Abstract
The involvement of ATP-sensitive K+ (KATP) channels in the atrophy of slow-twitch (MHC-I) soleus (SOL) and fast-twitch (MHC-IIa) flexor digitorum brevis (FDB) muscles was investigated in vivo in 14-day-hindlimb-unloaded (14-HU) rats, an animal model of disuse, and in vitro in drug-induced muscle atrophy. Patch-clamp and gene expression experiments were performed in combination with measurements of fibre diameters used as an index of atrophy, and with MHC labelling in 14-HU rats and controls. A down-regulation of KATP channel subunits Kir6.2, SUR1 and SUR2B with marked atrophy and incomplete phenotype transition were observed in SOL of 14-HU rats. The observed changes in KATP currents were well correlated with changes in fibre diameters and SUR1 expression, as well as with MHC-IIa expression. Half of the SOL fibres of 14-HU rats had reduced diameter and KATP currents and were labelled by MHC-I antibodies. Non-atrophic fibres were labelled by MHC-IIa (22%) antibodies and had enhanced KATP currents, or were labelled by MHC-I (28%) antibodies but had normal current. FDB was not affected in 14-HU rats and this is related to the high expression/activity of Kir6.2/SUR1 subunits characterizing this muscle phenotype. The long-term incubation of the control muscles in vitro with the KATP channel blocker glibenclamide (10−6m) reduced the KATP currents with atrophy and these effects were prevented by the KATP channel opener diazoxide (10−4m). The in vivo down-regulation of SUR1, and possibly of Kir6.2 and SUR2B, or their in vitro pharmacological blockade activates atrophic signalling in skeletal muscle. All these findings suggest a new role for the KATP channel as a molecular sensor of atrophy.
Introduction
Skeletal muscles are classified as slow- and fast-twitch phenotypes on the basis of their different speeds of contraction and different functions. At the molecular level, slow- and fast-twitch skeletal muscles can be distinguished by their complement of contractile proteins such as type I myosin heavy chain (MHC) in slow-twitch and MHC type IIa–IIb (IIx) in fast-twitch muscles, cellular metabolism, hormonal regulation and drug responses. Differences between muscle phenotypes in the expression and activity of sarcolemmal ion channels have also been demonstrated. For example, the lower activity of the voltage-dependent Na+ channel, ClC-1 chloride channel, and aquaporin-4 channel has been observed in slow-twitch muscles as compared with that measured in the fast-twitch phenotype (Desaphy et al. 2001; Frigeri et al. 2001; Pette, 2002; Pierno et al. 2002; Liu et al. 2004). By contrast, higher Ca2+-activated K+ (BK) channel activity has been observed in slow-twitch as compared with fast-twitch muscle (Tricarico et al. 2005).
An up-regulation of Na+, ClC-1, and aquaporin-4 channels has been observed in slow-twitch muscles of hindlimb-unloaded (HU) rats, an accepted animal model of hypogravity and muscle disuse which are conditions characterized by atrophy and myofibre phenotype transitions of skeletal muscle (Desaphy et al. 2001; Frigeri et al. 2001; Pierno et al. 2002). Slow-twitch muscles from these animals show reduced stretch-activated (SAC) and BK channel activities as contributing to lowering the resting intracellular Ca2+ concentration in type I fibres to levels resembling type II fibres. This is associated with deactivation of the Ca2+-dependent calcineurin pathway, which is a triggering mechanism for the slow-to-fast fibre transition in various conditions of disuse (Fraysse et al. 2003; Tricarico et al. 2005; Harridge, 2007). The Ca2+-dependent calmodulin/calcineurin pathway is indeed a known repressor of the slow-to-fast gene reprogramming in skeletal muscle (Fraysse et al. 2003; Harridge, 2007). Therefore, the activity of Na+, ClC-1, aquaporin-4, BK and stretch-activated channels appears to be dependent on phenotypic transition rather than atrophy.
Atrophy instead affects fast-twitch and slow-twitch muscles showing different degrees of damage depending on muscle type and function often leading in severe cases to an irreversible impairment of muscle function. This process is generally considered an imbalance between protein synthesis and degradation, in favour of the latter, which are under the control of several pathways and growth factors. Atrophy in skeletal muscle is known to be associated with a series of intracellular events involving deactivation of the PI3K/Akt/mTOR pathway, activation of the FOXO/atrogin and MurF1 genes with proteolysis and inhibition of protein synthesis (Kandarian & Jackman, 2006). Atrophy is also associated with apoptosis in slow-twitch muscle rather than in fast-twitch muscle in which lysosomal activity might prevail and the activation of proteolytic pathways could differ between slow and fast muscles (Dupont-Versteegden, 2006; Ferreira et al. 2007, 2008). However, not much is known about the membrane signals involved in this process in skeletal muscle.
More recently, we demonstrated that the molecular composition, biophysical properties and pharmacological responses of ATP-sensitive K+ (KATP) channels in skeletal muscle are phenotype-dependent and muscle-type specific (Kane et al. 2005; Tricarico et al. 2006; Wu et al. 2007). High KATP channel activity is observed in excised patches from fast-twitch muscle fibres as compared with slow-twitch muscle; differences in the surface KATP channel activity and properties have also been observed within fast-twitch muscle types suggesting that KATP channel activity, other than phenotype, is related to morphology or muscle-specific functions. The opening of KATP channels saves the intracellular energy pool, regulates glucose uptake into the fibres and contributes to the K+ ion efflux during strenuous muscle activity, and it is involved in the buffering of the energy pool during metabolic stress. In resting fibres, KATP channels contribute few millivolts to the resting potentials; however, KATP channel opening participates significantly in the fibre hyperpolarization following insulin stimulation (Tricarico et al. 1997). In fast-twitch muscle the KATP channel is involved in the delayed regulation of prolonged action potential firing thereby reducing the excitability of the fibres during muscle fatigue (Pedersen et al. 2009). KATP channels are complexes of inwardly rectifying K+ channels (Kir6.1 and Kir6.2) and sulfonylurea receptor subunits (SUR1, SUR2A and SUR2B). The Kir6.2/SUR2A subunits constitute the main KATP channel in different skeletal muscle phenotypes (Tricarico et al. 2006). Reduced/expression activity of SUR2A/Kir6.2 subunits is associated with the insulin-dependent fibre depolarization and paralysis in K+-depleted rats, and it maybe responsible for the observed reduced KATP channel activity recorded in the fibres from hypokalaemic periodic paralysis patients (Tricarico et al. 1999, 2008). Hybrid KATP channel complexes composed of SUR1 and SUR2B subunits contribute to functional channels in different muscle types. SUR1 is abundantly expressed in various fast-twitch muscle types including the flexor digitorum brevis (FDB) muscle and it is less expressed in slow-twitch soleus (SOL) muscle of the rat but the significance of this observation is currently unknown. Several reports demonstrated that SUR1- but not SUR2-expressing cells are more susceptible to apoptosis induced by SUR1 inhibitors such as glibenclamide and observed in the SUR1 knock-out mice as determined by monitoring cell detachment, nuclear condensation, DNA fragmentation, and caspase-3-like activity (Mandrup-Poulsen, 2001; Valentijn et al. 2004; Hambrock et al. 2006). SUR-1-induced apoptosis is also associated with activation of extracellular signal-regulated kinase (ERK) (Maedler et al. 2004). In pancreatic β cells, SUR1-selective blockers of KATP channels leads to apoptotic cell death, while SUR1 openers preserve cell integrity leading to the idea that down-regulation of the subunits or pharmacological blockade of this channel type is involved in the atrophy of pancreatic tissue (Mandrup-Poulsen, 2001; Maedler et al. 2004, 2005). However, the possible involvement of KATP channels in the atrophic signalling in skeletal muscle is not known.
Here we investigate the involvement of KATP channels in the atrophy of slow-twitch (MHC-I fibre type) SOL and fast-twitch (MHC-IIa fibre type) FDB muscles that are characterized by low and high Kir6.2/SUR1 channel activity, respectively. Patch-clamp/gene expression experiments on whole SOL and FDB muscles from 14-HU rats and controls were performed in combination with measurements of the muscle-to-body weight ratio, used as an index of atrophy, and with immunofluorescence staining of MHC isoforms. KATP channel activity, evaluation of MHC isoform expression and measurements of the fibre diameter were also performed on the same isolated SOL fibres from 14-HU rats. The capability of the KATP channel blocker glibenclamide in inducing ‘in vitro’ atrophy and apoptosis of SOL and FDB muscles from control rats, and the capability of the well-known KATP channel opener diazoxide in preventing these processes in the same muscles were also evaluated.
We found that KATP channels are down-regulated in atrophic MHC-I fibres but up-regulated in non-atrophic MHC-IIa fibres. The high Kir6.2/SUR1 activity protects FDB against atrophy. The reduced expression of Kir6.2, SUR1 or SUR2B subunits or their pharmacological blockade are associated with atrophy indicating that the KATP channel is a molecular sensor of this process in skeletal muscle.
Methods
Animal care and surgery
The experiments complied with the Italian guidelines for the use of laboratory animals, which conforms with the European Community Directive of 1986 (86/609/ECC). The experiments comply with the policies and regulations of The Journal of Physiology as described by Drummond (2009). In brief, deep anaesthesia was produced by a single intraperitoneal injection of urethane (1.2 g (kg body weight)−1). No neuromuscular blocking agents or nitric oxide were administered to the rats, since that would have interfered with our experiments using urethane for deep anaesthesia. An overdose of urethane was used to kill the animals. Experiments were also approved by the Italian Health Department (Art. 9 del Decreto Legislativo 116/92: Decreto no. 33/2000-B del Dipartimento degli alimenti e nutrizione e della sanità pubblica) and performed under the supervision of a local veterinary official. Male Wistar rats weighing 250–350 g (Charles River Lab., Calco, Italy) were randomly assigned to control or HU groups. To induce muscle unloading, the animals were suspended individually in a special cage for 14 days as previously described (Desaphy et al. 2001).
Whole muscle experiments were performed as follows: at the end of the period of unloading (14 days), SOL and FDB muscles were removed from the animals under deep anaesthesia. After muscle dissection, the animals were killed by an overdose of urethane. Muscles were rapidly dissected from controls (n= 6 rats; n= 12 muscles) and 14-HU rats (n= 10 rats; n= 20 muscles). The first group of muscles was used for patch-clamp experiments (n= 6 muscles from controls; n= 10 muscles from 14-HU rats). The contralateral muscles (second group) were frozen in liquid nitrogen promptly after surgical removal for mRNA analysis and for myosin isoform identification (n= 6 muscles from controls; n= 10 muscles from 14-HU rats). Muscles were carefully blotted before weighing. Muscles with evident damage and contraction were excluded from the experiments.
Single fibre experiments were also performed. Some isolated fibres from SOL muscles of controls (n= 14 fibres) and 14-HU rats (n= 35 fibres) were used for combined optical fibre diameter measurements, patch-clamp experiments and for the myosin isoform identification in the single fibres.
Patch-clamp experiments
Experiments were performed in inside-out configurations using the standard patch-clamp technique. Channel currents were recorded by using macropatches during voltage steps from 0 mV of holding potential to −60 mV membrane potential (Vm) immediately after excision, at 20–22°C, in the presence of KCl on both sides of membrane patches in the absence (control) or presence of ATP in the bath as previously described (Tricarico et al. 2006).
Fibre diameter measurements
Isolated fibres were equilibrated in normal Ringer solution (300 mosmol l−1) for 15 min at 25°C. The fibre diameter was determined using an ocular micrometer attached to a Zeiss Axiovert 10 inverted microscope (×10). For each fibre, three individual measurements were performed at three different points on the fibre. The same fibres were used for patch-clamp experiments and MHC subtype detection. Contracted fibres and fibres with blebs were disgarded from the analysis.
Real-time quantitative PCR on whole muscle
For each muscle sample, the total RNA was isolated using TRIzol reagent and treated with DNase I (4 units, 37°C, 1 h). The real-time quantitative PCR experiments were performed as previously described (Tricarico et al. 2006).
Myosin isoform identification in whole muscle and in single fibres
Immunofluorescence staining was performed as described previously (Frigeri et al. 1998, 2001). Briefly, SOL and FDB muscles from controls and 14-HU rats were dissected and rapidly frozen in isopentane cooled with liquid nitrogen. Cryostat cross-sections (5 μm) were incubated with types IIa, IIb (Frigeri et al. 1998) or I (1:500 dilution; Sigma, St Louis, MO, USA) MHC mouse monoclonal antibodies for 1 h at room temperature. After washing, sections were incubated for 1 h with fluorescein isothiocyanate (FITC)-coupled goat anti-mouse antibodies (1:100 dilution). Sections were examined with a Leica DMRXA photomicroscope equipped for epifluorescence, and digital images were obtained with a cooled CCD camera (Princeton Instruments, Princeton, NJ, USA). The type I, IIa and IIb fibres in SOL muscles were evidenced by the immunofluorescent staining of cryostat sections and the relative number determined.
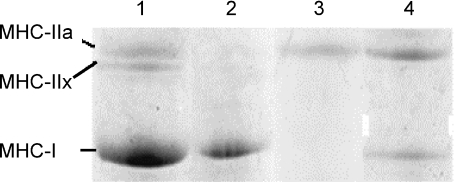
Following fibre diameter and patch-clamp measurements, some single fibres from SOL muscles were randomly collected using a microsyringe (B-D tuberculin syringe 27G1/2), rapidly stored at −20°C in cryovials and analysed blindly for their MHC expression profile. The criteria for fibre collection were: (1) fibres with no evident signs of advanced apoptosis as evidenced by surface blebs; (2) fibres with no evident contraction; (3) fibres that could be collected within 2 h of the enzymatic dissociation. The percentage of fibres discarded of controls and 14-HU rats were 10% and 13%, respectively. Separation and identification of MHC isoforms was performed as follows: the single fibres were taken and dissolved in Laemmli solution (Laemmli, 1970; Schiaffino et al. 1989; Pellegrino et al. 2003) and loaded on 8% polyacrylamide gels. Electrophoresis was run for 2 h at 200 V and then for 24 h at 250 V. Electrophoresis of single fibre segments were silver stained, as such staining, being more sensitive, is preferable when small amounts of protein are to be detected (Fig. 1). In the region of MHC isoforms, three major bands were separated that corresponded, in order of migration from the fastest to the slowest, to MHC-I or MHC-IIa and MHC-IIx. In relation to the presence of one or two bands in the MHC region, single fibres were classified as one of the following types: I, IIa, IIx (pure fibres) and I–IIa, IIa–IIx (mixed fibres) (Pellegrino et al. 2003).
Figure 1. Example of electrophoretic (SDS-PAGE) separation of myosin heavy chain (MHC) isoforms in bioptic samples of isolated fibres from 14-HU rats.
All samples were pure myosin extracted from single muscle fibre. Gels were Coomassie stained. Lane 1 shows a mixed rat fibre sample used as a reference; lane 2, pure slow type I fibre; lane 3, pure fast type IIa fibre; lane 4, hybrid type I–IIa fibre. No type IIx fibres were detected in our experiments.
Solutions
The normal Ringer solution used during muscle biopsy and for preparation of isolated fibres contained 145 × 10−3m NaCl, 5 × 10−3m KCl, 1 × 10−3m MgCl2, 0.5 × 10−3m CaCl2, 5 × 10−3m glucose and 10 × 10−3m 3-(N-morpholino) propanesulfonate (Mops) sodium salt and was adjusted to pH 7.2 with Mops acid. The patch-pipette solutions contained 150 × 10−3m KCl, 2 × 10−3m CaCl2 and 1 × 10−2m Mops (pH 7.2). The bath solution contained 150 × 10−3m KCl, 5 × 10−3m EGTA, and 1 × 10−2m Mops (pH 7.2).
Drug-dependent atrophy and KATP channel activity in muscles isolated from controls
SOL and FDB muscles were removed from the male control rats (body weight = 500 g) (n= 10 rats) under deep anaesthesia induced by intraperitoneal injection of urethane (1.2 g (kg body weight)−1). After muscle dissection, the animals were rapidly killed with an overdose of urethane. Intact muscles used for visual and stereomicroscope inspection were carefully pinned on Petri disks. Muscles damaged during dissection or showing contraction during the incubation period were discarded. All experiments were performed under 5% CO2–95% O2 atmosphere for the maintenance of aerobic conditions, at 37°C, and the muscles were incubated for 72 h with the drug solutions under investigation. At the end of the incubation period, all muscle samples were blotted on adsorbent paper, carefully weighed and rapidly frozen in liquid nitrogen. Muscles were incubated with Dulbecco's modified Eagle's medium (DMEM+) solution composed by 1X antibiotics (1%), l-glutamine (1%), FBS (10%) and enriched with glibenclamide at 10−6m concentration or diazoxide at 10−4m concentration, or with glibenclamide (10−6m) + diazoxide (10−4m). The observed values of total proteins and wet weight of the muscles were compared with those of the corresponding contralateral muscles from the same rats incubated with DMEM+ solution alone for 72 h. Samples from muscle homogenates were used for total protein content quantification using Bio-Rad protein assay (Bio-Rad Labs GmbH, München, Germany) and evaluation of the caspase-3 activity. Caspase-3 activity was measured using a colorimetric assay based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp-p-nitroaniline (Ac-DEVD-pNA) by caspase-3 resulting in the release of the pNA which has a high absorbance at 405 nm. The reagents and the CASP-3C kit used were supplied by Sigma (Milano). Some muscles incubated for 72 h with glibenclamide or diazoxide were used for patch-clamp experiments and fibre diameter measurements. Single fibres were obtained by enzymatic dissociation as previously described. KATP channel current recordings and measurements of the diameters of the fibres were performed in the absence of drugs.
Statistics
The fibre subtypes were identified on the basis of their combined statistically different fibre diameter and KATP current values as determined by Student's t test and on their reaction to the specific antibodies labelling the MHC-I or MHC-IIa isoforms. Inclusion criteria for grouping muscles and/or single fibres were the calculation of the significant differences between pairs of means of the KATP currents and fibre diameters. Significance between pairs of means were calculated by Student's paired t test. Significant differences was considered for P < 0.05 or less. Multiple correlation analysis was performed between three variables to calculate the coefficient of correlation (r) by solving the equation y=b0+b1x1+b2x2. While linear correlation analysis was performed by solving the equation y=b0+b1x1. Data evaluation and statistics were performed with Excel software.
Results
Muscle atrophy, slow-to-fast phenotype transition and expression activity of KATP channel subunits of SOL and FDB muscles from 14-HU rats
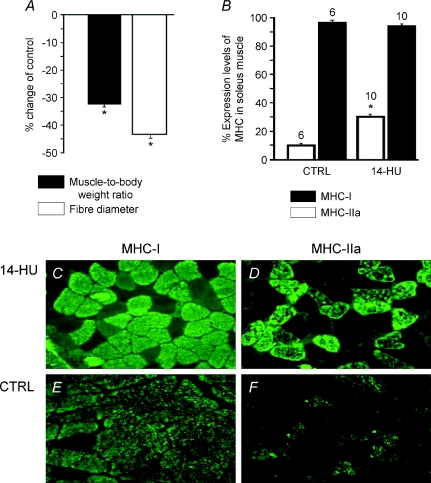
We previously demonstrated that 14-day-HU in rats leads to a significant reduction of the muscle-to-body weight ratio and of fibre diameter with respect to controls (Desaphy et al. 2005) and we confirmed this finding in our current experiments. The muscle-to-body weight ratio was 0.460 ± 0.04 mg g−1 (n= 6 muscles) and 0.345 ± 0.05 mg g−1 (n= 10 muscles) in control (n= 6 rats) and 14-HU rats (n= 10 rats), respectively, while the measured fibre diameter was 80.1 ± 12 μm (n= 94 fibres) and 60.3 ± 11 μm (n= 99 fibres) in control and 14-HU rats, respectively (Fig. 2A). Further, we observed a significant increase in expression of the fast type IIa MHC isoform in SOL muscles of 14-HU rats (n= 10 muscles) as compared to controls (n= 6 muscles), but no change in expression of the slow type I MHC isoform was observed in the same muscles (Fig. 2B, C, D, E and F). We should stress that the serial sections of the same muscles were simultaneously labelled by MHC IIa and MHC I antibodies suggesting co-expression of both types of MHC isoforms in the same fibres. These findings, in agreement with previous reports, indicate that a significant atrophy of SOL muscle is observed during 14-HU that is accompanied by an incomplete slow-to-fast phenotype transition of the muscle following disuse (Frigeri et al. 1998, 2001). In contrast, FDB muscles from the same animals were not affected by disuse. The muscle-to-body weight ratio was 0.360 ± 0.02 mg g−1 (n= 6 muscles) and 0.385 ± 0.05 mg g−1 (n= 10 muscles) in control and 14-HU rats, respectively, and the measured fibre diameter was 40.1 ± 9 μm (n= 34 fibres) and 38.1 ± 8 μm (n= 29 fibres) in control and 14-HU rats, respectively.
Figure 2. Percentage changes in myosin heavy chain (MHC) expression levels, fibre diameters and muscle-to-body weight ratio of slow-twitch soleus (SOL) muscle from 14-day-hindlimb-unloaded (14-HU) rats.
A, the mean muscle-to-body weight ratio was calculated for 14-HU rats (n= 10 rats) and normalized with respect to that of control rats (n= 6 rats). The body weights of the 14-HU rats and controls were 355 ± 30 g and 385 ± 30 g, respectively, at the end of the unloading period. The change to fibre diameter was calculated (n= 90 fibres) for the 14-HU rats and normalized with respect to that of control rats (n= 110 fibres). Disuse leads to a significant reduction of the muscle-to-body weight ratio and of fibre diameter. The muscles were rapidly excised from the bones of anaesthetized rats and carefully dried before weighing. B, percentage MHC isoform expression levels were evaluated on muscles from 14-HU and control (CTRL) rats. C, D, E and F, immunofluorescence staining for specific MHC isoforms expressed in SOL muscle sections from 14-HU rats and controls. Disuse leads to a significant increase in the expression levels of the fast type IIa MHC isoform; while no changes were observed in the expression levels of the slow type I MHC isoform. The numbers above the columns indicate the number of sampled muscles/rats. *Significant differences between data are evaluated by an unpaired Student's t test for P < 0.05 or less.
Patch-clamp experiments showed that the excision of macropatches into ATP-free solution produced a dramatic increase in inward currents in 38% and 35% of macropatches from SOL fibres of control and 14-HU rats, respectively. The total mean inward current recorded at −60 mV (Vm) after excision was −108.6 ± 11 pA (n= 94 patches) and −99.1 ± 32 pA (n= 99 patches) for SOL muscles of control and 14-HU rats, respectively. Exposure of macropatches excised from all fibres to intracellular ATP (5 × 10−3m) reduced current amplitudes. The total ATP-sensitive current was −86.5 ± 9 pA (n= 94 patches) and −80.56 ± 35 pA (n= 99 patches) in SOL fibres of control and 14-HU rats, respectively. A large variability in the KATP current amplitude was observed in the sampled patches from 14-HU rat fibres as compared with those of the controls. This suggested the presence of multiple current components contributing to the total mean current recorded in the fibres. Three groups of SOL muscles have been identified and grouped on the basis of their KATP channel activity and fibre diameter. The possible correlation of these parameters with the predominant muscle phenotype was also evaluated. The first group of muscles (n= 3 muscles) was characterized by a reduced KATP current and fibre diameter of −65.6 ± 3 pA (n= 31 patches) and 65.4 ± 3 μm (n= 31 fibres), respectively, and by a low expression level of the MHC type IIa isoform of 21.3 ± 4%. The second (n= 5 muscles) group was characterized by a significantly greater KATP current and fibre diameter of −75.34 ± 6 pA (n= 51 patches) and 75.96 ± 5 μm (n= 51 fibres), respectively (P < 0.05 or less) as compared with the first group, and an expression level of MHC type IIa of 27.6 ± 4%. The third group (n= 2 muscles) was characterized by a significantly enhanced KATP current and fibre diameter of −110.5 ± 9 pA (n= 17 patches) and 89.2 ± 4 μm, respectively (n= 17 fibres) (P < 0.05 or less) as compared with the others, and showed an expression level of MHC type IIa of 31%. A linear correlation between KATP current and fibre diameter values was observed (r= 0.848); multiple correlation analysis also revealed a correlation between KATP current, fibre diameter and percentage expression levels of MHC type IIa (r= 0.7413) (Table 1). While a low level of correlation was observed between the percentage expression levels of MHC IIa and KATP current values (r= 0.601).
Table 1.
Relationship between fibre diameter, fibre phenotype, relative expression levels of KATP channel subunits and KATP channel currents in soleus muscles from 14-HU rats
| SOL muscle (n fibres) | Fibre diameter (μm) | KATP currents (pA) | Percentage expression of MHC-II (fast type) | Percentage expression of MHC-I (slow type) | Kir6.2 relative expression | SUR1 relative expression | SUR2B relative expression |
|---|---|---|---|---|---|---|---|
| 1 (11) | 66 ± 9 | −65.3 ± 9 | 20.1 | 91.1 | 0.081 | 0.003 | 0.0057 |
| 2 (9) | 66 ± 4 | −67.4 ± 8 | 22.1 | 98.2 | 0.085 | 0.003 | 0.0071 |
| 3 (11) | 64 ± 3 | −65.5 ± 9 | 21.8 | 99 | 0.079 | 0.0032 | 0.0061 |
| 4 (11) | 73 ± 5 | −72.3 ± 7 | 29 | 97.2 | 0.083 | 0.0051 | 0.0067 |
| 5 (10) | 74 ± 6 | −73.4 ± 6 | 28.5 | 99 | 0.081 | 0.0053 | 0.0089 |
| 6 (11) | 75 ± 5 | −73.2 ± 6 | 28 | 97.3 | 0.075 | 0.0054 | 0.0078 |
| 7 (9) | 78 ± 3 | −78.1 ± 4 | 25 | 96.5 | 0.08 | 0.006 | 0.0067 |
| 8 (10) | 80 ± 6 | −81.1 ± 10 | 27.5 | 96.7 | 0.077 | 0.006 | 0.0061 |
| 9 (8) | 88 ± 7 | −100.1 ± 9 | 28 | 95.1 | 0.089 | 0.0062 | 0.0071 |
| 10 (9) | 90 ± 8 | −121.8 ± 10 | 34 | 99 | 0.101 | 0.009 | 0.0099 |
Linear correlation analysis gave a calculated r of 0.848 (b0= 59.88, b1=−1.84) between KATP currents and fibre diameters, and of 0.601 between KATP currents and percentage expression levels of MHC-IIa. Multiple correlation analysis gave a calculated r of 0.7413 (b0= 27.68, b1= 0.206, b2= 2.42) between fibre diameters, KATP currents and percentage expression levels of MHC-IIa of SOL. No correlation was found between fibre diameters, KATP currents and the percentage expression levels of MHC-I in the same muscle samples (r= 0.200). Multiple correlation analysis performed between the fibre diameters, KATP currents and the relative expression levels of SUR1 of SOL gave a coefficient of correlation (r) of 0.9335 (b0= 51.84, b1= 4.2 × 10−5, b2= 4515). A low correlation was observed between the fibre diameters, KATP currents and the relative expression levels of Kir6.2 (r= 0.600; b0= 15.59, b1= 4.79 × 10−4, b2= 720) and SUR2B subunits (r= 0.5413; b0= 49.14, b1= 4.69 × 10−5, b2= 3643).
In contrast, no change occurred in the KATP currents and fibre diameters recorded from FDB muscle fibres of 14-HU rats. The mean current amplitude and the fibre diameters were, respectively, −369.41 ± 11 pA (n= 29 patches) and 43.86 ± 5 μm (n= 29 fibres) in the controls, and −359.1 ± 8 pA (n= 25 patches) and 41.96 ± 5 μm (n= 25 fibres) in the FDB muscle fibres from 14-HU rats. No change in the percentage expression levels of MHC-IIa in this muscle phenotype was observed following the unloading period.
Quantitative real-time RT-PCR analysis performed on whole muscles demonstrated that the relative expression level of Kir6.2 mRNA was significantly reduced from 0.118 ± 0.09 in the SOL of controls (n= 6 muscles) to 0.0831 ± 0.013 in the SOL of 14-HU rats (n= 10 muscles) (P < 0.05 or less). The relative expression levels of SUR1 and SUR2B mRNA were also significantly reduced following disuse, respectively, from 0.0081 ± 0.0001 and 0.0151 ± 0.002 in the SOL of controls (n= 6 muscles) to 0.00522 ± 0.003 and 0.00721 ± 0.001 in the SOL of 14-HU rats (n= 10 muscles) (P < 0.05 or less). But no changes in the expression levels of Kir6.1 and SUR2A subunits were found in the same SOL muscles from 14-HU rats. Multiple correlation analysis performed between the fibre diameters, KATP currents and the expression levels of SUR1 of SOL showed high correlations between variables (r= 0.9335) (Table 1). A low level of correlation was observed between the fibre diameters, KATP currents and the relative expression levels of Kir6.2 (r= 0.600) and SUR2B (r= 0.5413) subunits. In contrast, the expression of all KATP channel subunits in the FDB muscles was not affected following 14 days of unloading of the rats.
KATP channel activity, MHC isoform identification and diameter measurements in single fibres isolated from SOL muscles of 14-HU rats and controls
To investigate the specific effects of atrophy and/or of the slow-to-fast phenotype transition on KATP channel properties, experiments were performed combining patch-clamp recording of channel activity, MHC isoform identification and fibre diameter measurements in the same isolated fibres. A similar number of fibres per SOL (n= 3–4 fibres) were randomly collected and grouped on the basis of their KATP currents and diameters, and their MHC was evaluated. Single fibre analysis revealed that 48.5% of the randomly sampled fibres (n= 35 fibres) isolated from all SOL muscles were atrophic following disuse. The atrophic fibres were of the MHC-I type, and showed reduced diameters of 47 ± 7 μm (n= 17 fibres) and KATP currents of −15 ± 4 pA (n= 17 patches) that were significantly lower than controls (P < 0.05).
A significant number of non-atrophic fibres (22%) were of the MHC-IIa type, and exhibited significantly enhanced KATP currents of −150 ± 12 pA (n= 8 patches, P < 0.05) as compared to controls, and normal diameters of 79 ± 11 μm (n= 8 fibres). The other non-atrophic fibres (28%) were of the MHC-I type, and showed KATP currents of −85 ± 10 pA (n= 10 patches) and diameters of 76 ± 5 μm (n= 10 fibres), thereby resembling those of controls.
Control fibres showed a mean diameter of 87 ± 3 μm (n= 14 fibres) with a KATP current amplitude of −78 ± 21 pA (n= 14 patches) and were of the MHC-I type.
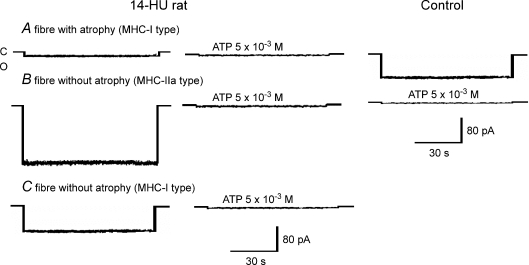
Three subtypes of fibre designated type A, B and C were observed. Fibre subtype A exhibited atrophy accompanied by a reduced KATP activity, but no slow-to-fast phenotype transition. Subtype B fibres showed no atrophy, but enhanced KATP channel activity and fast phenotype. Finally C fibres did not show any signs of atrophy or phenotype transition and exhibited normal KATP channel activity (Fig. 3).
Figure 3. KATP channel currents of SOL muscle fibres from control and 14-day-hindlimb-unloaded (14-HU) rats.
Sample traces of KATP channel currents recorded in excised macropatches from SOL fibres of 14-HU rats and controls during voltage steps from 0 mV holding potential to −60 mV (Vm) with 150 mm KCl on both sides of the membrane, at 20°C. C indicates closed channel levels; O indicates open channel levels. ATP applied on the internal side of the patches inhibited all types of currents. Three types of currents are represented from SOL fibres of 14-HU rats: the first is a sample trace of an atrophic fibre with diameter of 45 μm and KATP current amplitude of > −20 pA characterizing the fibre group named A; the second was not atrophic showing a diameter of 80 μm and a KATP current amplitude of −150 pA characterizing the fibre group named B; the third was not atrophic showing a KATP current amplitude of −85 pA and diameter of 76 μm characterizing the fibre group named C. The KATP current of a control fibre had an amplitude of −84 pA and fibre diameter of 76 μm.
Effects of pharmacological KATP modulators on KATP channel currents, fibre diameters, protein concentration/muscle weight and caspase-3 activities of control muscles
The involvement of the KATP channel in the atrophy of SOL and FDB muscles was further investigated by exposing ‘in vitro’ SOL or FDB muscles from control rats to glibenclamide and/or diazoxide, which are a known blocker and opener of KATP channels, respectively. The muscles were incubated for 72 h with DMEM+ solution used as controls, DMEM+ solution enriched with glibenclamide (10−6m) or diazoxide (10−4m), and glibenclamide (10−6m) + diazoxide (10−4m) solutions. Patch-clamp experiments showed that 72 h treatment of SOL with glibenclamide (10−6m) significantly reduced the KATP channel activity and diameters of the enzymatically isolated fibres. The KATP channel currents and fibre diameters were, respectively, −73 ± 5 pA (n= 16 patches) and 78.5 ± 7 μm (n= 16 fibres) in the DMEM+ solution and −24.5 ± 5 pA (n= 17 patches) (P < 0.05) and 59.5 ± 7 μm (n= 17 fibres) (P < 0.05) in DMEM+ solution enriched with glibenclamide (10−6m). The incubation of the SOL with glibenclamide (10−6m) + diazoxide (10−4m) solutions also prevented the reduction of the KATP channel currents and fibre diameters. We also found that glibenclamide was capable of significantly reducing the total protein content of SOL muscles by 10.38% and the ratio protein concentration/muscle wet weight, which is an index of atrophy, by 15.6% (Table 2). The incubation of SOL with glibenclamide + diazoxide solutions prevented the reduction of both total protein concentration and the ratio of protein concentration/muscle wet weight. Caspase-3 activity increased significantly in SOL muscle after 72 h incubation with glibenclamide and this effect was prevented by diazoxide (Table 2).
Table 2.
Effects of glibenclamide and diazoxide on protein concentration, protein concentration/weight of slow-twitch SOL muscles and caspase-3 activity of control rats
| Control muscles | Contralateral control muscles after 72 h incubation with DMEM+ | Muscles after 72 h incubation with DMEM+ and glibenclamide (10−6m) | Muscles after 72 h incubation with DMEM+, glibenclamide (10−6m) and diazoxide (10−4m) | Muscles after 72 h incubation with DMEM+ and diazoxide (10−4m) | |
|---|---|---|---|---|---|
| Protein | 2.11 ± 0.05 | 1.83 ± 0.06 | 1.64 ± 0.05* | 1.75 ± 0.04 | 1.79 ± 0.03 |
| concentration (μg μl−1) | (n= 7) | (n= 13) | (n= 7) | (n= 3) | (n= 3) |
| Protein concentration/ | 6.66 ± 0.03 | 5.56 ± 0.01 | 4.69 ± 0.01* | 5.59 ± 0.01 | 5.69 ± 0.02 |
| muscle weight (mg mg−1) | (n= 7) | (n= 13) | (n= 7) | (n= 3) | (n= 3) |
| Caspase-3 activity | 0 | 1.33± 0.09 | 1.73 ± 0.09* | 1.31 ± 0.08 | 1.32 ± 0.09 |
| (10−6μmol pNA min−1 ml−1) | (n= 7) | (n= 13) | (n= 7) | (n= 3) | (n= 3) |
Soleus (SOL) muscles from control rats were incubated for 72 h with the DMEM+, DMEM+ enriched with glibenclamide or diazoxide at 10−6m and 10−4m concentrations, respectively, under 5% CO2 atmosphere at 37°C. Control muscles are samples that were rapidly processed after dissection, and therefore not exposed to DMEM+ or other DMEM+ drug-enriched solutions. n in parentheses indicates the number of SOL muscles sampled. Values are mean ± standard deviation.
Controls vs. all other groups (significantly different for P < 0.05 or less).
Patch-clamp experiments showed that 72 h treatment of FDB with glibenclamide (10−6m) significantly reduced the KATP channel activity and diameter of the fibres. The KATP channel currents and fibre diameters were, respectively, −350.5 ± 19 pA (n= 18 patches) and 39 ± 7 μm (n= 18 fibres) in the DMEM+ solution and −19.16 ± 6 pA (n= 15 patches) (P < 0.05) and 25.3 ± 4 μm (n= 15 fibres) (P < 0.05) in DMEM+ solution enriched with glibenclamide (10−6m). The incubation of FDB with glibenclamide (10−6m) + diazoxide (10−4m) solutions also prevented the reduction of the KATP channel currents and fibre diameters. Treatment (72 h) of FDB muscles with glibenclamide (10−6m) also significantly reduced the total protein content by 16.9% and the ratio of protein concentration/muscle wet weight by 18.36% and these effects were prevented by diazoxide (10−4m) (Table 3). However, the activity of caspase-3 of the contralateral control FDB muscles after 72 h of incubation time with DMEM+ alone was undetectable as compared with that of the SOL muscles. Furthermore, the activity of caspase-3 of FDB muscles was not affected by glibenclamide or diazoxide.
Table 3.
Effects of glibenclamide and diazoxide on protein concentration, protein concentration/weight of fast-twitch FDB muscles and caspase-3 activity of control rats
| Control muscles | Contralateral control muscles after 72 h incubation with DMEM+ | Muscles after 72 h incubation with DMEM+ and glibenclamide (10−6m) | Muscles after 72 h incubation with DMEM+, glibenclamide (10−6m) and diazoxide (10−4m) | Muscles after 72 h incubation with DMEM+ and diazoxide (10−4m) | |
|---|---|---|---|---|---|
| Protein | 2.33 ± 0.06 | 2.01 ± 0.09 | 1.67 ± 0.07* | 1.91 ± 0.03 | 2.02 ± 0.03 |
| concentration (μg μl−1) | (n= 4) | (n= 9) | (n= 3) | (n= 3) | (n= 3) |
| Protein concentration/ | 8.56 ± 0.02 | 7.3 ± 0.03 | 5.96 ± 0.01* | 7.09 ± 0.02 | 7.29 ± 0.02 |
| muscle weight (mg mg−1) | (n= 4) | (n= 9) | (n= 3) | (n= 3) | (n= 3) |
| Caspase-3 activity | 0 | 0 | 0 | 0 | 0 |
| (μmol pNA min−1 ml−1) | (n= 4) | (n= 9) | (n= 3) | (n= 3) | (n= 3) |
Flexor digitorum brevis (FDB) muscles from control rats were incubated for 72 h with the DMEM+, DMEM+ enriched with glibenclamide or diazoxide at 10−6m and 10−4m concentrations, respectively, under 5% CO2 atmosphere at 37°C. Control muscles are samples that were rapidly processed after dissection, and therefore not exposed to DMEM+ or other DMEM+ drug-enriched solutions. n in parentheses indicates the number of FDB muscles sampled. Values are mean ± standard deviation.
Controls vs. all other groups (significantly different for P < 0.05 or less).
Incubation of SOL and FDB muscles for 72 h with DMEM+ and diazoxide (10−4m) did not restore the values of total protein concentrations and the ratio of protein concentration/muscle wet weight to controls, indicating that this compound is not capable of preventing the muscle atrophy that is not KATP dependent. The percentage reduction of KATP channel currents and diameters observed following 72 h treatment with DMEM+ in the absence or in the presence of glibenclamide, calculated with respect to the channel currents and diameters of control fibres, respectively, were −6.4% (SOL), −5.2% (FDB) and −10% (SOL), −11% (FDB) in the absence of drug; and −68.5% (SOL), −94.6% (FDB) and −31.6%(SOL), −42.3% (FDB) in the presence of glibenclamide. This suggested that a non-KATP-dependent process is responsible for about 10% of the atrophy in both SOL and FDB muscle fibres; while the KATP channel-dependent pathway is responsible for 21.6% and 31.3% of the atrophy observed in the SOL and FDB muscle fibres, respectively.
Discussion
In the present work we showed that the KATP channel is a key molecular component in the atrophic signalling in slow-twitch and fast-twitch muscles as investigated in the 14-day-hindlimb-unloaded rats, which are normally characterized by extensive atrophy of slow-twitch muscle and by an incomplete slow-to-fast phenotype transition of the fibres, and in an in vitro pharmacological model of muscle atrophy. This is supported by several findings; first, in the slow-twitch muscle from 14-HU rats a down-regulation of KATP channels has been observed. Reduced KATP channel current recorded in excised patches was indeed observed in atrophic fibres mostly in the MHC-I fibres (subtype A fibres). Second, the observed changes in the KATP channel currents correlate well with the changes in the fibre diameters, used as a cellular index of atrophy. Third, long-term in vitro incubation of the slow-twitch or fast-twitch muscles of control rats with glibenclamide, a selective blocker of the KATP channel, reduced either KATP channel currents recorded in excised patches and fibre diameters, and these effects were prevented by diazoxide, a well-known KATP channel opener.
The observed reduction of KATP channel activity in the muscles from 14-HU rats is due to the down-regulation of the genes of the KATP channel subunits. A significant reduction of relative expression levels of SUR1, Kir6.2 and SUR2B subunits was found in the slow-twitch muscle following disuse, with no changes in the SUR2A or Kir6.1 subunits. The observed changes in the fibre diameters and KATP channel currents correlate well with the relative expression levels of SUR1 of slow-twitch muscle, as demonstrated by the multiple correlation analysis performed between all these variables, while the low levels of correlation found between the fibre diameters, KATP channel currents and the relative expression levels of Kir6.2 or SUR2B subunits suggest a secondary role for these subunits in the atrophic processes. Furthermore, glibenclamide and diazoxide, which are capable in vitro of, respectively, inducing or preventing atrophy of skeletal muscle, bind with high affinity to SUR1 subunits but are low affinity ligands of SUR2 subunits. We therefore concluded that the SUR1 subunit of the KATP channel complex plays a major role in triggering the atrophic signalling in skeletal muscle. Whether this is the surface membrane SUR1 or the mitochondrial SUR1 subunit cannot be easily established from our data. The finding that the observed reduced expression of SUR1 and Kir6.2 subunits in our experiments is paralleled by the significant reduction of the KATP channel activity support the involvement of sarcolemmal rather than mitochondrial KATP channels in the atrophy of skeletal muscle, in contrast, to what is observed in other tissues (Debska et al. 2002; Ardehali & O’Rourke, 2005; Wu et al. 2007).
Atrophy in skeletal muscle is known to be associated with a series of events involving deactivation of the PI3K/Akt/mTOR pathway, activation of the FOXO/atrogin and MurF1 genes with proteolysis and inhibition of protein synthesis, and activation of apoptotic and/or lysosomal pathways (Kandarian & Jackman, 2006). In our experiments, the drug-induced atrophy of skeletal muscle observed ‘in vitro’ is associated with apoptosis as demonstrated by the fact that glibenclamide was able to enhance caspase-3-dependent activity in slow-twitch muscle and to reduce the ratio of protein concentration to muscle weight, an index of atrophy, and these effects were prevented by diazoxide. However, it seems that a caspase-3-independent mechanism plays a role in the drug-induced atrophy of fast-twitch muscle as demonstrated by the fact that glibenclamide is capable of reducing the ratio of protein concentration to muscle weight and this effect is prevented by diazoxide without leading to appreciable activation of caspase-3 in this muscle phenotype. This is in agreement with recent reports showing that atrophy is associated with caspase-3-dependent apoptosis in slow-twitch muscle rather than in fast-twitch muscle in which lysosomal activity might prevail, thereby supporting the idea that the activation of proteolytic pathways could differ between slow- and fast-twitch muscles (Dupont-Versteegden, 2006; Ferreira et al. 2007, 2008).
KATP channel activity is also dependent on muscle phenotypes as supported by the fact that the observed changes in the KATP channel currents, fibre diameters and the percentage expression levels of the MHC-IIa isoform in the slow-twitch muscle of 14-HU rats are correlated as determined by multiple coefficient correlation analysis. Furthermore, in the absence of atrophy an up-regulation of KATP channels is observed in fibres of slow-twitch muscles that were labelled by MHC-IIa antibodies (subtype B fibres). The KATP current values recorded in these fibres were in the range reported for the fast-twitch fibres leading to the idea that a shift from slow- to fast-twitch phenotypes occurred (Tricarico et al. 2006). The up-regulation of the KATP channel may have a protective role against atrophy and may explain the observed resistance of FDB muscles to the atrophic process. This is a fast-twitch muscle normally characterized by elevated KATP channel current levels sustained by high expression levels of SUR1 and Kir6.2 subunits other than SUR2 subunits (Tricarico et al. 2006). Several intracellular pathways may be affected by the up-regulation of the KATP channels seen in the MHC-IIa fibres such as the Ca2+–calmodulin calcineurin/NFAT/MEF2 pathway that normally triggers the slow-to-fast phenotype transition, the extracellular signal-regulated kinase (ERK) pathway that is involved in the apoptosis and up-regulation of the slow genes, and insulin-dependent PI3-Akt-kinase, a well-known hypertrophic signalling pathway in skeletal muscle (Fraysse et al. 2003; Tricarico et al. 2003; Maedler et al. 2004; Kandarian & Jackman 2006; Kane et al. 2006; Harridge, 2007).
However, we believe that the low level of correlation observed between the changes in KATP channel currents and percentage expression levels of the MHC-IIa isoform, and the existence in the slow-twitch muscles from 14-HU rats of two subpopulations of MHC-I fibres showing normal channel currents and diameters (subtype C) or reduced channel currents and diameters (subtype A fibres), would suggest a secondary role of the KATP channel in the muscle phenotype as compared with the atrophic process.
Therefore, down-regulation of SUR1/Kir6.2, and possibly of other KATP channel subtypes as well as their pharmacological blockade leads to atrophic signalling in slow-twitch and fast-twitch skeletal muscles. These findings taken together led us to propose a new role for the KATP channel as a molecular sensor of atrophy, and possibly of the slow-to-fast phenotype transitions associated with muscle disuse.
One limitation of our work is that the KATP current has been recorded in excised patch experiments that normally lead to loss of intracellular metabolites including ATP and other nucleotides without allowing the correlation between the energy conditions of the cell and other parameters such as atrophy and KATP channel activity. Previous work showed an increase in the ATP and lactate contents in isolated fibres from SOL muscle of 14-HU rats indicating a shift from the oxidative to glycolytic metabolism following 14 days HU (Grichko et al. 2000).
The observed reduced KATP activity in the atrophied SOL muscle fibres of 14-HU rats may have consequences on muscle physiology, for instance causing a slight fibre depolarization at rest as already reported, or may impair the fibre repolarization during prolonged AP firing (Pierno et al. 2007; Pedersen et al. 2009). These findings are of pathophysiological relevance as atrophy and myofibre phenotype transition often coexist in humans. Atrophy of MHC type IIx fibres is commonly observed in sprinters and body builders (Andersen & Aagaard, 2000), or in the elderly (sarcopenia) (Harridge, 2007). Vastus lateralis muscles from spinal cord-injured young patients, in contrast to those of healthy subjects, exhibit a high percentage of fibres expressing MHC-IIx, MHC-IIa or both, but almost no MHC type I fibres. Another condition of muscle disuse is that observed in humans during space flight and in experimental animals following simulated hypogravity and/or limb immobilization characterized by a reduction of strength and endurance, and the shortening of relaxation/contraction times with prolonged impairment of the muscle functionality. These findings may also have a therapeutic implication. Glibenclamide has been used for many years in the long-term treatment of type II diabetes and several reports have associated the use of this drug with pancreatic β cell death. The combined action of glibenclamide with other cytotoxic drugs with well-known atrophic effects, as commonly seen in the treatment of co-morbidity, may be associated with possible toxicodynamic drug–drug interaction in muscle.
Acknowledgments
This work was supported by the Italian Space Agency (project OSMA ‘Osteoporosis and Muscle Atrophy’).
Glossary
Abbreviations
- BK
maxi Ca2+-activated K+ channel
- FDB
flexor digitorum brevis
- 14-HU
14-day hindlimb unloading
- KATP
ATP-sensitive K+ channel
- Kir
inwardly rectifying K+ channel
- MHC
myosin heavy chain
- SOL
soleus
- SUR
sulfonylurea receptor
Author contributions
All authors contributed equally to the work. All authors approved the final version of the manuscript.
References
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ardehali H, O’Rourke B. Mitochondrial KATP channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debska G, Kicinska A, Skalska J, Szewczyk A, May R, Elger CE, Kunz WS. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim Biophys Acta. 2002;1556:97–105. doi: 10.1016/s0005-2728(02)00340-7. [DOI] [PubMed] [Google Scholar]
- Desaphy JF, Pierno S, Léoty C, George AL, Jr, De Luca A, Conte Camerino D. Skeletal muscle disuse induces fibre type-dependent enhancement of Na+ channel expression. Brain. 2001;124:1100–1113. doi: 10.1093/brain/124.6.1100. [DOI] [PubMed] [Google Scholar]
- Desaphy J-F, Pierno S, Liantonio A, De Luca A, Didonna MP, Frigeri A, Nicchia GP, Svelto M, Camerino D, Zallone A, Conte Camerino D. Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile. Neurobiol Dis. 2005;18:356–365. doi: 10.1016/j.nbd.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE. Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol. 2006;12:7463–7466. doi: 10.3748/wjg.v12.i46.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Neuparth MJ, Vitorino R, Appell HJ, Amado F, Duarte JA. Evidence of apoptosis during the early phases of soleus muscle atrophy in hindlimb suspended mice. Physiol Res. 2008;57:601–611. doi: 10.33549/physiolres.931272. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Vitorino R, Neuparth MJ, Appell HJ, Amado F, Duarte JA. Cellular patterns of the atrophic response in murine soleus and gastrocnemius muscles submitted to simulated weightlessness. Eur J Appl Physiol. 2007;101:331–340. doi: 10.1007/s00421-007-0502-z. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Desaphy J-F, Pierno S, De Luca A, Liantonio A, Mitolo CI, Conte Camerino D. Decrease in resting calcium and calcium entry associated with slow-to-fast transition in unloaded rat soleus muscle. FASEB J. 2003;17:1916–1918. doi: 10.1096/fj.02-1012fje. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Desaphy J-F, Pierno S, De Luca A, Conte Camerino D, Svelto M. Muscle loading modulates aquaporin-4 expression in skeletal muscle. FASEB J. 2001;15:1282–1284. doi: 10.1096/fj.00-0525fje. [DOI] [PubMed] [Google Scholar]
- Frigeri A, Nicchia GP, Verbavatz JM, Valenti G, Svelto M. Expression of aquaporin-4 in fast-twitch fibres of mammalian skeletal muscle. J Clin Invest. 1998;102:695–703. doi: 10.1172/JCI2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichko VP, Heywood-Cooksey A, Kidd KR, Fitts RH. Substrate profile in rat soleus muscle fibres after hindlimb unloading and fatigue. J Appl Physiol. 2000;88:473–478. doi: 10.1152/jappl.2000.88.2.473. [DOI] [PubMed] [Google Scholar]
- Hambrock A, de Oliveira Franz CB, Hiller S, Osswald H. Glibenclamide-induced apoptosis is specifically enhanced by expression of the sulfonylurea receptor isoform SUR1 but not by expression of SUR2B or the mutant SUR1 (M1289T) J Pharmacol Exp Ther. 2006;316:1031–1037. doi: 10.1124/jpet.105.097501. [DOI] [PubMed] [Google Scholar]
- Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol. 2007;92:783–797. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- Kandarian SC, Jackman RW. Intracellular signalling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodelling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca2+-dependent BK channel signalling complex by binding to β2 adrenergic receptor. EMBO J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced β-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501–506. doi: 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
- Maedler K, Størling J, Sturis J, Zuellig RA, Spinas GA, Arkhammar PO, Mandrup-Poulsen T, Donath MY. Glucose- and interleukin-1β-induced β-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T. Beta-cell apoptosis: stimuli and signalling. Diabetes. 2001;50:S58–S63. doi: 10.2337/diabetes.50.2007.s58. [DOI] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli FV, Flatman JA, Nielsen OB. Regulation of ClC-1 and KATP channels in action potential–firing fast twitch muscle fibres. J Gen Physiol. 2009;134:309–322. doi: 10.1085/jgp.200910290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MA, Canepari M, Rossi R, D’Antona G, Reggiani C, Bottinelli R. Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J Physiol. 2003;546:677–689. doi: 10.1113/jphysiol.2002.027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D. The adaptive potential of skeletal muscle fibres. Can J Appl Physiol. 2002;27:423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- Pierno S, Desaphy J-F, Liantonio A, De Bellis M, Bianco G, De Luca A, Frigeri A, Nicchia GP, Svelto M, Léoty C, George AL, Jr, Conte Camerino D. Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading induced muscle disuse. Brain. 2002;125:1510–1521. doi: 10.1093/brain/awf162. [DOI] [PubMed] [Google Scholar]
- Pierno S, Desaphy J-F, Liantonio A, De Luca A, Zarrilli A, Mastrofrancesco L, Procino G, Valenti G, Conte Camerino D. Disuse of rat muscle in vivo reduces protein kinase C activity controlling the sarcolemma chloride conductance. J Physiol. 2007;584:983–995. doi: 10.1113/jphysiol.2007.141358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Mallamaci R, Barbieri M, Conte Camerino D. Modulation of ATP-sensitive K+ channel by insulin in rat skeletal muscle fibres. Biochem Biophys Res Commun. 1997;232:536–539. doi: 10.1006/bbrc.1997.6320. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Conte Camerino D. Phenotype-dependent functional and pharmacological properties of BK channels in skeletal muscle: effects of microgravity. Neurobiol Dis. 2005;20:296–302. doi: 10.1016/j.nbd.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Liss B, Ashcroft FM, Lundquist AL, Desai RR, George AL, Jr, Conte Camerino D. Reduced expression of Kir6.2/SUR2A subunits explains KATP deficiency in K+-depleted rats. Neuromuscul Disord. 2008;18:74–80. doi: 10.1016/j.nmd.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Jr, Conte Camerino D. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci U S A. 2006;103:1118–1123. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D, Montanari L, Conte Camerino D. Involvement of 3Na+/2K+ ATP-ase and Pi-3 kinase in the response of skeletal muscle ATP-sensitive K+ channels to insulin. Neuromuscul Disord. 2003;13:712–719. doi: 10.1016/s0960-8966(03)00095-6. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Servidei S, Tonali P, Jurkat-Rott K, Conte Camerino DC. Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis. J Clin Invest. 1999;103:675–682. doi: 10.1172/JCI4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Zouq N, Gilmore AP. Anoikis. Biochem Soc Trans. 2004;32:421–425. doi: 10.1042/BST0320421. [DOI] [PubMed] [Google Scholar]
- Wu SN, Wu AZ, Sung RJ. Identification of two types of ATP-sensitive K+ channels in rat ventricular myocytes. Life Sci. 2007;80:378–387. doi: 10.1016/j.lfs.2006.09.042. [DOI] [PubMed] [Google Scholar]