Abstract
High doses of ionizing radiation clearly produce deleterious consequences in humans, including, but not exclusively, cancer induction. At very low radiation doses the situation is much less clear, but the risks of low-dose radiation are of societal importance in relation to issues as varied as screening tests for cancer, the future of nuclear power, occupational radiation exposure, frequent-flyer risks, manned space exploration, and radiological terrorism. We review the difficulties involved in quantifying the risks of low-dose radiation and address two specific questions. First, what is the lowest dose of x- or γ-radiation for which good evidence exists of increased cancer risks in humans? The epidemiological data suggest that it is ≈10–50 mSv for an acute exposure and ≈50–100 mSv for a protracted exposure. Second, what is the most appropriate way to extrapolate such cancer risk estimates to still lower doses? Given that it is supported by experimentally grounded, quantifiable, biophysical arguments, a linear extrapolation of cancer risks from intermediate to very low doses currently appears to be the most appropriate methodology. This linearity assumption is not necessarily the most conservative approach, and it is likely that it will result in an underestimate of some radiation-induced cancer risks and an overestimate of others.
The biological effects of low levels of radiation have been investigated and debated for more than a century. Little question exists that intermediate and high doses of ionizing radiation, say >100 mSv, given acutely or during a prolonged period, produce deleterious consequences in humans, including, but not exclusively, cancer. At lower doses, however, the situation is less clear. For example, most radiological examinations (Table 1) produce doses in the range from 3 to 30 mSv. Understanding the risks of low doses of radiation still has societal importance in relation to issues as varied as screening tests for cancer, the future of nuclear power, frequent-flyer risks, occupational radiation exposure, manned space exploration, and radiological terrorism. Some typical doses are given in Table 1 (1–6).
Table 1. Approximate mean doses relevant to societal low-dose radiation exposures and to low-dose radiation risk estimation.
| Approximate mean individual dose, mSv* | |
|---|---|
| Some societally relevant exposures | |
| Round-trip flight, New York to London | 0.1 |
| Single screening mammogram (breast dose) | 3 |
| Background dose due to natural radiation exposure | 3/yr |
| Dose (over a 70-year period) to 0.5 million individuals in rural Ukraine in the vicinity of the Chernobyl accident | 14 |
| Dose range over 20-block radius from hypothetical nuclear terrorism incident [FASEB scenario 1: medical gauge containing cesium (6)] | 3-30 |
| Pediatric CT scan (stomach dose from abdominal scan) | 25 |
| Radiation worker exposure limit (1) | 20/yr |
| Exposure on international space station | 170/yr |
| Some low-dose epidemiological studies | |
| A-bomb survivors [mean dose in LSS cohort (2)] | 200 |
| Medical x-rays [breast dose in scoliosis study (4)] | 100 |
| Nuclear workers [mean dose from major studies (5)] | 20 |
| Individuals diagnostically exposed in utero (3) | 10 |
In this article, absorbed doses in milligrays are numerically the same as equivalent organ doses in millisieverts. Absorbed dose is the physical quantity describing energy deposited per unit mass. For radiation protection purposes, equivalent dose and effective dose are used, which include a radiation-dependent weighting factor. For x-rays or γ-rays, 1 mGy = 1 mSv. FASEB, Federation of American Societies for Experimental Biology; CT, computed tomography; LSS, Life-Span Study.
All doses are effective whole-body doses with the exception of the medical exposures (mammography, CT scan, irradiation for scoliosis), which are to specific organs.
Compared with higher doses, the risks of low doses of radiation are likely to be lower, and progressively larger epidemiological studies are required to quantify the risk to a useful degree of precision. For example, if the excess risk were proportional to the radiation dose, and if a sample size of 500 persons were needed to quantify the effect of a 1,000-mSv dose, then a sample size of 50,000 would be needed for a 100-mSv dose, and ≈5 million for a 10-mSv dose (7, 8). In other words, to maintain statistical precision and power, the necessary sample size increases approximately as the inverse square of the dose. This relationship reflects a decline in the signal (radiation risk) to noise (natural background risk) ratio as dose decreases.
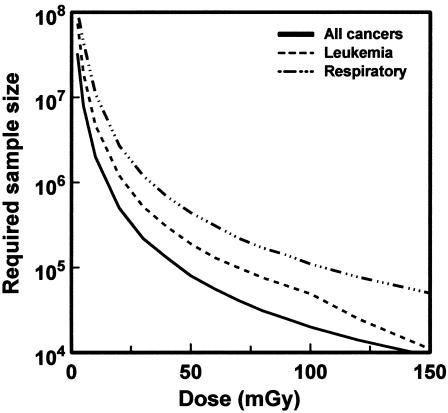
A more specific example is given in Fig. 1 (9). This shows, based on current estimates of the risks of low-dose radiation, the size of an exposed population that would need to be studied with lifetime follow-up to detect a significant increase in cancer mortality. Extraordinarily large studies are required to quantify the risks of very low doses of radiation.
Fig. 1.
Size of a cohort exposed to different radiation doses, which would be required to detect a significant increase in cancer mortality in that cohort, assuming lifetime follow-up (9).
Given these difficulties in quantifying low-dose risks, we address two specific questions. (i) What is the lowest dose of x- or γ-radiation for which convincing evidence of significantly elevated cancer risks in humans is available? (ii) What is the most appropriate way to extrapolate these risks to still lower doses?
We will focus largely on epidemiological studies of exposed human populations, although, of course, much ancillary information can be obtained from animal studies and from in vitro studies. In this way, we do not have to address the problems of extrapolating data from cells or laboratory animals to humans.
What Is the Lowest Dose for Which Good Epidemiological Evidence of Increased Carcinogenic Risks for Any Organ in Humans Is Available?
In estimating the lowest dose of x- or γ-radiation for evidence of increased cancer risks, it is important to make the distinction between acute exposures over a very short period (such as the atomic bomb exposures) and protracted exposures (such as occupational or fractionated exposure). In general, protracted exposures to x- or γ-radiation are associated with lower risks than those of an acute exposure to the same total dose, both for cancer and other endpoints (10, 11).
Acute Low-Dose Exposures. The epidemiological study with the highest statistical power for evaluating low-dose risks is the LSS cohort of atomic bomb survivors (2); because the cohort is large, follow-up is both complete and very long, and the survivors were exposed to a wide range of reasonably well characterized radiation doses. Although the atomic bomb survivor analyses have often been considered as high-dose studies, in fact, the mean dose in the exposed group in the LSS cohort is only 200 mSv, with >50% of the exposed individuals in the cohort (26,300 individuals) having doses <50 mSv. Cancer incidence (12), cancer mortality (2), and non-cancer-related mortality (2) have been studied, although almost half the exposed population, and a larger fraction of the individuals exposed as children, are still alive.
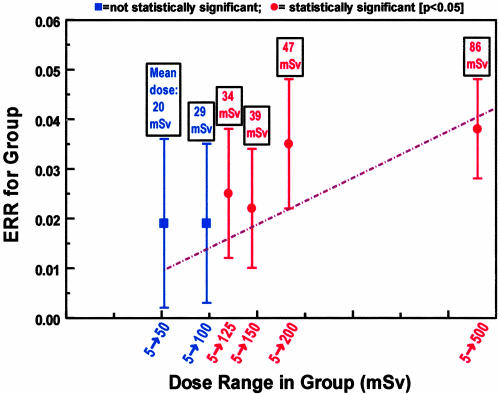
In the LSS study, organ dose estimates are available for all individuals included in the analysis, and the results are presented in dose-group categories; a comparison population is used that was sufficiently far from the explosions that their doses were <5 mSv. Fig. 2 shows low-dose risk estimates (2) for solid-cancer mortality in the atomic bomb survivors (1950–1997). The individuals in the dose category from 5 to 125 mSv (mean dose, 34 mSv) show a significant (P = 0.025) increase in solid-cancer-related mortality. It is possible that bias exists in these low-dose cancer-mortality risk estimates; for example, individuals nearer the blast might be more likely to have cancer recorded on their death certificates. Less potential for such bias exists in the cancer incidence studies, and the atomic bomb survivors in the dose range from 5 to 100 mSv (mean dose, 29 mSv) show a significantly increased incidence of solid cancer (P = 0.05) compared with the population who were exposed to <5 mSv (12).
Fig. 2.
Estimated excess relative risk (±1 SE) of mortality (1950–1997) from solid cancers among groups of survivors in the LSS cohort of atomic bomb survivors, who were exposed to low doses (<500 mSv) of radiation (2). The groups correspond to progressively larger maximum doses, with the mean doses in each group indicated above each data point. The first two data points (in blue) are not statistically significant (P = 0.15 and 0.3, respectively) compared with the comparison population who were exposed to <5 mSv, whereas the remaining four higher-dose points (in red) are statistically significant (P < 0.05). The dashed straight line represents the results of a linear fit (2) to all the data from 5 to 4,000 mSv (higher dose points are not shown).
The atomic bomb survivor risks discussed above represent an average over all exposed individuals. Good evidence documents that subpopulations are at greater or lower risk than the average, depending on age (13), genetic status (14), or other factors (15). In addition to the practical implications for population-wide radiation protection, low-dose studies on potentially radiosensitive subpopulations may result in a higher signal (risk) to noise (background) ratio, allowing low-dose risks to be more clearly established in these groups. One approach in this regard is to focus on in utero or childhood exposure; radiation risks are expected to be higher because of the higher proportion of dividing cells in younger individuals and also because of the longer lifespan available for a potential cancer to be expressed. Childhood cancer risks after prenatal x-ray exposure have been extensively studied: A detailed analysis of the many studies of childhood cancer risks from diagnostic in utero exposures concluded that a 10-mSv dose to the embryo and fetus does cause a significant and quantifiable increase in the risk of childhood cancer (3); Mole (16) has argued that the most reliable risk estimate from these studies comes from prenatal examinations in Britain during the period 1958–1961, for which the estimated mean fetal dose is 6 mSv and the odds ratio for childhood cancer deaths is 1.23 [95% confidence interval (CI) = 1.04–1.48].
Protracted Low-Dose Exposures. Much attention has been given to studies of large numbers of radiation workers who were chronically exposed to low radiation doses. Results have been reported from a pooled analysis of studies of nuclear workers in three countries [the United States, Canada, and the United Kingdom (UK) (17)], an enlarged UK study of nuclear workers (18), and studies of Canadian radiation workers (19, 20). These studies have been reviewed by Gilbert (5). Statistically significant excess cancer incidence and mortality risks for solid cancers were found in the Canadian studies (mean dose, 6.5 mSv). In contrast, neither the pooled analysis nor the UK study (both of which had higher mean doses, 40 and 30 mSv, respectively) showed a significant increase in solid cancer risk. However, all three studies found an increased risk for leukemia, which was statistically significant in the pooled study, borderline significant in the UK study, and nonsignificant in the Canadian studies.
As with the acute exposures, it is informative to examine childhood exposure, as the risks are expected to be higher and thus easier to quantify. The U.S. scoliosis cohort study (4) of females exposed under age 20 years to multiple diagnostic x-rays (mean breast dose, 108 mSv in 25 exposures) demonstrated a statistically significant increased risk for breast cancer [relative risk (RR) = 1.6; 95% CI = 1.1–2.6]; the excess risk remained significant when the analysis was limited to individuals with breast doses between 10 and 90 mSv.
Ron et al. (21) studied children who received fractionated irradiation of the scalp (five fractions; mean total thyroid dose, 62 mSv; dose range, 40–70 mSv); compared with matched, unirradiated comparison subjects, they showed a statistically significant increase in thyroid cancer risk (RR = 3.3; 95% CI = 1.6–6.7). Higher risks were seen when the age at exposure was limited to under 5 years (RR = 5.0; 95% CI = 2.7–10.3). A subsequent pooled analysis (22) of five cohort studies of thyroid cancer after childhood exposure to external radiation (four of these studies, including the scalp-irradiation study described above, were of fractionated exposure) showed clear evidence of an increased risk of thyroid cancer (RR = 2.5; 95% CI = 2–4) at a mean dose to the thyroid of 50 mSv (dose range, 10–90 mSv).
At still lower doses, an increase in leukemia risk is suggested (23) in children under age 5 years who were exposed to fallout from nuclear weapons testing (estimated fallout marrow dose, 1.5 mSv; RR = 1.11; 95% CI = 1.00–1.24). No individual doses were estimated in this study, but it is difficult to see how the biases that are common in ecologic studies could have affected the temporal correlation between the dose from fallout and the incidence of the disease. These results are consistent with an earlier case–control study (24) of leukemia in Utah in relation to fallout from the Nevada nuclear test site; here, a significant excess risk for acute leukemia was seen in individuals who died at younger than 20 years of age and who received bone-marrow doses from 6 to 30 mGy (odds ratio, 5.8; 95% CI = 1.6–22).
Summary of Doses at Which Clear Evidence of Cancer Risks Is Shown. For x- or γ-rays, good evidence of an increase in risk for cancer is shown at acute doses >50 mSv, and reasonable evidence for an increase in some cancer risks at doses above ≈5 mSv. As expected from basic radiobiology (10), the doses above which statistically significant risks are seen are somewhat higher for protracted exposures than for acute exposures; specifically, good evidence of an increase in some cancer risks is shown for protracted doses >100 mSv, and reasonable evidence for an increase in cancer risk at protracted doses above ≈50 mSv.
It seems unlikely that we will be able to directly estimate risks at significantly lower doses than these because of the practical limits of epidemiology discussed above. Of course, the fact that risks cannot be directly estimated at doses below, say, 5 mSv, does not imply any conclusion as to whether risks actually exist at these lower doses. As we discuss below, at lower doses inferences with regard to risk need to be based on understanding underlying mechanisms.
Extrapolation of Observed Risks to Lower Doses
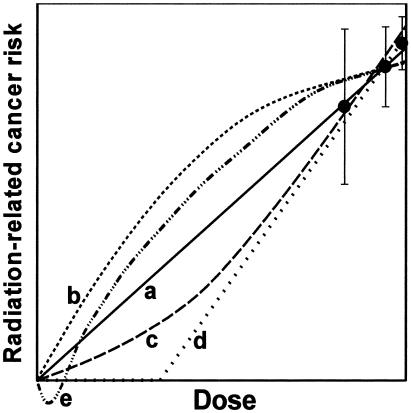
At doses below those where significant risks have been demonstrated in human populations [<50–100 mSv (protracted exposure) or 10–50 mSv (acute exposure)], we cannot use epidemiological data alone to establish the shape of the dose–response relation. All the dose–response relations shown in Fig. 3 are possible descriptors of low-dose radiation oncogenesis, and different endpoints may well exhibit differently shaped dose–response relations.
Fig. 3.
Schematic representation of different possible extrapolations of measured radiation risks down to very low doses, all of which could, in principle, be consistent with higher-dose epidemiological data. Curve a, linear extrapolation; curve b, downwardly curving (decreasing slope); curve c, upwardly curving (increasing slope); curve d, threshold; curve e, hormetic.
As we now discuss, scenarios exist where a linear extrapolation of risks from high to low doses could underestimate some low-dose risks (Fig. 3, curve b), and scenarios also exist where a linear extrapolation could overestimate some low-dose risks (Fig. 3, curves c–e). It is quite possible that many or all of these different scenarios apply, for differing endpoints.
Linear Dose–Response Relations (Fig. 3, Curve a). At the low and intermediate doses that are amenable to statistically meaningful analysis, a large amount of data are available, both from epidemiological and laboratory studies, that are consistent with a linear dose–response relation. The data have been extensively reviewed in a recent National Council on Radiation Protection and Measurements Report (ref. 25, p. 7), which concluded, “Although other dose–response relationships for the mutagenic and carcinogenic effects of low-level radiation cannot be excluded, no alternate dose–response relationship appears to be more plausible than the linear-nonthreshold model on the basis of present scientific knowledge.”
At still lower doses, which may not be amenable to direct study, the biophysical rationale for linearity (Fig. 3, curve a) relates to the unique, stochastic nature of ionizing-radiation energy deposition. The biophysical rationale is essentially as follows:
Direct epidemiological evidence demonstrates that an organ dose of 10 mGy of diagnostic x-rays is associated with an increase in cancer risk (3, 16).
At an organ dose of 10 mGy of diagnostic x-rays, most irradiated cell nuclei will be traversed by one or, at most, a few physically distant electron tracks. Being so physically distant, it is very unlikely that these few electron tracks could produce DNA damage in some joint, cooperative way; rather, these electron tracks will act independently to produce stochastic damage and consequent cellular changes.
Decreasing the dose, say by a factor of 10, will simply result in proportionately fewer electron tracks and fewer hit cells. It follows that those fewer cells that are hit at the lower dose will be subject to (i) the same types of electron damage and (ii) the same radiobiological processes as would occur at 10 mGy.
Thus, decreasing the number of damaged cells by a factor of 10 would be expected to decrease the biological response by the same factor of 10; i.e., the response would decrease linearly with decreasing dose. One could not expect qualitatively different biological processes to be active at, say, 1 mGy that were not active at 10 mGy, or vice versa. The argument suggests that the risk of most radiation-induced endpoints will decrease linearly, without a threshold, from ≈10 mGy down to arbitrarily low doses.
This biophysical argument for linearity considers radiation effects due to autonomous responses of individual cells. Even for clonal cancers, it may be that oncogenesis involves, in an essential way, interactions among different cells (26). Such multicellular interactions during oncogenesis would not be expected to alter linearity as long as the rate-limiting radiation damage step is a single-cell process; thus, e.g., the processing of a radiation-damaged cell within its microenvironment would not affect the linearity with dose of the final endpoint. However, linearity would not necessarily hold in the relevant dose range if multiple radiation-damaged cells influenced each other, either synergistically or antagonistically, although linearity would still hold at lower doses. Cooperative multicellular radiation effects that have been observed to date, such as bystander effects (27) and delayed instability (28, 29), show saturation at low doses, which, in turn, could underlie downwardly curving dose–response relations (Fig. 3, curve b; see below).
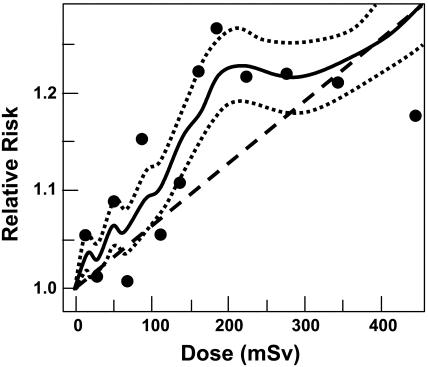
Scenarios in Which an Assumption of Linearity Underestimates Low-Dose Risks: Downwardly Curving Dose–Effect Relations (Fig. 3, Curve b). Evidence for the presence of downwardly curving (decreasing slope) dose–response relations, both from epidemiological and laboratory studies exists. The most recent low-dose atomic bomb survivor data for cancer mortality (Fig. 2) and cancer incidence (Fig. 4) both appear to exhibit this shape. Of course the shape of the dose–response curve at such low doses cannot be unequivocally established through epidemiological studies.
Fig. 4.
Estimated risks (relative to an unexposed individual) of solid cancer in atomic bomb survivors exposed to low radiation doses (12). Data points are placed at the mean of each dose category. The solid curve represents a weighted moving average of the points shown (dotted curves: ±1 SE), and the dashed straight line is a linear risk estimate computed from all the data in the dose range from 0 to 2,000 mSv. Age-specific cancer rates from 1958 to 1994 are used, averaged over follow-up and gender.
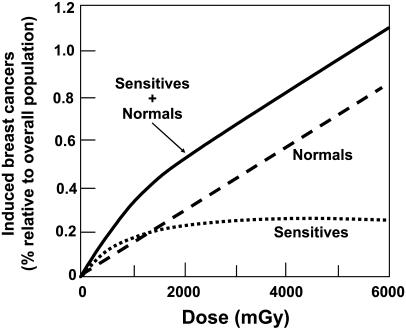
Such downwardly curving dose–response relations for human responses have been interpreted in several ways. The first way is the existence of small subpopulations of individuals within the total population who are hypersensitive to radiation (14). As an example, in the schematic in Fig. 5, we consider the significance of a very small subpopulation (in this hypothetical example, 0.25%) of sensitive individuals who are exceedingly sensitive to radiation-induced breast cancer. This sensitivity leads to the dose–response curve labeled “sensitives,” which will saturate at 0.25%. Combined with a linear dose–response relation for the “normal” population, the overall low-dose dose–response curve would then be downwardly curving. Some genetically based radiosensitive subpopulations have been identified, such as Atm (30–32) and Brca1 (33–35) heterozygotes, although the links with radiation-induced cancer sensitivity are still controversial (36, 37), and no radiosensitive populations have been identified to date with the frequency and hypersensitivity that would be needed to explain dose–response relations such as in Figs. 2 and 4.
Fig. 5.
Schematic representation of the potential effect of a small (0.25%) population of women, who are extremely sensitive for radiation-induced breast cancer, compared with the general (normal) population. Schematized is the number of radiation-induced breast cancers as a percentage of the overall population. The dose–risk relations for both the normal and the sensitive populations are assumed to be linear. Because the number of radiation-induced breast cancers in the sensitive population would saturate as the dose increases (because all the exposed women would have developed breast cancer), the dose–response for the whole population would be downwardly curving.
A second interpretation of downwardly curving dose–response relations (Fig. 3, curve b) is in terms of induced radioresistance, sometimes called adaptive response, in which a small “priming” radiation dose (typically 5–100 mGy) decreases the radiosensitivity to subsequent larger radiation exposures, perhaps by up-regulating some DNA repair mechanisms. The phenomenon has been reported for carcinogenesis (38), cellular inactivation (39), mutation induction (40), chromosome aberration formation (41), and in vitro oncogenic transformation (42). No evidence suggests that a priming dose can actually eliminate subsequent radioresponsiveness. Available data suggest that the induced radioresistance is transitory, lasting from 4 to 48 h, which suggests that the phenomenon could be of limited relevance for prolonged low-dose radiation exposures. In experiments in which the effect has been observed in human cells, considerable interindividual variation always occurs. It has been reported that the capacity for induced radioresistance decreases significantly with age (43).
As discussed above, a third interpretation is that downwardly curving dose–response relations (Fig. 3, curve b) are the result of bystander effects (27, 44). The bystander effect involves radiation-damaged cells sending out signals to adjacent cells that were not directly hit by the radiation; these signals can potentially result in oncogenic damage to the bystander cells (45). Bystander effects are characterized by a steep response at low doses, reflecting a large number of cells receiving a damage signal from adjacent radiation-damaged cells. At somewhat higher doses, however, the bystander effect saturates (because all relevant cells that can be affected are already affected), which results in a characteristic downwardly curving dose–response relation, such as Fig. 3, curve b (46). Bystander effects have been extensively demonstrated in the laboratory for α-radiation and, to a lesser extent, x-rays (44). Although evidence exists that bystander effects may be relevant to low-dose risks from radon (α particle) exposure (47), their relevance to low-dose x- or γ-ray risks has yet to be established.
Scenarios in Which an Assumption of Linearity Overestimates Low-Dose Risks: Threshold and Hormetic Responses (Fig. 3, Curves d and e). A threshold in dose (Fig. 3, curve d) implies that some dose exists below which the risk of a particular endpoint being induced is zero. A possible example is radiation-induced sarcoma (malignancies originating in connective tissue), which is rarely observed at low doses (48), potentially because noncycling connective-tissue cells need a large dose to stimulate them to cycle. Thus, for example, after radiotherapy a significant risk of secondary sarcomas exists in or near the high-dose (>50 Gy) treatment region, but not in distant organs exposed to low doses (49, 50). The different risk patterns for sarcomas and carcinomas is born out in the atomic bomb survivors (51), among whom a significant increase in bone-cancer mortality has not been observed (mean dose, 200 mSv; P = 0.4), but a significant increase in carcinomas, which originate in cells that are already cycling, is clearly seen (P < 10–4).
A hormetic response (Fig. 3, curve e) would occur if a given dose of radiation reduced the background incidence of some deleterious endpoint. Some experiments in animals have suggested that low and intermediate doses of radiation can enhance longevity (for a review, see ref. 52), a potentially hormetic response. As is often the case at low doses, the data are equivocal; e.g., Maisin et al. (53) report that 138 C57BL mice lived an average of 50 days longer than controls after exposure to an acute x-ray dose of 500 mGy; by contrast, in a much larger study, Storer et al. (54) report that 1,390 RFM mice lived an average of 75 days less than controls when exposed to the same acute dose of γ-rays.
In the animal experiments in which an increase in lifespan has been observed, the gain has generally not reflected a reduction in malignant disease, but rather an early reduction in mortality from infections and other nonmalignant diseases (52, 53). This finding suggests that a lifespan increase, if real, is less likely to be associated with a radiation-related stimulation of DNA-repair mechanisms (55), and more likely to be associated with a radiation-induced enhancement in the immune system (56).
Upwardly Curving Dose–Effect Relations (Fig. 3, Curve c). Upwardly curving (increasing slope) dose–effect relations (Fig. 3, curve c) provide a good description of acute dose–effect relations for radiation-induced leukemia in humans (2), and also of acute dose–effect relations for chromosome aberration induction (57). Such dose–response data have been extensively analyzed by using mechanistically motivated models such as linear-quadratic and related approaches (58), or by modeling competition between different recombinational processes (59). These upwardly curving dose–effect models generally reduce to simple linear models at sufficiently low doses or dose rates (59, 60).
Summary
Above doses of 50–100 mSv (protracted exposure) or 10–50 mSv (acute exposure), direct epidemiological evidence from human populations demonstrates that exposure to ionizing radiation increases the risk of some cancers. Table 1 puts these numbers into the context of the radiation doses to which individuals are or might be exposed. The methodological difficulties inherent in low-dose epidemiological studies suggest that it is unlikely that we will be able to directly and precisely quantify cancer risks in human populations at doses much below 10 mSv. Our inability to quantify such risks does not, however, imply that the corresponding societal risks are necessarily negligible; a very small risk, if applied to a large number of individuals, can result in a significant public health problem.
At present, we cannot be sure of the appropriate dose–response relation to use for risk estimation at very low doses. Mechanistic arguments exist for suggesting that a linear extrapolation of risks to very low doses is appropriate, but testing such arguments at very low doses is not easy. However, the alternate models shown in Fig. 3, although applicable for some endpoints, are less credible than the linear model as a generic descriptor of radiation carcinogenesis at low doses and low dose rates.
The reader is reminded that this article addresses the risks of low doses of x- and γ-rays. For densely ionizing radiations, as from radon progeny, mechanistic and epidemiological evidence appear to lead to similar conclusions regarding the credibility of the linear model for the estimation of low-dose risk (61, 62).
In summary, given our current state of knowledge, the most reasonable assumption is that the cancer risks from low doses of x- or γ-rays decrease linearly with decreasing dose. In light of the evidence for downwardly curving dose responses (see Figs. 2 and 4), this linear assumption is not necessarily the most conservative approach, as sometimes has been suggested (63, 64), and it is likely that it will result in an underestimate of some radiation risks and an overestimate of others. Given that it is supported by experimentally grounded, quantifiable, biophysical arguments, a linear extrapolation of cancer risks from intermediate to very low doses currently appears to be the most appropriate methodology.
Acknowledgments
This work was supported in part by the U.S. Department of Energy Low-Dose Radiation Research Program.
Abbreviations: CI, confidence interval; LSS, Life-Span Study; RR, relative risk.
References
- 1.International Commission on Radiological Protection (1991) 1990 Recommendations of the International Commission on Radiological Protection (Pergamon, Oxford).
- 2.Preston, D. L., Shimizu, Y., Pierce, D. A., Suyama, A. & Mabuchi, K. (2003) Radiat. Res. 160, 381–407. [DOI] [PubMed] [Google Scholar]
- 3.Doll, R. & Wakeford, R. (1997) Br. J. Radiol. 70, 130–139. [DOI] [PubMed] [Google Scholar]
- 4.Doody, M. M., Lonstein, J. E., Stovall, M., Hacker, D. G., Luckyanov, N. & Land, C. E. (2000) Spine 25, 2052–2063. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert, E. S. (2001) Am. J. Epidemiol. 153, 319–322. [DOI] [PubMed] [Google Scholar]
- 6.Kelly, H. (2002) Pub. Interest Rep. J. Fed. Am. Sci. 55, 1–10. [Google Scholar]
- 7.Pochin, E. E. (1976) Health Phys. 31, 148–151. [PubMed] [Google Scholar]
- 8.Land, C. E. (1980) Science 209, 1197–1203. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council (1995) Radiation Dose Reconstruction for Epidemiologic Uses (Natl. Acad. Press, Washington, DC).
- 10.National Council on Radiation Protection and Measurements. (1980) Influence of Dose and Its Distribution inTime on Dose-Response Relationships for Low-LET Radiations (NCRP, Washington, DC), Report No. 64.
- 11.Brenner, D. J. (1999) Radiat. Res. 151, 225–229. [PubMed] [Google Scholar]
- 12.Pierce, D. A. & Preston, D. L. (2000) Radiat. Res. 154, 178–186. [DOI] [PubMed] [Google Scholar]
- 13.Pierce, D. A. (2002) J. Radiol. Prot. 22, A147–A154. [DOI] [PubMed] [Google Scholar]
- 14.International Commission on Radiological Protection (1999) Genetic Susceptibility to Cancer (Pergamon, Oxford), ICRP Publ. No. 79.
- 15.Herold, D. M., Hanlon, A. L. & Hanks, G. E. (1999) Int. J. Radiat. Oncol. Biol. Phys. 43, 475–479. [DOI] [PubMed] [Google Scholar]
- 16.Mole, R. H. (1990) Br. J. Cancer 62, 152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardis, E., Gilbert, E. S., Carpenter, L., Howe, G., Kato, I., Armstrong, B. K., Beral, V., Cowper, G., Douglas, A., Fix, J., et al. (1995) Radiat. Res. 142, 117–132. [PubMed] [Google Scholar]
- 18.Muirhead, C. R., Goodill, A. A., Haylock, R. G., Vokes, J., Little, M. P., Jackson, D. A., O'Hagan, J. A., Thomas, J. M., Kendall, G. M., Silk, T. J., et al. (1999) J. Radiol. Prot. 19, 3–26. [DOI] [PubMed] [Google Scholar]
- 19.Ashmore, J. P., Krewski, D., Zielinski, J. M., Jiang, H., Semenciw, R. & Band, P. R. (1998) Am. J. Epidemiol. 148, 564–574. [DOI] [PubMed] [Google Scholar]
- 20.Sont, W. N., Zielinski, J. M., Ashmore, J. P., Jiang, H., Krewski, D., Fair, M. E., Band, P. R. & Letourneau, E. G. (2001) Am. J. Epidemiol. 153, 309–318. [DOI] [PubMed] [Google Scholar]
- 21.Ron, E., Modan, B., Preston, D., Alfandary, E., Stovall, M. & Boice, J. D., Jr. (1989) Radiat. Res. 120, 516–531. [PubMed] [Google Scholar]
- 22.Ron, E., Lubin, J. H., Shore, R. E., Mabuchi, K., Modan, B., Pottern, L. M., Schneider, A. B., Tucker, M. A. & Boice, J. D., Jr. (1995) Radiat. Res. 141, 259–277. [PubMed] [Google Scholar]
- 23.Darby, S. C., Olsen, J. H., Doll, R., Thakrar, B., Brown, P. D., Storm, H. H., Barlow, L., Langmark, F., Teppo, L. & Tulinius, H. (1992) Br. Med. J. 304, 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens, W., Thomas, D. C., Lyon, J. L., Till, J. E., Kerber, R. A., Simon, S. L., Lloyd, R. D., Elghany, N. A. & Preston-Martin, S. (1990) J. Am. Med. Assoc. 264, 585–591. [PubMed] [Google Scholar]
- 25.National Council on Radiation Protection and Measurements (2001) Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing Radiation (NCRP, Bethesda), Report No. 136.
- 26.Barcellos-Hoff, M. H. (2001) J. Mammary Gland Biol. Neoplasia 6, 213–221. [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa, H. & Little, J. B. (1999) Radiat. Res. 152, 552–557. [PubMed] [Google Scholar]
- 28.Ullrich, R. L. & Davis, C. M. (1999) Radiat. Res. 152, 170–173. [PubMed] [Google Scholar]
- 29.Little, J. B. (2000) Carcinogenesis. 21, 397–404. [DOI] [PubMed] [Google Scholar]
- 30.Hall, E. J., Schiff, P. B., Hanks, G. E., Brenner, D. J., Russo, J., Chen, J., Sawant, S. G. & Pandita, T. K. (1998) Cancer J. Sci. Am. 4, 385–389. [PubMed] [Google Scholar]
- 31.Smilenov, L. B., Brenner, D. J. & Hall, E. J. (2001) Cancer Res. 61, 5710–5713. [PubMed] [Google Scholar]
- 32.Swift, M., Morrell, D., Massey, R. B. & Chase, C. L. (1991) N. Engl. J. Med. 325, 1831–1836. [DOI] [PubMed] [Google Scholar]
- 33.Buchholz, T. A., Wu, X., Hussain, A., Tucker, S. L., Mills, G. B., Haffty, B., Bergh, S., Story, M., Geara, F. B. & Brock, W. A. (2002) Int. J. Cancer 97, 557–561. [DOI] [PubMed] [Google Scholar]
- 34.Rothfuss, A., Schutz, P., Bochum, S., Volm, T., Eberhardt, E., Kreienberg, R., Vogel, W. & Speit, G. (2000) Cancer Res. 60, 390–394. [PubMed] [Google Scholar]
- 35.Xia, F. & Powell, S. N. (2002) Semin. Radiat. Oncol. 12, 296–304. [DOI] [PubMed] [Google Scholar]
- 36.Broeks, A., Russell, N. S., Floore, A. N., Urbanus, J. H., Dahler, E. C., van't Veer, M. B., Hagenbeek, A., Noordijk, E. M., Crommelin, M. A., van Leeuwen, F. E., et al. (2000) Int. J. Radiat. Biol. 76, 693–698. [DOI] [PubMed] [Google Scholar]
- 37.Shafman, T. D., Levitz, S., Nixon, A. J., Gibans, L. A., Nichols, K. E., Bell, D. W., Ishioka, C., Isselbacher, K. J., Gelman, R., Garber, J., et al. (2000) Genes Chromosomes Cancer 27, 124–129. [PubMed] [Google Scholar]
- 38.Bhattacharjee, D. & Ito, A. (2001) In Vivo 15, 87–92. [PubMed] [Google Scholar]
- 39.Joiner, M. C., Marples, B., Lambin, P., Short, S. C. & Turesson, I. (2001) Int. J. Radiat. Oncol. Biol. Phys. 49, 379–389. [DOI] [PubMed] [Google Scholar]
- 40.Ueno, A. M., Vannais, D. B., Gustafson, D. L., Wong, J. C. & Waldren, C. A. (1996) Mutat. Res. 358, 161–169. [DOI] [PubMed] [Google Scholar]
- 41.Wolff, S. (1998) Environ. Health Perspect. 106, Suppl 1, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzam, E. I., Raaphorst, G. P. & Mitchel, R. E. (1994) Radiat. Res. 138, S28–S31. [PubMed] [Google Scholar]
- 43.Gadhia, P. K. (1998) Mutagenesis 13, 151–152. [DOI] [PubMed] [Google Scholar]
- 44.Ballarini, F., Biaggi, M., Ottolenghi, A. & Sapora, O. (2002) Mutat. Res. 501, 1–12. [DOI] [PubMed] [Google Scholar]
- 45.Sawant, S. G., Randers-Pehrson, G., Geard, C. R., Brenner, D. J. & Hall, E. J. (2001) Radiat. Res. 155, 397–401. [DOI] [PubMed] [Google Scholar]
- 46.Brenner, D. J., Little, J. B. & Sachs, R. K. (2001) Radiat. Res. 155, 402–408. [DOI] [PubMed] [Google Scholar]
- 47.Brenner, D. J. & Sachs, R. K. (2002) Int. J. Radiat. Biol. 78, 593–604. [DOI] [PubMed] [Google Scholar]
- 48.White, R. G., Raabe, O. G., Culbertson, M. R., Parks, N. J., Samuels, S. J. & Rosenblatt, L. S. (1993) Radiat. Res. 136, 178–189. [PubMed] [Google Scholar]
- 49.Brenner, D. J., Curtis, R. E., Hall, E. J. & Ron, E. (2000) Cancer 88, 398–406. [DOI] [PubMed] [Google Scholar]
- 50.Kuttesch, J. F., Jr., Wexler, L. H., Marcus, R. B., Fairclough, D., Weaver-McClure, L., White, M., Mao, L., Delaney, T. F., Pratt, C. B., Horowitz, M. E., et al. (1996) J. Clin. Oncol. 14, 2818–2825. [DOI] [PubMed] [Google Scholar]
- 51.Pierce, D. A., Shimizu, Y., Preston, D. L., Vaeth, M. & Mabuchi, K. (1996) Radiat. Res. 146, 1–27. [PubMed] [Google Scholar]
- 52.Upton, A. C. (2001) Crit. Rev. Toxicol. 31, 681–695. [DOI] [PubMed] [Google Scholar]
- 53.Maisin, J. R., Gerber, G. B., Vankerkom, J. & Wambersie, A. (1996) Radiat. Res. 146, 453–460. [PubMed] [Google Scholar]
- 54.Storer, J. B., Serrano, L. J., Darden, E. B., Jr., Jernigan, M. C. & Ullrich, R. L. (1979) Radiat. Res. 78, 122–161. [PubMed] [Google Scholar]
- 55.Pollycove, M. & Feinendegen, L. E. (1999) C. R. Acad. Sci. Ser. III 322, 197–204. [DOI] [PubMed] [Google Scholar]
- 56.Xu, Y., Greenstock, C. L., Trivedi, A. & Mitchel, R. E. (1996) Radiat. Environ. Biophys. 35, 89–93. [DOI] [PubMed] [Google Scholar]
- 57.Cornforth, M. N., Bailey, S. M. & Goodwin, E. H. (2002) Radiat. Res. 158, 43–53. [DOI] [PubMed] [Google Scholar]
- 58.Sachs, R. K., Hahnfeld, P. & Brenner, D. J. (1997) Int. J. Radiat. Biol. 72, 351–374. [DOI] [PubMed] [Google Scholar]
- 59.Cucinotta, F. A., Nikjoo, H., O'Neill, P. & Goodhead, D. T. (2000) Int. J. Radiat. Biol. 76, 1463–1474. [DOI] [PubMed] [Google Scholar]
- 60.Brenner, D. J., Hlatky, L. R., Hahnfeldt, P. J., Huang, Y. & Sachs, R. K. (1998) Radiat. Res. 150, 83–91. [PubMed] [Google Scholar]
- 61.National Research Council (1999) Health Effects of Exposure to Radon: BEIR VI (Natl. Acad. Press, Washington, DC). [PubMed]
- 62.Puskin, J. S. (2003) Health Phys. 84, 526–532. [DOI] [PubMed] [Google Scholar]
- 63.Kellerer, A. M. (2000) Radiat. Environ. Biophys. 39, 17–24. [DOI] [PubMed] [Google Scholar]
- 64.Sinclair, W. K. (1981) Radiology 138, 1–9. [DOI] [PubMed] [Google Scholar]