Abstract
Until recently the brain was studied almost exclusively by neuroscientists and the immune system by immunologists, fuelling the notion that these systems represented two isolated entities. However, as more data suggest an important role of the immune system in regulating the progression of brain aging and neurodegenerative disease, it has become clear that the crosstalk between these systems can no longer be ignored and a new interdisciplinary approach is necessary. A central question that emerges is whether immune and inflammatory pathways become hyperactivated with age and promote degeneration or whether insufficient immune responses, which fail to cope with age-related stress may contribute to disease. We try to explore here the consequences of gain- versus loss-of-function with an emphasis on microglia as sensors and effectors of immune function in the brain and we discuss the potential role of the peripheral environment in neurodegenerative diseases.
Keywords: neurodegenerative disease, neuroimmunology, neuroinflammation, microglia
Introduction
More than a half century ago Peter Medawar noted a curious phenomenon. When heterologous tissues were transplanted into the CNS he noticed they were spared from immunological rejection (Medawar, 1948). This finding led him to speculate that the brain and immune system existed as two isolated systems. Until recently, this dogma was generally accepted and brain immune responses were only studied in a few “immune-mediated” diseases. However, epidemiological studies revealing that long-term use of anti-inflammatory drugs reduces the risk for Alzheimer’s and Parkinson’s disease by roughly half hinted at a role for inflammation in classical age-related neurodegeneration (Chen et al., 2005; McGeer et al., 1996; Vlad et al., 2008). Growing evidence now suggests that the brain and immune system are intricately connected and engage in significant crosstalk to maintain homeostasis. Indeed, immune cells and mediators are routinely found in the CNS under normal and pathological states, while neurons are capable of interacting with and regulating immune cells. Neurodegenerative diseases also exhibit extensive microglial activation (the immune cells of the brain). Elegant studies combining mouse models that have various immune defects (developed by immunologists) with transgenic models for neurodegenerative diseases further implicate the immune system in disease progression by showing that lymphocytes play a role in PD, amyotrophic lateral sclerosis (ALS), and possibly AD. Interestingly, normal aging also exhibits immune activation and cell infiltration in the brain. Despite these commonalities, the role of the immune system in aging and neurodegenerative disease remains unclear. Here we discuss some of the new and exciting findings that provide insight into how the immune system affects neurodegenerative disease and aging. Specifically, we will discuss how the immune system is activated and regulated in an aged and injured brain and whether impaired immune function may lead to the accumulation of protein aggregates in neurodegenerative disease.
Microglia: a sensor of brain injury and aging
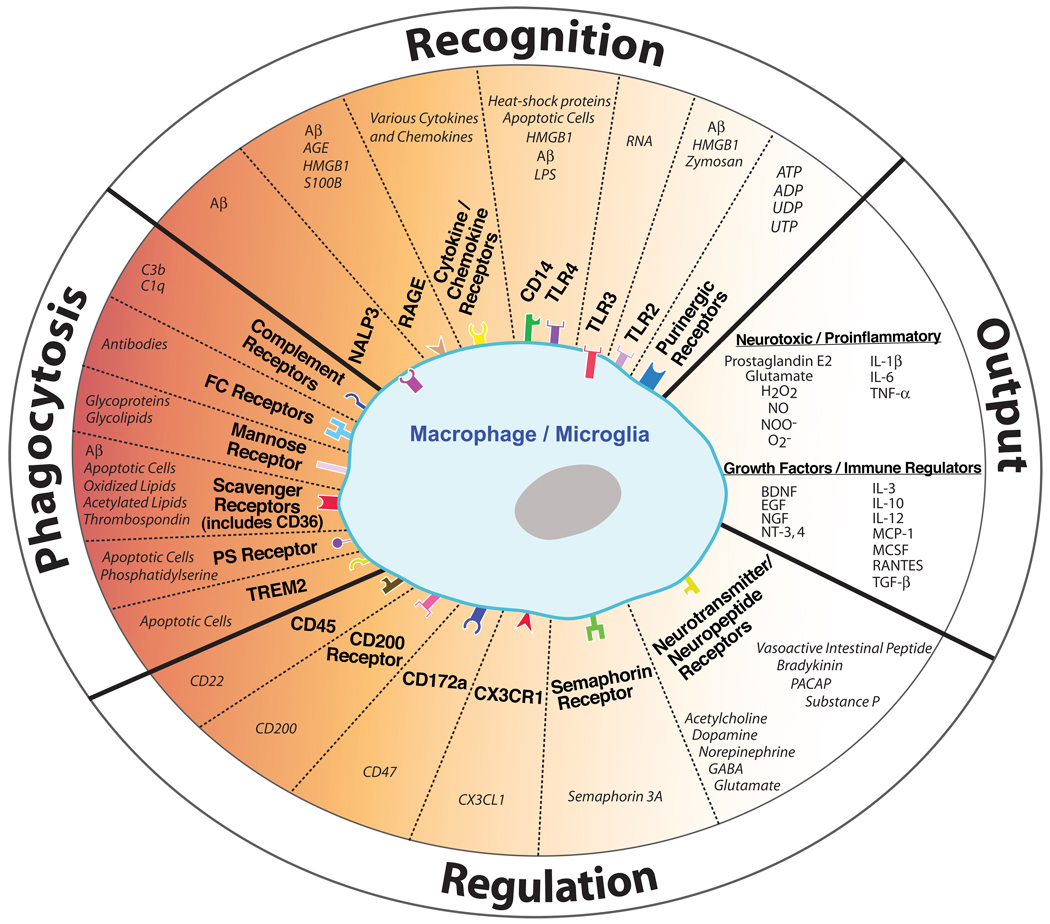
A basic feature of all living organisms is the ability to detect potentially harmful stimuli and react accordingly. In the brain, glial cells constantly survey the microenvironment for noxious agents or injurious processes (Nimmerjahn et al., 2005). This is primarily done by microglia, a type of glial cell derived from myeloid precursors in the bone marrow that populate the CNS during development. Microglia are the resident immune cells of the brain and are endowed with numerous receptors capable of detecting physiological disturbances (See Figure 1). When neurons are injured in aging or neurodegeneration, microglia become activated via the release of ATP, neurotransmitters, growth factors or cytokines, ion changes in the local environment, or loss of inhibitor molecules displayed by healthy neurons (Hanisch and Kettenmann, 2007). Microglia are also alerted that something is wrong when they encounter molecules not normally found in the healthy CNS, such as blood clotting factors, intracellular constituents released by necrotic cells (i.e., RNA, DNA), externalized phosphatidylserine on apoptotic cells, immunoglobulin-antigen complexes, opsonizing complement, abnormally folded proteins (i.e., protein aggregates), or pathogen-related structures (Hanisch and Kettenmann, 2007). Upon activation, microglia may proliferate and undergo a morphological transformation from a ramified to amoeboid appearance. Based on the activation stimuli, microglia are informed of the encountered problem and instructed to act in an appropriate manner and perform a defined task (e.g., clear apoptotic cells or abnormal proteins). If the disturbance is relatively minor (e.g., supporting a stressed neuron), microglia may secrete anti-inflammatory cytokines and supportive growth factors. If the disturbance poses a serious threat, such as a pathogen invasion, microglia may release toxic factors to kill the pathogen and recruit help by releasing proinflammatory cytokines (Figure 1). Depending on the environment in which microglia are activated, they can either take on a “classically activated” (also called M1) phenotype or an “alternatively activated” (also called M2) phenotype (Mantovani et al., 2004). M2 microglia are typically considered less inflammatory than M1 cells and are characterized by reduced nitric oxide production and increased anti-inflammatory cytokine production. Accordingly, microglial heterogeneity exists during neurodegenerative disease and may influence disease outcome (Colton et al., 2006; Maier et al., 2008). Given the continuum of potential microglial responses, how a given insult is interpreted can mean the difference between a beneficial outcome or a detrimental outcome if the response is either too aggressive or too passive.
Figure 1. Regulation of microglia/macrophages in the CNS.
Microglia/macrophages are activated following the stimulation of various recognition or phagocytic receptors. This activation state is subsequently controlled by neurons, which secrete or express numerous regulatory ligands. The end result of these interactions is the release of cytokines, neurotoxic substrates, and/or growth factors by microglia/macrophages, or the activation of cellular pathways including phagocytosis. Aberrant function of these pathways can result in significant degeneration during aging or disease. For an extensive review of cytokines and chemokines recognized by microglia, refer to (Hanisch, 2002). Aβ, amyloid-β; ADP/ATP, adenosine di/triphosphate; AGE, advanced glycation end product; BDNF, brain-derived neurotrophic factor; EGF, epidermal growth factor; GABA, gamma-aminobutyric acid; H2O2, hydrogen peroxide; HMGB1, High-mobility group box 1; IL-1β, interleukin 1β; LPS, lipopolysaccharide; MCP-1, monocyte chemotactic protein-1; MCSF, macrophage colony-stimulating factor; NGF, nerve growth factor; NO, nitric oxide; NOO−, peroxynitrite; NT-3,4, neurotrophin-3,4; O2−, superoxide; PACAP, pituitary adenylate cyclase-activating peptide; PS, phosphatidylserine; RAGE, receptor for advanced glycation end products; RANTES, regulated upon activation, normal T cell expressed and secreted; TGF-β, transforming growth factor-β; TLR, toll-like receptor; TNFα, tumour necrosis factor-α; TREM2, triggering receptor expressed by myeloid cells-2; UDP/UTP, uridine di/triphosphate. Receptors displayed inside the cell represent intracellular receptors. Different receptor shapes are not meant to represent actual receptor structures.
PAMPs and DAMPs sound the alarm
In order to detect and interpret potential insults, the immune system has evolved an ingenious set of receptors that detect small molecular motifs consistently found on pathogens or factors associated with tissue damage. Detection of these exogenous pathogen-associated molecular patterns (PAMPs) or endogenous danger-associated molecular patterns (DAMPs) is accomplished by a vast array of highly conserved pattern recognition receptors (Janeway and Medzhitov, 2002; Seong and Matzinger, 2004). While toll-like receptors (TLRs) have received the most attention for their ability to recognize both classes of these molecular patterns (see below), many other receptors that recognize specific molecular patterns have been described (e.g., complement, mannose, scavenger, C-type lectin, nucleotide-binding oligomerization domain (Nod)-like (including NALP3 and NOD2), and retinoic acid-inducible gene I (RIG-I)-like helicase receptors) (Palm and Medzhitov, 2009). Additional receptors also exist that appear to selectively recognize DAMPs, including CD24 and receptor for advanced glycation end-products (RAGE) (Arancio et al., 2004; Chen et al., 2009).
To date, 13 TLRs have been described which collectively recognize various PAMPs essential for pathogen survival (reviewed by (Iwasaki and Medzhitov, 2004)). TLRs are expressed on a variety of immune cells, including macrophages, dendritic cells, B cells, specific types of T cells, and even non-immune cells such as epithelial cells, fibroblasts, and neurons. Stimulation of TLRs induces a well-characterized signalling cascade, culminating in the activation of nuclear factor κB and subsequent transcriptional activation of numerous pro-inflammatory genes, encoding cytokines, chemokines, complement proteins, enzymes (such as cyclooxygenase 2 and the inducible form of nitric oxide synthase), adhesion molecules and immune receptors (Nguyen et al., 2002). In face of an invading pathogen, these factors allow for a robust and coordinated immune response capable of eradicating infectious pathogens. While this approach is highly effective for eliminating pathogens, it comes at the expense of extensive bystander injury. Indeed, CNS microinjections of the TLR 2 or 4 ligands zymosan and LPS, respectively, cause robust glial activation and elicit substantial neurodegeneration (Lehnardt et al., 2003; Popovich et al., 2002). This becomes increasingly problematic when one considers that TLRs can also be engaged by DAMP-containing molecules released by CNS trauma or disease (e.g., heat-shock proteins (HSPs), high-mobility-group box protein 1 (HMGB1), fibrinogen, hypomethylated mammalian DNA, and RNA released from necrotic cells) (Kariko et al., 2004; Leadbetter et al., 2002; Ohashi et al., 2000; Smiley et al., 2001; Viglianti et al., 2003). In support of this, TLR4-deficient mice have smaller infarct volumes and better functional outcomes than control mice in a sterile model of experimental stroke (Caso et al., 2007). One explanation for this finding is that HSP60, a molecule released from CNS cells undergoing necrotic or apoptotic cell death, activates microglia via TLR4 and causes enhanced nitric oxide production and neuronal cell death (Lehnardt et al., 2008). Interestingly, TLR2 and -4 are upregulated on cerebral cortical neurons in response to ischemia/reperfusion injury and their absence protects primary neuron cultures from energy deprivation-induced cell death (Tang et al., 2007). Given these observations it is tempting to speculate that the concerted increase in expression of multiple TLRs in the aging brain (Berchtold et al., 2008; Letiembre et al., 2007) may generate a hypersensitive state of glia and neurons and thus magnify potential bystander injury. Exactly how neuronal TLRs promote neurodegeneration and the identity of their ligands is currently unclear. Additional studies are required to elucidate these mechanisms using more precise neuron-specific TLR deficient mice.
While ischemia and subsequent necrosis may initiate neuroinflammation and provoke further neurodegeneration in models of acute neurological disease and trauma, they are unlikely to play a role in chronic neurodegenerative disorders where necrotic conditions are uncommon. Instead, abnormal protein assemblies can function as a possible trigger for cellular stress and neuroinflammation. Most neurodegenerative disorders are associated with the accumulation of abnormal protein assemblies (proteopathies) (Orr and Zoghbi, 2000; Sherman and Goldberg, 2001; Walker and LeVine, 2000) some of which result from specific genetic mutations. A common feature of these abnormal protein aggregates, which may occur inside cells or in the extracellular space, is the existence of β-sheets, as detected by staining with Congo red or related dyes and referred to as amyloid. Interestingly, bacteria produce similar amyloidogenic aggregates on their cell surface that bind Congo red (Chapman et al., 2002; Hammer et al., 2007). This begs the question of whether amyloid structures act as PAMPs and are interpreted as invading pathogens that elicit an aggressive neurotoxic response. In line with this hypothesis, Aβ is known to bind the TLR4 co-receptor CD14 (Fassbender et al., 2004; Liu et al., 2005) and can trigger microglia to secrete nitric oxide, interleukin (IL)-6 and other neurotoxic factors (Walter et al., 2007). Consistent with this, levels of tumour necrosis factor (TNF)-α and macrophage inflammatory protein (MIP)-1β are significantly higher in the brains of AD mice with wild-type TLR4 than their mutant TLR4 AD counterparts (Jin et al., 2008). On the other hand, TLR-mediated activation of microglia may also aid in the clearance of Aβ. Studies in AD mouse models with mutant TLR2 or TLR4 suggest deficiencies in TLR signalling causes cognitive impairments with a concomitant increase in Aβ deposition (Richard et al., 2008; Tahara et al., 2006). Likewise, acute administration of the TLR4 ligand LPS can promote Aβ clearance (DiCarlo et al., 2001), and studies in mouse models of ALS suggest that TLR signalling is protective in disease progression (Kang and Rivest, 2007). Whether these receptors normally exacerbate neurodegeneration or guide the clearance of protein aggregates is still largely unknown and many studies, particularly with Aβ, do not consider the aggregation state of the peptide. It is thus likely that different receptors will be engaged by monomers, oligomers, or fibrillar assemblies of Aβ and other amyloidogenic peptides eliciting different types of responses in microglia (and other cells).
The complex life of complement
Complement proteins represent another line of defence in recognizing PAMPs and DAMPs and act as secreted pattern recognition receptors. Using a collagen-like receptor binding domain and multiple globular domains, complement binds molecular patterns on microbes, dying cells, and abnormal protein aggregates (Tenner, 1999). Although the liver is the major source of complement, neurons and glia can make nearly all of the 30 different proteins involved in the complement cascade. During AD (Akiyama et al., 2000; Gasque et al., 2000), HD (Singhrao, 1999), PD (Yamada et al., 1992), and prion disease (Dandoy-Dron et al., 1998) levels of various complement components increase. An increase in complement proteins C1q, mannose binding lectin, and C3 can all initiate neuroinflammatory processes by activating inflammatory cells, promoting their migration, upregulating phagocytosis, and facilitating lysis by the membrane attack complex (Holers, 1996; Song et al., 2000). In AD, complement products, including the membrane attack complex, colocalize with amyloid plaques and tangle bearing neurons (Webster et al., 1997). APP mouse models where C3 activation is inhibited, via overexpressing soluble complement receptor-related protein y (sCrry), find an increase in Aβ deposition and an accumulation of degenerating neurons (Wyss-Coray et al., 2002). In support of this, APP mice with a complete knockout of C3 also show increased Aβ and neuronal degeneration, accompanied by changes in microglial activation (Maier et al., 2008). These data suggest C3 may work with microglia to effectively clear Aβ. Alternatively, C3 may act in the periphery to clear circulating Aβ by creating a peripheral sink, as previously proposed (DeMattos et al., 2001). Indeed, studies show that Aβ binds to complement receptor 1 on red blood cells in a C3-dependent manner and that AD patients in their earliest stages have significantly less Aβ bound to red blood cells (Rogers et al., 2006). Because the genetic manipulations were systemic in the previously mentioned studies (Wyss-Coray et al., 2002) (Maier et al., 2008), this theory cannot be ruled out. Studies using sCrry under the GFAP or hepatocyte-specific transthyretin promoters could shed light on this question.
But again, complement proteins like other immune factors can have detrimental effects. APP mice lacking complement C1q had less neuronal damage than complement sufficient mice suggesting a role for C1q in neuronal integrity (Fonseca et al., 2004). Indeed, C1q and C3 have recently been shown to mediate synaptic pruning during development and in a model of glaucoma opening the possibility that complement factors may contribute to aberrant elimination of synapses in neurodegenerative diseases (Stevens et al., 2007).
Microglia in neurodegeneration: repairman or reckless killer?
Maybe 90% of published articles on microglia in neurodegeneration link activation of these cells with pro-inflammatory cytokine production and execution of neuronal cell death. Thus, microglia are thought to be responsible for killing dopaminergic cells in PD (Mount et al., 2007) and forebrain neurons in AD, and they have been shown to contribute to neurodegeneration in SOD1 transgenic mouse models for ALS (Boillee et al., 2006). Microglial-derived factors capable of inducing neuron death are numerous and range from reactive oxygen species to pro-inflammatory cytokines (see Figure 1). TNF-α is one such factor that has received much attention because of its ability to promote PD progression (McCoy et al., 2006), while TNF receptor 1 knockout protects against AD- and PD-like disease in mice (He et al., 2007; Sriram et al., 2002). Inhibiting the neurotoxic potential of pro-inflammatory cytokines is also likely to contribute to the beneficial effects of NSAIDs on neurodegenerative diseases. Interestingly, ablating reactive microglia by 50% in a model of ALS has no effect on motor neuron degeneration, suggesting deleterious microglial function may be context dependent (Gowing et al., 2008). Clearly in most cases hyperactivated microglia can be deleterious and must be regulated to prevent neuronal damage.
Keeping the beast in check: neurons as tamers of microglia
Interestingly, the brain has evolved various safety measures to keep microglia in check (Figure 1). These mechanisms allow neurons to downregulate microglial function until neuronal health is compromised. For example, CD200 is a membrane glycoprotein expressed by neurons that interacts with its cognate receptor on microglia (CD200R) to deliver regulatory signals. When mice are genetically deficient in CD200, microglial reactivity is enhanced under normal and pathological conditions (i.e., EAE and facial nerve axotomy) (Hoek et al., 2000). Similar to CD200, the integrin-associated protein CD47 is expressed by neurons and when bound to its receptor on microglia (SIRPα; CD172a) inhibits TNF-α release and phagocytosis (Oldenborg et al., 2001; Smith et al., 2003). These regulatory molecules appear to be relevant to humans as well given that both CD47 and CD200 are downregulated in the center of chronic active and inactive human MS lesions, suggesting a possible role in propagating microglial activation and proinflammatory cytokine release in MS lesions (Koning et al., 2007). Fractalkine (CX3CL1) is another ligand expressed by neurons that restricts microglial activation when bound to its receptor (CX3CR1) (Cardona et al., 2006). This ligand-receptor interaction is absent when neurons die or are damaged, resulting in hyper-responsive microglia. Accordingly, in mouse models of Parkinson’s disease or ALS, lack of CX3CR1 leads to extensive neuronal cell loss (Cardona et al., 2006). Microglia also express CD14, CD36, and phosphatidylserine receptors that, when bound to specific ligands on apoptotic cells (i.e., neurons), trigger inhibitory intracellular signals that reduce nitric oxide and proinflammatory cytokine release, while concomitantly increasing anti-inflammatory cytokine production (Savill et al., 2002). Triggering receptor expressed by myeloid cells-2 (TREM2) is an additional receptor on microglia that promotes phagocytosis and retards inflammation upon binding unknown ligands on apoptotic cells (Hsieh et al., 2009). When neurons are stressed, they can secrete Semaphorin 3A and induce apoptosis of activated microglia, which express upregulated levels of the semaphorin receptors plexin-A1 and neuropilin-1 (Majed et al., 2006). This response is thought to protect neurons from further microglia-mediated damage. Neurons also release the anti-inflammatory neuropeptide CD22 from their nerve terminals, which regulates microglia activation and proinflammatory cytokine production via its receptor CD45 (Mott et al., 2004). Various secreted neurotransmitters have also shown similar modulatory effects on microglia (Pocock and Kettenmann, 2007) and suppressing spontaneous neuronal activity increases immune reactivity (Neumann et al., 1996). It should be noted that these regulatory mechanisms may prevent damage, but the extent to which they inhibit inherent repair mechanisms is unclear. More sophisticated tetracycline-inducible promoters or pharmacological tools are required to address whether intervening with these processes at defined time points may provide significant therapeutic benefit.
Do changes in brain and microglial function with age promote neurodegeneration?
Although microglia are typically regarded as robust pro-inflammatory minutemen, microglia existing within an aging environment may be a different beast altogether. Aging is the strongest risk factor for neurodegenerative diseases and while aging is not considered a disease, it results in a significant increase in glial activation (see Figure 2), complement factors, inflammatory mediators, and brain atrophy (Lu et al., 2004; Streit et al., 2008; West et al., 1994). Microarrays of aged human and mouse brains extend these findings by showing that genes related to cellular stress and inflammation increase with age while genes related to synaptic function/transport, growth factors, and trophic support decrease (Lee et al., 2000; Lu et al., 2004). These global changes paint a bleak picture of the aged brain and suggest neurons encounter increased challenges with age, but receive reduced support. To make matters worse, neurogenesis also decreases with age- possibly as a result of factors secreted by activated microglia (i.e., IL-6) (Reviewed in detail in Carpentier and Palmer, this issue; also see Monje et al., 2003). It is unclear why inflammation increases with age, but genetic studies suggest an important role of DNA damage caused by increased reactive oxygen species (Lu et al., 2004). Also unclear is to what extent aging affects the ability of microglia to respond or contribute to neuronal loss. Despite morphological and phenotypic changes that indicate microglial activation (Figure 2), it has been proposed that microglia may actually become dysfunctional and enter a senescent state with age (Streit et al., 2008). Such a state may cause microglia to secrete diminished levels of neurotrophic factors and to downregulate phagocytic function. This, coupled with increased secretion of inflammatory mediators, may lead to neuronal loss and inefficient clearance of toxic protein aggregates in neurodegenerative disease.
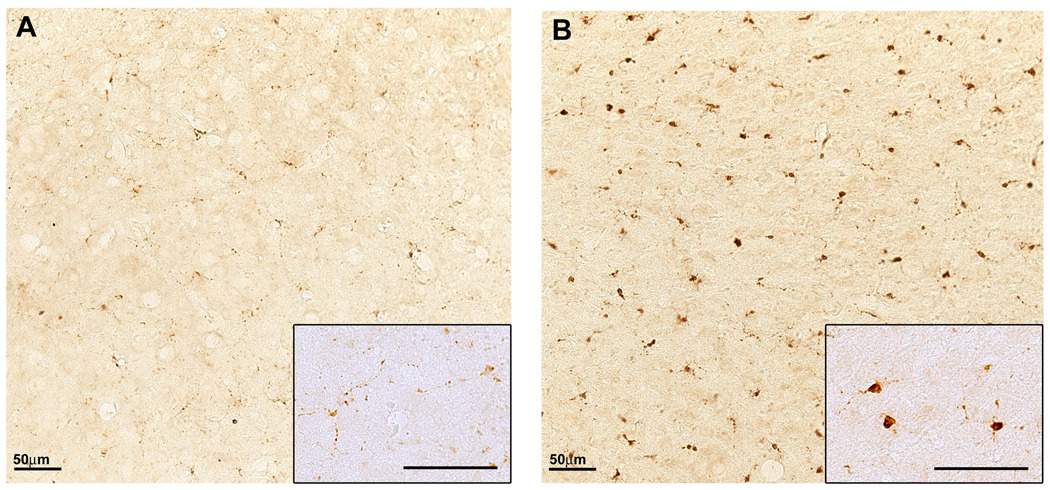
Figure 2. Increased microglial activation in the aged brain.
Immunostaining for the endosomal/lysosomal enzyme CD68, a marker of the microglia/macrophage lineage, shows a prominent increase in the neocortex of 24-month-old C57BL/6 mouse (B) compared with a 6-month-old mouse (A) consistent with increased microglial activation in aged brains. Inserts show individual microglia under higher magnification where hypertrophied cell bodies are evident in aged brains (compare insert A to B). Scale bars represent 50µm under 20 or 40× magnification.
Impaired trash removal as a risk factor for neurodegeneration?
The accumulation of protein aggregates in neurodegenerative diseases may occur from enhanced production of abnormal proteins or abnormal folding. Alternatively, accumulating protein aggregates may result from insufficient clearance. Recent evidence suggests impaired autophagy is one mechanism that influences the clearance of protein aggregates and neurodegenerative disease pathogenesis (Pickford et al., 2008; Ravikumar et al., 2004). Autophagy (also known as macroautophagy) is the major pathway involved in the degradation of long-lived proteins (Klionsky and Emr, 2000). Autophagy is highly conserved in all species and cell types studied thus far. It is initiated by the formation of a cup-shaped isolation membrane around cytosolic components, which eventually encloses to form a double membrane vesicle (two bi-layered membranes) (Wang and Klionsky, 2003). This autophagosome undergoes several microtubule-dependent maturation events before eventually fusing with lysosomes, where cytosolic contents are degraded (Berg et al., 1998). Autophagy is impaired in AD (Nixon et al., 2005) and is implicated in the clearance of protein aggregates associated with AD, PD, and HD (Pickford et al., 2008; Ravikumar et al., 2004; Webb et al., 2003). The exact mechanism by which protein aggregates are targeted during autophagy is unclear. One possible link for intracellular aggregates is the protein, sequestosome 1 (SQSTM1, also known as p62), which binds both ubiquitin (protein aggregates are often poly-ubiquitinated) and the autophagosome protein MAP1LC3 (Pankiv et al., 2007). Also unclear is which cell type(s) are influenced by autophagy to regulate levels of protein aggregates. Given that many protein aggregates have an intraneuronal component that may precede extracellular deposition, neurons are likely a target of critical importance (for further review see (Jaeger and Wyss-Coray, 2009)). However, emerging evidence also indicates a link between autophagy and phagocytic function in microglia/macrophages (see below).
Extracellular clearance of debris is classically attributed to phagocytes such as the peripheral macrophage, which literally means “big eater” (derived from the Greek words makros "large" + phagein "eat"). These bone marrow derived cells accomplish clearance of debris via phagocytosis, a receptor-mediated process whereby engagement of specific receptors (e.g., PAMPs and DAMPs) triggers actin polymerization and engulfment into specialized vesicles called phagosomes. The phagosome is internalized and fuses with lysosomes where its contents are degraded. Evidence for autophagy interacting with phagocytosis was first discovered in studies utilizing Mycobacterium tuberculosis, which is a bacterium that persists in macrophage phagosomes by interfering with phagolysosome biogenesis (Gutierrez et al., 2004). Promoting autophagy in infected macrophages caused colocalization of the autophagy effectors LC3 with phagosomes and suppressed intracellular survival of mycobacteria (Gutierrez et al., 2004). Using more elegant methods to establish a connection between autophagy and phagocytosis in macrophages, proteomic analysis on the membrane fraction of latex bead-containing phagosomes revealed the presence of LC3-II (Shui et al., 2008). Recent studies have strengthened this connection and additionally suggested that TLR-2 and -4 stimulation during macrophage phagocytosis recruits LC3 to the phagosome membrane and assists in phagosome maturation (Sanjuan et al., 2007). It is now clear that various TLR ligands activate autophagy in macrophages (Delgado et al., 2008; Xu et al., 2007). Because TLRs can recognize pathogenic protein aggregates, collaboration between autophagy and phagocytosis may represent a newly recognized innate defense mechanism for eliminating abnormal protein aggregates. Therefore, deficits in autophagy, as seen in AD (Pickford et al., 2008), may actually have mutually exclusive effects on the clearance of intraneuronal aggregates by macroautophagy and extracellular aggregates by phagocytosis.
While this newly emerging collaboration between autophagy and phagocytosis is intriguing in the context of neurodegeneration, triggering classical phagocytosis in the CNS may also directly improve clearance of aggregates. During acute CNS injury, when the blood-brain-barrier (BBB) is severely compromised and robust inflammation is elicited, blood-derived macrophages (known as monocytes) infiltrate the CNS and contribute to the pathological sequelae. Unfortunately, CNS-resident microglia and blood-derived monocytes entering the CNS take on similar morphological and phenotypic profiles, currently making the two cell populations almost indistinguishable. Chronic neurodegenerative disease (e.g., AD and PD) causes only subtle inflammation and the contribution of blood-derived monocytes is controversial (see below). However, what is clear is that microglia and astrocytes are activated and produce an array of inflammatory mediators, while upregulating phagocytic activity (Aldskogius et al., 1999). Using in vivo multiphoton imaging, the specific kinetics of this response were recently revealed (Meyer-Luehmann et al., 2008). This study showed Aβ plaques form rapidly within 24 hrs and subsequently recruit activated microglia to the plaques within 1–2 days, dispelling previous notions that microglia may actually contribute to plaque formation. Instead, mounting evidence proves that microglia are capable of taking up Aβ. Indeed, amyloid fibrils have been detected in microglia cultured with Aβ and in AD brains (Frackowiak et al., 1992; Wegiel and Wisniewski, 1990). Increased microglial activation is also associated with reduced Aβ accumulation in human APP transgenic mice (Herber et al., 2004; Wilcock et al., 2004; Wyss-Coray et al., 2001).
Microglia are capable of taking up soluble Aβ via macropinocytosis (Mandrekar et al., 2009) and insoluble Aβ aggregates via phagocytosis. Following internalization, soluble Aβ appears to be readily degraded (Mandrekar et al., 2009), but dense insoluble aggregates may be more difficult for microglia to handle. Indeed, mouse microglia phagocytose large amounts of Aβ in vitro, but the majority of internalized Aβ is undegraded 72 hrs after uptake (Paresce et al., 1997). In fact, when cultured with primary dog microglia, undigested Aβ (obtained from human AD patients) can be detected in phagosomes for up to 19 days (Frackowiak et al., 1992). However, not all proteins ingested by microglia universally experience impaired degradation. 70–80% of internalized non-amyloid proteins (e.g., acetylated low-density lipoprotein or alpha2-macroglobulin) are degraded and released by microglia within 4hrs (Paresce et al., 1997). Undegraded Aβ is also released by microglia (Chung et al., 1999), suggesting that detection of Aβ within microglia may not prove its clearance. As a result, the effectiveness of microglia in amyloid clearance has come into question. Recent studies suggest microglia are ineffective during neurodegenerative disease because they are ill equipped for the job. In a mouse model of AD, old PS1-APP mice have a two- to five-fold decrease in Aβ-binding scavenger receptors and Aβ-degrading enzymes, despite increases in proinflammatory cytokines (Hickman et al., 2008). When AD mice are treated with the anti-inflammatory drug minocycline, microglial activation and IL-6 levels are reduced, but levels of various Aβ species are unchanged (Fan et al., 2007). Despite a lack of Aβ clearance, minocycline-treated mice show a significant improvement in behavioral performance. Thus, microglia may only play a minor role in amyloid clearance, but may become activated by protein aggregates and contribute to secondary degeneration via release of neurotoxic factors. But clearly, the consequences and most importantly, the significance of microglial interactions with Aβ in AD remain unclear. This is in part due to the plethora of different experimental settings used in published studies including different types and sources of Aβ assemblies (which may recruit different receptors on microglia), different cell types (neonatal vs adult primary cells, or cell lines), and cell culture versus in vivo. To develop disease relevant findings putative Aβ receptors need to be tested in loss and gain-of-function animal models, and ideally in more than one AD animal model.
Cerebral infiltration of immune cells in neurodegeneration
In models where effective amyloid clearance is observed it has recently been attributed to infiltrating monocytes or perivascular macrophages, which seem to readily degrade amyloid (Hawkes and McLaurin, 2009; Majumdar et al., 2008). This disparity may be explained by microglial lysosomes being less acidic than macrophage lysosomes, resulting in reduced activity of lysosomal enzymes (Majumdar et al., 2007). Indeed, treating microglia with macrophage colony stimulating factor (M-CSF) acidifies microglial lysosomes to levels comparable to macrophages and facilitates Aβ degradation (Majumdar et al., 2007). Although CNS infiltrating monocytes appear beneficial for amyloid clearance, their role in human neurodegenerative disease remains unclear. This has been a source of controversy because initial studies implicating blood-derived monocytes in promoting Aβ clearance used a bone marrow irradiation model to track GFP monocytes into the diseased brain (Simard et al., 2006). This model is not ideal because irradiation itself may damage the BBB and activate the peripheral immune system, thus augmenting cellular infiltration. More recent studies using parabiosis (a technique that allows shared vasculature between two mice) showed that when wildtype mice are joined with GFP mice, GFP monocytes do not enter the brain under normal conditions or after CNS insult (Ajami et al., 2007). Only when mice encounter irradiation and bone marrow transplants do bone marrow-derived monocytes infiltrate the brain. A complementary study also showed that bone marrow-derived cells do not contribute to microglial populations unless the brain is irradiated (Mildner et al., 2007). This study provided further insight by characterizing a specific bone marrow-derived cell population that infiltrates the brain after irradiation (Ly-6Chi CCR2+ cells) and identified the importance of the chemokine receptor CCR2 in this migration. In support of this concept, recent studies in a model of liver injury and peripheral inflammation suggested that fluorescently tagged monocytes can infiltrate the brain in a chemokine receptor CCR2-dependent manner (D'Mello et al., 2009). While such studies are technically challenging and need to be confirmed they indicate that specific signalling pathways may allow the CNS to recruit monocytes from the periphery.
Additional studies indicate the chemokine CCL2 and its receptor CCR2 play a key role in regulating the infiltration of peripheral monocytes into the brain. Lack of CCR2 in mice results in reduced accumulation of microglia/monocytes in the brain and accelerates disease progression and Aβ deposition in a mouse model of AD (El Khoury et al., 2007). Because microglia from CCR2−/− mice do not exhibit aberrant proliferation (El Khoury et al., 2007), it was postulated that changes in microglia accumulation are a consequence of monocyte infiltration. Conversely, overexpressing CCL2 in the brain results in increased microglial accumulation (Yamamoto et al., 2005). However, overexpressing CCL2 also results in increased Aβ accumulation which may be due to a prominent increase in mouse ApoE (Yamamoto et al., 2005), a factor known to independently enhance Aβ deposition (Bales et al., 1999). Recent studies also suggest infiltrating monocytes may originate from a subpopulation of CD11c monocytes when TGFβ signalling is impaired (Town et al., 2008). These cells appear to infiltrate and clear Aβ in a mouse model of AD (Town et al., 2008). While CD11c expressing cells may indeed come from the periphery, recent reports show that CD11c microglia exist within the CNS under pathological and non-pathological conditions and may provide a pool for endogenous expansion in the absence of TGFβ signalling (Bulloch et al., 2008; Chiu et al., 2008).
In summary, flow cytometry has been used to describe several profiles including CD45highCD11b+, Ly-6ChighCCR2+, and CD45+CD11b+CD11c+ (Mildner et al., 2007; Sedgwick et al., 1991; Town et al., 2008) which might identify monocytes that have recently infiltrated the brain. However, because microenvironmental changes in the CNS during an insult may alter expression profiles of microglia, resulting in profiles that resemble peripheral monocytes (Ponomarev et al., 2005), there is still doubt about the extent of monocyte recruitment into the CNS and the role these cells play in disease. Genetic models that prevent monocyte trafficking (e.g., P-selectin and α4 integrin knockouts; (D'Mello et al., 2009; Kerfoot et al., 2006)) might offer an opportunity to better define the role of resident microglia vs. infiltrating monocytes in neurodegenerative disease models.
While this debate over monocytes infiltrating the brain continues various other peripheral immune cells are clearly present during neurodegenerative disease (see table 1). Maybe most surprisingly, T cells have been detected in nearly all neurodegenerative diseases studied, albeit in small numbers (See table 1). And while these cells are seen as the culprit in diseases such a MS, new data in chronic neurodegeneration paint a more complex picture. Using a series of sophisticated genetic studies three independent groups showed recently in ALS models that mSOD1 mice lacking T cells show accelerated motorneuron disease while adoptive transfer of T cells ameliorated disease (Banerjee et al., 2008; Beers et al., 2008; Chiu et al., 2008). Meanwhile, in a mouse model of Parkinson’s disease, dopaminergic cell death is markedly attenuated in the absence of T cells (Brochard et al., 2009). Similarly, mice lacking T and B cells are resistant to prion disease following intraperitoneal or intracerebral ME7 scrapie injection (Fraser et al., 1996). In this model, immune cells may cause degeneration, but may also be responsible for replication and transfer of the prion particles to the CNS (Blattler et al., 1997; Fraser et al., 1996). Interestingly, even if immune cells do not directly access CNS parenchyma, new studies suggest T cells and polymorphonuclear leukocytes may promote seizures and subsequent degeneration by binding to the luminal side of cerebral blood vessels (Fabene et al., 2008). Consequently, inhibiting leukocyte-vascular interactions via blocking or deleting vascular adhesion molecules markedly reduced seizures. While this concept is seemingly tangential to common neurodegenerative disease, the fact that AD mice and certain patients show underlying seizure activity lends relevance to the possibility of these mechanisms contributing to disease pathogenesis (Amatniek et al., 2006; Palop et al., 2007). Currently it is unclear whether Aβ aberrantly excites the neuro-circuitry or recruits immune cells to CNS vasculature where interactions promote seizures, but this could be experimentally tested using APP mice deficient in vascular adhesion molecules.
Table 1.
Cellular Infiltration in Neurological Disease
| Disease | Protein Aggregates |
Infiltrating Cell Type |
Beneficial or Detrimental |
Experimental model |
Irradiation | Reference |
|---|---|---|---|---|---|---|
| ALS | mSOD1 | Monocytes | Role unknown | mSOD1 | Yes | (Kang and Rivest, 2007) |
| CD4+ T Cells | + | mSOD1 | No | (Beers et al., 2008; Chiu et al., 2008) |
||
| Human | N/A | (Engelhardt et al., 1993) | ||||
| CD8+ T Cells | Role unknown | mSOD1 | No | (Beers et al., 2008; Chiu et al., 2008) |
||
| Human | N/A | (Engelhardt et al., 1993) | ||||
| NK Cells | Role unknown | mSOD1 | No | (Chiu et al., 2008) | ||
|
Alzheimer’s Disease |
Aβ, Tau |
Monocytes | + | APPswe/PS1, APP23 |
Yes | (Malm et al., 2005; Simard et al., 2006; Stalder et al., 2005) |
| Tg2576/TGFβ DNR | No | (Town et al., 2008) | ||||
| T cells | + | Human | No | (Rogers et al., 1988; Togo et al., 2002) |
||
| APP/IFN-γ | (Monsonego et al., 2006) | |||||
|
Huntington’s Disease |
Huntingtin | No reported studies found |
Mhtt, human | |||
|
Parkinson’s Disease |
α-synuclein | Monocytes | Role unknown | MPTP | Yes | (Kokovay and Cunningham, 2005; Rodriguez et al., 2007) |
| CD4+ T cells | − | MPTP, human | No | (Brochard et al., 2009; Kurkowska-Jastrzebska et al., 1999) |
||
| CD8+ T cells | Role unknown | MPTP, human | No | (Brochard et al., 2009; Kurkowska-Jastrzebska et al., 1999; McGeer et al., 1988) |
||
|
Prion Disease |
Prp-amyloid | Monocytes | Role unknown | scrapie | Yes | (Priller et al., 2006; Williams et al., 1995) |
| T Cells (CD4,8) |
− | Scrapie | No | (Betmouni et al., 1996) | ||
| SCID + intracerebral ME7 |
Yes | (Blattler et al., 1997; Fraser et al., 1996) |
||||
| Aging | Reelin | T cells (CD4,8) |
Role unknown | Aged Lewis rat, mice |
No | (Bradl et al., 2005; Knuesel et al., 2009) |
Soluble immune factors allow crosstalk between the periphery and CNS
If peripheral immune cells can be mobilized to the CNS during neurodegenerative disease, the CNS and periphery must engage in crosstalk that likely involves soluble factors. As mentioned above, CCL2 may function as a chemoattractant for peripheral monocytes (El Khoury et al., 2007) but many other factors could participate in regulating interactions between CNS and periphery. Such communication could involve proteins classically assigned to the immune system including acute phase proteins, complement, cytokines, and chemokines although these communication factors can be the source or target of many other tissues including the brain. Indeed, many classical immune regulatory factors are produced by glial cells or neurons in the CNS and increased during injury. On the other hand neurodegenerative changes in the brain appear to be associated with changes in the peripheral immune system. For example, freshly isolated peripheral blood mononuclear cells (PBMCs) from AD patients produce higher levels of IL-1β, IL-6, and oncostatin M than cells from non-demented controls (Reale et al., 2005). Using more global approaches, microarray analysis of PBMCs and lymphocytes from AD patients show a prominent gene dysregulation when compared to age-matched controls (Fiala et al., 2007; Kalman et al., 2005; Maes et al., 2007). Similarly, changes in patterns of soluble immune mediators and other communication factors in the periphery have been linked to AD, HD, and PD and were used to predict disease progression (Bjorkqvist et al., 2008; Ray et al., 2007; Scherzer et al., 2007). For example, elevated IL-6 levels were observed in preclinical HD mutation carriers up to 16 years before the onset of motor abnormalities (Bjorkqvist et al., 2008), and changes in the levels of 18 cellular communication factors in plasma predicted AD progression several years prior to clinical manifestation (Ray et al., 2007). Indeed, some of these 18 proteins have been linked mechanistically to AD or AD-like disease in mice although others may be unrelated or false positive findings. Overall, the significance of changes in immune function and expression of soluble mediators the periphery in neurodegeneration is still unclear. However, expanding the search for changes in cellular communication factors (which we dubbed the cellular communicome) in neurodegenerative disease to hundreds of proteins and increasing the number of patient samples could provide support for systemic dysregulation of biological pathways. If successful, proteins or biological pathways, which change as a result of neurodegeneration could become targets for treatments, and groups of proteins might be used as diagnostic signatures for patient selection or for therapy monitoring.
Currently it is unknown whether altered peripheral communication factors in neurodegenerative disease are actively produced in the periphery, derived from the CNS, or both. It is also unclear whether these changes in peripheral immune factors are merely a correlate of CNS degeneration or actually impact disease progression. Emerging evidence is beginning to suggest the latter; that altering peripheral inflammation during neurodegenerative disease can significantly alter disease course (Perry et al., 2007). In support of this, studies show that systemic inflammatory challenges in mouse models of ALS, optic nerve crush, PD, and prion disease lead to exaggerated CNS inflammation and a significant increase in neurodegeneration, culminating in accelerated disease progression (Cunningham et al., 2005; Frank-Cannon et al., 2008; Nguyen et al., 2004; Palin et al., 2008). Similarly, in humans with AD, systemic infections and elevated plasma levels of IL-1β are both associated with an increased rate of cognitive dysfunction (Holmes et al., 2003). Chronic systemic expression of IL-1β in mouse models of PD enhances CNS inflammation and neurodegeneration (Godoy et al., 2008). In light of these findings, it is tempting to speculate that chronic peripheral inflammation may accelerate neurodegenerative disease. This theory may explain the reported epidemiological benefits of NSAIDs in AD and PD (Chen et al., 2005; McGeer et al., 1996; Vlad et al., 2008) where individuals may have an underlying inflammatory condition, but the lack of a beneficial effect in clinical trials where individuals with ancillary inflammatory conditions are excluded. If true, determining which types of chronic inflammation (i.e., arthritis, inflammatory bowel disease, infections, etc.) exacerbate neurodegenerative disease and finding effective treatments for these disorders will be critical for reducing overall disease progression.
Apart from intercellular communication factors antibodies have recently received much attention as peripheral immune molecules with a possible role in AD and possibly other neurodegenerative diseases. Stimulating the production of Aβ antibodies by active immunization with synthetic Aβ (Schenk et al., 1999) or administering monoclonal Aβ antibodies (DeMattos et al., 2001; Levites et al., 2006) is being actively pursued as a potential treatment for AD and is discussed in the accompanying review by Nitsch and colleagues. Interestingly, antibodies against Aβ occur naturally in blood and CSF in free form or in complex with Aβ, both in AD patients and healthy individuals (reviewed in (Szabo et al., 2008). Although titers of these antibodies are low, naturally occurring antibodies recognizing known toxic oligomeric Aβ and amyloidogenic non-Aβ species are abundant in healthy humans and non-human primates and decrease with age and advancing AD (Britschgi et al., 2009). Plasma IgGs are capable of reducing some of Aβ’s neurotoxicity and plasma Aβ antibodies may facilitate the clearance of Aβ but more studies are necessary to understand the role of these antibodies in vivo.
Summary
The past few years have generated new links between the brain and immune system at a fervent pace and to a growing number of neuroscientists names of immune molecules have become familiar, if not interesting. Given the emergence of unexpected links between the immune system and CNS, neuroscientists and immunologists should take a humble step back and consider the factors they study (e.g., neurotransmitters or cytokines) as just that – factors in a complex organism that function based on their sequence and structure, not their anointed “immune” or “neuro” classification. Indeed, emerging studies highlighting the role of “immune molecules” such as complement and class I major histocompatibility complex in CNS development and plasticity (Huh et al., 2000; Stevens et al., 2007); and see Review article in this issue by Boulanger), or “CNS factors” such as neurotransmitters shaping immune function (Pocock and Kettenmann, 2007) attest to the disregard of proteins for classification. Moreover, while there is clearly strong support for a role of classical inflammatory cytokines such as TNF-α in neurodegeneration and a toxic gain-of-function by microglia, there are exciting experiments involving immune activation in neurodegeneration that leave room for loss-of-function paradigms. Apart from vaccines, however, medicine has clearly been more successful in inhibiting the immune system rather than activating it and caution must be taken when attempting to boost microglia or macrophage function. In the meantime, we need to understand the molecular changes in immune cell function that occur with normal healthy aging, be it in the brain or in the peripheral immune system.
Acknowledgements
The authors would like to thank Kristina Kigerl, Kira Irving, and Philipp Jaeger for their insightful review of the manuscript. Additionally, the authors thank Saul Villeda and Jian Luo for their assistance with the aged microglia images. This work was supported by the Department of Veterans Affairs, NIH grant AG27505, AG30144 (TWC) and the Hillblom Foundation (KL, TWC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neuroinflammation Working Group. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldskogius H, Liu L, Svensson M. Glial responses to synaptic damage and plasticity. J. Neurosci. Res. 1999;58:33–41. [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. Embo J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS ONE. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105:15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattler T, Brandner S, Raeber AJ, Klein MA, Voigtlander T, Weissmann C, Aguzzi A. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature. 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009 doi: 10.1016/j.neuron.2009.09.001. this issue. [DOI] [PubMed] [Google Scholar]
- Bradl M, Bauer J, Flugel A, Wekerle H, Lassmann H. Complementary contribution of CD4 and CD8 T lymphocytes to T-cell infiltration of the intact and the degenerative spinal cord. Am J Pathol. 2005;166:1441–1450. doi: 10.1016/S0002-9440(10)62361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britschgi M, Olin CO, Johns HT, Takeda-Uchimura Y, LeMieux M, Rufibach K, Rajadas J, Zhang H, Tomooka B, Robinson WH, et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0904866106. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, Kaunzner UW, Liu K, Lindquist R, Nussenzweig MC, et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carpentier P, Palmer T. Immune influence on neural stem cell regulation and function. Neuron. 2009 doi: 10.1016/j.neuron.2009.08.038. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, Brown RH, Jr, Carroll MC. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandoy-Dron F, Guillo F, Benboudjema L, Deslys J-P, Lasmézas C, Dormont D, Tovey MG, Dron M. Gene expression in scrapie. Cloning of a new scrapie–responsive gene and the identification of increased levels of seven other mRNA transcipts. J. Biol. Chem. 1998;273:7691–7697. doi: 10.1074/jbc.273.13.7691. [DOI] [PubMed] [Google Scholar]
- Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart J-C, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Aβ load in APP+PS1 transgenic mice. Neurobiol. Aging. 2001;22:1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch. Neurol. 1993;50:30–36. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Navarro MG, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, et al. The LPS receptor (CD14) links innate immunity with Alzheimer's disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J Neurosci. 2004;24:6457–6465. doi: 10.1523/JNEUROSCI.0901-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992;84:225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O'Brien DE, Casey B, et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H, Brown KL, Stewart K, McConnell I, McBride P, Williams A. Replication of scrapie in spleens of SCID mice follows reconstitution with wild-type mouse bone marrow. J Gen Virol. 1996;77(Pt 8):1935–1940. doi: 10.1099/0022-1317-77-8-1935. [DOI] [PubMed] [Google Scholar]
- Gasque P, Dean YD, McGreal EP, Beek JV, Morgan BP. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology. 2000;49:171–186. doi: 10.1016/s0162-3109(00)80302-1. [DOI] [PubMed] [Google Scholar]
- Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson's disease. Brain. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G, Philips T, Van Wijmeersch B, Audet JN, Dewil M, Van Den Bosch L, Billiau AD, Robberecht W, Julien JP. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J Neurosci. 2008;28:10234–10244. doi: 10.1523/JNEUROSCI.3494-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Hammer ND, Schmidt JC, Chapman MR. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc Natl Acad Sci U S A. 2007;104:12494–12499. doi: 10.1073/pnas.0703310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol. 2007;178:829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Roth LM, Wilson D, Wilson N, Mason JE, Morgan D, Gordon MN. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Holers VM. Complement. In: Rich RR, editor. Clinical Immunology Principles and Practice. St. Louis, Missouri: Mosby–Year Book; 1996. pp. 363–391. [Google Scholar]
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta S, Niemi E, Yenari M, Nakamura MC, Seaman WE. A Role for TREM2 Ligands in the Phagocytosis of Apoptotic Neuronal Cells by Microglia. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jaeger PA, Wyss-Coray T. All-you-can-eat: autophagy in neurodegeneration and neuroprotection. Mol Neurodegener. 2009;4:16. doi: 10.1186/1750-1326-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman J, Kitajka K, Pakaski M, Zvara A, Juhasz A, Vincze G, Janka Z, Puskas LG. Gene expression profile analysis of lymphocytes from Alzheimer's patients. Psychiatr Genet. 2005;15:1–6. doi: 10.1097/00041444-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J Cell Biol. 2007;179:1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, D'Mello C, Nguyen H, Ajuebor MN, Kubes P, Le T, Swain MG. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology. 2006;43:154–162. doi: 10.1002/hep.21003. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Nyffeler M, Mormede C, Muhia M, Meyer U, Pietropaolo S, Yee BK, Pryce CR, LaFerla FM, Marighetto A, Feldon J. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol Aging. 2009;30:697–716. doi: 10.1016/j.neurobiolaging.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Cunningham LA. Bone marrow-derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson's disease. Neurobiol Dis. 2005;19:471–478. doi: 10.1016/j.nbd.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Koning N, Bo L, Hoek RM, Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol. 2007;62:504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Lee C-K, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat. Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, Murphy MP, Golde TE. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer's amyloid peptide. Brain. 2005;128:1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Maes OC, Xu S, Yu B, Chertkow HM, Wang E, Schipper HM. Transcriptional profiling of Alzheimer blood mononuclear cells by microarray. Neurobiol Aging. 2007;28:1795–1809. doi: 10.1016/j.neurobiolaging.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majed HH, Chandran S, Niclou SP, Nicholas RS, Wilkins A, Wing MG, Rhodes KE, Spillantini MG, Compston A. A novel role for Sema3A in neuroprotection from injury mediated by activated microglia. J Neurosci. 2006;26:1730–1738. doi: 10.1523/JNEUROSCI.0702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Chung H, Dolios G, Wang R, Asamoah N, Lobel P, Maxfield FR. Degradation of fibrillar forms of Alzheimer's amyloid beta-peptide by macrophages. Neurobiol Aging. 2008;29:707–715. doi: 10.1016/j.neurobiolaging.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, Lobel P, Maxfield FR. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18:1490–1496. doi: 10.1091/mbc.E06-10-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, Fisher Y, Owens T, Weiner HL. Abeta-induced meningoencephalitis is IFN-{gamma}-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5048–5053. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Boucraut J, Hahnel C, Misgeld T, Wekerle H. Neuronal control of MHC class II inducibility in rat astrocytes and microglia. Eur J Neurosci. 1996;8:2582–2590. doi: 10.1111/j.1460-9568.1996.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: The missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Reversing neurodegeneration: A promise unfolds. Cell. 2000;101:1–4. doi: 10.1016/S0092-8674(00)80617-0. [DOI] [PubMed] [Google Scholar]
- Palin K, Cunningham C, Forse P, Perry VH, Platt N. Systemic inflammation switches the inflammatory cytokine profile in CNS Wallerian degeneration. Neurobiol Dis. 2008;30:19–29. doi: 10.1016/j.nbd.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Hoyvarde Clausen T, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of Ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007 doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer's disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Priller J, Prinz M, Heikenwalder M, Zeller N, Schwarz P, Heppner FL, Aguzzi A. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J Neurosci. 2006;26:11753–11762. doi: 10.1523/JNEUROSCI.2275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13 doi: 10.1038/nm1653. 1369–1362. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer's disease patients. Exp Gerontol. 2005;40:165–171. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Alvarez-Erviti L, Blesa FJ, Rodriguez-Oroz MC, Arina A, Melero I, Ramos LI, Obeso JA. Bone-marrow-derived cell differentiation into microglia: a study in a progressive mouse model of Parkinson's disease. Neurobiol Dis. 2007;28:316–325. doi: 10.1016/j.nbd.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Rogers J, Li R, Mastroeni D, Grover A, Leonard B, Ahern G, Cao P, Kolody H, Vedders L, Kolb WP, Sabbagh M. Peripheral clearance of amyloid beta peptide by complement C3-dependent adherence to erythrocytes. Neurobiol Aging. 2006;27:1733–1739. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Eklund AC, Morse LJ, Liao Z, Locascio JJ, Fefer D, Schwarzschild MA, Schlossmacher MG, Hauser MA, Vance JM, et al. Molecular markers of early Parkinson's disease based on gene expression in blood. Proc Natl Acad Sci U S A. 2007;104:955–960. doi: 10.1073/pnas.0610204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: A cell biologist thinks about neurodegenerative disease. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Shui W, Sheu L, Liu J, Smart B, Petzold CJ, Hsieh TY, Pitcher A, Keasling JD, Bertozzi CR. Membrane proteomics of phagosomes suggests a connection to autophagy. Proc Natl Acad Sci U S A. 2008;105:16952–16957. doi: 10.1073/pnas.0809218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Singhrao SK. Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp. Neurol. 1999;159:362–376. doi: 10.1006/exnr.1999.7170. [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Smith RE, Patel V, Seatter SD, Deehan MR, Brown MH, Brooke GP, Goodridge HS, Howard CJ, Rigley KP, Harnett W, Harnett MM. A novel MyD-1 (SIRP-1alpha) signaling pathway that inhibits LPS-induced TNFalpha production by monocytes. Blood. 2003;102:2532–2540. doi: 10.1182/blood-2002-11-3596. [DOI] [PubMed] [Google Scholar]
- Song W-C, Sarrias MR, Lambris JD. Complement and innate immunity. Immunopharmacology. 2000;49:187–198. doi: 10.1016/s0162-3109(00)80303-3. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. Faseb J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid beta peptide. Autoimmun Rev. 2008;7:415–420. doi: 10.1016/j.autrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner AJ. Membrane receptors for soluble defense collagens. Curr. Opin. Immunol. 1999;11:34–41. doi: 10.1016/s0952-7915(99)80007-7. [DOI] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, LeVine H. The cerebral proteopathies - Neurodegenerative disorders of protein conformation and assembly. Mol. Neurobiol. 2000;21:83–95. doi: 10.1385/MN:21:1-2:083. [DOI] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65–76. [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Webster S, Lue L-F, Brachova L, Tenner AJ, McGeer PL, Terai K, Walker DG, Bradt B, Cooper NR, Rogers J. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol. Aging. 1997;18:415–421. doi: 10.1016/s0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Wisniewski HM. The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol. 1990;81:116–120. doi: 10.1007/BF00334499. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol Dis. 2004;15:11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Williams AE, Ryder S, Blakemore WF. Monocyte recruitment into the scrapie-affected brain. Acta Neuropathol. 1995;90:164–169. doi: 10.1007/BF00294316. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu G, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc. Natl. Acad. Sci. USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84:100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Horiba M, Buescher JL, Huang D, Gendelman HE, Ransohoff RM, Ikezu T. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]