. Ocular coloboma and dorsoventral neuroretinal patterning defects in Lrp6 mutant eyes. Developmental Dynamics. 2008;237:3681–9. doi: 10.1002/dvdy.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Duester G. Effect of retinoic acid signaling on Wnt/beta-catenin and FGF signaling during body axis extension. Gene Expr Patterns. 2009;9:430–435. doi: 10.1016/j.gep.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


