Neutrophils and mononuclear phagocytes migrate from blood vessels to various tissue sites after exposure to a variety of stimuli, including antigens, bacterial products, cytokines, and oxidized low-density lipoproteins (1, 2). The ability of these cells to traverse the endothelium and become activated at only specific sites of tissue injury is critical for modulating the host inflammatory response. A better understanding of the signaling pathways in neutrophils and monocytes that are regulated by both soluble factors and the extracellular matrix will be important in further defining the mechanisms that control this process.
One of the most important cytokines involved in the regulation of the host defense is the human granulocyte–macrophage colony-stimulating factor (GM-CSF), which is produced by activated T cells, macrophages, endothelial cells, and fibroblasts (1, 2). GM-CSF initiates its function by binding to its heterodimeric receptor present on myeloid progenitors and mature monocytes, neutrophils, eosinophils, basophils, and dendritic cells (3–6). Distinct GM-CSF-activated signaling pathways (7–9) are critical in regulating the proliferation, differentiation, and maturation of myeloid cells and stimulating macrophage proliferation (10, 11). In addition, GM-CSF primes the respiratory burst and enhances the effector function of mature granulocytes and mononuclear phagocytes.
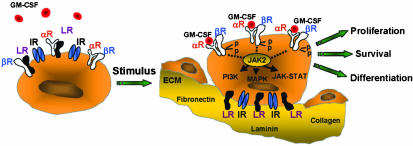
In this issue of PNAS, Chen et al. (12) demonstrate that the laminin receptor, which is expressed on the surface of myeloid cells and mononuclear phagocytes, binds to the GM-CSF receptor to prevent its activation in the absence of tissue injury. Binding of the laminin receptor to extracellular matrix proteins such as laminin relieves this inhibition to stimulate GM-CSF-mediated signal transduction. This is an important observation that provides a potential mechanism to help explain how host defenses can be regulated by the extracellular matrix at sites of tissue injury (Fig. 1). To place this work in its appropriate context, we will review our current knowledge of GM-CSF signaling and discuss how these new observations impact our understanding of this pathway.
Fig. 1.
Proposed mechanism of GM-CSF receptor signal modulation by the laminin receptor (LR). Circulating granulocytes and monocytes are not activated by low levels of GM-CSF due to the binding of the LR to the GM-CSF receptor α (αR) and β (βR) subunits. On transendothelial migration, these cells attach to the basement membrane via interactions of the LR and the integrin receptor (IR) with extracellular matrix (ECM) proteins such as laminin, fibronectin, and collagen. This process relieves the inhibitory effects of the LR on GM-CSF signaling and increases the proliferation, survival, and maturation of myeloid cells and augments their host defense function.
The GM-CSF receptor is a member of the cytokine receptor superfamily that includes the receptors for G-CSF, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-11, IL-15, growth hormone, erythropoietin, prolactin, and leptin (13). These receptors consist of multiple subunits, one of which is cytokine specific. For example, the GM-CSF receptor is comprised of two subunits, designated α and β, whose association is increased by GM-CSF binding (3, 4). The isolated α receptor binds to GM-CSF with low affinity and forms a complex with the β receptor to create the high affinity receptor. Evidence suggests that the GM-CSF receptor exists as an α2β2 tetrameric complex in the ligand-bound state although the role of a simple αβ complex cannot be ruled out (1). The organization of the GM-CSF receptor is similar to the receptors for two other hematopoietic growth factors, IL-3 and IL-5 (5, 6). Each of these receptors comprises a unique ligand-specific α-subunit and a shared β-subunit, which is the more critical of the subunits in cytokine-mediated signaling. However, the α receptor is required for signaling by the β receptor and is also necessary for regulating cell proliferation and differentiation (10). Given this similar pattern of receptor organization, it is not surprising that IL-3, IL-5, and GM-CSF can stimulate these related cytokine pathways. For example, changes in the levels of IL-3 or IL-5 can alter GM-CSF-mediated signaling via competition for the common β-subunit (13, 14).
Neither the GM-CSF receptor nor other members of the cytokine receptor superfamily possess intrinsic enzymatic activity to mediate their intracellular signaling (1, 13). The organization of these receptors includes extracellular domains characterized by 200-aa modules containing two fibronectin-like beta barrel structures, a single transmembrane domain, and an intracellular domain (15). The α subunit consists of a cytoplasmic domain of limited size, whereas the β subunit consists of a more extensive intracellular domain with multiple tyrosine residues that are targets for phosphorylation by tyrosine kinases including Janus kinase (JAK), to result in the recruitment of Src homology 2 (SH2)-containing proteins to this receptor subunit (14). After ligand binding and the assembly of the high affinity GM-CSF receptor, tyrosine kinases such as JAK2 are recruited to the intracellular domain of the β receptor to activate the JAK/signal transducer and activator of transcription (STAT), ras/mitogen-activated protein (MAP) kinase and phosphatidylinositol 3-kinase (PI3-kinase) pathways, leading to increases in the proliferation, maturation, survival, and activation of myeloid and monocyte lineages (1, 7–9, 13). Because a subset of GM-CSF receptors preexists as heterodimers in the absence of ligand binding (14), mechanisms are likely operative that prevent the activation of this receptor in the absence of tissue injury. Data presented in the article by Chen et al. demonstrate that the binding of the laminin receptor to the GM-CSF α- and β-subunits is involved in attenuating activation of this receptor in the presence of low levels of its ligand.
The laminin receptor is a non-integrin cell surface receptor that helps to mediate high affinity interactions between cells and components of the extracellular matrix such as laminin, fibronectin, collagen, and elastin (16–19). This receptor is evolutionarily conserved and has significant homology to ribosomal proteins with its carboxyl-terminal domain facilitating its interaction with extracellular matrix proteins including laminin (18). The laminin receptor is a 37-kDa protein that is acylated by fatty acids to result in a 67-kDa homodimer that mediates its binding to laminin and other extracellular matrix proteins (17). The expression of this receptor is induced by both cytokines and inflammatory mediators and laminin itself, suggesting that a positive feedback loop may link the presence of laminin with the expression of its receptor (18). In addition, a variety of cancers overexpress this receptor, and this result correlates with increased tumor aggressiveness and metastatic behavior (20, 21). It is interesting to note that the laminin receptor also serves as the receptor for the cellular prion protein (PrP).
The laminin receptor does not function alone, but rather in conjunction with other receptors, to facilitate its interactions with laminin and other components of the extracellular matrix. For example, a physical association of the laminin receptor and α6β4 integrins has been demonstrated, and the expression of the laminin receptor and specific integrins is coregulated (22). Studies suggest that the interaction of the laminin and integrin receptors with the extracellular matrix is subject to dynamic regulation that alters their interactions and function (19).
Hematopoietic signaling is activated by growth factors and cellular interactions with the extracellular matrix.
Chen et al. (12) present data that suggest that components of the extracellular matrix interact with the laminin receptor to regulate cytokine receptor signaling. A well characterized role of the extracellular matrix in regulating cell signaling has been demonstrated through its interactions with integrins to initiate a focal adhesion kinase (FAK)-mediated process that activates the MAP kinase pathway and enhances cell motility (23). In the present study, laminin and fibronectin were demonstrated to stimulate GM-CSF-mediated signal transduction. The observation that GM-CSF signaling can be activated by the interactions of laminin and fibronectin with the laminin receptor is consistent with previous observations that demonstrate that neutrophil binding to laminin and fibronectin markedly enhances the GM-CSF-mediated respiratory burst (24).
The current study has several important implications. First, it defines an additional regulatory mechanism that is operative in regulating GM-CSF signaling. Second, it provides evidence that changes in the extracellular matrix can influence host defense responses. Finally, it provides an explanation of how neutrophils and monocytes can be activated at specific sites of tissue injury as they traverse the vascular wall. This work suggests that, in response to tissue injury, GM-CSF signaling of neutrophils and macrophages is activated by a binary response involving both soluble growth factors and the interactions of cells with the extracellular matrix.
With any new observations, a variety of additional questions are raised. For example, which of the GM-CSF subunits, α or β, is the major target for interaction with the laminin receptor? In addition to the laminin receptor, are specific integrins also involved in the regulation of GM-CSF receptor signaling? Does the laminin receptor mediate GM-CSF signaling in multiple cell lineages and is IL-3 and IL-5 receptor signaling also regulated by similar mechanisms? Finally, are there coordinate patterns of regulation of the laminin receptor and specific cytokine receptors in pathologic conditions such as cancer and atherosclerosis? Future studies will be necessary to address the interesting and important observations elucidated by Chen et al. in this study.
See companion article on page 14000.
References
- 1.Guthridge, M. A., Stomski, F. C., Thomas, D., Woodcock, J. M., Bagley, C. J., Berndt, M. C. & Lopez, A. F. (1998) Stem Cells 16, 301–313. [DOI] [PubMed] [Google Scholar]
- 2.Gasson, J. C. (1991) Blood 77, 1131–1145. [PubMed] [Google Scholar]
- 3.Gearing, D. P., King, J. A., Gough, N. M. & Nicola, N. A. (1989) EMBO J. 8, 3667–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashida, K., Kitamura, T., Gorman, D. M., Arai, K., Yokota, T. & Miyajima, A. (1990) Proc. Natl. Acad. Sci. USA 87, 9655–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavernier, J., Devos, R., Cornelis, S., Tuypens, T., Van der Heyden, J., Fiers, W. & Plaetinck, G. (1991) Cell 66, 1175–1184. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura, T., Sato, N., Arai, K. & Miyajima, A. (1991) Cell 66, 1165–1174. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki, K., Hino, M., Hato, F., Tatsumi, N. & Kitagawa, S. (1999) Blood 93, 341–349. [PubMed] [Google Scholar]
- 8.Quelle, F. W., Sato, N., Witthuhn, B. A., Inhorn, R. C., Eder, M., Miyajima, A., Griffin, J. D. & Ihle, J. N. (1994) Mol. Cell. Biol. 14, 4335–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhar-Mascareno, M., Chen, J., Zhang, R. H., Carcamo, J. M. & Golde, D. W. (2003) J. Biol. Chem. 278, 11107–11114. [DOI] [PubMed] [Google Scholar]
- 10.Matsugachi, T., Zhao, Y., Lilly, M. B. & Kraft, A. S. (1997) J. Biol. Chem. 272, 17450–17498. [DOI] [PubMed] [Google Scholar]
- 11.Doyle, S. E. & Gasson, J. C. (1998) Blood 92, 867–876. [PubMed] [Google Scholar]
- 12.Chen, J., Cárcamo, J. M., Bórquez-Ojeda, O., Erdjument-Bromage, H., Tempst, P. & Golde, D. W. (2003) Proc. Natl. Acad. Sci. USA 100, 14000–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D' Andrea, R. J. & Gonda, T. J. (2000) Exp. Hemat. 28, 231–243. [DOI] [PubMed] [Google Scholar]
- 14.Woodcock, J. M., McClure, B. J., Stomski, F. C., Elliott, M. J., Bagley, C. J. & Lopez, A. F. (1997) Blood 90, 3005–3017. [PubMed] [Google Scholar]
- 15.Bazan, J. F. (1990) Proc. Natl. Acad. Sci. USA 87, 4670–4674.2162053 [Google Scholar]
- 16.Castronovo, V., Claysmith, A. P., Barker, K. T., Cioce, V., Krutzsch, H. C. & Sobel, M. E. (1991) Biochem. Biophys. Res. Commun. 177, 177–183. [DOI] [PubMed] [Google Scholar]
- 17.Landowski, T. H., Dratz, E. A. & Starkey, J. R. (1995) Biochemistry 34, 11276–11287. [DOI] [PubMed] [Google Scholar]
- 18.Menard, S., Castronovo, V., Tagliabue, E. & Sobel, M. E. (1997) J. Cell Biochem. 67, 155–165. [PubMed] [Google Scholar]
- 19.Aumailley, M. & Gayraud B. (1998) J. Mol. Med. 76, 253–265. [DOI] [PubMed] [Google Scholar]
- 20.Barsky, S. H., Rao, C. N., Williams, J. E. & Liotta, L. A. (1984) J. Clin. Invest. 74, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martignone, S., Menard, S., Bufalino, R., Cascinelli, N., Pellegrini, R., Tagliabue, E., Andreola, S., Rilke, F. & Colnaghi, M. I. (1993) J. Natl. Cancer Inst. 85, 398–402. [DOI] [PubMed] [Google Scholar]
- 22.Ardini, E., Tagliabue, E., Magnifico, A., Buto, S., Castronovo, V., Colnaghi, M. I. & Menard, S. (1997) J. Biol. Chem. 4, 2342–2345. [DOI] [PubMed] [Google Scholar]
- 23.Hsia, D. A., Mitra, S. K., Hauck, C. R., Streblow, D. N., Nelson, J. A., Ilic, D., Huang, S., Li, E., Nemerow G. R., Leng, J., et al. (2003) J. Cell Biol. 160, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan, C. F. (1989) Blood 73, 301–306. [PubMed] [Google Scholar]