Abstract
Rational
Although THC-induced elevations in accumbal dopamine levels are believed to play an important role in the abuse-related effects of cannabis, little direct evidence has been provided that the dopaminergic system is involved in the psychotropic effects of THC.
Objectives
To investigate whether drugs activating or blocking the dopaminergic system modulate the discriminative effects of THC.
Methods and Results
In rats that had learned to discriminate 3 mg/kg of THC from vehicle injections, the indirect dopaminergic agonists cocaine and amphetamine, the D1-receptor agonist SKF-38393, and the D2-receptor agonists quinpirole and apomorphine did not produce significant THC-like discriminative effects. However, both cocaine and amphetamine and D2-, but not the D1-, receptor agonists, augmented THC discrimination. Neither the D1-receptor antagonist SCH-23390 nor the D2-receptor antagonist raclopride reduced the discriminative effects of THC, even at doses that significantly depressed baseline operant responding. However, the D2-, but not the D1-, antagonist counteracted the augmentation of THC’s discriminative effects produced by cocaine and amphetamine. We hypothesized that release of anandamide by activation of D2 receptors was responsible for the observed augmentation of THC discrimination. This hypothesis was supported by two findings. First, the cannabinoid CB1-receptor antagonist rimonabant blocked quinpirole-induced augmentation of THC discrimination. Second, inhibition of anandamide degradation by blockade of fatty acid amide hydrolase (FAAH) augmented the THC-like effects of quinpirole.
Conclusions
Dopamine does not play a major role in THC discrimination. However, activation of the dopaminergic system positively modulates the discriminative effects of THC, possibly through D2-induced elevations in brain levels of anandamide.
Keywords: Cannabis, THC, endocannabinoid, dopamine, behavior, psychostimulants, rats
INTRODUCTION
Systemic administration of the psychoactive ingredient in cannabis, delta-9-tetreahydrocannabinol (THC), increases firing of dopaminergic neurons in the midbrain (Diana et al. 1998; French 1997; French et al. 1997) and increases extra-cellular levels of dopamine in the nucleus accumbens (Chen et al. 1991) especially in its ventro-medial part, the shell (Tanda et al. 1997). These increases in dopaminergic activity are considered critical in the mediation of the reinforcing effects of all drugs of abuse, including THC (Gardner and Vorel 1998; Solinas et al. 2008; Solinas et al. 2007d; Tanda and Goldberg 2003).
Drug-discrimination procedures allow the study of mechanisms through which drugs of abuse produce central effects that are important for the maintenance of drug-taking behavior and serve as a preclinical model of subjective reports of drug effects by humans (Solinas et al. 2006b). In recent studies, we investigated the role of opioid (Solinas and Goldberg 2005; Solinas et al. 2004) and cholinergic (Solinas et al. 2007a; Solinas et al. 2007b) systems in the discriminative effects of THC. We found that interactions between opioid and cannabinoid systems might be related to the ability of THC to increase extracellular levels of beta-endorphin in the ventral tegmental area (Solinas et al. 2004), while interactions between cholinergic and cannabinoid systems could be related to elevated brain levels of the endogenous cannabinoid anandamide produced by activation of nicotinic receptors (Solinas et al. 2007b). Here, we used drug discrimination procedures to investigate the possibility that the dopamine system modulates the discriminative effects of THC and to explore possible mechanisms underlying these interactions.
The effects of dopamine are mediated, to a large extent, by two subtypes of dopamine receptors: the D1-like and D2-likereceptors (Sealfon and Olanow 2000). D1 receptors (D1 and D5) are positively coupled to adenyl cyclase and stimulate cAMP formation, whereas D2 receptors (D2, D3 and D4) are negatively coupled to the enzyme. In this manuscript we will simply use the terms D1 and D2 receptors to indicate the two main sub-types of dopamine receptors without specifically addressing the further specific subdivision. In the nucleus accumbens, both D1 and D2 subtypes of dopamine receptors are present and, although there is evidence for co-localization in the same neurons (Aizman et al. 2000), D1 and D2 receptor levels substantially differ in distinct neuronal populations that project to different brain regions (Aubert et al. 2000; Steiner and Gerfen 1998). Activation of D1 and D2 receptors has been shown to have either similar effects, synergistic effects or, in some instances, considerably different and even opposite effects (Self 2004).
Interactions between cannabinoid and dopaminergic systems appear to be bidirectional and complex (Solinas et al. 2008; van der Stelt and Di Marzo 2003). Several studies have shown that strong interactions and reciprocal modulation between cannabinoid and dopaminergic systems exist under both physiological conditions and pathological conditions such as Parkison’s and Huntington disease (van der Stelt and Di Marzo 2003). Some data suggest that cannabinoid and dopaminergic systems have opposing functions and that dopamine, acting on D2, but not D1, receptors, increases extracellular levels of the endogenous cannabinoid anandamide (Giuffrida et al. 1999), which serves as a negative feedback for subsequent dopamine release and is involved in striatal long-term depression (LTD) (Centonze et al. 2004; Kreitzer and Malenka 2007). However, other data suggest that, in some instances, the two systems can potentiate each other. For example, we recently found that anandamide, like other cannabinoid CB1-receptor agonists (Tanda et al. 1997), increases extracellular dopamine levels in the nucleus accumbens (Solinas et al. 2006a; Solinas et al. 2007c). Therefore, it was of interest to investigate whether dopaminergic drugs could modulate the discriminative effects of THC and whether the modulation would be an augmentation or antagonism of THC’s effects in rats trained to discriminate THC. We first used the indirectly-acting dopamine agonists cocaine and amphetamine, which elevate extra-cellular levels of dopamine, in order to establish a general role for dopamine in THC discrimination. We then used selective agonists and antagonists for dopamine D1 and D2 receptors to better dissect the role these receptor subtypes play in THC discrimination. We found that dopamine D2, but not D1, receptor activation augmented THC discrimination. Finally, we tested the hypothesis that augmentation of THC discrimination was mediated by a D2-induced increase in brain levels of anandamide. This hypothesis was supported by reversing the augmentation of THC discrimination by dopamine agonists with the CB1-receptor antagonist rimonabant, and by enhancing the THC-like discriminative effects of D2 agonists with URB-597, an inhibitor of fatty acid amide hydrolase (FAAH), which prevents anandamide degradation and increases its levels in the brain (Kathuria et al. 2003).
METHODS
Subjects
Male Sprague-Dawley rats (Charles River, Wilmington, Mass., USA) were experimentally naive at the start of the study. For drug-discrimination studies, 11 rats initially weighting 350–380 g were housed individually. Rats’ weights were gradually reduced to approximately 80% of free feeding by limiting daily access to food before the start of drug-discrimination training sessions. Once drug-discrimination sessions were started, weight was maintained at about 80% of free feeding by giving about 15 g of food pellets shortly after the end of each daily session. All rats were housed in a temperature-and humidity-controlled room and were maintained on a 12-h light/dark cycle; the lights were on from 6:45 a.m. to 6:45 p.m. Experiments were conducted during the light phase. Animals used in this study were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and all experiments were conducted in accordance with the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse (NIDA), National Institutes of Health and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Drug-discrimination apparatus and procedure
Standard operant conditioning chambers (Coulbourn Instruments, Lehigh Valley, Pa., USA) were used. Each chamber contained two levers, separated by a recessed tray into which a pellet dispenser could deliver 45 mg food pellets (F0021; Bioserv, Frenchtown, N.J., USA). Each press of a lever with force of 0.4 N through 1 mm was recorded as a response and was accompanied by an audible click. The operant conditioning chambers were controlled by computers using the MED Associates MED-PC software package (Med Associates Inc., East Fairfield, Vt., USA). Rats were trained under a discrete-trial schedule of food-pellet delivery to respond on one lever after injection of a training dose of 3 mg/kg THC and on the other lever after injection of 1 ml/kg of THC vehicle. Injections of THC or vehicle were given intraperitoneally (i.p.) 30 min before the start of the session. For the entire duration of the experiment, for the present group of rats, each rat was assigned to one chamber. Other groups of rats were run in the same chambers and no specific precaution was taken to avoid interferences by odor cues. However, treatments and schedules in the other groups of rats were completely independent from those in the present experiments, making it unlikely that odor cues interfered with performance in the present experiments.
At the start of the session, a white house light was turned on and in its presence the rats were required to make ten consecutive responses (fixed-ratio 10 schedule of food delivery; FR10) on the lever appropriate to the pre-session treatment in order to obtain a food pellet. The completion of ten consecutive responses on the correct lever produced delivery of a 45 mg food pellet and initiated a 45-s time-out during which lever-press responses had no programmed consequences and the chamber was dark. Responses on the incorrect lever had no programmed consequences other than to reset the FR requirement to ten on the correct lever. After each time-out, the white house light was again turned on and the next trial began. Each session ended after completion of 20 fixed-ratio trials or after 30 min elapsed, whichever occurred first. Lever assignment was counterbalanced among rats so that for half of the rats the THC-appropriate lever was the right lever and for the other half of the rats it was the left lever.
Discrimination-training sessions were conducted five days per week under a double alternation schedule (i.e. DDVVDDVV etc., D=drug, THC; V=Vehicle). Training continued until there were eight consecutive sessions during which rats completed at least 90% of their responses during the session on the correct lever and no more than four responses occurred on the incorrect lever during the first trial. Test sessions were then initiated. Test sessions were identical to training sessions with the exception that ten consecutive responses on either one of the two levers led to the delivery of food pellets. Similar to training sessions, a maximum of 20 pellets in 30 min was available during test sessions. Switching responding from one lever to the other lever reset the ratio requirement. Once the test phase began, a single alternation schedule was introduced and test sessions were usually conducted on Tuesdays and Fridays. Thus, a 2-week sequence starting on Monday was: DTVDTVTDVT (T=test). In this way, test sessions occurred with equal probability after vehicle and drug sessions. Test sessions were conducted only if the criterion of 90% accuracy and not more than four incorrect responses during the first trial was maintained in the two preceding training sessions. If the criterion was not met, training sessions were conducted until the criterion was again attained. The first test sessions consisted of different doses of the training drug in order to establish a THC dose-response curve. Afterwards, tests with other compounds, alone and in combination with THC, began.
Two measures were analyzed: 1) percentage of total lever-presses made on the THC lever, that gives a quantitative indication of how much the drug or the combination of drugs tested produced discriminative effects similar to those of the 3 mg/kg training dose of THC; 2) overall rate of lever-press responding, that gives an indication of any disruption of motor responses produced by the drug or the combination of drugs tested. When rates of responding were significantly reduced compared to basal levels, administrations of higher doses of that specific drug or combination of drugs were normally avoided.
Drugs
Delta-9-THC (National Institute on Drug Abuse, Baltimore, Md., USA) 50 mg/ml in ethanol was dissolved in a solution 40% w/v of β-hydroxy-cyclodextrine (RBI-Sigma; St. Louis, MO, USA). Cocaine hydrochloride and d-amphetamine hydrochloride (National Institute on Drug Abuse, NIH, USA), apomorphine, quinpirole, SKF-38393, SCH-23390 and Raclopride (RBI-Sigma) were dissolved in sterile saline. Rimonabant (SR141716; N-piperidino-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methylpyrazole-3-carboxamide) (National Institute on Drug Abuse, NIH, USA) was dissolved in a vehicle containing Tween 80, 2%, ethanol, 2%, and saline 96%. We used a rimonabant dose of 1 mg/kg which blocks both the discriminative (Solinas et al. 2003) and reinforcing effects of THC in rats (Zangen et al. 2006). URB-597 was a gift of Drs. A. Duranti, A. Tontini and G. Tarzia, and was dissolved in a vehicle containing 20% dimethyl sulphoxide (DMSO) in saline. We used a URB-597 dose of 0.3 mg/kg which does not produce THC-like effects by itself but potentiates the discriminative and dopamine releasing effects of anandamide (Gobbi et al. 2005; Solinas et al. 2007c). All drugs were injected i.p. in a volume of 1.0 ml/kg. Dopaminergic antagonists were administered 45 min before the session (15 min before THC), dopaminergic agonists were administered 15 min before the session (15 min after THC), URB-597 was administered 40 min before the session, and rimonabant was administered 60 min before the session. All doses and treatments were chosen on the basis of published studies using these compounds in drug discrimination procedures. We have previously shown that the vehicle used for dissolving rimonabant and URB-597 produces no THC-lever selection (Solinas et al. 2003; Solinas et al. 2007c).
Data analysis
Discriminative-stimulus data were expressed as the percentage of the total responses (emitted on both levers) that were made on the THC-appropriate lever during the entire test session. Response-rate data were expressed as responses per second averaged over the session, with responding during time-out periods not included in calculations. The data from sessions during which rats did not complete at least one fixed-ratio were excluded from analysis of drug-lever selection. All results are presented as group means (±SEM). Statistical analysis of the ability of compounds to produce generalization to the discriminative effects of the training dose of THC was done using one-way ANOVA for repeated measures in comparison with vehicle treatments, followed, when appropriate, by the Dunnet’s post-hoc test. A probability value of p<0.05 was considered significant.
ED50 values for each compound or combination were obtained by nonlinear regression analysis with a sigmoidal dose-response (variable slope) equation, using GraphPad Prism 3 software (GraphPAD Software, San Diego, CA, USA). The equation was:
with the bottom and top values kept constant at 0% and 100%, respectively. Curves were considered parallel when their slopes did not differ significantly and dose-response curves were considered significantly different when 95% confidence intervals of ED50 values did not overlap. Statistical analysis of the effect of any treatment on rates of responding was done by using one-way ANOVA for repeated measures in comparison with vehicle treatment, followed, when appropriate, by the Dunnet’s post-hoc test.
RESULTS
Dopaminergic indirect antagonists do not produce THC-like discriminative effects
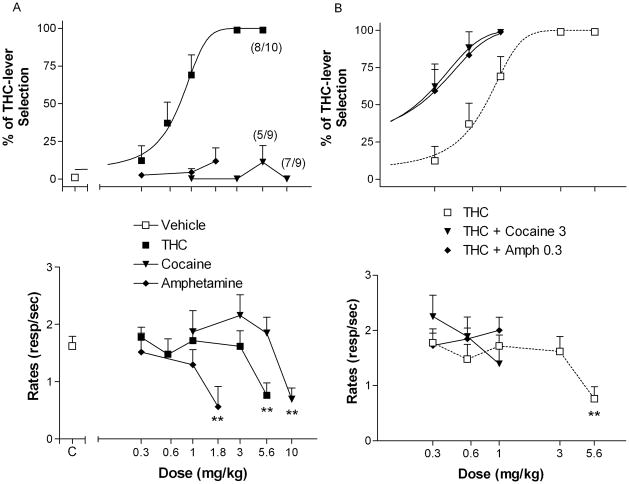
Rats learned to discriminate the 3 mg/kg training dose of THC (about 100% THC-lever selection, Fig. 1, upper panel) from injections of vehicle (about 0% THC-lever selection, Fig. 1, upper panel, bottom left corner) and the discriminative effects of THC were dose dependent (Fig. 1A, upper panel), with an ED50 value of 0.76 (95% confidence intervals = 0.59 to 0.92). The highest dose of THC tested (5.6 mg/kg) significantly decreased rates of responding (Fig. 1A, lower panel) [F(5,50) = 3.69, p < 0.01] as compared to vehicle control levels (Fig. 1A, lower panel, left side). A replication of the THC dose-response curve (data not shown), obtained at the end of the experiments, demonstrated that the ability of rats to discriminate THC did not change over time, as shown by no significant difference from the initial dose-response curve [ED50= 0.81, 95% confidence intervals = 0.60 to 1.02].
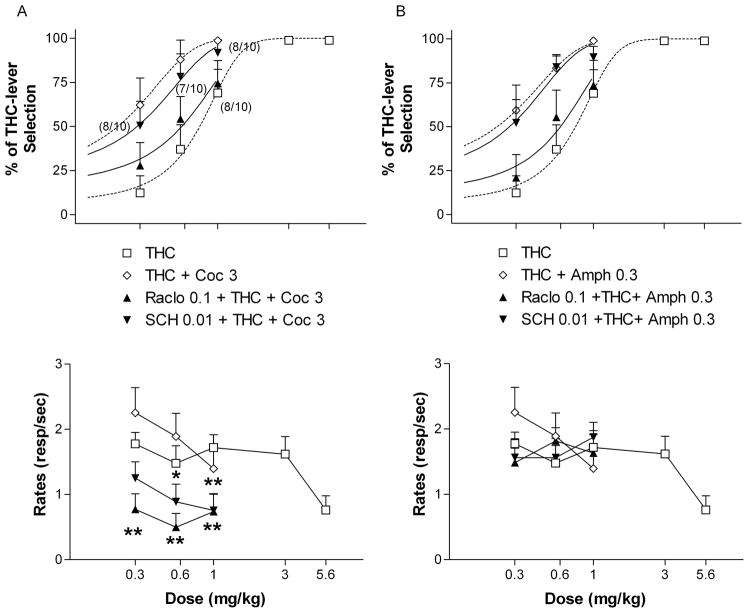
Figure 1.
Effects of cocaine and amphetamine in rats trained to discriminate 3 mg/kg of THC from THC vehicle (A) and effects of selected doses of cocaine (3 mg/kg) and amphetamine (0.3 mg/kg) on the dose-response curve for THC discrimination (B). Ordinates: overall percentage of responses on the lever associated with THC administration (upper panels) and overall rate of lever pressing expressed as responses per seconds (lower panels) averaged over the entire session. Abscissae: dose in mg/kg (log scale). Results represent means±SEM from 9–10 rats. C = control value for THC vehicle alone. Repeated measures ANOVA followed by post-hoc Dunnet’s test: **: p<0.01 compared to vehicle. Numbers in parenthesis at higher doses indicate the number of rats that completed at least one fixed ratio during the session over the total number of rats in which the dose was tested. Dose-response curve data for THC are the same for A and B.
Neither cocaine (1–10 mg/kg) nor amphetamine (0.3–1.8 mg/kg) produced significant generalization to the discriminative effects of THC (<20% THC-lever selection, Fig. 1A, upper panel), even at doses that significantly depressed rates of responding [F(4,32) = 6.13, p < 0.01 for cocaine and [F(3,24) = 4.86, p < 0.01 for amphetamine] (fig. 1A, lower panel). Saline injections did not produce any THC-lever selection (data not shown).
Dopaminergic indirect agonists augment discriminative effects of low doses of THC
Cocaine, at a dose of 3.0 mg/kg, significantly shifted to the left (i.e. augmented) the dose-response curve for THC discrimination (Fig. 1B, upper panel) [ED50 = 0.21, 95% confidence intervals = 0.031 to 0.46]. Similarly, amphetamine at a dose of 0.3 mg/kg, significantly shifted to the left the dose-response curve for THC discrimination (Fig. 1B, upper panel) [ED50 = 0.22, 95% confidence intervals = 0.015 to 0.43]. Combinations of cocaine or amphetamine with different doses of THC did not significantly alter rates of responding (Fig. 1B, lower panel).
Effects of dopaminergic direct agonists in rats trained to discriminate 3 mg/kg of THC
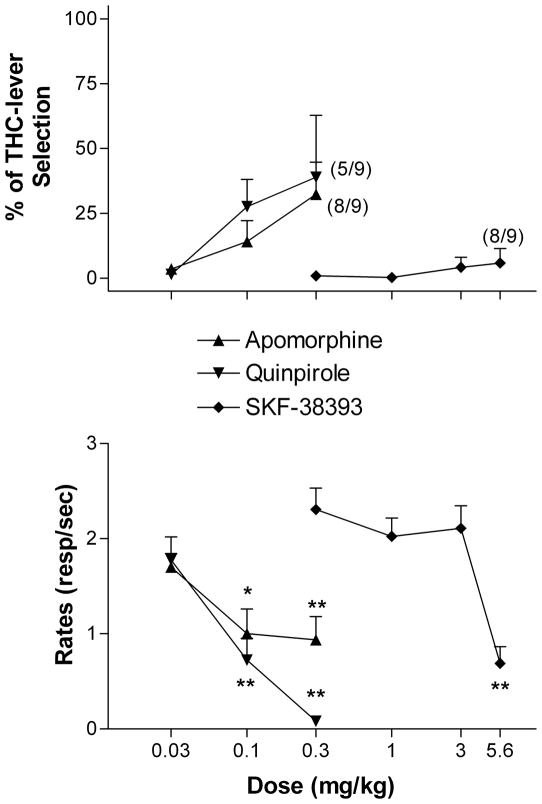
We then investigated the ability of selective dopamine D1 (SKF-38393) and D2 (quinpirole and apomorphine) receptor agonists to produce THC-like discriminative effects. SKF-38393 (0.3–5.6 mg/kg) produced only vehicle-like responding, i.e. THC-lever selection never exceeded 10%, even at doses that significantly decreased rates of responding [F(4,32) = 17.21, p < 0.0001] (Fig. 2). Surprisingly, both quinpirole (0.03–0.3 mg/kg) and apomorphine (0.03–0.3 mg/kg) produced some THC-like responding (35–40% at the highest dose tested) (Fig. 2, upper panel). However, generalization did not reach statistical significance and appeared at doses that dramatically reduced rates of responding and, in fact, completely disrupted responding in some animals [quinpirole, F(3,24)=15.96, p < 0.0001; apomorphine, F(3,24) = 3.73, p < 0.05] (Fig. 2, lower panel).
Figure 2.
Effects of the dopamine D2 agonists apomorphine and quinpirole, and the dopamine D1 agonist SKF-38393 in rats trained to discriminate 3 mg/kg of THC from vehicle. Ordinates: overall percentage of responses on the lever associated with THC administration (upper panels) and overall rate of lever pressing expressed as responses per seconds (lower panels) averaged over the entire session. Abscissae: dose in mg/kg (log scale). Results represent means±SEM from 9–10 rats. Repeated measures ANOVA followed by post-hoc Dunnet’s test: * and **: p<0.05 and p<0.01 compared to vehicle. Numbers in parenthesis at higher doses indicate the number of rats that completed at least one fixed ratio during the session over the total number of rats in which the dose was tested.
Dopamine D2 receptor agonists, but not D1 receptor agonists, augment discriminative effects of low doses of THC
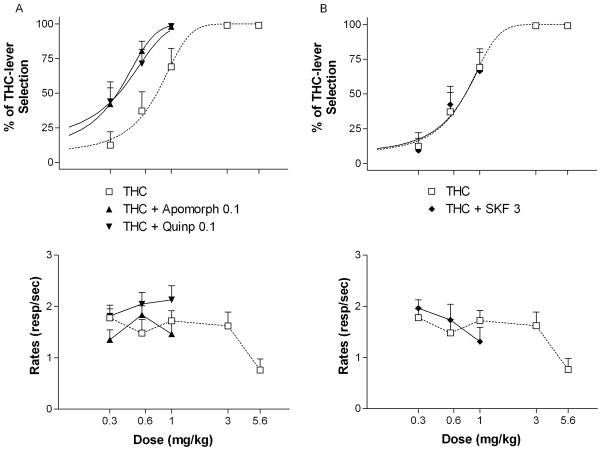
Both apomorphine (0.3 mg/kg) and quinpirole (0.3 mg/kg) significantly shifted to the left (i.e. augmented) the dose-response curve for THC discrimination (Fig. 3A, upper panel) [ED50 = 0.35, 95% confidence intervals = 0.26 to 0.43 for apomorphine and ED50 = 0.36, 95% confidence intervals = 0.21 to 0.50 for quinpirole]. Quinpirole and apomorphine in combination with THC did not significantly alter rates of responding (Fig. 4A, lower panel). In contrast, combinations of 3 mg/kg of SKF-38393 with different doses of THC did not significantly modify the dose-response curve for THC discrimination (Fig. 3B, upper panel) and did not produce changes in rates of responding (Fig. 3B, lower panel).
Figure 3.
Effects of selected doses of apomorphine (apomorph, 0.1 mg/kg), quinpirole (quinp, 0.1 mg/kg) (A), and SKF-38393 (SKF, 3 mg/kg) (B) on the discriminative effects of THC. Ordinates: overall percentage of responses on the lever associated with THC administration (upper panels) and overall rate of lever pressing expressed as responses per seconds (lower panels) averaged over the entire session. Abscissae: dose of THC in mg/kg (log scale). Results represent means±SEM from 10 rats. Repeated measures ANOVA followed by post-hoc Dunnet’s test: *: p<0.05 compared to vehicle. Dose-response curve data for THC are the same as shown in Fig. 1.
Figure 4.
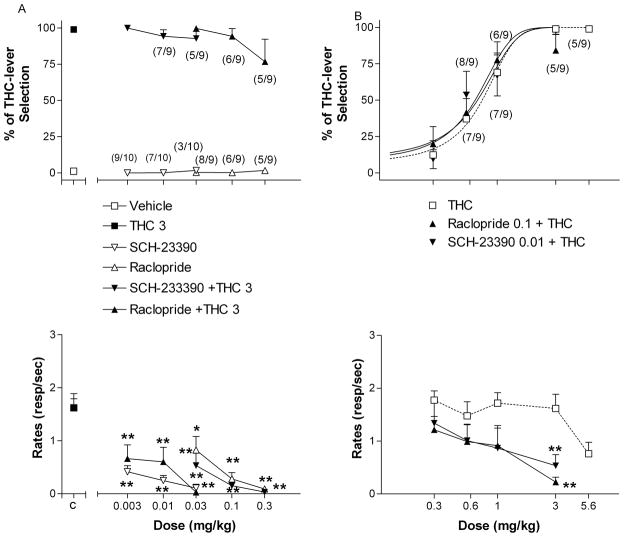
Effects of the dopamine D2 antagonists raclopride and the dopamine D1 antagonist SCH-23390 on the discriminative effects of the 3 mg/kg training dose of THC (A), and effects of selected doses of raclopride (0.1 mg/kg) and SCH-23390 (0.01 mg/kg) on the dose-response curve for THC discrimination (B). Ordinates: overall percentage of responses on the lever associated with THC administration (upper panels) and overall rate of lever pressing expressed as responses per seconds (lower panels) averaged over the entire session. Abscissae: dose in mg/kg (log scale). C = control values for 3 mg/kg THC and vehicle alone. Results represent means±SEM from 9 rats. Repeated measures ANOVA followed by post-hoc Dunnet’s test: **: p<0.01 compared to vehicle. Numbers in parentheses at higher doses indicate the number of rats that completed at least one fixed ratio during the session over the total number of rats in which the dose was tested. Dose-response curve data for THC are the same as shown in Fig. 1.
Effects of dopaminergic antagonists in rats trained to discriminate 3 mg/kg of THC
Subsequently, we investigated the ability of SCH-23390, a selective dopamine D1 receptor antagonist, and raclopride, a selective D2 receptor antagonist, to modulate the discriminative effects of THC. Neither SCH-23390 (0.003–0.03 mg/kg) nor raclopride (0.03–0.3 mg/kg) produced THC-like effects (Fig. 4A, upper panel) but both produced significant decreases in rates of responding (F) (Fig. 4A, lower panel) [F(3,24) = 39.43, p < 0.0001 and [F(3,27) = 13.36, p < 0.0001 for raclopride]. In addition, neither one of these dopaminergic antagonists significantly reduced the discriminative effects of the 3 mg/kg training dose of THC (> 80% of THC-lever selection) (Fig. 4A, upper panel) but rates of responding were significantly decreased by both compounds [F(3,24) = 13.56, p < 0.0001 for SCH-23390 and F(3,21) = 24.13, p < 0.0001 for raclopride] (Fig. 4A, lower panel).
We also tested the effects of SCH-23390 and raclopride on the dose response curve for THC discrimination. Neither SCH-23390 (0.01 mg/kg) nor raclopride (0.1 mg/kg) significantly modified the dose-response curve for THC discrimination (Fig. 4B, upper panel) [ED50 = 0.64, 95% confidence intervals = 0.41 to 0.88 for SCH-23390 and ED50 = 0.56, 95% confidence intervals = 0.33 to 0.79 for raclopride], but they both produced significant decreases in rates of responding [F(4,32) = 2.92, p < 0.05 for SCH-23390 and F(4,32) = 4.20, p < 0.01 for raclopride] (Fig. 4B, lower panel).
Augmentation of THC discrimination by cocaine and amphetamine depends on D2, but not D1, receptor activation
A dose of 0.1 mg/kg of raclopride completely blocked the leftward shift in THC discrimination produced by cocaine (3.0 mg/kg) [ED50 = 0.57, 95% confidence intervals = 0.33 to 0.81] (Fig. 5A, upper panel) or amphetamine (0.3 mg/kg) [ED50 = 0.60, 95% confidence intervals = 0.35 to 0.85] (Fig. 5B, upper panel). In contrast, SCH-23390 (0.01 mg/kg) did not block the cocaine- [ED50 = 0.29, 95% confidence intervals = 0.06 to 0.51] (Fig. 5A, upper panel) or amphetamine-induced augmentation of THC discrimination [ED50 = 0.27, 95% confidence intervals = 0.10 to 0.44] (Fig. 5B, upper panel). Both raclopride and SCH-23390 in combination with cocaine produced a decrease in rates of responding [F(3,27) = 4.48, p < 0.05 for SCH-23390 and F(3,27) = 3.93, p < 0.05 for raclopride] (Fig. 5A, lower panel). In contrast, raclopride and SCH-23390 in combination with amphetamine did not alter rates of responding (Fig. 5B, lower panel).
Figure 5.
Effects of raclopride (Raclo) and SCH-23390 (SCH) on A) cocaine- (Coc), and B) amphetamine-induced (Amph), augmentation of the discriminative effects of THC. Ordinates: overall percentage of responses on the lever associated with THC administration (upper panels) and overall rate of lever pressing expressed as responses per seconds (lower panels) averaged over the entire session. Abscissae: dose in mg/kg (log scale). Results represent means±SEM from 10 rats. Repeated measures ANOVA followed by post-hoc Dunnet’s test: *: p<0.05 and **: p<0.01 compared to vehicle. Numbers in parentheses at higher doses indicate the number of rats that completed at least one fixed ratio during the session over the total number of rats in which the dose was tested. Dose-response curve data for THC are the same as shown in Fig. 1. In addition, dose-response curve data for THC + Coc and THC + Amph are the same as in Fig. 1B.
Quinpirole-induced augmentation of THC discrimination depends on CB1 receptor activation
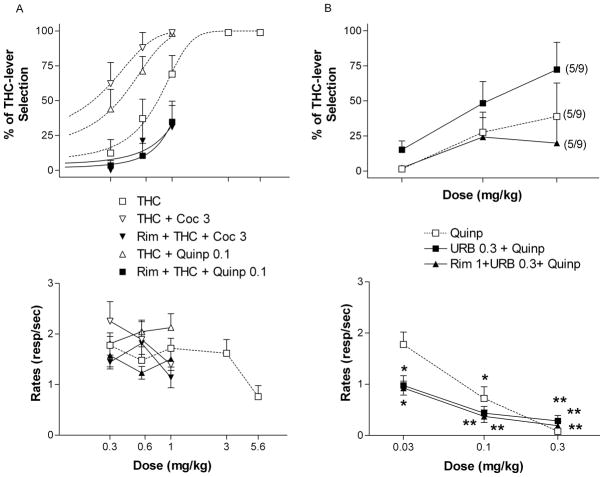
To test whether the effects of dopaminergic agonists were due to release of anandamide induced by stimulation of D2 receptors, we first tried to block quinpirole-mediated augmentation of THC discrimination with the cannabinoid CB1 antagonist rimonabant. A 1 mg/kg dose of rimonabant completely reversed cocaine and quinpirole-induced augmentation of THC discrimination [ED50 = 1.29, 95% confidence intervals = 0.49 to 2.10 for Rim + THC + cocaine and ED50 = 1.18, 95% confidence intervals = 0.78 to 1.57 for Rim + THC + quinpirole] without affecting rates of responding (Fig. 6A). It should be noted that rimonabant not only blocked the augmentation of THC discrimination by the dopaminergic agonists but tended to produce a rightward-shift in the THC discrimination curve, which is consistent with its reported ability to block THC’s discriminative effects (Solinas et al. 2003).
Figure 6.
Effects of the cannabinoid CB1 antagonist rimonabant (Rim) on augmentation of the discriminative effects of THC induced by cocaine (Coc) and quinpirole (Quinp) (A) and effects of a combination of the FAAH inhibitor URB-597 (URB) with quinpirole (Quinp) (B). Ordinates: overall percentage of responses on the lever associated with THC administration (upper panels) and overall rate of lever pressing expressed as responses per seconds (lower panels) averaged over the entire session. Abscissae: dose in mg/kg (log scale). Results represent means±SEM from 10 rats for panel A and 9 rats for panel B. Repeated measures ANOVA followed by post-hoc Dunnet’s test: *: p<0.05 and **: p<0.01 compared to vehicle. Numbers in parentheses at higher doses indicate the number of rats that completed at least one fixed ratio during the session over the total number of rats in which the dose was tested. Dose-response curve data for THC are the same as shown in Fig. 1 and quinpirole data in fig. 6B are the same as shown in fig. 2. In addition, dose-response curve data for THC + Coc and THC + Quinp are the same as in Fig. 1B.
Quinpirole produces significant THC-like effects when metabolic degradation of anandamide is inhibited by URB-597
Then, we tested the hypothesis that in the presence of the FAAH inhibitor URB-597, stimulation of D2 receptors would produce significant THC-like effects. When quinpirole was administered after a 0.3 mg/kg dose of URB-597, it produced significant THC-like effects compared to vehicle injections [F(3,12) = 8.85, p < 0.01] (Fig. 6B). On the other hand, quinpirole plus URB-597 produced no significant THC-like effects when CB1 receptors were blocked by 1 mg/kg of rimonabant, supporting the hypothesis that these effects of quinpirole are mediated by cannabinoid CB1 receptors (Fig. 6B). Combinations of URB-597 and quinpirole and of rimonabant, URB-597 and quinpirole produced significant decreases in rates of responding [F(3,24) = 15.40, p < 0.0001 for URB + Quinp and F(3,24) = 21.74, p < 0.0001 for Rim + URB + Quinp]
DISCUSSION
In this study, we found that dopamine does not mediate the discriminative effects of THC but does positively modulate the effects of low doses of THC. These results indicate that dopamine and cannabinoid systems can interact to enhance the psychotropic effects of cannabinoids. However, dopaminergic modulation was somewhat asymmetric, because dopaminergic agonists augmented, but dopaminergic antagonists did not antagonize, the discriminative effects of THC. This suggests that THC-induced dopamine release does not account for the psychotropic effects of THC studied with drug-discrimination procedures, and is not the mechanism underlying the observed augmentation. Instead, release of anandamide induced by stimulation of dopamine D2-like receptors appears to be, at least in part, responsible for the augmentation of the discriminative effects of THC by dopaminergic agonists.
Neither the direct dopaminergic agonists SKF-38393, apomorphine or quinpirole, nor the indirect agonists cocaine and amphetamine, produced significant THC-like discriminative effects. These results agree with previous findings in rats that amphetamine does not produce cannabinoid CB1-like discriminative effects (Browne and Weissman 1981; Jarbe et al. 2009). In addition, the indirect dopaminergic agonist methylphenidate does not lead to THC-lever selection in humans (Lile et al. 2009). It should be noted that a low level of generalization to the cannabinoid agonist AM1346 was found by Jarbe and colleagues with a high 3 mg/kg dose of amphetamine that produced significant disruption of operant behavior (Jarbe et al. 2009). We did not test this high dose of amphetamine because of the appearance of significant depression of baseline food-reinforced behavior at a lower 1.8 mg/kg dose of amphetamine. This effect of a high dose of amphetamine resembles the effects of the dopamine D2 agonists, quinpirole and apomorphine, which tended to produce THC-like discriminative effects at high doses that also produced significant disruption of food-reinforced behavior. It could be argued that quinpirole and apomorphine produced some THC-like effects by non-specifically disrupting stimulus control. However, THC effects are rather specific to stimulation of CB1 receptors and do not appear to be mediated by general psychological states associated with intoxication (Wiley 1999). In fact, the effects of dopamine D2 agonists in combination with URB-597 were reversed by rimonabant suggesting that this drug combination produced THC-like effects mediated by activation of CB1 receptors.
Consistent with a previous finding that haloperidol does not block the discriminative effects of THC (Browne and Weissman 1981), we also found that dopaminergic antagonists did not block the discriminative effects of THC. This is also consistent with a recent report showing that in humans the dopamine antagonist haloperidol worsens THC-induced cognitive deficits but does not alter THC subjective effects (D’Souza et al. 2008). Since high doses of dopamine receptor antagonists could not be tested for effects on THC discrimination because they almost completely eliminated baseline food-reinforced behavior, it could be argued that the lack of effect of the dopaminergic antagonists in this study was due to the use of doses that were too low. However, it is unlikely that the doses of dopamine antagonists tested were not high enough, because the discriminative effects of cocaine and amphetamine are blocked by doses of SCH-23390 and raclopride similar to those used in this study (Costanza et al. 2001; Filip and Przegalinski 1997). Furthermore, doses of raclopride that failed to shift the dose response curve for THC completely reversed the augmentation of THC’s discriminative effects produced by cocaine and amphetamine. Thus, THC-induced increases in brain dopamine levels (Chen et al. 1991; Tanda et al. 1997) and/or stimulation of dopamine receptors do not appear to be the primary mechanisms underlying the discriminative effects of THC.
In contrast to these negative findings with dopaminergic antagonists, we found that both direct stimulation of dopamine D2 receptors, or indirect stimulation of D2 receptors by drugs such as cocaine and amphetamine which increase extra-cellular levels of dopamine, augmented the discriminative effects of low doses of THC, suggesting synergistic or additive effects between cannabinoid and dopaminergic activation. Although both cocaine and amphetamine non-selectively increase extra-cellular levels of several monoamines, the effects of these drugs on THC discrimination appeared to be mediated mostly by dopamine because 1) their effects were reversed by dopamine D2 antagonists and 2) because selective inhibitors of noradrenaline (desipramine) and serotonin (fluoxetine) transporters do not augment THC discrimination under similar conditions (Solinas et al. unpublished results). Facilitatory interactions between cannabinoid and dopamine systems would be consistent with the finding that self-administration of cocaine under progressive ratio schedules is reduced in mice genetically engineered to lack CB1 receptors (Soria et al. 2005) and that self-administration of methamphetamine is increased by administration of anandamide, and its synthetic analogue methandandamide (Vinklerova et al. 2002). However, a number of studies have found no evidence for positive cannabinoid-dopamine interactions, some studies have found antagonistic interactions between cannabinoids and dopamine (Solinas et al. 2008; van der Stelt and Di Marzo 2003), and a recent study found that cannabinoid CB1 and dopamine D2 receptors form heterodimers with reciprocal antagonistic interactions (Marcellino et al. 2008). These discrepancies highlight the fact that different behavioral effects can be mediated differently. Moreover dopamine-induced release of endocannabinoids, cannabinoid-induced release of dopamine, and direct interactions between dopamine and cannabinoid signaling pathways, can together function as a mechanism for fine tuning of brain activity and behavioral output (Solinas et al. 2008).
The asymmetric modulation of THC’s discriminative effects by dopaminergic compounds is different from the modulation of THC’s discriminative effects by opioid compounds (Solinas and Goldberg 2005; Solinas et al. 2004) but is similar to the modulation by acetylcholinergic nicotinic and muscarinic compounds (Solinas et al. 2007b). In fact, the dopaminergic augmentation of THC discrimination in the present study appears to be mediated, at least in part, by a dopamine D2-induced formation of endogenous anandamide, since shifts to the left of THC dose-response curves by cocaine or quinpirole were blocked by the CB1 receptor antagonist rimonabant and quinpirole only produced THC-like discriminative effects when anandamide degradation was blocked by URB-597. This proposed mechanism is put forward based only on behavioral experiments and further studies will be needed to provide definitive evidence. However, it should be noted that our hypothesis is supported by previous findings that activation of dopamine D2, but not D1, receptors increases anandamide levels in the nucleus accumbens (Giuffrida et al. 1999). In addition, a dopamine D2-induced increase in anandamide levels could explain why cocaine-induced reinstatement of drug-seeking behavior is blocked by rimonabant (De Vries et al. 2001).
Inhibitors of FAAH enzymes have recently been proposed as novel medications for several psychiatric diseases such as anxiety, depression and cannabis dependence (Clapper et al. 2009). The fact that, when FAAH enzymes were inhibited by URB-597, dopamine agonists produced THC-like effects indicates that some of the effects of psychostimulants could be increased by inhibitors of FAAH. However, it is not yet clear whether FAAH inhibition would increase, decrease or have little effect on the abuse liability of psychostimulants. In the case of nicotine, although URB-597 increased the ability of nicotine to produce THC-like discriminative effects (Solinas et al. 2007b), it decreased nicotine abuse-related behavioral and neurochemical effects in rats (Scherma et al. 2008), suggesting potential utility in treating tobacco dependence. Further studies are needed to investigate conditions under which FAAH inhibitors might reduce abuse-related effects of cocaine and amphetamine. However, in self-administration studies with squirrel monkeys, URB-597 neither augmented nor attenuated the reinforcing effects of cocaine (Justinova et al. 2008).
In conclusion, we found that dopamine does not mediate, but can modulate the discriminative effects of THC. Increased anandamide signaling may play a role in modulating the discriminative effects of THC, as well as the discriminative and reinforcing effects of other psychotropic drugs such as cocaine, amphetamine and nicotine.
Acknowledgments
This research was supported by the Centre National de la Recherche Scientifique (CNRS) and the University of Poitiers, France, and the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, Maryland, USA.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–30. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981;21:227S–234S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology. 2004;29:1488–97. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL. Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: an in vivo microdialysis study. Neurosci Lett. 1991;129:136–80. doi: 10.1016/0304-3940(91)90739-g. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology 56 Suppl. 2009;1:235–43. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza RM, Barber DJ, Terry P. Antagonism of the discriminative stimulus effects of cocaine at two training doses by dopamine D2-like receptor antagonists. Psychopharmacology (Berl) 2001;158:146–53. doi: 10.1007/s002130100872. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2008;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998;10:2825–30. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Przegalinski E. The role of dopamine receptor subtypes in the discriminative stimulus effects of amphetamine and cocaine in rats. Pol J Pharmacol. 1997;49:21–30. [PubMed] [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–62. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–52. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–33. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–63. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–5. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Li C, Liu Q, Makriyannis A. Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide analog. Psychopharmacology (Berl) 2009;203:229–39. doi: 10.1007/s00213-008-1199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–7. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Delta9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2009;203:241–50. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, Tanganelli S, Muller CE, Fisone G, Lluis C, Agnati LF, Franco R, Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–23. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, Justinova Z, Mikics E, Haller J, Medalie J, Stroik J, Barnes C, Yasar S, Tanda G, Piomelli D, Fratta W, Goldberg SR. Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008;327:482–90. doi: 10.1124/jpet.108.142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealfon SC, Olanow CW. Dopamine receptors: from structure to behavior. Trends Neurosci. 2000;23:S34–40. doi: 10.1016/s1471-1931(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 47 Suppl. 2004;1:242–55. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Involvement of mu-, delta- and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology (Berl) 2005;179:804–12. doi: 10.1007/s00213-004-2118-x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–83. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006a;98:408–19. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006b;1:1194–206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007a;27:5615–20. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Tanda G, Wertheim CE, Fratta W, Goldberg SR. Nicotinic facilitation of delta9-tetrahydrocannabinol discrimination involves endogenous anandamide. J Pharmacol Exp Ther. 2007b;321:1127–34. doi: 10.1124/jpet.106.116830. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007c;321:370–80. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007d;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9-tetrahydrocannabinol. Eur J Neurosci. 2004;19:3183–92. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–80. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–34. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–50. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Vinklerova J, Novakova J, Sulcova A. Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. J Psychopharmacol. 2002;16:139–43. doi: 10.1177/026988110201600204. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–60. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–7. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]