Abstract
This study was undertaken to investigate neuromuscular transmission in regions of the inflamed colon in which motility is disrupted. Propulsive motility was evaluated in segments of control guinea pigs and those treated 6 days previously with trinitrobenzene sulfonic acid. Intracellular recordings were then obtained from circular muscle cells to examine excitatory and inhibitory junction potentials (EJPs and IJPs). In inflamed preparations, propulsion of fecal pellets was temporarily halted or obstructed at sites of mucosal damage, whereas the propulsive motility was linear in control colons. The amplitudes of evoked and spontaneous IJPs were significantly reduced in ulcerated regions of inflamed preparations, but EJPs were comparable to controls. Pharmacological dissection of the IJP revealed that the purinergic component was reduced, while the nitrergic IJP was slightly increased. Furthermore, the reduction in the purinergic IJP in inflamed preparations persisted in the presence of hexamethonium, suggesting that the deficit involved the inhibitory motor neuron and/or smooth muscle. Nerve fibre density was not altered in the circular muscle, and pre-contracted rings of inflamed colon relaxed normally to ATP, suggesting that the deficit involves altered ATP release and/or degradation. The P2Y1 receptor antagonist MRS2179 slowed propulsive motility indicating that decreased purinergic neuromuscular transmission could contribute to the inflammation-induced motor deficit. We conclude that purinergic inhibitory neuronal input to the circular muscle is selectively reduced in regions of the colon in experimental colitis where the mucosa is damaged, and this is likely to contribute to altered motility in colitis by diminishing downstream relaxation during the peristaltic reflex.
Introduction
Intestinal motor function is regulated by intrinsic neural reflexes that result in coordinated contractions and relaxations of smooth muscle that serve to mix and propel luminal contents. The most extensively investigated motor reflex in the gut is the peristaltic reflex, which was originally described by Bayliss and Starling in the canine small and large intestines (Bayliss & Starling, 1899, 1900) and by Trendelenburg in the guinea pig small intestines (Trendelenburg, 2006). Luminal contents activate the peristaltic reflex via the release of serotonin and other mediators from enterochromaffin cells that act as sensors and/or through stretch-induced activation of enteric neurons (Heredia et al. 2009). Intrinsic sensory neurons in turn activate ascending interneurons, which selectively synapse on excitatory motor neurons, as well as descending interneurons, which synapse on inhibitory motor neurons. The net result is contraction above and relaxation below the level of the stimulus, and the generation of a pressure gradient that transports the luminal contents along the intestines. Colitis in the guinea pig disrupts propulsive motility (Linden et al. 2003b; Krauter et al. 2007b), and in humans with ulcerative colitis, the motor response to a meal is significantly reduced (Coulie et al. 2001), but the underlying mechanisms for these motor deficits have not been resolved.
Previous studies by us and by others have demonstrated that inflammation leads to alterations in several elements of the peristaltic circuitry. For example, myenteric afterhyperpolarizing (AH) neurons are hyperexcitable in the inflamed colon (Linden et al. 2003b) and in the small intestine (Nurgali et al. 2007) of guinea pigs treated with trinitrobenzene sulfonic acid (TNBS), as well as in the small intestine of Trichinella spiralis infected guinea pigs (Palmer et al. 1998; Chen et al. 2007). As compared to AH neurons from healthy animals, AH neurons in the inflamed colon fire more action potentials during a prolonged depolarizing current pulse, generate more spontaneous activity, and exhibit a smaller afterhyperpolarization and facilitated synaptic transmission (Linden et al. 2003b). Another neuroplastic change that has been observed in the inflamed colon is that synaptic potentials are facilitated in synaptic (S) neurons, which serve as interneurons and motor neurons (Linden et al. 2003b; Krauter et al. 2007a). However, it is yet not clear whether and how output from the nervous system to the smooth muscle is altered in the inflamed intestine.
Output from the enteric nervous system to the intestinal smooth muscle can be detected as neuromuscular excitatory and inhibitory junction potentials (EJPs and IJPs) (Furness, 1969). The junction potentials occur spontaneously and can be evoked by electrical stimulation or mucosal stroking. There is morphological and physiological evidence from the mouse that interstitial cells of Cajal (ICC) are the principal targets of the motor neurons, and that they spread the synaptic signals to the smooth muscle syncytium via gap junctions (Ward et al. 2006). In the colon, the EJP is predominantly mediated by acetylcholine acting at muscarinic receptors (Spencer & Smith, 2001), but high frequency stimulation evokes the release of tachykinins that activate neurokinin receptors (Zagorodnyuk et al. 1993). The IJP has both rapid and slow components. The rapid component of the IJP is mediated by P2Y1 receptors and is widely thought to be transmitted by ATP (Mutafova-Yambolieva et al. 2007; Wang et al. 2007; King & Townsend-Nicholson, 2008), although there is evidence that β-nicotinamide is also involved (Mutafova-Yambolieva et al. 2007). This component of the IJP is sensitive to apamin (Vladimirova & Shuba, 1978; Bywater & Taylor, 1986; Crist et al. 1992; Spencer & Smith, 2001), a component of honeybee venom, which inhibits small conductance Ca2+-activated K+ (SK) channels (Stocker, 2004). The slow component of the IJP is nitrergic, and can be blocked by nitric oxide synthase inhibitors (Watson et al. 1996; Spencer et al. 2001). In general, due to the organization of peristaltic circuitry in the bowel, EJPs can be activated and studied by stimulating aboral to the recording electrode, while stimulating the preparation oral to the recording electrode evokes IJPs, although the polarity of these events is not unequivocal.
The aim of this study was to evaluate excitatory and inhibitory neuromuscular transmission in regions of TNBS-inflamed guinea pig distal colon that exhibited disrupted motility. To accomplish this, propulsive motility of fecal pellets was evaluated, and subsequent intracellular recordings were obtained from circular muscle cells in areas exhibiting transient or complete obstruction of pellet propulsion, which were found to be primarily in regions where the mucosa was damaged. Spontaneous and evoked EJPs and IJPs were evaluated in circular muscle cells from normal and inflamed preparations. The results of this study indicate that the purinergic component of the IJP was selectively attenuated in circular muscle cells of the TNBS-inflamed guinea pig distal colon.
Methods
Animal preparations
Albino guinea pigs of either sex weighing 250–350 g (Charles River, Montreal, Canada) were housed in microfilter cages, given free access to food and water, and maintained on a 12 h: 12 h light-dark cycle. To induce colitis, guinea pigs were anaesthetized with isoflurane (4% induction, 2% maintenance in oxygen), and analgesia was confirmed by assessing a lack of limb withdrawal to paw pressure. A polyethylene catheter was inserted rectally a distance of 7 cm and 0.3 ml of TNBS (Fluka, Milwaukee, WI; 25 mg ml−1) in 30% ethanol was injected into the lumen. Animals that were given the TNBS treatment were weighed each day and monitored twice daily for signs of pain or distress such as lack of mobility, vocalization upon handling, and unkempt appearance. Also, if an animal was found to lose ≥ 20% of its weight, it would be killed, but no unplanned killings were necessary in the current study. On day 6 following TNBS administration, animals were killed by isoflurane overdose and exsanguination, and the distal colon removed. Severity of colitis was assessed by weight loss, and by determination of a macroscopic damage score based on wall thickness and presence of ulceration, hyperaemia, and/or diarrhoea. Ulceration was defined as disruption of the mucosal surface that was visible macroscopically or with the aid of a dissecting microscope. All methods used in this study were approved by the University of Vermont's Institutional Animal Care and Use Committee (IACUC), and all experiments were carried out at the University of Vermont. The experiments comply with The Journal of Physiology's policy (Drummond, 2009).
Motility analyses
Segments of distal colon were pinned at either end in a Sylgard lined organ bath and allowed to equilibrate for 30 min in aerated circulating Krebs solution at 37°C (mm: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose, 8; aerated with 95% O2–5% CO2; all from Sigma, St Louis, MO, USA) with a flow rate of 10 ml min−1. To localize and ascertain the extent of impeded or obstructed peristalsis in inflamed colon, an epoxy coated pellet was introduced into the oral end to initiate peristalsis and a camera positioned above the chamber recorded instantaneous movement of the pellet, allowing qualitative analysis of peristalsis over a 5 cm segment of distal colon (Gastrointestinal Motility Monitor, Med Associates, St Albans, VT, USA). Three to five trials of peristalsis were performed for each animal with a 5 min recovery period between each run.
Intracellular recording
Following motility analysis, the distal colon was opened along the mesenteric border and pinned flat in a Sylgard lined recording chamber. The mucosa and submucosa were dissected away, leaving the circular muscle layer exposed. Tissue was continuously bathed in 37°C aerated Krebs solution with nifedipine (2 μm) to prevent muscle contraction. Glass microelectrodes were loaded with 0.5 m KCl, and then backfilled with 2.0 m KCl, and had resistances ranging from 80 to 140 MΩ. Circular muscle was visualized at ×200 through an inverted microscope (Nikon Diaphot, Melville, NY, USA). Transmembrane potential was measured with an Axoclamp-2A amplifier (Axon Instruments, Union City, CA, USA) and signals were acquired and analysed with PowerLab Chart (version 5.01, ADInstruments, Castle Hill, NSW, Australia).
Junction potentials were evoked using pairs of stainless steel transmural electrodes positioned above and below and spanning the width of the tissue section. Since synaptic facilitation has been reported in TNBS colitis (Linden et al. 2003b; Krauter et al. 2007a), recordings were made at distances of 10 mm and 2 mm from stimulating electrodes in order to include events that are more likely to include multisynaptic circuitry as well as those primarily due to motor neuron stimulation. To record evoked IJPs that mediate descending relaxation, the recording electrode was positioned aborally. However polarity alone was not sufficient to prevent overlap of inhibitory and excitatory junction potentials in the presence of nifedipine alone, and therefore evoked IJPs were recorded in the presence of atropine (1 μm) to block muscarinic receptors. Purinergic and nitrergic components of the IJP were isolated by addition of apamin (250 nm, Sigma-Aldrich) and NG-nitro-l-arginine (l-NNA; 100 μm, Sigma-Aldrich) to the bathing solution.
Transmural stimulation consisted of single 500 ms pulses over voltages of 10, 20, 30, 40 and 50 V to span the range from undetectable response to maximal response. In order to simplify the presentation of data, only the 50 V stimulus is presented; however, the trend and significance of responses were maintained across lower voltages. The voltage series was repeated for each cell for four to five repetitions, during which no substantial variation in responses was observed.
Signals were acquired and analysed using the Peak Analysis Module within PowerLab Chart (version 5.01, ADInstruments). For consistency of peak detection, signals were passed through a low-pass digital filter set at 50 Hz. Peaks were detected using presets for General Sine Shape (negative peaks) for IJPs and General Sine Shape for EJPs. Each peak was visually inspected using Analysis View to be certain that artifacts were not included. Parameters evaluated included resting membrane potential, junction potential amplitude, and width at 50% maximum amplitude. Data were exported to Excel and averages for each cell were calculated for each parameter at each voltage.
Myographic analyses
Myograph studies were designed to evaluate contractile and relaxive responses to direct application of neurochemical mediators. Initial motility analyses consisted of three trials to localize regions of interrupted motility. Colons were then placed on ice to prevent contraction. Intact rings of equal width were cut using two straight-blade razors spaced 2 mm apart; one ring taken at the ulcer, and one ring each from regions oral and aboral to the ulcer. Mucosa and submucosa were left intact to preserve as much tissue architecture as possible. Rings were mounted directly onto hooks of force transducers on a MyoMed myograph (Med Associates). Rings were lowered into water-jacketed chambers containing 8 ml 37°C Krebs solution, bubbled with 95% O2–5% CO2, and treated from the outset with 300 nm tetrodotoxin to block neural activity. Transducers were set at the minimum height required to keep the ring from falling off the hooks. After tissue had warmed for 10 min, transducers were tared and tension applied to an initial load of 1 g. Rings were allowed to equilibrate for 30 min during which they would accommodate to the initial load, ending at a resting force between 0.2 g and 0.3 g. Bathing solution was refreshed every 15 min.
Relaxatory responses
Following evaluation of contractile response, rings were precontracted with 10 mm bethanechol until a steady state of contraction developed (approximately 5 min after addition of bethanechol). Concentration–effect curves were generated for ATP by cumulative addition of 1 mm, 10 mm, 100 mm, 1 mm and 10 mm ATP. ATP was washed out and it was verified that rings still contracted in response to 10 mm bethanechol.
Data were analysed for peak amplitude response to electric field stimulation, and for mean amplitude over a 2 min period for each drug application using MyoViewer (Med Associates). Following recording, tissue was fixed overnight in 2% paraformaldehyde, 0.2% picric acid at 4°C. After washing 3 × 15 min in phosphate-buffered saline (PBS), ring thickness and diameter were measured, and tissue fixed weight was recorded to allow for normalization.
Immunohistochemistry for analysis of nerve fibre densities
Sections of intact control and inflamed distal colon were fixed in 2% paraformaldehyde, 0.2% picric acid overnight at 4°C. After 3 × 15 min washes in PBS, tissue was cryoprotected overnight in 30% sucrose in PBS at 4°C. Each colon was cut into 5 mm segments and embedded in OCT for cryostat sectioning. Sections 20 μm thick were cut and mounted on glass slides coated with 2% aminopropyltriethoxysilane in acetone (APTEX; Sigma). Sections were blocked for 2 h at room temperature with 4% normal horse serum in PBS containing 0.5% Triton X-100. Then sections were incubated overnight at room temperature in PBS with 4% horse serum and 0.5% Triton X-100, and primary antibody. For motor neuron fibre density a 1: 500 dilution of mouse anti-PGP9.5 (Biogenesis, Pool, UK), a 1: 100 dilution of goat anti-choline acetyltransferase (ChAT, Chemicon), a 1: 400 dilution of rabbit anti-nitric oxide synthase (NOS; Transduction Labs, BD Biosciences, San Jose, CA, USA), and a 1: 800 dilution of mouse anti-smooth muscle actin (Sigma). Unbound primary antibody was removed with 3 × 15 min washes in PBS. Sections were incubated for 2 h in PBS with 0.5% Triton X-100 and secondary antibodies: for motor neuron fibre density a 1: 100 dilution of AMCA-conjugated donkey anti-mouse (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), a 1: 100 dilution of FITC-conjugated donkey anti-goat (Jackson), and a 1: 500 dilution of Cy3-conjugated donkey anti-rabbit (Jackson). For nuclear staining, tissue was incubated 15 min in DAPI at 0.1 μg ml−1 (Sigma). Sections were washed 3 × 15 min in PBS, quickly rinsed with deionized water, then mounted with Citifluor and coverslipped.
Data analysis
Statistical analyses were performed using GraphPad Prism software (v. 5.0a for Macintosh, GraphPad Software, San Diego, CA, USA). Differences between control and inflamed groups were determined by unpaired Student's t test for inflamed versus non-inflamed preparations, a paired t test for evaluating propulsive motility before and after addition of a purinergic agonist, and a two-way ANOVA with Bonferroni post hoc analysis for myograph studies. A P value of < 0.05 was considered to be statistically significant. For motility experiments n values represent the number of animals tested; for electrophysiological experiments n values represent the number of cells tested; for myograph experiments n values represent the number of rings tested. Data presented are means ±s.e.m. for n animals.
Results
Propulsive motility is disrupted in ulcerated regions in TNBS colitis
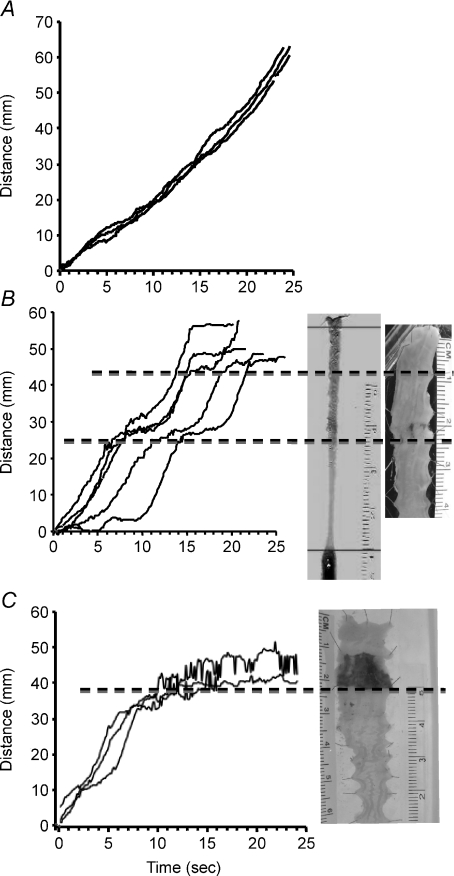
Initial evaluation of propulsive motility in the TNBS-inflamed guinea pig distal colon involved determining the total time elapsed as a fecal pellet progressed over an isolated segment of distal colon. The use of the GIMM system allows continuous analysis of propulsive motility as it traverses the segment of colon. In TNBS-inflamed preparations, propulsive motility was impeded specifically at an ulcer in colons from 66 out of 73 animals tested, with progress of the fecal pellet being temporarily halted in 51 preparations and completely halted in colons from 15 of the animals (Fig. 1). The rate of motility was significantly increased 2 cm oral and aboral to the ulcerated region, as compared to control values (control, 1.9 ± 0.07 mm s−1, n= 72; TNBS oral to ulcer, 2.5 ± 0.11 mm s−1, n= 60, P≤ 0.01; TNBS aboral to ulcer, 3.6 ± 0.2 mm s−1, n= 34, P≤ 0.01).
Figure 1. Propulsive motility is linear in colons from non-treated animals, but is disrupted in ulcerated regions 6 days following TNBS administration.
A, data from a normal colon illustrating the progress of a fecal pellet over time in 3 consecutive trials. B, graph illustrating data from a TNBS-treated animal in which propulsive motility was temporarily halted in ulcerated regions. This pattern was observed in 51 of 73 (70%) animals tested. C, graph illustrating data from a TNBS-treated animal in which propulsive motility was completely obstructed at the site of an ulcer. This pattern was observed in 15 of 73 (21%) animals tested.
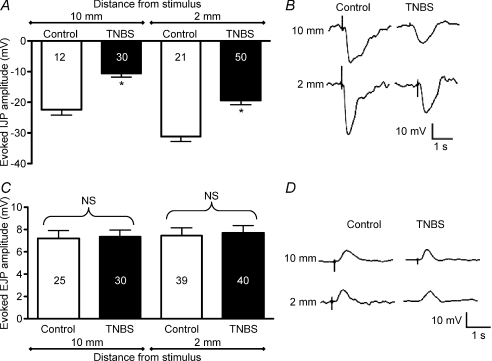
The amplitude of inhibitory junction potentials is reduced in ulcerated regions, but that of excitatory junction potentials is unchanged
To determine whether neuromuscular communication is altered in the ulcerated regions of the TNBS-inflamed distal colon, evoked and spontaneous inhibitory and excitatory junction potentials (IJPs and EJPs) were evaluated while recording from circular muscle cells in normal preparations and in the ulcerated regions of TNBS-inflamed preparations. Evoked responses were generated by transmural stimulation at distances of 2–10 mm oral to, or aboral to, an ulcerated region while recording from a circular muscle cell with an intracellular microelectrode. Nifedipine (2.0 μm) was present in the bathing solution to eliminate slow wave depolarizations, and IJPs were evaluated in the presence of atropine (1.0 μm), which blocked EJPs, and EJPs were studied in the presence of apamin (250 nm) plus l-NNA (100 μm), which inhibited IJPs.
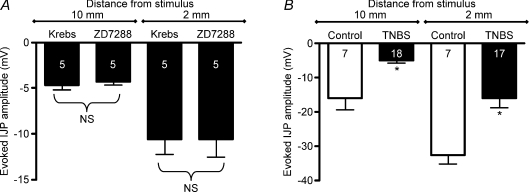
Transmural stimulation at both 10 mm and 2 mm oral to the recording electrode evoked IJPs with significantly smaller amplitudes in ulcerated regions compared with control colon (Fig. 2A and B; P < 0.0001). In contrast, EJPs evoked by transmural stimulation 2–10 mm aboral to the impaled smooth muscle cell were comparable in control vs. TNBS-inflamed preparations (Fig. 2C and D; 2 mm, P= 0.78; 10 mm, P= 0.84).
Figure 2. Evoked inhibitory junction potentials (IJPs) are reduced at an ulcer, but excitatory junction potentials (EJPs) are unchanged.
Junction potentials were evoked by transmural electrical field stimulation 2–10 mm oral (IJPs) or aboral (EJPs) from the impaled circular muscle cell, which in TNBS-treated preparations, was at the site of an ulcer. A, graph showing IJP amplitude in ulcerated regions versus control with transmural stimulation at distances of 10 mm and 2 mm oral to the recording electrode. B, representative traces for IJPs at 10 mm (top traces) and 2 mm (bottom traces). C, graph illustrating EJP amplitude in ulcerated regions versus control with transmural stimulation at distances of 10 mm and 2 mm oral to the recording electrode. D, representative traces for EJPs at 10 mm (top traces) and 2 mm (bottom traces).
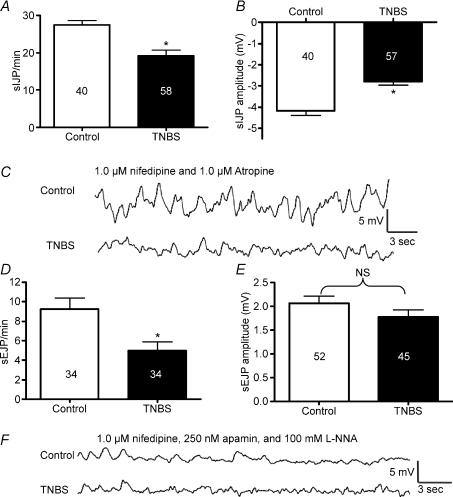
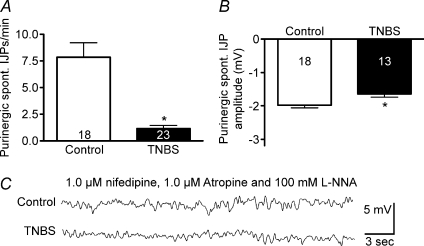
In the presence of atropine (1 μm) to block EJPs, the frequency of spontaneous IJPs was significantly lower in inflamed compared with control colon (Fig. 3A and C; P < 0.0001) and sIJP amplitude was significantly decreased (Fig. 3B and C; P < 0.0001).
Figure 3. The frequencies of spontaneous IJPs and EJPs, and the amplitudes of spontaneous IJPs were reduced in TNBS-inflamed preparations.
A and B, quantification of spontaneous IJP frequency (A) and amplitude (B) in ulcerated regions versus control. C, representative traces illustrating spontaneous IJPs. D and E, quantification of spontaneous EJP frequency (D) and amplitude (E) in ulcerated regions versus control. F, representative traces illustrating spontaneous EJPs.
The frequency of spontaneous EJPs (sEJPs) was significantly decreased in inflamed compared with control colon (Fig. 3D and F; P < 0.0001), but the sEJP amplitude was not significantly altered (Fig. 3E and F; P > 0.05).
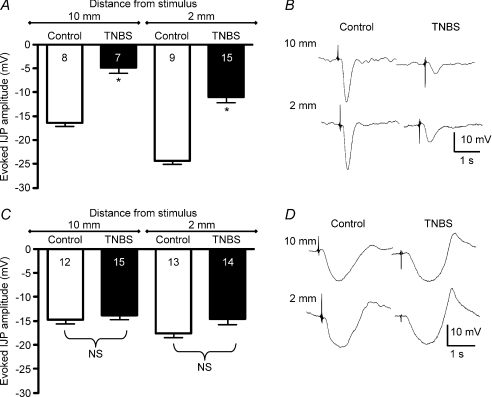
The purinergic component of the IJP is selectively reduced in the ulcerated region of TNBS-inflamed colon
The IJP is primarily composed of a relatively rapid, apamin-sensitive, purinergic component that is mediated by P2Y1 receptors, and a slow l-NNA-sensitive, nitrergic component that involves cyclic GMP activation. To determine whether one or both of these components was decreased in the ulcerated region of inflamed preparations, IJPs were evaluated in the presence of apamin (250 nm) or l-NNA (100 μm) to isolate the nitrergic and purinergic components, respectively. In the presence of l-NNA, the purinergic component of the IJP was significantly reduced in ulcerated regions compared with control tissue (Fig. 4A and B; P < 0.0001 for each pair). In the presence of atropine (1 μm) plus apamin, no differences were detected in the apamin-insensitive, nitrergic component of evoked IJPs that were activated by transmural stimulation at distances of 10 mm or 2 mm from the recording site in the ulcerated region (Fig. 4C and D).
Figure 4. The purinergic component of the IJP is reduced in TNBS-inflamed preparations, but the nitrergic component of the IJP is unchanged.
A, graph illustrating evoked purinergic IJP amplitudes in the presence of l-NNA. B, representative recordings of IJPs in the presence of l-NNA. C, graph of evoked nitrergic IJPs in the presence of apamin. D, representative traces of IJPs in the presence of apamin. *P < 0.0001.
This pharmacological approach was also used to evaluate whether inflammation differentially affects the purinergic and/or nitrergic components of spontaneous IJPs. In the presence of atropine plus l-NNA (100 μm) to isolate the purinergic component, spontaneous IJPs were significantly reduced in inflamed tissue both in frequency (Fig. 5A and C; P < 0.0001) and in amplitude (Fig. 5B and C; P < 0.02). In the presence of apamin (250 nm) to isolate the nitrergic component of the IJP, no difference was detected in frequency of spontaneous IJPs between control and TNBS-treated colons (control 20.5 ± 0.9 min−1, n= 14, TNBS, 21.4 ± 0.9 min−1, n= 47; P= 0.73), but there was a slight increase in nitrergic IJP amplitude in inflamed tissue (control −3.8 ± 0.2 mV, n= 14, TNBS, −4.8 ± 0.2 mV, n= 47; P < 0.05).
Figure 5. The frequency and amplitude of spontaneous purinergic IJPs were reduced in TNBS-inflamed preparations.
A and B, quantification of spontaneous purinergic IJP frequency (A) and amplitude (B) in ulcerated regions versus control. C, representative traces illustrating spontaneous purinergic IJPs.
Suppression of AH neuron excitability and interneuronal synaptic activity does not restore inhibitory junction potential amplitude
In the guinea pig distal colon, TNBS-induced inflammation is associated with enhanced excitability and increased spontaneous activity of AH neurons, as well as facilitation of interneuronal synaptic activity (Linden et al. 2004; Krauter et al. 2007a). Therefore, we tested whether IJPs were affected by altering the excitability of AH neurons and/or by inhibiting interneuronal myenteric fast synaptic communication.
The increase in excitability of myenteric AH neurons in the TNBS-inflamed guinea pig distal colon involves an increase in a hyperpolarization-activated cation conductance that is mediated by hyperpolarization-activated, cyclic nucleotide gated, non-selective cation (HCN) channels (Linden et al. 2003b). Therefore the effect of the HCN channel blocker, ZD7288, on the evoked IJP was evaluated in the ulcerated region of the TNBS-inflamed distal colon. Addition of 10 μm ZD7288 to the bathing solution had no detectable effect on IJPs evoked by stimulation at distances of 2 mm or 10 mm oral to the ulcerated region (Fig. 6A).
Figure 6. The attenuation of the IJP in the ulcerated region does not appear to involve TNBS-induced AH neuron hyperexcitability or facilitation of interneuronal synaptic activity.
A, graph of data from TNBS-treated preparations illustrating that the amplitude of the IJP is not increased by the HCN channel blocker, ZD7288 (10 μm), which should increase the afterhyperpolarization in AH neurons and decrease their excitability. B, graph illustrating that the TNBS colitis-induced attenuation of the evoked IJP is not reversed by inhibition of interneuronal synaptic transmission with 300 μm hexamethonium. *P < 0.002.
To determine whether the decrease in the IJP amplitude in the ulcerated region of TNBS-inflamed preparations involves the neural circuitry of myenteric ganglia, synaptic transmission was blocked to examine the level of motor neuron innervation. In the presence of atropine (1 μm) plus hexamethonium (300 μm), evoked IJPs were still significantly reduced in the ulcerated region of TNBS-inflamed preparations as compared with control tissue (Fig. 6B; P < 0.005). Previous studies have demonstrated a pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS)-sensitive, purinergic component to interneuronal fast synaptic transmission in the ileum (Galligan, 2002), and this has also been demonstrated in myenteric ganglia of the distal colon (Krauter et al. 2007a). Therefore, IJPs were evaluated in the presence of hexamethonium plus PPADS (30 μm). In these preparations, the amplitude of the IJP was still reduced in ulcerated versus control tissue (2 mm distance: control, 11.5 ± 1.5 mV, n= 6; TNBS, 4.0 ± 0.7 mV, n= 11; P < 0.0001; 10 mm distance: control, 23.8 ± 1.5 mV, n= 6; TNBS, 14.4 ± 1.8, n= 12; P < 0.005). Collectively, these findings indicate that the inflammation-induced suppression in the inhibitory neuromuscular response involves the motor unit itself, and not the enteric neural circuitry upstream of the inhibitory motor neuron.
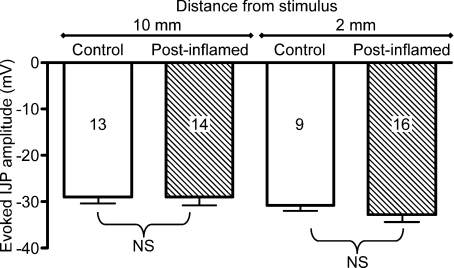
The inflammation-induced attenuation of the IJP amplitude recovers following resolution of inflammation
Previously, we have demonstrated that inflammation is undetectable 28 days following administration of TNBS, but that enhanced AH neuron excitability and synaptic facilitation persist for at least 56 days (Krauter et al. 2007b). Therefore, junction potentials were evaluated at the 56 day time point to determine whether the inflammation induced suppression of the IJP persists after inflammation has resolved. The amplitude of IJPs evoked by stimulation at distances of 10 mm or 2 mm was not different in 56-day post-inflamed animals compared with weight matched controls (Fig. 7; 2 mm, P= 0.38; 10 mm, P= 0.99). The frequency of spontaneous IJPs was comparable to weight matched controls at the 56 day time point (control, 34.3 ± 1.6 events min−1, n= 16; TNBS, 30.1 ± 1.5, n= 23; P= 0.13), but spontaneous IJPs were significantly larger in the preparations from the TNBS treated animals (control, −4.6 ± 0.33 mV events min−1, n= 16; TNBS, −6.1 ± 0.33 mV; P < 0.004). Collectively, these data indicate that the deficit in the IJP does not persist following recovery from inflammation, but it is not clear why spontaneous IJPs are larger at the post-inflammatory time point.
Figure 7.
At a 56 day post-TNBS treatment time point, which is 4 weeks after inflammation can be detected, the amplitude of the evoked IJP is comparable to that detected in weight-matched control preparations
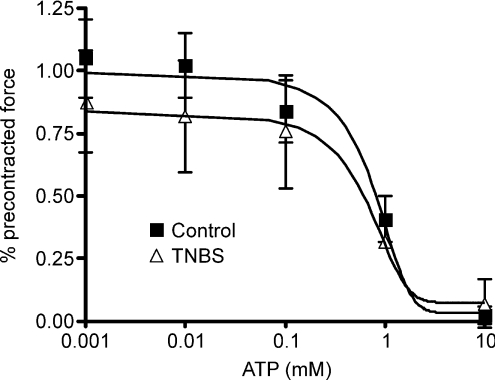
Responsiveness of muscle strips to ATP is unchanged in the ulcerated region
To determine if a decrease in the responsiveness of the circular smooth muscle to ATP could be responsible for the attenuation of the IJP in TNBS-inflamed preparations, the effect of ATP on pre-contracted muscle strips was evaluated in rings of distal colons from control and the ulcerated region of inflamed preparations. Contractile responses to 10 μm bethanechol were comparable between control tissue and inflamed tissue rings (data not shown; P > 0.05), and there was no difference in the relaxatory response to ATP at any of the concentrations tested (Fig. 8). The potencies of the ATP responses were similar in the control and TNBS-treated preparations (control IC50, 0.64 mm; TNBS IC50, 0.48 mm).
Figure 8. The concentration–effect curves for ATP-induced relaxations of colonic rings that were pre-contracted with bethanechol were comparable.
No significant differences were detected between control and TNBS treated colons at any given ATP concentration (ANOVA; n= 4 per group).
The density of circular muscle innervation was not altered in the ulcerated region
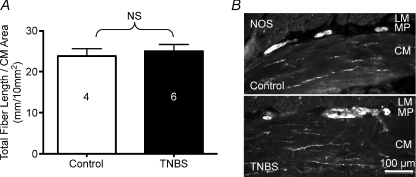
A reduction in neuronal innervation density ICC could be a factor in decreased inhibitory neuromuscular transmission, so motor neuron fibre density was determined. Neuronal fibre densities within the circular muscle layer, as measured by ChAT and NOS immunoreactivity, were not significantly altered between control and ulcerated colon sections (Fig. 9A and B; P= 0.63). Other studies in rat TNBS- and dinitrobenzene sulfonic acid-induced colitis have found a normal innervation density despite an early reduction and smooth muscle hypertrophy (Sanovic et al. 1999; Lourenssen et al. 2005) whereas a study of TNBS colitis in rat has shown increasing neuronal loss and reduced innervation density with increasing proximity to an ulcer (Marlow & Blennerhassett, 2006). In the latter study, tissue integrity was lost throughout the entire thickness of the colon, whereas the muscularis propria remains intact in the guinea pig model (see Fig. 9B).
Figure 9. The density of nerve fibres in the circular muscle layer is comparable in the normal colon as compared to the ulcerated region of the TNBS-inflamed colon.
A, graph illustrating measurements of nerve fibres immunostained with antisera directed against NOS and choline acetyltransferase. Immunostaining for both antigens were observed together in circular muscle fibre bundles. B, micrographs of NOS immunoreactivity in sections of normal and inflamed distal colon.
Propulsive motility is slowed by inhibition of P2Y1 receptors
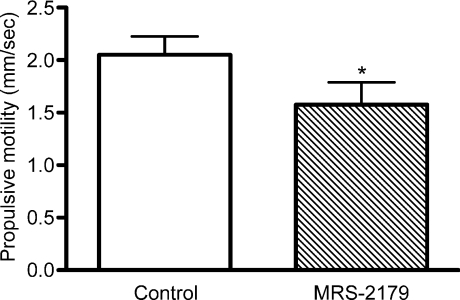
Purinergic neuromuscular transmission in the guinea pig colon is mediated by P2Y1 receptors. Therefore, propulsive motility was evaluated in the presence of a P2Y1 receptor antagonist to test whether a reduction in the purinergic component of the IJP affects propulsive motility. In the presence of MRS-2179 (3 μm), the rate of propulsive motility was significantly slower than the rate in the presence of normal Krebs solution (Fig. 10; P < 0.002, n= 10). These findings suggest that the suppression of the IJP could contribute to the deficit in propulsive motility in the ulcerated regions of the TNBS-inflamed colon.
Figure 10. Inhibition of P2Y1 receptors decreases propulsive motility in the distal colon.
The P2Y1 receptor is known to mediate inhibitory purinergic neurotransmission in colonic smooth muscle. To test the effects of blockade of this receptor, motility experiments were performed on colons from non-treated animals (n= 10) with bath application of the selective P2Y1 receptor antagonist, MRS-2179 (3 μm). Graph demonstrates a significant decrease in propulsive motility. *P < 0.002, paired t test.
Discussion
Inflammation of the intestines leads to changes at many points within the peristaltic reflex circuitry, including increased serotonin availability, AH neuron hyperexcitability, and interneuronal synaptic facilitation (Mawe et al. 2009). We have previously shown that motility in colon is slowed in animals with colitis (Linden et al. 2003a), consistent with clinical findings (Reddy et al. 1991). The purpose of this investigation was to determine how neuromuscular transmission is altered in regions of the TNBS-inflamed colon that have disrupted motility. Our findings indicate that propulsive motility is temporarily halted or obstructed in ulcerated regions, and that in these same regions, descending purinergic inhibitory neuromuscular transmission is selectively attenuated.
Several lines of evidence indicate that the attenuation of purinergic inhibitory neuromuscular transmission that we observed is not directly related to previously described changes in the components of the peristaltic reflex circuit, but rather the deficit appears to be situated somewhere in the motor neuron–ICC–smooth muscle motor unit. In the myenteric plexus of the TNBS-inflamed colon, hyperexcitability of AH neurons involves an increase in the hyperpolarization-activated current (Ih), which reduces the magnitude of the afterhyperpolarization, and inhibition of this current dampens the excitability of these neurons (Linden et al. 2003b). In the current study, inhibition of HCN channels with ZD7288 failed to restore purinergic IJPs to their control amplitude in inflamed preparations. Also, when inter-neuronal fast excitatory synaptic transmission was inhibited with hexamethonium to remove the enteric neural circuitry from the evoked neuromuscular responses, the amplitude of the purinergic IJP in the ulcerated region of inflamed preparations was unchanged.
Another line of evidence supporting the concept that the reduction in purinergic neuromuscular transmission does not involve previously identified changes upstream in the myenteric circuitry is related to the relative persistence of these inflammation-induced changes. Long-term studies have demonstrated that AH neuron hyperexcitability and synaptic facilitation persist for at least 4 weeks beyond recovery from inflammation (56 days following TNBS) (Krauter et al. 2007b). However, at this post-inflammatory time point, the purinergic IJP is restored to the control amplitude. Collectively, these findings indicate that the disruption in purinergic neuromuscular transmission is unrelated to AH neuron hyperexcitability or interneuronal synaptic facilitation. Furthermore, it is possible that different mechanisms are involved, with longer-term transcriptional changes leading to the neuronal plasticity within the ganglia, and more transient post-translational changes causing the suppression of inhibitory neuromuscular transmission.
We hypothesized that the reduction in purinergic neuromuscular transmission that was detected in the current study contributes to the interruption of propulsive motility that occurs in ulcerated regions of the TNBS-inflamed distal colon. The descending limb of the peristaltic reflex is thought to relax the bowel downstream from the site of stimulation, thus creating a receiving segment for the luminal contents to easily flow into. Purinergic neuromuscular transmission in the guinea pig colon is mediated by P2Y1 receptors (Mutafova-Yambolieva et al. 2007; Wang et al. 2007; King & Townsend-Nicholson, 2008). In the current study, inhibition of P2Y1 receptors decreased the rate of propulsive motility, suggesting that decreased purinergic neuromuscular transmission could at least, in part, contribute to the disrupted motility observed in TNBS colitis. Other factors such as physical changes in the tissue and altered muscle function likely contribute to disrupted motility as well.
The studies reported here do not rule out the possibility that altered mucosal serotonin signalling and/or neuroplasticity in the ganglia could also contribute to dysmotility in the inflamed colon. The peristaltic reflex is activated by serotonin release from enterochromaffin cells (Heredia et al. 2009), and involves coordinated activities of ascending and descending limbs of the peristaltic circuitry. However, the neuromuscular signals that were studied here were evoked by electrical field stimulation, which could over-ride the effects of increased serotonin availability in the mucosa, AH neuron hypersensitivity, and synaptic facilitation. On the other hand, data presented here do not support the previously proposed concept (Mawe et al. 2009) that increased spontaneous activity results in ongoing uncoordinated activation of inhibitory and excitatory neuromuscular activity in the inflamed region. If this were the case, one would expect to detect an increase in the frequencies of spontaneous IJPs and EJPs in the ulcerated region, but while spontaneous IJPs were smaller in amplitude, the frequencies of IJPs and EJPs in the inflamed preparations were comparable to controls. Future studies involving more physiological means of stimulating the peristaltic circuitry will be required to test whether inflammation-induced changes upstream of the motor neurons affect neuromuscular signalling in the motor circuitry of the inflamed colon.
Another issue that remains to be resolved is the mechanism responsible for a selective reduction in purinergic transmission without changes in nitrergic or cholinergic transmission. Given that the deficit is likely to lie in the circular muscle motor unit, it stands to reason that it could involve: (1) a decreased responsiveness of the ICC and/or smooth muscle to purinergic stimulation; (2) a decreased ability of the ICC network to transmit the purinergic signal to the smooth muscle; and/or (3) a decrease in ATP release from the inhibitory motor neurons and/or enhanced degradation of interstitial ATP. The finding that ATP-induced relaxation of pre-contracted rings of ulcerated distal colon exhibited a concentration–response curve that is comparable to that of non-inflamed preparations indicates that the ICC-circular muscle syncytium is capable of responding to purinergic stimulation. Furthermore, if there were a disruption in the ability of the ICC network to mediate neuromuscular signalling, one would expect to detect suppression of ascending cholinergic signals as well as descending nitrergic signals, in addition to the disruption of descending purinergic signals. This interpretation leaves a disruption in ATP release and/or enhanced breakdown as the most likely explanation for the suppression of purinergic neuromuscular transmission in inflamed regions of the distal colon. It is possible that inflammatory mediators affect mitochondrial function, and therefore ATP synthesis, in the ulcerated region of the TNBS-inflamed colon. This hypothesis is supported by the restoration of purinergic IJPs following the recovery from inflammation. Another possibility is that there may be increased expression of ectonucleotidases, which are responsible for the breakdown of ATP (Duarte-Araujo et al. 2009; Neshat et al. 2009), as has been demonstrated in the purinergic sympathetic regulation of submucosal blood vessels (Neshat et al. 2009). However, in the submucosal vessel preparation, responses to exogenous ATP were diminished, whereas in the current investigation, circular muscle relaxations were found to be unchanged.
In conclusion, these findings demonstrate that propulsive motility is attenuated in the ulcerated region of the TNBS inflamed colon, and that this disruption is associated with a decrease in the purinergic component of the descending, inhibitory limb of the peristaltic reflex circuit. Of particular interest is the finding that the purinergic component of the IJP was selectively affected, with no detectable change in nitrergic neuromuscular transmission. This is contrary to the change in inhibitory neuromuscular signalling that has been reported in the jejunum of diabetic mice, where nitrergic neuromuscular responses are attenuated, but purinergic transmission is unaffected (Zandecki et al. 2008). The decrease in propulsive motility detected in the presence of a P2Y1 receptor antagonist suggests that attenuated purinergic neuromuscular transmission is involved in the disruption in motility in the ulcerated region, and this could be a contribute to altered motility in inflammatory bowel disease.
Acknowledgments
This work was supported by NIH grant DK62267 and a grant from the Crohn's and Colitis Foundation of Canada. K.A.S. is an Alberta Heritage Foundation for Medical Research Medical Scientist and the CCFC Chair in IBD Research at the University of Calgary. The authors have no conflicts of interest to declare.
Glossary
Abbreviations
- AH
afterhyperpolarizing
- ChAT
choline acetyl transferase
- EJP
excitatory junction potential
- GIMM
gastrointestinal motility monitor
- HCN
hyperpolarization-activated, cyclic nucleotide gated, non-selective cation channels
- ICC
interstitial cell of Cajal
- IJP
inhibitory junction potential
- l-NNA
NG-nitro-l-arginine
- NOS
nitric oxide synthase
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate
- TNBS
trinitrobenzene sulfonic acid
Author contributions
D.S., C.C., J.R. and J.H. performed the experiments, and all authors contributed to the analysis and interpretation of the data. D.S., K.S. and G.M. conceived the study and designed the experiments. D.S. and G.M. generated the original draft of the manuscript, and all authors contributed to critical revisions and ultimately approved the final version. The experiments were carried out at the University of Vermont.
References
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and the innervation of the large intestine. J Physiol. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Taylor GS. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol. 1986;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Suntres Z, Palmer J, Guzman J, Javed A, Xue J, Yu JG, Cooke H, Awad H, Hassanain HH, Cardounel AJ, Christofi FL. Cyclic AMP signalling contributes to neural plasticity and hyperexcitability in AH sensory neurons following intestinal Trichinella spiralis-induced inflammation. Int J Parasitol. 2007;37:743–761. doi: 10.1016/j.ijpara.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Coulie B, Camilleri M, Bharucha AE, Sandborn WJ, Burton D. Colonic motility in chronic ulcerative proctosigmoiditis and the effects of nicotine on colonic motility in patients and healthy subjects. Aliment Pharmacol Ther. 2001;15:653–663. doi: 10.1046/j.1365-2036.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Araujo M, Nascimento C, Timoteo MA, Magalhaes-Cardoso MT, Correia-de-Sa P. Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons. Br J Pharmacol. 2009;156:519–533. doi: 10.1111/j.1476-5381.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol. 1969;205:549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther. 2008;324:1055–1063. doi: 10.1124/jpet.107.131169. [DOI] [PubMed] [Google Scholar]
- Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol. 2007a;581:787–800. doi: 10.1113/jphysiol.2007.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007b;19:990–1000. doi: 10.1111/j.1365-2982.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen J-X, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003a;285:G207–216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol. 2004;557:191–205. doi: 10.1113/jphysiol.2004.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003b;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenssen S, Wells RW, Blennerhassett MG. Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp Neurol. 2005;195:497–507. doi: 10.1016/j.expneurol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Marlow SL, Blennerhassett MG. Deficient innervation characterizes intestinal strictures in a rat model of colitis. Exp Mol Pathol. 2006;80:54–66. doi: 10.1016/j.yexmp.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol Motil. 2009;21:481–491. doi: 10.1111/j.1365-2982.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshat S, deVries M, Barajas-Espinosa AR, Skeith L, Chisholm SP, Lomax AE. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol. 2009;296:G399–405. doi: 10.1152/ajpgi.90450.2008. [DOI] [PubMed] [Google Scholar]
- Nurgali K, Nguyen TV, Matsuyama H, Thacker M, Robbins HL, Furness JB. Phenotypic changes of morphologically identified guinea-pig myenteric neurons following intestinal inflammation. J Physiol. 2007;583:593–609. doi: 10.1113/jphysiol.2007.135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Wong-Riley M, Sharkey KA. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am J Physiol Gastrointest Liver Physiol. 1998;275:G922–935. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ., Jr Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–1297. doi: 10.1016/0016-5085(91)90079-z. [DOI] [PubMed] [Google Scholar]
- Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999;155:1051–1057. doi: 10.1016/S0002-9440(10)65207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Spatial and temporal coordination of junction potentials in circular muscle of guinea-pig distal colon. J Physiol. 2001;535:565–578. doi: 10.1111/j.1469-7793.2001.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Trendelenburg P. Physiological and pharmacological investigations of small intestinal peristalsis. Translation of the article ‘Physiologische und pharmakologische Versuche uber die Dunndarmperistaltik’, Arch. Exp. Pathol. Pharmakol. 81, 55–129, 1917. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:101–133. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- Vladimirova IA, Shuba MF. [Effect of strychnine, hydrastine and apamin on synaptic transmission in smooth muscle cells] Neirofiziologiia. 1978;10:295–299. [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1483–1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–159. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MJ, Bywater RA, Taylor GS, Lang RJ. Effects of nitric oxide (NO) and NO donors on the membrane conductance of circular smooth muscle cells of the guinea-pig proximal colon. Br J Pharmacol. 1996;118:1605–1614. doi: 10.1111/j.1476-5381.1996.tb15581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V, Santicioli P, Maggi CA. Tachykinin NK1 but not NK2 receptors mediate non-cholinergic excitatory junction potentials in the circular muscle of guinea-pig colon. Br J Pharmacol. 1993;110:795–803. doi: 10.1111/j.1476-5381.1993.tb13882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandecki M, Vanden Berghe P, Depoortere I, Geboes K, Peeters T, Janssens J, Tack J. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil. 2008;20:818–828. doi: 10.1111/j.1365-2982.2008.01091.x. [DOI] [PubMed] [Google Scholar]