Abstract
Preterm delivery occurs in approximately 10% of all pregnancies. Prenatal exposure to synthetic glucocorticoids (sGCs) reduces the incidence of respiratory distress syndrome (RDS) in these babies. Therefore, administration of multiple courses of sGCs became common practice. Animal and human studies have demonstrated that multiple courses of sGCs can have long-term effects. While the majority of animal studies have been undertaken in male offspring, it is emerging that there are profound sex differences in the consequences of prenatal sGC exposure. To our knowledge, no studies have determined the effects of prenatal sGC exposure on hypothalamic–pituitary–adrenal (HPA) axis function in female offspring while accounting for reproductive cycle status, or determined if there are effects on pregnancy parameters. Pregnant guinea pigs were administered three courses of betamethasone (Beta), dexamethasone (Dex) or vehicle on gestational days 40/41, 50/51 and 60/61. In adulthood (age range: postnatal days 126–165), basal and activated HPA axis function were assessed at various stages of the reproductive cycle. The female offspring were then mated and underwent an undisturbed pregnancy. Females were killed in the luteal phase of the reproductive cycle following litter weaning, and molecular analysis undertaken. In the luteal phase, Beta-exposed females exhibited significantly lower basal salivary cortisol levels (P < 0.05). Dex-exposed females also exhibited significantly lower basal salivary cortisol levels during the luteal phase (P < 0.05), but increased basal salivary cortisol levels during the ostrous phase (P < 0.01). The Beta-exposed females exhibited increased glucocorticoid receptor (GR) mRNA expression in the CA1/2 region of the hippocampus (P < 0.05) and MC2R mRNA in the adrenal cortex (P < 0.05). The Dex-exposed animals exhibited higher hippocampal GR and mineralocorticoid receptor (MR) mRNA levels (P < 0.05). Beta-exposed females showed reduced fecundity (P < 0.05). In Dex-exposed females there was a lower male to female sex ratio. In conclusion, prenatal sGC exposure affects HPA axis activity, in a cycle-dependent manner, and long-term reproductive success. The clinical implications of the findings on endocrine function and pregnancy in females are profound and further follow-up is warranted in human cohorts. Furthermore, we have shown there are considerable difference in phenotypes between the Beta- and Dex-exposed females and the specific endocrine and maternal outcome is contingent on the specific sGCs administered during pregnancy.
Introduction
Preterm labour (less than 37 weeks) occurs in approximately 10% of all pregnancies, and about 50% of all babies born under 32 weeks are affected by respiratory distress syndrome (RDS) (National Institutes of Health Consensus Development Panel, 2001). Prenatal exposure to synthetic glucocorticoids (sGCs) reduces the incidence of RDS (Liggins & Howie, 1972). Due to the efficacy of such treatment in reducing neonatal morbidity and mortality, together with the difficulty in diagnosing preterm labour, multiple courses of sGCs became common practice (Quinlivan et al. 1998; Brocklehurst et al. 1999). As a result, a very large cohort of children has been exposed to sGCs, often repeatedly, and many of these children were born at normal term. Both prospective animal studies and retrospective human studies have shown that multiple courses of sGCs can have long-term effects on brain structure, behaviour and endocrine function (French et al. 1999; Banjanin et al. 2004; French et al. 2004; Owen & Matthews, 2007).
Glucocorticoids (GCs) are essential for normal brain development, but exposure to sGCs modifies normal development and can lead to a permanent alteration in both structure and function of the brain (Kapoor et al. 2008). The fetal brain contains high levels of glucocorticoid receptors (GRs) and as such represents a key target for sGCs (Andrews & Matthews, 2000; Owen & Matthews, 2003; Owen et al. 2005). We and others have shown that prenatal exposure to sGCs can lead to lifelong alterations in regulation of hypothalamic–pituitary–adrenal (HPA) axis function in the offspring of several species including the rat, sheep, guinea pig and non-human primate (Levitt et al. 1996; Sloboda et al. 2002; Hauser et al. 2007; Sloboda et al. 2007). In a number of studies, alterations in HPA function have been associated with modification of corticosteroid receptor expression at sites of glucocorticoid feedback (hippocampus, hypothalamus and pituitary) in the juvenile and adult brain (Liu et al. 2001; Banjanin et al. 2004; Sloboda et al. 2008).
More recent studies have identified that the nature of the HPA axis phenotype in offspring following maternal exposure to sGCs in late gestation depends on the age at which assessment is undertaken. An elegant series of studies in the sheep has demonstrated that multiple courses of sGCs in late gestation lead to young (1-year-old) sheep that exhibit increased basal and activated adrenocortical function, but that these same animals exhibit reduced adrenocortical activity as mature adults (3 years) (Sloboda et al. 2002, 2007). We have shown in the guinea pig that maternal treatment with repeated courses of dexamethasone (Dex) led to young adult male offspring that exhibited pronounced hypocortisolaemia (Liu et al. 2001), but that the magnitude of these effects decreased with advancing age (Banjanin et al. 2004).
To date, the majority of animal studies have been undertaken in male or mixed sex offspring. However, it is emerging that there are profound sex differences in the long-term consequences of prenatal sGC exposure in the rat and guinea pig (Liu et al. 2001; O’Regan et al. 2004). It has recently been demonstrated in female guinea pigs born to mothers that had been stressed during pregnancy (and therefore exposed to excess endogenous glucocorticoids) that modification or programming of the HPA axis was dependent on the stage of the reproductive cycle at which analysis was undertaken. Furthermore, these animals demonstrated differences in sex steroids and ostrous behaviour, suggesting that there may be impairments in fertility in these animals (Kapoor & Matthews, 2008). To our knowledge, no studies have determined the effects of prenatal sGC exposure on subsequent pregnancy in offspring.
The Royal College of Obstetricians and Gynaecologists recommend that betamethasone (Beta) be used as the sGC for the management of preterm labour (RCOG Guideline 7, 2004). Therefore, while Beta is the most frequently administered sGC, there is currently no definitive consensus on the use of Beta or Dex (American College of Obstetricians and Gynecologists Committee on Obstetric Practice, 2008). In the current study, we wished to assess the effects of repeated sGC exposure on HPA axis function and basic reproductive function in adult female guinea pigs. Within our study design, we also compared effects of the Beta and Dex in the preparations most commonly administered to pregnant women, and determined whether the sGC effects on HPA activity changed as a function of reproductive cycle. Finally, we assessed molecular regulation of the HPA axis during the luteal phase of the reproductive cycle.
Methods
Animals
Female guinea pigs (400–500 g) (Hartley strain; Charles River Canada, St Constant, Quebec, Canada) were mated in our animal facility as described previously (Dean & Matthews, 1999). This method produces accurately time-dated pregnant guinea pigs. Food (Guinea Pig Chow 5025; Ralston Purina International, Leis Pet Distributing Inc., Wellesley, Ontario, Canada) and water were available ad libitum. The animals were kept in a 12 h light–12 h dark cycle, with lights off at 19.00 h. Room temperature was 23°C. We use the guinea pig as a model for these studies because it has a long gestation (68 days) and gives birth to mature young that exhibit a similar pattern of fetal brain development to humans (Dobbing & Sands, 1970). In addition, the guinea pig has a regular 16 day reproductive cycle with an actively secreting corpus luteum, similar to the situation in humans (Stockard & Papanicolaou, 1917; Donovan & Lockhart, 1972). All studies were performed according to protocols approved by the Animal Care Committee at the University of Toronto, in accordance with the Canadian Council for Animal Care, and are reported according to journal standard (Drummond, 2009).
Treatments
Pregnant guinea pigs were subcutaneously (s.c.) injected (three courses; each course two injections, 24 h apart) with Beta (Betaject; betamethasone sodium phosphate and betamethasone acetate 3mg in aqueous vehicle; Sabex, Boucherville, QC, Canada; 1 mg kg−1; 6 mg ml−1; n= 8), Dex (Dexamethasone 2 (dexamethasone sodium phosphate in 2% benzyl alcohol), Austin Vetoquinol, Lavaltrie, Canada; 1 mg kg−1; n= 8) or vehicle (saline; n= 8) on gestational days (gd) 40 and 41 (period of rapid neurogenesis), 50 and 51 (peak brain growth), and 60 and 61 (period of rapid myelination) (Dobbing & Sands, 1970). We have previously shown that maternal s.c. vehicle injection into the intrascapulary region does not cause activation of the maternal HPA axis in late gestation in animals that are accustomed to being handled (A. Kapoor & S. G. Matthews, unpublished observation). Pregnant animals were housed separately during pregnancy and in the perinatal period, but animals were in visual, olfactory and auditory contact with other animals at all times. The dose (1 mg kg−1) of Beta and Dex used in this study is comparable to the dose used in pregnant women (approximately 0.25 mg kg−1) because the guinea pig GR has a fourfold lower affinity for synthetic glucocorticoid (Keightley et al. 1998). Animals were allowed to deliver undisturbed. There was no effect of repeated synthetic glucocorticoid exposure on gestation length, litter size, birth weight and sex ratio (data not reported). Due to the scope and size of the study, only female offspring were assessed in the current study (control, n= 12; Beta, n= 11; Dex, n= 14). Animals were weaned at postnatal day (pnd) 25 and pair-housed in clear polycarbonate cages. Reproductive cycle status was monitored by visual inspection beginning on pnd 25 as previously described (Kapoor & Matthews, 2008).
Endocrine testing
In adulthood, (age range: pnd 126–165), basal salivary cortisol levels were assessed in the guinea pigs at 08.00 h, 12.00 h and 16.00 h during the ostrous (day 0), mid-luteal (day 8) and late-luteal (day 11) phases of the reproductive cycle. The salivary cortisol response to a strobe light was assessed on days following basal cortisol measurement, on reproductive cycle days 1, 9 and 12. Animals were exposed to a high frequency strobe light for 30 min in a dark environment as described previously (Kapoor & Matthews, 2005, 2008). In all animals, the stress exposure was initiated at 11.00 h and saliva samples were collected prior to the initiation of the stressor (0 min), immediately after completion of the stress exposure (30 min) and during the recovery phase at 60 and 120 min. Saliva was used to measure cortisol in females as we have previously demonstrated that the catheterization surgery required to collect blood disrupts normal reproductive cyclicity.
Pregnancy in offspring
Following endocrine testing (age range: 157–196), female guinea pigs were mated with non-experimental males. The number of attempts to conceive was recorded as number of cycles from the first attempted mating to conception. Once a successful pregnancy was established by sustained closure of the vaginal membrane, adrenocortical function was assessed. Salivary samples for cortisol were taken on gd 30, 40, 50 and 60. Following delivery, gestation length, the number of pups per litter (F2 generation), their sex and birth weight were recorded.
Tissue and plasma collection
Following weaning of the F2 offspring, the F1 mothers were killed by decapitation during the mid-luteal phase of the cycle at 12.00 h. Trunk blood was collected and centrifuged (10 000 g, 30 s, 4°C) and plasma was stored at −20°C until further analysis. The brain, pituitary, adrenal glands and ovaries were rapidly dissected, weighed and frozen (−80°C). Prior to freezing brains, were sagitally hemisected at an angle and slightly off midline, such that the left block contained the left hippocampus and the entire hypothalamus. The right hippocampus was dissected from the remaining block.
Endocrine analysis
Salivary cortisol (Salimetrics LLC, State College, PA, USA), plasma oestradiol (MP Biomedicals, Orangeburg, NY, USA) and plasma testosterone (Neogen Corp., Lexington KY, USA) were measured using enzyme-linked immunosorbant assays (ELISAs) as previously described (Banjanin et al. 2004; Kapoor & Matthews, 2008). Plasma cortisol (ICN Pharmaceuticals Inc., Medicorp, Montreal, QC, Canada) and plasma ACTH (ICN Pharmaceuticals) were measured using radioimmunoassays (RIAs) as previously described (Banjanin et al. 2004; Kapoor & Matthews, 2008). For all ELISAs and RIAs, samples were run in the same assay to negate interassay bias. The intra-assay coeffiecient of variation was <6% for all assays.
In situ hybridization
The method of in situ hybridization (ISH) has been described in detail previously (Matthews & Challis, 1995; Matthews, 1998). Briefly, coronal cryosections (10 μm) of brain, pituitary and left adrenals were mounted on poly-l-lysine coated slides, dried and fixed with paraformaldehyde (4%). The antisense oligonucleotide probes for mineralocorticoid receptor (MR), GR, corticotrophin releasing hormone (CRH), arginine vasopressin (AVP), pro-opiomelanocortin (POMC), adrenocorticotrophin receptor (MC2R), side chain cleavage enzyme, P450scc (CYP11A1), 11β-hydroxylase, steroidogenic enzyme cytochrome P450C17 (CYP 17), steroidogenic factor-1 (SF-1) and steroidogenic acute regulatory protein (StAR) have been previously described (Dean & Matthews, 1999; McCabe et al. 2001; Owen & Matthews, 2003, 2007; Banjanin et al. 2004). The relative optical density (ROD) of signal on the autoradiographic film was quantified after subtraction of background values as previously described (Owen & Matthews, 2003). All analyses were undertaken on an average of 9–12 sections/gene/animal. A section was excluded from analysis if its integrity was diminished. GR mRNA levels were measured in the hippocampus (CA1/2, CA3 and CA 4 regions), dentate gyrus (DG), hypothalamic paraventricular nucleus (PVN) and anterior lobe of the pituitary. MR mRNA levels were determined in the hippocampus (as for GR) and in the DG. CRH and AVP mRNA levels were determined in the PVN. POMC mRNA levels were measured in the anterior and intermediate lobes of the anterior pituitary (Banjanin et al. 2004). MC2R, StAR, CYP 17, SF-1 and CYP 11A1 mRNA levels were all determined across all zones of the adrenal cortex (Owen & Matthews, 2007). In all cases, the medulla was excluded from the analysis and an average of the autoradiographic signal across the entire adrenal cortex was calculated. Given that the boundaries between the zones were difficult to consistently delineate visually, we did not attempt to differentiate between different zones of the adrenal cortex for the purposes of the analysis.
Western blot analysis
GR and MR protein expression in the hippocampus was determined using Western blotting as described previously (Owen & Matthews, 2003; Kalabis et al. 2005). The membranes were first incubated with primary MR antibody (rabbit polyclonal, sc-11412, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:400 dilution, 1 h at 23°C) in 5% skim milk with phosphate buffered saline with tween (PBS-T). The membranes were then washed in PBS-T followed by incubation with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1: 5000 dilution, 1 h, 23°C; NEN, Boston, MA, USA). Restore Western Blot Stripping buffer (30 min, 23°C; Pierce, MJS Bioynx, Mississauga, ON, Canada) was used to strip the membranes, which were then re-probed with GR antibody (rabbit polyclonal sc-1002, Santa Cruz Biotechnology; 1: 500 dilution, 1 h at 23°C) and β subunit of G protein (Gβ; 1: 5000 dilution, rabbit polyclonal sc-261; Santa Cruz Biotechnology). Computerized image analysis was used to measure the ROD of the bands (MR ∼110 kDa, GR ∼96 kDa), which were standardized against the Gβ signal (36 kDa).
Statistical analysis
All data were expressed as means ± standard error of the mean (s.e.m.). For all tests, significance was set at P < 0.05. Statistical analysis was performed using SPSS v. 16.0 (SPSS Inc., Chicago, IL, USA). Basal, stress-induced, and pregnancy salivary cortisol levels were analysed using a linear mixed model with prenatal sGCs and time as fixed effects and dam as a random effect. Salivary cortisol total area under the curve (AUC) (for assessment of basal adrenocortical function), net AUC (120 min; to assess adrenocortical responsiveness to a stressor), organ weights, the number of cycles to conceive, gestation length, number of pups per litter, pup weight, plasma hormone analysis, gene expression and Western Blot data were analysed using a linear mixed model with prenatal sGCs as a fixed effect and dam as a random effect. If significance was found, the data were further analysed using pairwise comparisons. Sex ratio was analysed by Fisher's exact test. In any of the tests, if the variance was significantly different, the data were log-transformed and reanalysed.
Results
Basal salivary cortisol
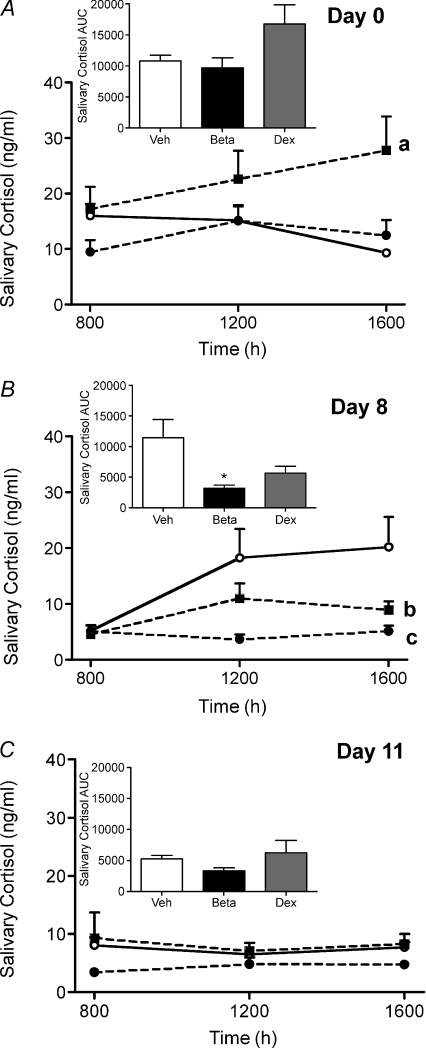
Analysis of basal salivary cortisol levels revealed that during the ostrous phase (day 0) of the reproductive cycle there was a significant effect of prenatal sGC exposure (P < 0.05; Fig. 1A). Pairwise comparison demonstrated that the Dex-exposed females exhibited significantly higher basal salivary cortisol levels over time compared to vehicle females (P < 0.01; Fig. 1A). Analysis of the AUC of basal salivary cortisol levels revealed no effect of prenatal Beta or Dex exposure on net total basal salivary cortisol levels (Fig. 1A inset). Analysis during the mid-luteal phase (day 8) of the reproductive cycle demonstrated there was a significant effect of prenatal sGC exposure (P < 0.05; Fig. 1B). Further analysis revealed that salivary cortisol levels were reduced in Beta- (P < 0.01) and Dex- (P < 0.05) exposed females. There was also an effect of time of day (P < 0.01) and an interaction between prenatal sGC exposure and time of day (P < 0.05). Beta-exposed females exhibited significantly lower salivary cortisol levels at 12.00 h and 16.00 h (P < 0.01). Total basal salivary cortisol AUC was also significantly lower in the Beta-exposed females (P < 0.05; Fig. 1B inset). During the late-luteal phase (day 11) of the reproductive cycle there was no effect of prenatal sGC exposure on salivary cortisol levels or on the total AUC (Fig. 1C inset).
Figure 1. Basal salivary cortisol concentrations.
Basal salivary cortisol concentrations at 08.00 h, 12.00 h and 16.00 h on day 0 (ostrous phase) (A), day 8 (mid-luteal phase) (B) and day 11 (late-luteal phase) (C) of the reproductive cycle in adult female guinea pig offspring whose mothers had been injected with vehicle (Veh; open circles, continuous line; n= 12) or betamethasone (Beta; 1 mg kg−1, filled circles, dashed line; n= 11) or dexamethasone (Dex; 1 mg kg−1, filled squares, dashed line; n= 14) on days 40/41, 50/51 and 60/61 of gestation. Inset, total area under the curve. Data are presented as means ±s.e.m.aP < 0.01 vehicle vs. Dex over time, bP < 0.05 vehicle vs. Dex over time, cP < 0.01 vehicle vs. Beta over time, *P < 0.05 vs. vehicle.
Salivary cortisol response to a stressor
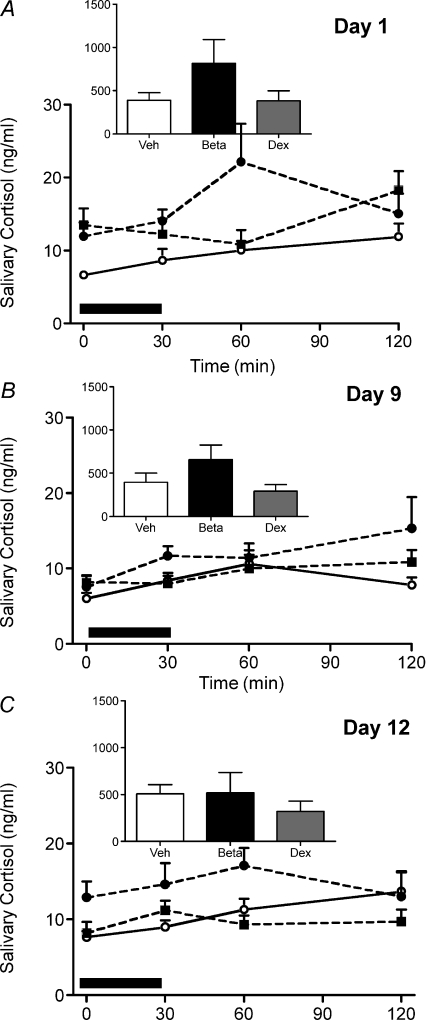
During the ostrous phase (day 1) of the reproductive cycle there was an effect of prenatal sGC exposure (P < 0.05; Fig. 2A) on the cortisol response to the strobe light exposure, but no effect of a specific sGC by pairwise comparison. There was no effect of prenatal sGC exposure on the net AUC (Fig. 2A inset). During the mid-luteal phase (day 9) there was a significant effect of time on the salivary cortisol response to the strobe light (P < 0.01; Fig. 2B), but no effect of prenatal sGC exposure. During the late-luteal phase (day 12) there was a significant effect of prenatal sGC exposure (P < 0.05), but again no effect of a specific sGC by pairwise comparison. There was also a significant effect of time (P < 0.05; Fig. 2C).
Figure 2. Salivary cortisol response to a strobe light stressor.
Salivary cortisol was analysed before (time 0), immediately after exposure to the stressor (30 min) and during the recovery phase (60 and 120 min) on day 1 (A), day 9 (B) and day 12 (C) of the reproductive cycle in adult female guinea pig offspring whose mothers had been injected with vehicle (Veh; open circles, continuous line; n= 12) or betamethasone (Beta; 1 mg kg−1, filled circles, dashed line; n= 11) or dexamethasone (Dex; 1 mg kg−1, filled squares, dashed line; n= 14) on days 40/41, 50/51 and 60/61 of gestation. Net area under the curve inset. Data are presented as means ±s.e.m.
Plasma ACTH, cortisol and sex steroids
Blood was collected for analysis at the time of killing. There was no effect of prenatal exposure to either Beta or Dex on mid-luteal plasma levels of ACTH (vehicle (Veh), 20.84 ± 5.23; Beta, 19.57 ± 1.97; Dex, 29.60 ± 4.02 pg ml−1). However, there was a trend towards decreased plasma cortisol levels in the Beta-exposed females (P= 0.07; Veh, 26.06 ± 5.83; Beta, 13.78 ± 2.85; Dex, 29.75 ± 6.37 ng ml−1), consistent with the reduced salivary cortisol at this time.
There was a significant effect of prenatal sGC exposure on plasma oestradiol levels in the luteal phase, with Beta- but not Dex-exposed females exhibiting lower plasma oestradiol concentrations (P < 0.05; Veh, 44.88 ± 1.71; Beta, 34.70 ± 1.90; Dex, 39.61 ± 4.43 pg ml−1). There was no effect of prenatal exposure to either Beta or Dex on plasma testosterone levels (Veh, 0.34 ± 0.04; Beta, 0.35 ± 0.05; Dex, 0.45 ± 0.10 ng ml−1) in adult female offspring during the mid-luteal phase of the reproductive cycle.
Pregnancy parameters
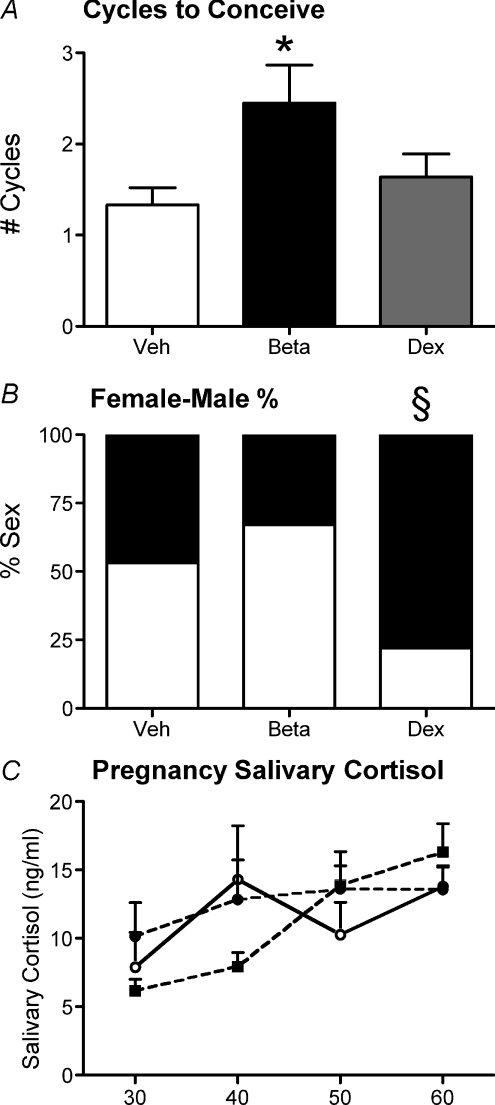
There was no effect of prenatal sGC exposure on the age that the females began cycling (Veh, 34.1 ± 1.5; Beta, 35.8 ± 1.9; Dex, 32.7 ± 0.7 days) and the average length of their cycles (Veh, 15.9 ± 0.2; Beta, 15.9 ± 0.2; Dex, 16.0 ± 0.2 days). At the time of mating, Beta-exposed females required a significantly greater number of cycles to successfully conceive (P < 0.05; Fig. 3A). There was no effect of prenatal exposure to either Beta or Dex on pre-conception weights (Veh, 769.9 ± 20.8; Beta, 792.6 ± 18.2; Dex, 780.9 ± 14.5 grams), litter size (Veh, 3.2 ± 0.2; Beta, 2.4 ± 0.2; Dex 2.8 ± 0.3), pregnancy duration (Veh, 69.6 ± 0.3; Beta, 70.3 ± 0.4; Dex, 70.0 ± 0.3 days) or birth weights (males: Veh, 102.2 ± 1.9; Beta, 107.6 ± 3.1; Dex 101.3 ± 4.2; females: Veh, 99.9 ± 3.3; Beta, 110.2 ± 6.2; Dex, 96.5 ± 2.6 grams). However, Dex-exposed females had a significantly greater proportion of females in their litters compared to vehicle females as determined by Fisher's exact test (Fig. 3B). There was no effect of prenatal sGC exposure on salivary cortisol levels during pregnancy (Fig. 3C).
Figure 3. Pregnancy parameters.
Pregnancy parameters in adult female guinea pig offspring whose mothers had been injected with vehicle (Veh; n= 12), betamethasone (Beta; 1 mg kg−1; n= 11) or dexamethasone (Dex; 1 mg kg−1; n= 14) on days 40/41, 50/51 and 60/61 of gestation. A, number of reproductive cycles needed for successful conception. B, percentage of female (filled bar) and male (open bar) offspring in litters of first pregnancies of vehicle, Beta and Dex females. C, salivary cortisol levels on gestational days 30, 40, 50 and 60 in first pregnancy of vehicle (open circles, continuous line), Beta (filled circles, dashed line) and Dex (filled squares, dashed line) females. *P < 0.05 vehicle vs. Beta. §Significant difference in the female/male ratio compared to vehicle and Beta.
Organ weights
There was no significant effect of prenatal sGC exposure on post-pregnancy body weights. However, Beta-exposed females exhibited significantly higher brain weights (P < 0.01). There was no effect of prenatal sGC exposure on any other organ weights (Table 1).
Table 1.
Organ weights (means ±s.e.m.) of female guinea pig offspring whose mothers were treated with betamethasone (Beta; 1 mg kg−1), dexamethasone (Dex 1 mg kg−1) or vehicle (Veh) injections on gestational days 40/41, 50/51 and 60/61 of pregnancy
| Veh (12) | Beta (11) | Dex (14) | |
|---|---|---|---|
| Body | 833.8 ± 20.2 | 840.2 ± 22.5 | 838.9 ± 21.3 |
| Brain | 4.17 ± 0.06 | 4.41 ± 0.05 ** | 4.20 ± 0.05 |
| Pituitary | 0.019 ± 0.0005 | 0.018 ± 0.0009 | 0.017 ± 0.0005 |
| Left adrenal | 0.29 ± 0.01 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| Left kidney | 2.41 ± 0.04 | 2.36 ± 0.09 | 2.61 ± 0.10 |
| Left ovary | 0.072 ± 0.006 | 0.068 ± 0.007 | 0.077 ± 0.008 |
Animal numbers are indicated in parentheses. **P < 0.01 vs. vehicle.
HPA-related gene and protein expression
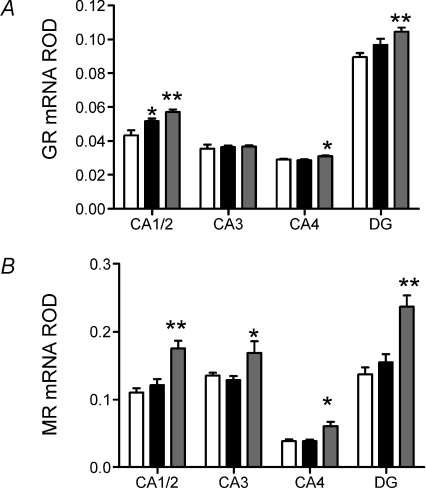
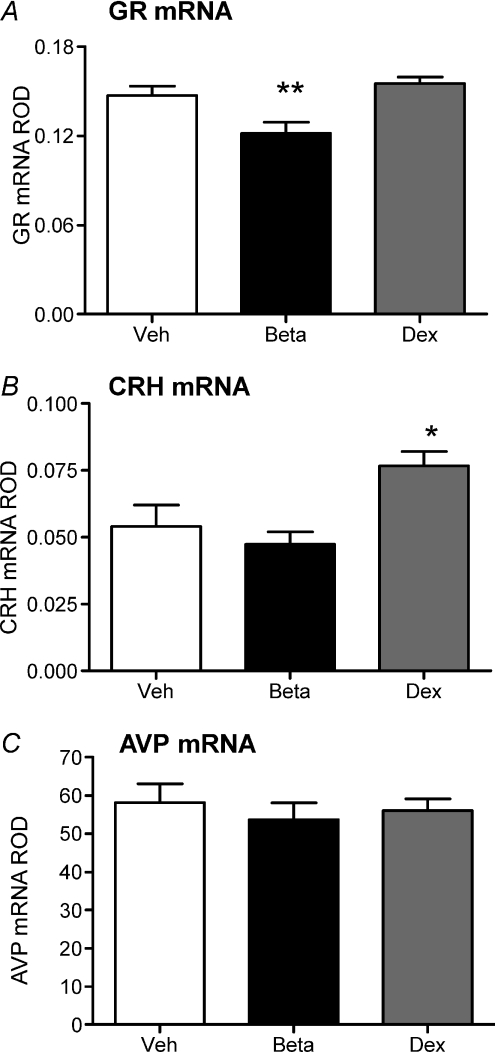
Molecular analysis was undertaken in the mid-luteal phase of the reproductive cycle, once the F2 offspring had been weaned. Dex-exposed females exhibited significantly higher GR mRNA in the CA1/2 (P < 0.01) and CA4 (P < 0.05) regions of the hippocampus, and the DG (P < 0.01). These females also exhibited significantly higher MR mRNA in the CA1/2 (P < 0.01) and CA4 (P < 0.05) regions of the hippocampus and the DG (P < 0.01; Fig. 4B). Prenatal Beta-exposed females exhibited significantly higher GR mRNA in the CA1/2 region of the hippocampus, but there was no effect on MR mRNA levels (P < 0.05; Fig. 4A). There was no effect of either prenatal Beta or prenatal Dex exposure on hippocampal expression of MR or GR protein (MR: Veh, 0.28 ± 0.06; Beta, 0.36 ± 0.07; Dex, 0.30 ± 0.08; GR: Veh, 0.35 ± 0.09; Beta, 0.41 ± 0.09; Dex, 0.28 ± 0.05).
Figure 4. Hippocampol corticosteroid receptor gene expression.
Relative optical density (ROD) (mean ±s.e.m.) of GR mRNA (A) and MR mRNA (B) in CA1/2, CA3, CA4, and dentate gyrus (DG) of the hippocampus following in situ hybridization in adult female guinea pig offspring whose mothers had been injected with vehicle (Veh; white bar; n= 12), betamethasone (Beta; 1 mg kg−1; black bar; n= 11) or dexamethasone (Dex; 1 mg kg−1; grey bar; n= 14) on days 40/41, 50/51 and 60/61 of gestation. *P < 0.05, significant difference between prenatal treatment groups. Exposure time for GR mRNA was 21 days and for MR mRNA was 14 days. *P < 0.05 vehicle vs. Beta or Dex. **P < 0.01 vehicle vs. Beta or Dex.
In the hypothalamic PVN, Beta-exposed females exhibited decreased GR mRNA (P < 0.01), but there was no effect of prenatal Dex exposure on GR mRNA levels compared to vehicle females (Fig. 5A). Dex- but not Beta-exposed females exhibited significantly higher CRH mRNA in the PVN (P < 0.05; Fig. 5B). There was no effect of either Beta or Dex exposure during gestation on AVP mRNA (Fig. 5C). There was no effect of prenatal Beta or Dex exposure on GR mRNA levels (Veh, 0.091 ± 0.003; Beta, 0.093 ± 0.003; Dex, 0.097 ± 0.003) or POMC mRNA levels (Veh, 1.0 ± 0.1; Beta, 1.2 ± 0.6; Dex, 1.5 ± 0.2) in the anterior pituitary.
Figure 5. Hypothalamic gene expression.
Relative optical density (ROD) (mean ±s.e.m.) of GR mRNA (A), CRH mRNA (B) and AVP mRNA (C) in hypothalamic paraventricular nucleus following in situ hybridization in adult female guinea pig offspring whose mothers had been injected with vehicle (Veh; white bar; n= 12), betamethasone (Beta; 1 mg kg−1; black bar; n= 11) or dexamethasone (Dex; 1 mg kg−1; grey bar; n= 14) on days 40/41, 50/51 and 60/61 of gestation. *P < 0.05, significant difference between prenatal treatment groups. Exposure time for GR mRNA was 21 days, for CRH mRNA was 35 days and for AVP mRNA was 8 h. *P < 0.05 vehicle vs. sGC. **P < 0.01 vehicle vs. Beta or Dex.
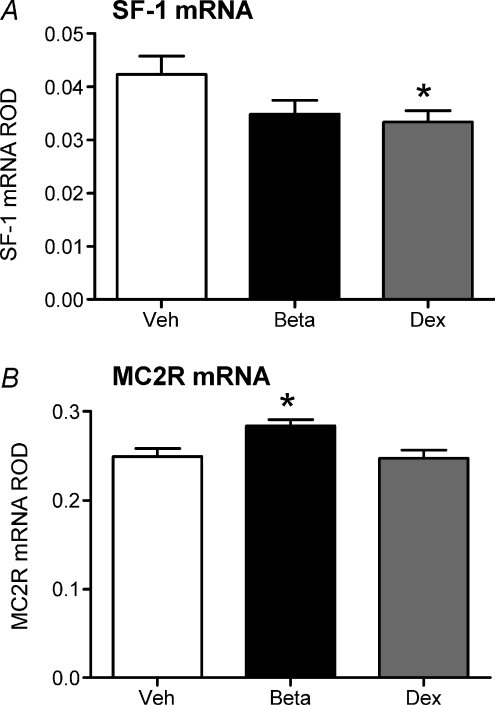
In the adrenal cortex, Dex-exposed females exhibited significantly decreased expression of SF-1 mRNA (P < 0.05; Fig. 6A) and Beta-exposed females exhibited significantly higher expression of MC2R mRNA (P < 0.05; Fig. 6B). There was no effect of prenatal Beta or Dex exposure on StAR (Veh, 0.40 ± 0.05; Beta, 0.42 ± 0.03; Dex, 0.36 ± 0.04), CYP17 (Veh, 4.5 ± 0.2; Beta, 4.6 ± 0.3; Dex, 3.9 ± 0.2) or CYP11A1 (Veh, 0.27 ± 0.03; Beta, 0.24 ± 0.02; Dex, 0.26 ± 0.01).
Figure 6. Adrenocortical gene expression.
Relative optical density (ROD) (mean ±s.e.m.) of steroidogenic factor 1 (SF-1) mRNA (A) and MC2R mRNA (B) in the adrenal cortex following in situ hybridization in adult female guinea pig offspring whose mothers had been injected with vehicle (Veh; white bar; n= 12), betamethasone (Beta; 1 mg kg−1; black bar; n= 11) or dexamethasone (Dex; 1 mg kg−1; grey bar; n= 14) on days 40/41, 50/51 and 60/61 of gestation. Exposure time for SF-1 and MC2R mRNA was 10 days. *P < 0.05 vehicle vs. Beta or Dex.
Discussion
In the current study, we have shown that there are long-term effects of repeated prenatal sGC exposure in female guinea pigs, but the phenotype is dependent on the specific sGC administered. Both Beta- and Dex-exposed females exhibited reproductive cycle-dependent effects on HPA axis activity, but the Dex-exposed females also exhibited profound differences in molecular regulation of the HPA axis at the level of the hippocampus and a sex ratio bias towards females in their first litter. For the first time in any species, we have shown that females exposed to Beta prenatally exhibited reduced fecundity.
The potential impact of these findings in human populations is profound as the high incidence of preterm labour (approximately 10%), together with difficulty in its diagnosis, means that around 25% of all babies exposed to sGCs in utero deliver at normal term. Prospective studies are currently underway examining the effects of repeated sGC administration during pregnancy in human cohorts. A large Australian trial found that infants born of mothers administered repeated doses of sGCs exhibited decreased RDS and severe lung disease compared to those whose mothers had received a single course (Crowther et al. 2006). Based on those results, the authors concluded that multiple courses were beneficial for respiratory function in preterm infants. In contrast to the study by Crowther et al., data from the Canadian multiple courses of antenatal corticosteroids for preterm birth (MACS) trial demonstrated that multiple courses of betamethasone administered to pregnant women every 14 days resulted in decreased weight, length and head circumference of the neonate at birth without additional benefits on respiratory function (Murphy et al. 2008). The short term benefits of multiple vs. a single course of sGCs remain controversial, but studies are emerging from the human cohorts demonstrating long-term effects of multiple courses of sGCs compared to a single course, such as reduced attention and increased impulsivity (Crowther et al. 2007; Pesonen et al. 2009).
Numerous animal studies have demonstrated that there are long-term effects of repeated sGC exposure during gestation on growth and endocrine function. In vervet monkeys of mixed sex, repeated Dex exposure resulted in attenuated postnatal growth, impaired glucose tolerance and hyperinsulinaemia, increased diastolic and systolic blood pressure, increased reward behaviour and decreased social motivation (de Vries et al. 2007; Hauser et al. 2008). In guinea pigs, juvenile male offspring (pnd 10) born to mothers treated with Beta on gd 40/41, 50/51 and 60/61 exhibited a reduced cortisol response to maternal separation, increased pituitary proopiomelanocortin (POMC) and CRH mRNA and decreased adrenal CYP17 mRNA (Owen & Matthews, 2007). Young adult male guinea pig offspring (pnd 70) born to mothers treated with Dex on gd 40/41, 50/51 and 60/61 exhibited decreased basal and stress induced cortisol levels (Liu et al. 2001). This was associated with increased hippocampal MR mRNA and increased plasma testosterone levels. In a further study, we showed that older adult male offspring (pnd 140) that had been exposed to the same repeated Dex regiment exhibited increased blood pressure, and continued to demonstrate increased hippocampal MR mRNA expression (Banjanin et al. 2004). In contrast, young adult female guinea pig offspring that had been exposed to multiple courses of Dex prenatally exhibited increased basal and stress-induced plasma cortisol levels during the ostrous and early luteal phases of the reproductive cycle (Liu et al. 2001). However, in the latter study, animals were catheterized, and we have subsequently shown that catheterization results in disruption of the female reproductive cycle in the guinea pig (S. Banjanin, S. G. Matthews et al. unpublished observations). To our knowledge there have been no other studies that have determined the long-term endocrine outcomes of prenatal sGC exposure in female offspring while accounting for reproductive cycle status.
In the present study, we found that the programming effects of in utero exposure to Beta or Dex on HPA axis function in females was dependent on the stage of the reproductive cycle. During the ostrous phase, Dex-exposed females exhibited significantly higher levels of basal salivary cortisol throughout the day. In line with the basal cortisol data from day 0, the Dex-exposed females appeared to exhibit higher levels of salivary cortisol in the sample prior to initiation of the strobe light stressor. Therefore, the magnitude of the response to the stressor was similar to that of the vehicle females. It is well established that oestradiol leads to increased HPA axis function (Viau, 2002). In rats, corticosterone responses to stress are highest during pro-ostrous, when oestrogen levels peak (Viau & Meaney, 1991). Furthermore, ovariectomized rats with oestrogen replacement exhibited an enhanced and prolonged ACTH and corticosterone response to physical stress compared with females not given oestrogen replacement (Handa et al. 1994). Therefore it is possible that plasma oestradiol levels were higher in the Dex-exposed females and this led to the heightened HPA axis activity observed. However, we were unable to measure ostrous phase oestradiol levels in the current study as the females were killed during the luteal phase of the reproductive cycle.
During the mid-luteal phase of the reproductive cycle both Beta- and Dex-exposed females exhibited decreased basal salivary cortisol levels. Molecular analysis of the brains of these animals revealed that during the luteal phase the Dex-exposed females exhibited increased GR and MR mRNA in all regions of the hippocampus, which would facilitate increased negative feedback and thus the lower cortisol levels observed. Despite the elevation of GR and MR mRNA in virtually all of the hippocampal subfields, GR and MR protein levels in whole hippocampal homogenates were not increased compared to vehicle females. This latter finding was quite surprising as the increase in hippocampal corticosteroid receptor mRNA in the Dex-exposed female offspring was substantial. This inconsistency could be due to the fact that whole hippocampal homogenates were used to measure protein, and this may have masked an increase in GR and MR protein in the specific hippocampal subfields. Alternatively, there may be a disconnection of transcription and translation in the animals exposed to sGCs prenatally; further work is required to investigate these possibilities. In agreement with the decreased basal cortisol levels during the luteal phase of the cycle, adrenal SF-1 mRNA was significantly lower in the Dex-exposed females. SF-1 is a transcription factor that upregulates many of the enzymes involved in cortisol biosynthesis (Simmonds et al. 2001; Li et al. 2004). A reduction in SF-1 could potentially lead to the reduction in cortisol levels observed in the Dex-females, through reduced drive of steroidogenic enzymes. The Beta-exposed females also exhibited increased GR mRNA in the hippocampus, but this was confined to the CA1/2 region in this group. While this effect was not as profound as that in the Dex-exposed females, increased GR mRNA would facilitate increased negative feedback and thus the lower basal salivary cortisol levels observed. Again, in contrast to the mRNA data, there was no significant effect on GR protein levels. GR mRNA was significantly lower in the hypothalamic PVN in the Beta-exposed females. While this would first appear counterintuitive based on the reduction in basal adrenocortical activity identified in these animals during the luteal phase of the cycle, it is possible that there has been a reduction in hypothalamic glucocorticoid sensitivity, in an attempt to restore normal HPA axis function. The Beta females also exhibited significantly higher adrenal MC2R mRNA. Again, this is counterintuitive to the reduction in salivary cortisol observed during the luteal phase, but could again be a compensatory mechanism by which the reduced circulating cortisol levels act to upregulate the MC2R to increase endogenous cortisol production. This possibility requires further investigation.
The Beta females exhibited significantly higher brain weight compared to the control females. Brain sparing is when the developing fetus redistributes blood flow to sustain and protect vital organs leading to differential fetal growth patterns, such as a large brain to body ratio. Brain sparing as a result of prenatal sGC exposure has been demonstrated in rats and is common in cases of intrauterine growth restriction (IUGR) (Slotkin et al. 1993; Lumbers et al. 2001; Dressino et al. 2002). In the current model, it is not clear whether the increase in brain weight in the Beta females is reflective of brain sparing as all organ measurements were taken during adulthood. However, it would be clinically important to determine in the fetus whether Beta, but not Dex, is a potent enough glucocorticoid to lead to brain sparing in cases of glucocorticoid administration for the risk of preterm labour.
In the current study, we found no effect of prenatal synthetic glucocorticoid exposure on the timing of puberty in the female offspring. This is in contrast to studies in the rat that showed that females whose mothers were treated with dexamethasone during pregnancy exhibited a delay in puberty (Smith & Waddell, 2000). However, there are some major differences with the methodology used in the study by Smith & Waddell and those used in the current study such as differences in species, dose and route of administration of the sGCs and other postnatal outcomes in the offspring. In spite of the specific differences, it is emerging from animal studies that sGC exposure in utero does affect the reproductive axis and should be investigated in human follow-up studies of prenatal sGC exposure.
Perhaps one of the most novel findings in the present study is that Beta-exposed female guinea pigs required a significantly greater number of cycles for successful conception, indicating reduced fecundity in these animals. This may result from either a reduction in fertility or decreased sexual receptivity in the Beta-exposed females. Early studies in the guinea pig showed that ovariectomized females required administration of oestradiol followed by the administration of progesterone to induce sexual behaviour (Young et al. 1939). The amount of oestrogen required to induce sexual receptivity is less than that needed to induce vaginal ostrous. We used vaginal opening as the marker for ostrous, and therefore decreased oestradiol levels during the ostrous phase of the reproductive cycle in Beta-exposed females seems an unlikely explanation. Interestingly, these early studies also demonstrated that thyroidectomized animals administered exogenous thyroxine exhibited increased sexual behaviour compared to intact females (Young et al. 1935). As all females were killed during the luteal phase of the ostrous cycle we were unable to conduct thorough hormone assessments, which may have provided evidence for reduced fertility. The salivary cortisol response to stress tended to be higher in the Beta-exposed females during the ostrous phase of the reproductive cycle. Whether this heightened stress responsiveness led to differences in behaviour and sexual receptivity, which subsequently affected fecundity is unknown. It is also possible that the Beta-exposed females exhibited increased aggression towards the male, thereby preventing copulatory advances. Observation of the female–male interaction during mating, and measures of aggression and sexual behaviour are required to further to investigate the reduced fecundity in the Beta-exposed females.
Dex-exposed females delivered an increased proportion of females in the litter of their first pregnancy with no effect on litter size. The dominant theory in evolutionary biology is the Trivers–Willard hypothesis, which states that where one sex has more variable reproductive success, mothers in good condition would be advantaged by producing more of that sex, whereas mothers in poor condition would be advantaged by producing more of the reproductively stable sex (Trivers & Willard, 1973). Indeed, this has been experimentally demonstrated in a black-backed gull, where decreasing the quality of the maternal environment skewed the sex ratio towards females, the sex with the higher survival prospects (Nager et al. 1999). Since guinea pigs are a polygynous species, these data suggest that the exposure to Dex during gestation signalled that the environment into which the litter would be born would be unfavourable. An increase in maternal testosterone levels during pregnancy is thought to be the key factor underlying changes in sex ratio (Grant, 2007). Although we did not find any effect of sGC exposure during gestation on luteal phase plasma testosterone levels, we were unable to assess testosterone levels during pregnancy in the sGC-exposed females. There are a number of methods by which sex ratios can be controlled by mammals such as differential sperm transport, events at conception, zygote transport, implantation, genomic imprinting and fetal resorption (Peaker & Taylor, 1996). Clearly further studies are required to confirm and extend this unexpected observation
The Royal College of Obstetricians and Gynaecologists recommend that Beta be used as the sGC for treatment of RDS in cases of preterm labour (RCOG Guideline 7, 2004). This recommendation was based on a meta-analysis by Crowley et al., which demonstrated that Beta, but not Dex decreased the incidence of cystic periventricular leucomalacia in preterm infants born between 24 and 31 weeks of gestation. Therefore, while Beta is the most frequently administered sGC, there is no definitive consensus on whether Beta or Dex should be the sGC of choice for the management of preterm labour (American College of Obstetricians and Gynecologists Committee on Obstetric Practice, 2008). Moreover, recent studies have identified that repeated courses of Beta administered during late gestation affects behaviour in children (Crowther et al. 2007; Pesonen et al. 2009). From the current study, one of the most profound findings is the differences in phenotype depending on whether Beta or Dex was the sGC administered during gestation. Beta and Dex are steriomers of one another. They both bind specifically to the GR and not the MR, and pass rapidly across the placenta to the fetus as they are both poor substrates for placental 11β-hydroxysteroid dehydrogenase (Matthews et al. 2002; Roberts & Dalziel, 2006). For the current study, we have mimicked the clinical situation by administering Beta as a combination of betamethasone sodium phosphate and betamethasone acetate and Dex as dexamethasone sodium phosphate (Brownfoot et al. 2008). The difference in formulation between the two sGCs leads to differences in the pharmacokinetic properties and may be responsible for the differences in outcomes observed. The half-life of Beta in the fetal circulation is around 12 h and 6 h in maternal circulation whereas that of Dex is 2.4–3.6 h in maternal circulation (Liggins & Howie, 1972; Tsuei et al. 1980; Kream et al. 1983; Ballard & Ballard, 1995). Additionally, Beta and Dex have different affinities for GR, 5.4-fold greater and 7.1-fold greater, respectively, compared to cortisol (Ballard & Ballard, 1995). Further studies investigating the mechanism of action of the two sGCs are required to determine how each specifically affects fetal development.
In conclusion, the present study has demonstrated that there are long-term effects of prenatal sGC exposure on HPA axis activity and regulation in adult female guinea pigs. For the first time, we have demonstrated that these changes are dependent on the stage of the reproductive cycle and there are effects on fecundity. We have also demonstrated that the outcomes are different depending on whether Beta or Dex was the sGC administered during pregnancy. This highlights the importance of long-term follow-up in children who were treated with either sGC. Understanding the mechanisms by which endocrine function can be programmed during early life will help in the development of new therapeutic approaches and management of high risk pregnancies.
Acknowledgments
We would like to acknowledge the assistance of Dawn Owen and Alice Kostaki. This study was funded by the Canadian Institutes for Health Research (FRN-97736; to S.G.M.).
Glossary
Abbreviations
- AVP
arginine vasopressin
- AUC
area under the curve
- Beta
betamethasone
- CRH
corticotrophin releasing hormone
- Dex
dexamethasone
- DG
dentate gyrus
- ELISA
enzyme-linked immunosorbant assay
- GR
glucocorticoid receptor
- HPA
hypothalamic–pituitary–adrenal
- ISH
in situ hybridization
- MC2R
adrenocorticotrophin receptor
- MR
mineralocorticoid receptor
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- RIA
radioimmunoassay
- RDS
respiratory distress syndrome
- ROD
relative optical density
- SF-1
steroidogenic factor-1
- sGC
synthetic glucocorticoid
- StAR
steroidogenic acute regulatory protein
- Veh
vehicle
Author contributions
S.G.M., E.D. and A.K. contributed to the conception and design of experiments. S.G.M., E.D., A.K. and J.L. contributed to the execution, analysis and interpretation of experiments. L.D. and A.K. wrote the initial draft of the manuscript and S.G.M., contributed to writing and revising the manuscript. All authors approved the final version of the manuscript.
References
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG committee opinion no. 402: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2008;111:805–807. doi: 10.1097/AOG.0b013e318169f722. [DOI] [PubMed] [Google Scholar]
- Andrews MH, Matthews SG. Regulation of glucocorticoid receptor mRNA and heat shock protein 70 mRNA in the developing sheep brain. Brain Res. 2000;878:174–182. doi: 10.1016/s0006-8993(00)02735-9. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic–pituitary–adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. doi: 10.1111/j.1471-0528.1999.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008;4:CD006764. doi: 10.1002/14651858.CD006764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2002;4:CD000065. doi: 10.1002/14651858.CD000065. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS, Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: A randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117:1058–1067. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/s0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Donovan BT, Lockhart AN. Gonadal hormones and the control of ovulation in the guinea-pig. J Endocrinol. 1972;55:599–607. doi: 10.1677/joe.0.0550599. [DOI] [PubMed] [Google Scholar]
- Dressino V, Orden B, Oyhenart EE. Sexual responses to intrauterine stress: Body and brain growth. Clin Exp Obstet Gynecol. 2002;29:100–102. [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: Size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: Effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Grant VJ. Could maternal testosterone levels govern mammalian sex ratio deviations? J Theor Biol. 2007;246:708–719. doi: 10.1016/j.jtbi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hauser J, Dettling-Artho A, Pilloud S, Maier C, Knapman A, Feldon J, et al. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148:1813–1822. doi: 10.1210/en.2006-1306. [DOI] [PubMed] [Google Scholar]
- Hauser J, Knapman A, Zurcher NR, Pilloud S, Maier C, Diaz-Heijtz R, et al. Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys. Endocrinology. 2008;149:6343–6355. doi: 10.1210/en.2008-0615. [DOI] [PubMed] [Google Scholar]
- Kalabis GM, Kostaki A, Andrews MH, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: Fetal protection. Biol Reprod. 2005;73:591–597. doi: 10.1095/biolreprod.105.042242. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Prenatal stress modifies behaviour and hypothalamic-pituitary-adrenal function in female guinea pig offspring: Effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008;149:6406–6415. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behaviour by synthetic glucocorticoids. Brain Res Rev. 2008;57:586–595. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Keightley MC, Curtis AJ, Chu S, Fuller PJ. Structural determinants of cortisol resistance in the guinea pig glucocorticoid receptor. Endocrinology. 1998;139:2479–2485. doi: 10.1210/endo.139.5.5982. [DOI] [PubMed] [Google Scholar]
- Kream J, Mulay S, Fukushima DK, Solomon S. Determination of plasma dexamethasone in the mother and the newborn after administration of the hormone in a clinical trial. J Clin Endocrinol Metab. 1983;56:127–133. doi: 10.1210/jcem-56-1-127. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Li LA, Chang YC, Wang CJ, Tsai FY, Jong SB, Chung BC. Steroidogenic factor 1 differentially regulates basal and inducible steroidogenic gene expression and steroid synthesis in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. 2004;91:11–20. doi: 10.1016/j.jsbmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: Sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Yu ZY, Gibson KJ. The selfish brain and the barker hypothesis. Clin Exp Pharmacol Physiol. 2001;28:942–947. doi: 10.1046/j.1440-1681.2001.03554.x. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Brain Res Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. Levels of pro-opiomelanocortin and prolactin mRNA in the fetal sheep pituitary following hypoxaemia and glucocorticoid treatment in late gestation. J Endocrinol. 1995;147:139–146. doi: 10.1677/joe.0.1470139. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo-pituitary-adrenal (HPA) development, and life after birth. Endocr Res. 2002;28:709–718. doi: 10.1081/erc-120016991. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): A randomised controlled trial. Lancet. 2008;372:2143–2151. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci U S A. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: Repeat courses – National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol. 2001;98:144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–867. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- Owen D, Banjanin S, Gidrewicz D, McCabe L, Matthews SG. Central regulation of the hypothalamic-pituitary-adrenal axis during fetal development in the guinea-pig. J Neuroendocrinol. 2005;17:220–226. doi: 10.1111/j.1365-2826.2005.01294.x. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function in juvenile guinea pigs. J Neuroendocrinol. 2007;19:172–180. doi: 10.1111/j.1365-2826.2006.01517.x. [DOI] [PubMed] [Google Scholar]
- Peaker M, Taylor E. Sex ratio and litter size in the guinea-pig. J Reprod Fertil. 1996;108:63–67. doi: 10.1530/jrf.0.1080063. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Lano A, Peltoniemi O, Hallman M, Kari MA. Antenatal betamethasone and fetal growth in prematurely born children: Implications for temperament traits at the age of 2 years. Pediatrics. 2009;123:e31–37. doi: 10.1542/peds.2008-1809. [DOI] [PubMed] [Google Scholar]
- Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by australian obstetricians – a survey of clinical practice. Aust N Z J Obstet Gynaecol. 1998;38:1–7. doi: 10.1111/j.1479-828x.1998.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- Simmonds PJ, Phillips ID, Poore KR, Coghill ID, Young IR, Canny BJ. The role of the pituitary gland and ACTH in the regulation of mRNAs encoding proteins essential for adrenal steroidogenesis in the late-gestation ovine fetus. J Endocrinol. 2001;168:475–485. doi: 10.1677/joe.0.1680475. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, McCook EC, Seidler FJ. Glucocorticoids regulate the development of intracellular signalling: enhanced forebrain adenylate cyclase catalytic subunit activity after fetal dexamethasone exposure. Brain Res Bull. 1993;32:359–364. doi: 10.1016/0361-9230(93)90200-u. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Li S, Doherty D, Nitsos I, Challis JR, et al. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am J Physiol Endocrinol Metab. 2007;292:E61–70. doi: 10.1152/ajpendo.00270.2006. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Li S, Matthews SG, Challis JR, Newnham JP. Expression of glucocorticoid receptor, mineralocorticoid receptor, and 11β-hydroxysteroid dehydrogenase 1 and 2 in the fetal and postnatal ovine hippocampus: Ontogeny and effects of prenatal glucocorticoid exposure. J Endocrinol. 2008;197:213–220. doi: 10.1677/JOE-07-0375. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 2000;141:2422–2428. doi: 10.1210/endo.141.7.7541. [DOI] [PubMed] [Google Scholar]
- Stockard CR, Papanicolaou GN. A rhythmical ‘heat period’ in the guinea-pig. Science. 1917;46:42–44. doi: 10.1126/science.46.1176.42. [DOI] [PubMed] [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Tsuei SE, Petersen MC, Ashley JJ, McBride WG, Moore RG. Disporition of synthetic glucocorticoids. II. dexamethasone in parturient women. Clin Pharmacol Ther. 1980;28:88–98. doi: 10.1038/clpt.1980.136. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Young WC, Dempey EW, Hagquist CW, Boling JL. Sexual behaviour and sexual receptivity in the female guinea pig. J Comp Physiol Psychol. 1939;27:49–68. [Google Scholar]
- Young WC, Dempsey EW, Myers HI. Cyclic reproductive behaviour in the female guinea pig. J Comp Physiol Psychol. 1935;19:131–335. [Google Scholar]