Abstract
Experimentally-induced hyperglycemia by prolonged glucose infusion allows investigation of the effects of sustained stimulation of the pancreatic β-cell on insulin secretion and sensitivity. Hormonal responses to a meal following prolonged glucose infusions have not been investigated. To determine if a 48-h glucose infusion alters hormonal responses to a test meal as well as food intake and hunger in normal weight individuals, 16 subjects (8 men, 8 women, age 18–30 y, mean BMI=21.7±1.6 kg/m2) were infused for 48-h with either saline (50 ml/h) or 15% glucose (200 mg/m2/min). Subjects ingested a 600 kcal mixed nutrient meal 3-h after infusion termination. Blood samples were taken during the 48-h and for 4 hours following food ingestion. The 48-h glucose infusion elicited a metabolic profile of a glucose intolerant obese subjects, with increased plasma glucose, insulin and leptin (all P<0.01) and increased HOMA-IR (P<0.001). During meal ingestion, early insulin secretion was increased (P<0.05) but postprandial glucose (P<0.01) and insulin (P<0.01) excursions were lower following the glucose infusion. Postprandial plasma triglyceride concentrations were increased after glucose compared with saline. Food intake and hunger ratings were not different between the two conditions. Plasma leptin levels were inversely correlated with hunger (P<0.03) in both conditions and with food intake (P<0.003) during the glucose condition only. Thus, a 48-h glucose infusion does not impair postprandial hormonal responses, alter food intake or hunger in normal weight subjects. The glucose-induced increases in plasma leptin result in a stronger inverse relationship between plasma leptin and hunger as well as food intake. These data are the first to demonstrate a relationship between leptin and hunger in normal weight, non-calorically restricted human subjects.
Keywords: leptin, insulin, food intake, insulin sensitivity, triglycerides
INTRODUCTION
Experimentally-induced hyperglycemia has been used as a research tool to investigate the effects of chronically elevated blood glucose levels on insulin secretion and sensitivity. While in vitro studies suggest that sustained high levels of glucose result in glucose toxicity and impaired insulin secretion 1, in vivo studies involving 24-h to 48-h glucose infusions indicate that prolonged glucose exposure can lead to hyperinsulinemia 2;3 and in some cases, increased insulin sensitivity 4. Discrepancies in results are most likely due to the release of neurotransmitters and peptides which may influence insulin secretion during the in vivo experiments 5 but would not be present in the in vitro experiments. Neural factors contributing to an increase in insulin secretion after prolonged glucose exposure have been implicated in both animal 6;7 and human studies 8 but the effect of the elevated glucose levels on other hormones that could potentially influence insulin sensitivity has not been investigated.
An additional factor contributing to differential responses between the in vitro and in vivo experiments is the glucose concentrations to which the β-cell is exposed as glucose levels are typically lower in the in vivo studies. Glucose levels differ even more substantially between animal and human experiments. For example, while in animals, plasma glucose concentrations can be increased to within the diabetic range (> 300 mg/dl) following prolonged glucose infusion 6;7, in humans, due to limitations in the rates and concentrations of the infusates, plasma glucose levels are only marginally elevated, typically within 100–120 mg/dl 8. In non-diabetic, normal weight subjects, plasma glucose levels are maintained within this marginally elevated level by compensatory insulin release. Therefore, the glucose infusion paradigm in humans is not necessarily representative of a diabetic metabolic profile but instead resembles that of obese, glucose intolerant individuals with mild hyperglycemia and significant hyperinsulinemia.
To date, studies examining the effects of 48-h glucose infusions have focused primarily on insulin responses to standardized intravenous tests for assessing insulin secretion and sensitivity using either the frequently sampled intravenous glucose tolerance test 4;8 or a graded glucose infusion 3. However, the consequences of prolonged glucose infusion on subsequent hormonal responses to a physiologically-relevant stimulus such as a mixed nutrient meal have not been evaluated. Furthermore, little data are available regarding the effects of consistently elevated glucose and insulin levels on other hormones and metabolic fuels that may contribute to glucose homeostasis and insulin sensitivity. In the present experiment, we have evaluated the effect of a 48-h glucose infusion on hormonal and metabolic responses during the infusion as well as responses to a mixed nutrient meal at the end of the infusion in healthy normal weight subjects. In addition, as previous studies suggest that elevated levels of glucose reduce food intake 9, we measured ad libitum food intake and hunger/appetite ratings during the 48-h infusion period to determine if normal, healthy individuals exhibit compensation for the large increase in available calories during the glucose infusion. These data provide interesting insights into the consequences of prolonged mild elevations in plasma glucose and insulin such as may occur during overfeeding conditions on food intake, hunger and post-prandial responses to a test meal. The effects of the prolonged 48-h glucose infusion on the plasma insulin and glucose levels during the 48-h period have been previously reported but all other data presented in this paper are new 10.
METHODS
Subjects
Sixteen lean subjects (8 males and 8 females) 18–30 years of age (mean 22.3±3.7 yr) with body mass indexes (BMI) ranging from 18.1 to 24 kg/m2 (mean 21.7±1.6 kg/m2) and a percent body fat of 16.9±5.4 % (range 7–18 % for men and 16–26 % for women) as determined by bioelectrical impedance, participated in this study. Subjects were recruited through newspaper advertisements and each subject provided written informed consent before entering the study. Two women entered the study but then dropped out and their data are not included in the data set. After a telephone interview to assess eligibility, subjects underwent a physical examination, including an electrocardiogram and a medical history to ensure they had no chronic illnesses including: diabetes, hypertension or abnormal heart rhythms. Smokers, subjects taking prescription medications and subjects with a family history of diabetes or hypertension were also excluded from the study. A blood sample was drawn after an overnight fast to evaluate clinical blood chemistries and subjects were permitted to participate if fasting plasma glucose was < 110 mg/dl), hemoglobin levels > 12 mg/dl, and blood pressure was < 140/90 mmHg. In order to control for the effect of the menstrual cycle, all experimental testing in women took place within the follicular phase of their menstrual cycle. These studies were approved by the Institutional Review Board of the University of Pennsylvania.
Experimental Protocol
Each subject underwent two experimental conditions administered in a counter-balanced order over a 2-month period. Each experimental condition involved a 3-night stay in the hospital and either a 48-hour glucose infusion or a 48-hour saline infusion. Three hours after termination of the saline and glucose infusions, the subjects ingested a standardized a test meal.
On the evening before each experimental stay, the subjects arrived at the General Clinical Research Center (GCRC) at the Hospital of the University of Pennsylvania at 5:00 p.m. Subjects were given dinner at 6:00 p.m. and a snack at 8:00 p.m., after which they remained fasted until the following morning. During their stay in the GCRC, the subjects were permitted to eat three meals and a snack per day (ad libitum) but the meals were at fixed time and they were only allowed to consume food items prepared by the GCRC kitchen and selected from a pre-set menu. Subjects were permitted to walk around the GCRC but not allowed to leave the unit.
At 7:00 a.m., following an overnight fast, two intravenous (i.v.) catheters were inserted in opposite arms, and an intravenous infusion of either saline (0.9 % saline at a rate of 50 ml/hour) or glucose (15 % dextrose solution at a rate of 200 mg/m2/min) was initiated and sustained for a 48-h period. One i.v. line was used for infusion and the other for blood sampling. Glucose infusions were supplemented with 8 meq/l of KPh to prevent hyperinsulinemia-induced hypokalemia. Blood was collected in tubes containing EDTA, and the samples were kept on ice for not longer than 1 h. Samples were then centrifuged and stored at −80°C for later assay.
Food Intake, Hunger Ratings and Taste Measurements
To determine if the glucose infusion altered sweet taste perception, a taste test was conducted after 24-h of glucose infusion prior to breakfast. This time was selected as to not to interfere with measurements of hunger ratings and of insulin responses to meal ingestion conducted on the second day of the glucose or saline infusions. Subjects were asked to taste 6 different concentrations of sucrose solutions (0.4%, 0.85, 1.6%, 4.0%, 8.0% and 16%) by keeping the solution briefly in their mouth and then expectorating it. They were then asked to rate them for sweet intensity and likeness, each on a nine point scale.
Patients admitted to the GCRC are permitted to select foods from a rotating menu with numerous meals offered. Many of these meals are composed of mixed nutrients within a single meal such as stew, lasagna etc and if it is often difficult to ascertain precise macronutrient intake from these meals. For example, subjects may pick out certain items within a mixed nutrient meal. Therefore, in order to be able to accurately determine food intake during the 48-h infusion periods, a specific menu was developed. The menu, illustrated in Table 1, offered a large variety of single foods for breakfast, lunch, dinner and snack. Subjects were told they could select as few or as many foods as they wished and that they could request more than one portion of each food. Unknown to the subjects, all foods were weighed prior to presentation of the meals and then following food intake. Macronutrient composition of the individual foods was obtained either from the manufacturer or from the Processor nutrient database made by ESHA, version 8.22. Hunger was determined using a visual analogue scale ranging from 0–9 asking the following question “How hungry do you feel right now?”
Table 1.
Foods offered for ad libitum diet during 48-h infusion periods
| Breakfast | Lunch | Dinner | Snacks |
|---|---|---|---|
| Eggs | Grilled cheese sandwich (wheat or white) | Baked chicken | Apple |
| Cream of wheat | Macaroni and cheese | Baked fish | Orange |
| Oatmeal (various flavors) | Veggie Burger | Grilled cheese | Bananas |
| Cereal (cold, various brands) | Turkey and/or ham sandwich | Macaroni -cheese | Grapes |
| Bacon | Side salad with choice of dressing | Veggie Burger | Ice cream (various flavors) |
| Turkey Bacon | Steamed rice | Hamburger | Jello |
| Toast (white, wheat) | Green beans | Turkey/Ham sandwich | Pudding (various flavors) |
| English muffin | Mashed potatoes | Side salad | Pound cake |
| Bagel | Carrots | Steamed rice | Milk (skim, whole, 2%) |
| Apple | Kaiser roll | Green beans | Juice (various) |
| Orange | Bread (wheat or white) | Mashed potatoes | Spring water |
| Bananas | Apple | Carrots | Decaffeinated Coffee/Tea |
| Grapes | Orange | Kaiser roll | Cream cheese |
| Milk (skim, whole, 2%) | Bananas | Bread (wheat or white) | Butter |
| Juice (various) | Grapes | Ice cream (various flavors) | Margarine |
| Spring water | Ice cream (various flavors) | Jello | Ketchup |
| Decaffeinated Coffee/Tea | Jello | Pudding (various flavors) | Jelly |
| Cream cheese | Pudding (various flavors) | Pound cake | Salt |
| Butter | Pound cake | Milk (skim, whole, 2%) | Pepper |
| Margarine | Milk (skim, whole, 2%) | Juice (various) | Mustard |
| Ketchup | Juice (various) | Spring water | Mayonnaise |
| Jelly | Spring water | Decaffeinated Coffee/Tea | |
| Salt | Decaffeinated Coffee/Tea | Cream cheese | |
| Pepper | Cream cheese | Butter | |
| Mustard | Butter, Margarine | Margarine | |
| Mayonnaise | Ketchup | Ketchup | |
| Jelly | Jelly | ||
| Salt, pepper | Salt, pepper | ||
| Mustard | Mustard | ||
| Mayonnaise | Mayonnaise |
Meal Challenge
Three hours after the termination of the glucose or saline infusions and following a 15 min period of baseline sampling, subjects were given a standardized mixed nutrient meal to evaluate the effect of prolonged glucose infusion on hormonal and metabolic responses to a physiological stimulus. A three hour delay prior to the meal challenged was used to avoid the effects of the glucose infusion on pancreatic β-cell “memory” and this length of time has been previously identified as adequate 11. Subjects were allotted 15 minutes to consume the meal. The energy content of the meal was 600 kcal and was composed of typical breakfast items including a grilled cheese sandwich, corn flakes with milk and sugar and orange juice. The macronutrient content was 15% protein, 60% carbohydrate and 25% fat. This diet has been used in previous studies 12 and was found to be acceptable to men and women in terms of both palatability and quantity.
To obtain arterialized venous blood, an intravenous line was inserted into a dorsal hand vein in a retrograde manner and the hand heated in a thermoregulated box. The catheter was kept patent by a slow infusion of saline. Subjects sat quietly for 30 min to acclimatize to the insertion of the catheters. Starting at 10:00, blood samples were taken at the following time points, −15, −10, −5, 0, 2, 4,6, 8, 10, 12, 14, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 195, 210, 225, 240 post-meal ingestion. The meal was given at time =0. Each blood collection involved a 4 ml sample collected into a Vacutainer containing EDTA and a 3 ml sample collected into a Vacutainer containing heparin for norepinephrine and epinephrine analysis. Samples for catecholamine analysis were centrifuged and the plasma mixed in a 96:4 ratio of plasma to 5N perchloric acid. The samples were then re-centrifuged and the supernatant removed and frozen at −80 C. Samples for hormonal analysis were centrifuged and the plasma was separated and stored in aliquots at −80 C within one hour of collection.
Biochemical Analysis
Plasma immunoreactive insulin, cortisol and pancreatic polypeptide were measured in duplicate by double-antibody radioimmunoassays. The antibodies were purchased from Linco Research Inc., MO. The insulin antibody used is the human-specific antibody from Linco with no crossreactivity (< 0.2%) to human proinsulin or the primary circulating split form. Analysis of insulin and leptin were performed by the Diabetes Research Center of the University of Pennsylvania. The inter-assay coefficient of variation for insulin is 8% and the intra-assay variation is 6%. Plasma glucose was analyzed with a YSI automated glucose/lactate monitor and free fatty acids and triglycerides were determined by enzymatic assay using the WAKO kit at the Monell Chemical Senses Center, Philadelphia PA. Adiponectin was measured in the laboratory of Dr. Peter Havel, UC Davis with a radioimmunoassay kit specific for human adiponectin (HADP-61HK; Linco Research, St. Charles, MO) after 1:500 dilution. Samples were analyzed in two batches, with an intra-assay coefficient of variation (CV) 1.8–6.2% and interassay CV 6.9–9.3% for duplicates over a range of adiponectin concentrations.
Statistical Analysis
To determine differences between variables during the 48-h infusion period, paired T-tests were conducted on mean levels over the 48-h period. To determine significant differences during the meal challenge, two approaches were used: 1) multiple analysis of variance (MANOVA) was conducted to determine if there were significant time and treatment interactions over the 240 minute post-prandial period and 2) areas under the curve (AUC) were determined by calculating AUCs over baseline. This was done by subtracting the mean baseline value (first four blood samples) from the amount of hormone or metabolite released at each time point. Early (cephalic) phase insulin release was determined as insulin released between 0 and 10 min after the beginning of the meal ingestion. Post-prandial insulin AUCs were calculated from 10–240 min post-ingestion for a total of 230 min. These data were then plotted and the area under the curve calculated by a point-to-point method using computer software (Origin, Northhampton, MA). Using the areas, paired T-tests were performed to determine if there were statistically significant differences between treatments. Statistical significance was P<0.05.
RESULTS
Plasma substrate and hormone concentrations during 48-h infusions of glucose or saline
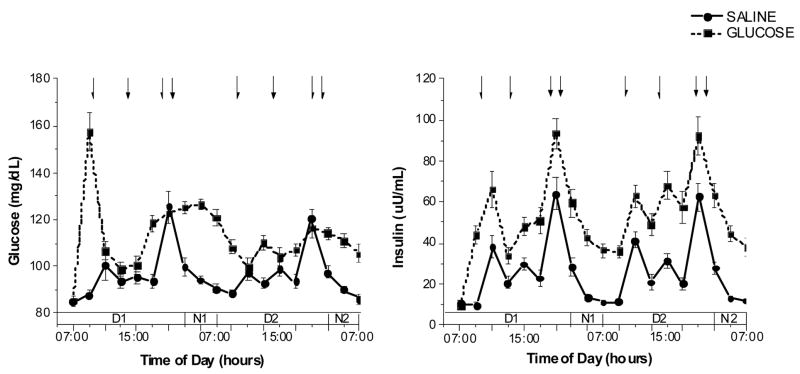
The infusion of glucose initially resulted in a transient marked increase of plasma glucose concentrations to a mean 160 mg/dl. Thereafter, plasma glucose declined and was increased by approximately 16% over the 48-h period compared with saline infusion. Mean glucose levels over 48-h were 96.3±6.8 mg/dL during the saline infusion compared with 112.6±6.7 mg/dL during the glucose infusion (P<0.0001; Fig. 1, left hand graph). These relatively modest increases in plasma glucose are in the range of those observed in subjects with impaired fasting glucose and were achieved by the sustained increases in plasma insulin. Plasma insulin levels were increased two-fold during the glucose infusion to an average of 52.0±13.9 uU/mL compared with during 25.8±7.3 uU/mL saline infusion (P<0.0001; Fig. 1, right hand graph). The insulin and glucose data during the 48-h period have been reported in a previous publication on heart rate variability 10 but all other data in this paper have not been previously published.
Figure 1.
Fig. 1, A. (left graph): Plasma glucose levels (mean ± standard error, n=16) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Arrows indicate time of meals (09:15, 13:15, 18:15hr) and evening snack (20:15 hr). Treatment × time interaction: F(18,252)=14.1, P<0.001
Fig. 1, B. (right graph): Plasma insulin levels (mean ± standard error, n=16) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Arrows indicate time of meals (09:15, 13:15, 18:15hr) and evening snack (20:15 hr). Treatment × time interaction: F(18,198)=2.1, P<0.01
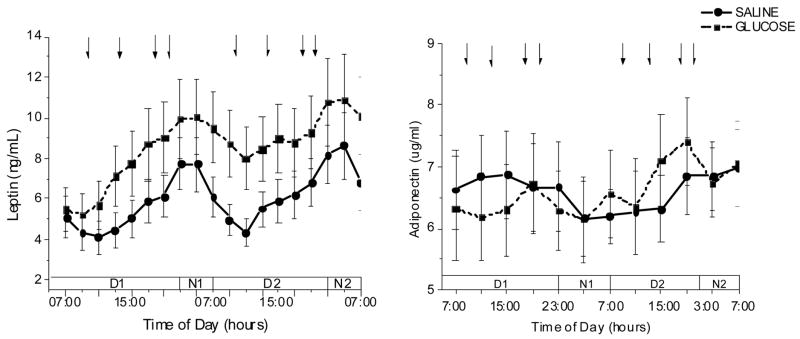
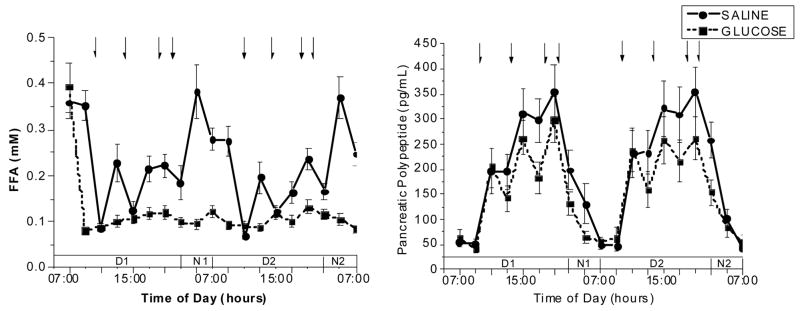
A progressive increase of plasma leptin concentrations was observed following the glucose infusion compared with saline with a mean increase of 31% of the 48-h infusion period (5.9±4.2 ng/ml, saline vs. 8.5±6.5 ng/ml, glucose, P<0.002; Fig. 2, left hand graph. Plasma adiponectin concentrations were not different over the course of the glucose infusion compared with the saline infusion (glucose; 6.6±1.9, saline; 6.6±2.2 ug/ml, glucose) (Figure 2, right hand graph). In contrast, the sustained increases in plasma insulin resulted in a complete suppression of free fatty acids during the 48-h glucose infusion period (0.12±0.03 mM glucose) compared to the saline infusion (0.23±0.06 mM, saline vs., P< 0.00003; Fig. 3, left hand graph). Intravenous glucose infusion has been demonstrated to acutely decrease circulating pancreatic polypeptide (PP) concentrations 13. In the present experiment, although PP does increase as expected in response to meal ingestion under both study conditions, mean plasma PP levels were significantly lower over the course of the glucose compared with the saline infusion (glucose; 196.4±103.1, saline, vs. 156.4±82.1 glucose, P<0.002, Figure 3, right hand graph).
Figure 2.
Fig. 2, A. (left graph): Plasma leptin levels (mean ± standard error, n=16) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Arrows indicate time of meals (09:15, 13:15, 18:15hr) and evening snack (20:15 hr). Treatment × time interaction: F(18,216)=5.1, P<0.001
Fig. 2, B. (right graph): Plasma adiponectin levels (mean ± standard error, n=10) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Arrows indicate time of meals (09:15, 13:15, 18:15hr) and evening snack (20:15 hr). Treatment × time interaction: F(18,99)=1.9, P<0.05
Figure 3.
Fig. 3, A. (left graph): Plasma free fatty acids levels (mean ± standard error, n=16) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Arrows indicate time of meals (09:15, 13:15, 18:15hr) and evening snack (20:15 hr). Treatment × time interaction: F(18,234)=9.2, P<0.001
Fig. 3, B. (right graph): Plasma pancreatic polypeptide levels (mean ± standard error, n=16) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Arrows indicate time of meals (09:15, 13:15, 18:15hr) and evening snack (20:15 hr). Treatment × time interaction: F(18,144)=2.5, P<0.001
Effect of prior 48-h glucose and saline infusions on circulating substrate and hormone responses to ingestion of a standard meal
Three hours after the termination of the glucose infusion plasma glucose concentrations had returned to baseline (83.8±7.2 mg/dL, glucose vs. 81.8±7.4 mg/dL, saline). Plasma insulin levels remained moderately elevated but within the normal range following the glucose infusion (14.6±4.6 uU/mL, glucose vs. 9.4±2.6 uU/mL, saline, P<0.0001). HOMA-IR (homeostatic model assessment-insulin resistance 14) values calculated from the fasting levels of glucose and insulin ((FPI (mU/ml)*FPG (mmol/l))/22.5) indicated a significant increase in insulin resistance as HOMA-IR values increased from 1.9+0.6 three hours after termination of the saline infusion and 3.0+0.9 three hours after the glucose infusion (P<0.0001).
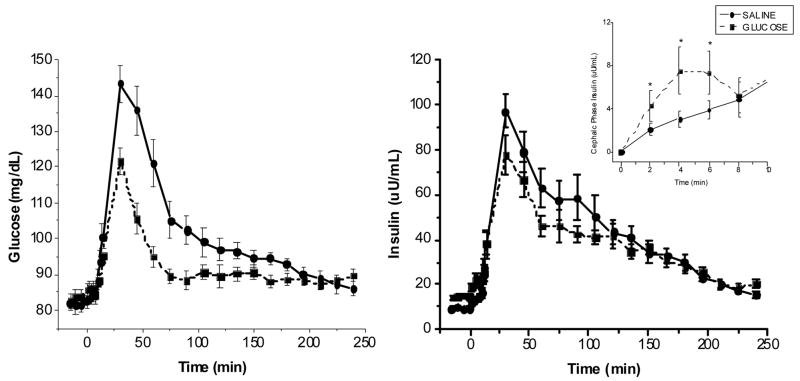
During the meal challenge, plasma glucose excursions (Figure 4 left hand graph) were significantly decreased following the glucose infusion compared to the saline infusion (treatment × time interaction, F(25, 50)=3.8, P<0.001). No treatment or treatment by time effects were found for plasma insulin (Figure 4, right hand graph). However, when the areas under the curve (AUCs) for the 4 hours after the meal were calculated, significant differences in glucose and insulin were found. The post-prandial AUC for plasma glucose was significantly decreased following the 48-glucose infusion compared to the saline condition (1922±1432 mg/dl/230min, glucose vs. 4776± 2141 mg/dl/230 min saline, P< 0.0001) as were the insulin AUCs (5539± 2204 uU/ml/230min, glucose vs. 8084±4305 uU/ml/230min, saline, P< 0.004) (Table 2). In addition, the AUCs were determined for the pre-absorptive early phase period (0–10 min). Early phase insulin (Inset Figure 4) was increased following the glucose infusion (52.4±45.9 uU/ml/10 min) compared to saline (34.2±26.8 uU/ml/10min, P<0.05) despite no significant differences in early phase AUC for glucose between the two conditions (Table 2).
Figure 4.
Fig. 4, A. (left graph): Plasma glucose levels (mean ± standard error, n=16) following the ingestion of a standardized mixed nutrient meal, 3 hours after the termination of the infusion in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Treatment × time interaction: F(25,150)=3.8, P<0.001
Fig. 4, B. (right graph): Plasma insulin levels (mean ± standard error, n=16) following the ingestion of a standardized mixed nutrient meal, 3 hours after the termination of the infusion in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Treatment × time interaction: F(25,125)=1.2, P=0.22 Inset: Early phase insulin response (0–10 min post ingestion) difference from mean baseline values (mean ± standard error, n=16) following the ingestion of a standardized mixed nutrient meal, 3 hours after the termination of the infusion in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Treatment effect F (1,11)=P<0.001, post-hoc analysis, *P<0.05.
Table 2.
Area under the curve (AUC; mean ± standard deviation, n=16) for the early phase (0–10 min) and post-prandial phase (10–240 min) during the meal challenge test following the 48-h glucose or saline infusion.
| 48-h Saline Infusion | 48-h Glucose Infusion | |||
|---|---|---|---|---|
| Early Phase (0–10 min) | Post-prandial (10–240 min) | Early Phase (0–10 min) | Post-prandial (10–240 min) | |
| AUC Glucose (mg/dl/time) | 25.3±25.5 | 4776±2141 | 14.1±22.9 | 2085±1342* |
| AUC Insulin (uU/ml/time) | 34.2±26.8 | 8084±4305 | 52.4±45.9* | 5539±2204* |
| AUC Pancreatic Polypeptide (pg/ml/time) | 2119±1110 | 42878±23088 | 1763±1189 | 46045±32016 |
| AUC Triglycerides (mg/dl) | 29.9±10.2 | 1100.9±557.8 | 25.2±11.9 | 6553.9±1212.3* |
P<0.05, significantly different from 48-h saline infusion condition.
Plasma triglycerides were measured in a subset of subjects (n=7) during the meal challenge (Table 3). Significant elevations in post-prandial triglyceride levels were observed following the glucose infusion compared to the saline (6554±3207 mg/dl/230min glucose vs. 1101±1476 mg/dl/230min saline, P<0.02). Interestingly, plasma free fatty acid concentrations remained suppressed three hours after termination of the glucose infusion (data not shown). No significant differences between glucose and saline treatments in the calculated AUCs for pancreatic polypeptide (Table 2), norepinephrine or epinephrine (data not shown) were observed.
Table 3.
Intensity and hedonic ratings of varying concentrations of sucrose solutions following 24-h of saline and glucose infusions (mean ± standard error).
| Percent Sucrose Solution | Intensity Rating | Hedonic Rating | ||
|---|---|---|---|---|
| Saline Infusion | Glucose Infusion | Saline Infusion | Glucose Solution | |
| 0.4 | 1.5±0.2 | 1.8±0.3 | 3.4±0.5 | 3.9±0.6 |
| 0.8 | 1.9±0.2 | 2.1±0.2 | 3.4±0.6 | 3.5±0.6 |
| 1.6 | 3.0±0.4 | 2.9±0.3 | 3.0±0.5 | 4.0±0.5 |
| 4.0 | 4.5±0.4 | 5.1±0.4 | 3.9±0.5 | 4.1±0.4 |
| 8.0 | 6.7±0.3 | 6.9±0.4 | 4.9±0.5 | 5.1±0.5 |
| 16 | 7.4±0.4 | 7.3±0.4 | 4.9±0.6 | 5.0±0.6 |
Ad libitum food intake, hunger ratings and sweet taste perceptions during 48-h infusions of glucose or saline
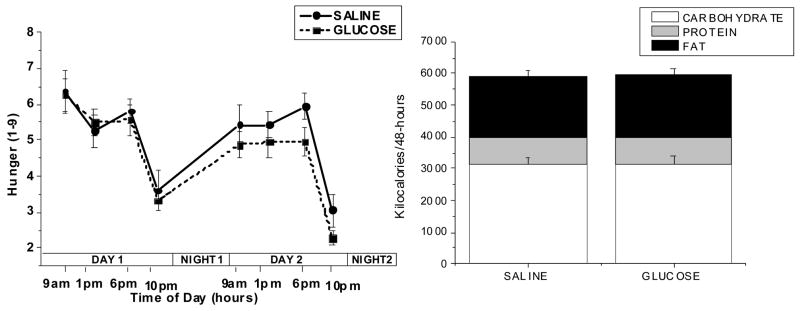
The mean number of kilocalories supplied by the glucose infused over each 24-h period was 2021±216 kcal. The large number of calories infused as glucose (>4000 over 48 hours) had no effect on the subject’s self-reported hunger over the 48-h period (Figure 5 left hand graph) although a trend towards a decrease was evident by the end of the 48-h glucose infusion. No significant differences were found between the mean caloric intake during the 48-h glucose infusion compared to the saline infusion periods (5773±484 kcal/48-h, saline vs 5840±491 kcal/48-h, glucose) (Figure 5 right hand graph). Similarly, no significant differences were found between the individual macronutrients selected on the days of glucose infusion compared to saline infusion: protein (206±19 g/48-h, saline vs. 207±15 g/48-h, glucose), carbohydrate (784±56 g/48-h, saline vs. 789±55 g/48-h, glucose), fat (214±27 g/48-h saline vs. 218±26 g/48-h, glucose). Food intake during each of the two days and during individual meals was also analyzed and no significant differences were found (data not shown). Thus, despite the large number of calories being infused and the sustained elevations in plasma glucose, insulin, and leptin concentrations, subjects did not decrease their caloric intake during glucose infusion.
Figure 5.
Fig. 5, A. (left graph): Hunger ratings (mean ± standard error, n=16) during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period. Before each meal (09:00, 13:00, and 18:00hr) and at 22:00hr, subjects were asked to rate their hunger on a scale from 1 to 9 where 1 was “not hungry at all” and 10 was “extremely hungry”.
Fig. 5, B. (right graph): Total macronutrient content of the food ingested during the 2 experimental trials in which either saline (50 ml/hour) or 15% dextrose (200 mg/m2/min) was infused for a 48-h period.
Twenty-four hours of glucose infusion had no effect on sweet taste perception or on the hedonic likeness rating of sweet solutions (Table 3).
Correlations between hormonal and behavioral measures
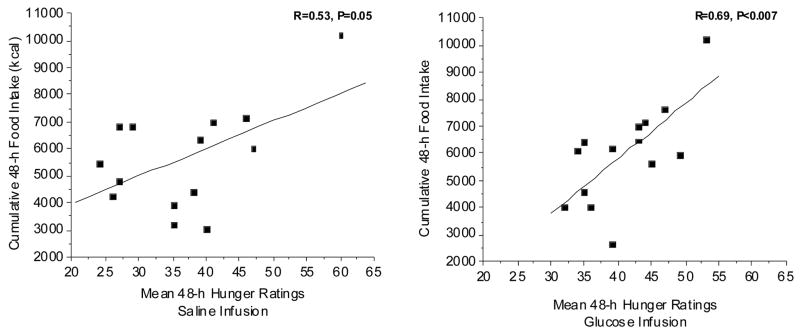
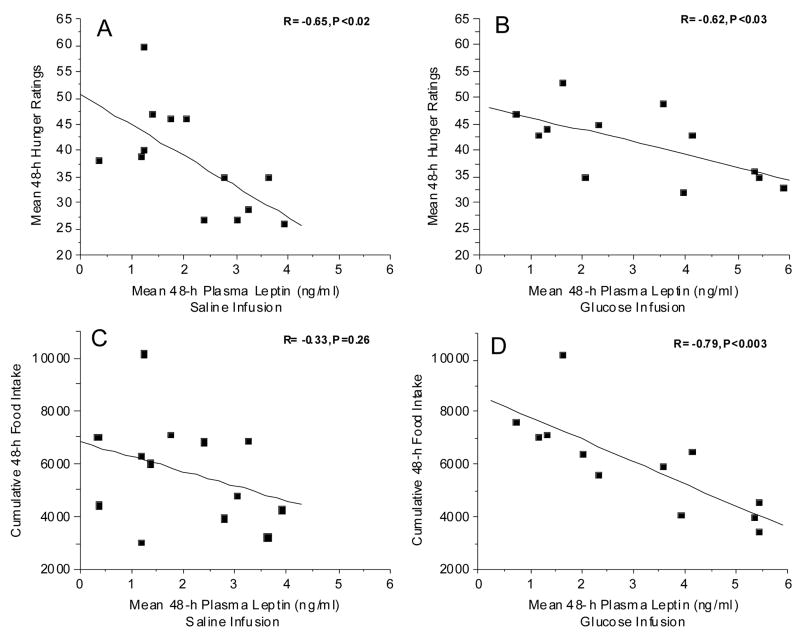
We examined the relationship between the hormonal responses during the 48-h infusions and the behavioral responses of food intake and hunger. Figure 6 illustrates the relationship between hunger and cumulative food intake over the 48-h infusion periods. In the present study, a positive correlation between hunger and food intake was observed during the saline infusion (Fig. 6, left graph) but this relationship just barely reached statistical significance (p=0.05). In contrast, during the glucose infusion, the variability decreased and the correlation became highly significant (P<0.007; Fig. 6, right graph). Although one would assume that hunger ratings should correlate with food intake, this relationship is often not observed in human studies. Similarly, the relationship between food intake and plasma leptin levels was enhanced during the glucose infusion. No significant relationship between leptin and food intake was observed during saline infusion (Fig. 7, upper left graph) but during the glucose infusion which increased plasma leptin levels (Fig. 2, left graph), food intake decreased with increasing leptin (R= −0.79, P<0.003). Thus, despite no significant differences in overall food intake between the two experimental conditions, induced increases in plasma leptin levels by the glucose infusion, tightened the relationship between food intake and leptin levels. A significant inverse correlation was observed between leptin and hunger, independent of treatment (Fig. 7, left and right graphs). If the data from the two treatments are combined, the overall inverse correlation between hunger and leptin levels was found to be R=−0.48, P<0.01. To our knowledge, this is first demonstration of a relationship between leptin and hunger ratings as well as leptin and food intake, in a non-calorically restricted non-obese population.
Figure 6.
Fig. 6, A. (left graph): Relationship between mean of 16 hunger ratings taken over the 48-h period of saline infusion and the amount of ad libitum kilocalories ingested over the same 48-h time period. R=0.52, P=0.51.
Fig. 6, B. (right graph): Relationship between mean of 16 hunger ratings taken over the 48-h period of glucose infusion and kilocalories ingested over the same 48-h time period. R=0.68, P<0.007*.
Figure 7.
Fig. 7. A (left, upper graph): Relationship between mean of 16 hunger ratings taken over the 48-h period of saline infusion and the mean of 38 plasma leptin samples taken over the same 48-h period. R= −0.65, P=0.02*. B (right, upper graph): Relationship between mean of 16 hunger ratings taken over the 48-h period of glucose infusion and the mean of 38 plasma leptin samples taken over the same 48-h period. R= −0.62, P=0.03*. C. (left, lower graph): Relationship between kilocalories ingested over the 48 h period of saline infusion and the mean of 38 plasma leptin samples taken over the same 48-h period. R= −0.33, P= 0.26. C. (right, lower graph): Relationship between kilocalories ingested over the 48 h period of glucose infusion and the mean of 38 plasma leptin samples taken over the same 48-h period. R= −0.79, P<0.003*.
Surprisingly, no statistically significant correlations were found between insulin and food intake (R=0.38, P=0.15, saline infusion; R=0.21, P=0.44, glucose infusion) or glucose and food intake (R=0.31, P=0.25, saline; R=0.22, P=0.41, glucose infusion). However, the quantity of food ingested during the 48-h period was inversely correlated with the post-prandial insulin response to the meal, i.e. the greater the kilocalorie intake over the 48-h period, the lower the post-prandial insulin response. This relationship held true independent of whether saline (R=−0.60, P<0.04) or glucose (R= −0.59, P<0.02) was infused. Food intake over the 48-h period was also inversely correlated with post-prandial glucose levels during the saline infusion (R= −0.68, P<0.01) but during the glucose infusion, this relationship was disrupted (R= −0.12, P=0.55). If individual macronutrient intakes over the 48-h period were correlated with post-prandial insulin levels, both carbohydrate intake in grams (R=−0.62, P<0.01) and fat intake in grams (R=−0.65, P<0.01) were statistically significant during the saline infusion but only fat intake remained significantly correlated during the glucose infusion (R= −0.50, P<0.02).
DISCUSSION
In the present study, we investigated the effects of prolonged mild hyperglycemia induced by a 48-h glucose infusion on hormonal responses, food intake and hunger during the infusion period and subsequently on post-prandial responses to a standard test meal. Prolonged glucose infusion produced modest elevations of plasma glucose in the range of 110 mg/dl, coupled with a two-fold increase of plasma insulin concentrations compared with saline infusion. Circulating levels of leptin were also elevated during the 48-h glucose infusion similar to those observed after more short term infusions of exogenous insulin and glucose 15;16. This metabolic profile of hyperinsulinemia, hyperleptinemia coupled with mildly elevated plasma glucose levels is similar to that of obese, glucose intolerant individuals with moderate insulin resistance. However, insulin resistance is typically associated with elevations in free fatty acids (FFAs) which in this experimental manipulation were completely suppressed by the glucose-induced hyperinsulinemia. Furthermore, plasma adiponectin concentrations, which are usually decreased in obese insulin resistant subjects 17–19, were not significantly different during the insulin and saline infusions, most likely due to the low body fat of the participating subjects 19. Thus, this experimental paradigm uncouples the glucose homeostatic parameters, glucose and insulin and to some degree, leptin, the production of which is dependent on insulin mediated glucose metabolism, from those variables often associated specifically with increased body adiposity such as elevated FFA and decreased adiponectin.
Three hours after the glucose infusion, despite insulin and glucose levels within the normal range, the HOMA-IR, a parameter of insulin sensitivity suggested an induction of insulin resistance by the glucose infusion. However, if the 48-h glucose infusion induced insulin resistance, one would predict a larger post-prandial increase of insulin in response to the test meal. Instead, we observed a substantial decrease of post-prandial glucose excursions and a modest reduction of postprandial insulin secretion in response to meal ingestion after the glucose infusion compared with the saline infusion. The discrepancy between the predicted response based on the HOMA which is derived from static insulin and glucose values and the actual dynamic insulin responses to a physiologically-relevant challenge reveal the limitation of HOMA as an assessment of insulin resistance. We postulate that the attenuation in post-prandial glucose levels may be a consequence of an increase in early phase insulin release (Figure 4, inset) which has been shown to play an important role in glucose tolerance 20–23. In fact, our laboratory has demonstrated that the presence of early phase insulin secretion can lower post-prandial glucose levels by 30% in normal weight and obese subjects. The increase in early phase insulin release may be a consequence of an induction of vagal efferent activity at the level of the pancreas or due to a recruitment of insulin vesicles to the perimeter of the B-cell 24 as a consequence of the prolonged glucose infusion, thereby facilitating rapid early phase insulin secretion in response to the meal challenge.
The increase of plasma triglyceride levels beginning ~2 hours after the test meal was the only metabolic parameter associated with lipid metabolism that was altered by the prolonged glucose infusion (Table 2). The increase in post-prandial triglycerides may have been due to an increase in triglyceride synthesis as a consequence of induction of lipogenic enzymes such as fatty acid synthase that are known to be induced in response to insulin and glucose 25. As elevated post-prandial triglycerides are associated with insulin resistance and atherosclerosis 26;27, the change in triglyceride response may be an early metabolic alteration to hypercaloric conditions and that precedes the development of insulin resistance. Furthermore, triglycerides inhibit leptin transport across the blood-brain barrier, thereby contributing to the development of insulin resistance 28
During the infusions periods, hunger and food intake were positive correlated, a relationship which became more tightly coupled and more significant during the glucose infusion condition (Figure 6). However, despite the large number of calories infused (~4000 kcal) over the 48-h period, we found no significant decreases in hunger ratings or the quantity of food or macronutrients content the subjects consumed ad libitum (Figure 5). Elevations in glucose are thought to reduce food intake 29 while declines in blood glucose are postulated to stimulate food intake 30. Glucose infused at 25% of basal caloric intake significantly reduced food intake in baboons 31 by 2–3 days although the infusion took place for a substantially longer time period (12–21 days).. In humans, the effect of acute glucose infusions (2–5 h) are equivocal with some studies reporting an increase in hunger and food intake 32 while others report no effect 33 Healthy subjects undergoing chronic glucose infusion (5–6 days) reduced their food intake to adequately compensate for the percentage of infused nutrients 9 although in this study, subjects were restricted to only ingesting a commercial liquid diet, thereby limiting sensory stimulation. In contrast, the subjects in the present study were provided with a large array of foods from which they could select their meals and under these conditions, subjects ate very similar quantities and composition of food irrespective of the infusate.
Energy regulation theories predict that subjects would compensate for the infused calories by decreasing their food intake. Both insulin 34 and leptin 18;35 are known to function as signals to the central nervous system in the long-term regulation of food intake and energy homeostasis. We found significant negative correlations between the mean of the plasma leptin levels over the 48-h period and the mean of the hunger ratings over the same period (Figure 7, upper graphs), that is as leptin increased, hunger ratings declined. To our knowledge, a significant correlation between increasing leptin and decreasing hunger has not been previously reported in a non-energy restricted population. The two studies reporting significant relationships between leptin and hunger 36 or leptin and satiety 37 were both in normal weight or obese women, respectively on calorically restricted diets resulting in weight loss. Other studies reporting a lack of the relationship between leptin and hunger were typically conducted in acute, meal to meal studies 38;39. Furthermore, in our normal weight subjects, the increase in leptin induced by the glucose infusion, resulted in a very strong negative correlation between leptin and food intake (Fig. 7, B) that was not evident during the saline infusion conditions (Fig. 7A). Thus, this normal weight population which would not be expected to exhibit central leptin resistance was responsive to the increase in leptin although this was not manifested during the 48-h period of testing. We would speculate that if the glucose infusion was continued, a reduction in food intake may have been evident. It should be noted that this is a small group of subjects and perhaps, a larger group would have provided the statistical power to overcome the large variation in food intake notable in human populations. Furthermore, measurement of food intake in humans is extremely limited as accurate intake can only be assessed in a laboratory situation. Conversely, eating behavior is most likely not “normal” in a laboratory setting. No significant correlation was found between mean 48-h insulin values and food intake although surprisingly, the quantity of total kilocalories ingested was inversely correlated to the post-prandial insulin response. .
In conclusion, we found that a 48-h glucose infusion elicits a metabolic profile of mild hyperglycemia, hyperinsulinemia and hyperleptinemia. Post-prandial glucose excursions and insulin secretion were attenuated in response to ingestion of a standard meal following the glucose infusion compared with the saline infusion. The reduction in post-prandial glucose may be a consequence of an increase in early phase insulin release as suggested by some previous studies, although additional experiments are need to determine if this the mediating mechanism. We also found that when given the opportunity to freely select and ingest food ad libitum that human subjects do not compensate for infused calories provided by glucose over a 48 hour period and do not reduce their food intake despite sustained elevations glucose, insulin, and leptin within the physiological range. However, significant inverse correlations between food intake and plasma leptin levels as well as hunger and leptin were found for the first time in a normal weight, freely eating group of subjects. These data highlight the importance of investigating behavioral responses to physiological stimuli in as close to “real-world” conditions as possible and to recognize that adaptations to over-nutrition are likely to require prolonged periods of time to manifest and may be blunted, particularly in individuals prone to weight gain such as obese, glucose intolerant, insulin resistant subjects.
Acknowledgments
We would like to acknowledge Dr. Beverly Tepper who suggested evaluating sweet taste responses during the glucose infusions. In addition, we acknowledge the help of the nurses of the General Clinical Research Center of the University of Pennsylvania, the expert technical assistance of Dr. Heather Collins at the Diabetes Research Center of the University of Pennsylvania and Huonglan Nguyen at the Monell Chemical Senses Center and James Graham in the Department of Nutrition at University of California, Davis. This work was supported by NIH grants; DK58003-07 (K.T.), DK-19525 and M01-RR00042. Dr. Havel’s research is supported by NIH grants: DK-58108, AT-002599, AT-003645, and HL-075675 and the American Diabetes Association.
Support: NIH DK58003-07 (K.T.), DK-19525, MO1-RR00042
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen L. Teff, Monell Chemical Senses Center and the Institute of Diabetes, Obesity and Metabolism, Dept. of Medicine, University of Pennsylvania, Philadelphia
Maja Petrova, Monell Chemical Senses Center, Philadelphia, PA
Peter J. Havel, Department of Nutrition, University of California, Davis, CA
Raymond R. Townsend, Dept. of Medicine, University of Pennsylvania, Philadelphia, PA PA
Reference List
- 1.Bonner-Weir S, Trent D, Weir G. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71:1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward K, Halter J, Beard J, Porte D. Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol. 1984;246:E405–E411. doi: 10.1152/ajpendo.1984.246.5.E405. [DOI] [PubMed] [Google Scholar]
- 3.Byrne MM, Sturis J, Polonsky KS. Insulin secretion and clearance during low-dose graded glucose infusion. Am J Physiol. 1995;268:E21–E27. doi: 10.1152/ajpendo.1995.268.1.E21. [DOI] [PubMed] [Google Scholar]
- 4.Kahn S, Bergman R, Schwartz M, Taborsky G, Porte D. Short-term hyperglycemia and hyperinsulinemia improve insulin action but do not alter glucose action in normal humans. Am J Physiol. 1992;262:E518–E523. doi: 10.1152/ajpendo.1992.262.4.E518. [DOI] [PubMed] [Google Scholar]
- 5.Laury M, Takao F, Bailbe D, Penicaud L, Portha B. Differential effects of prolonged hyperglycemia on in vivo and in vitro insulin secretion in rats. Endocrinology. 1991;128:2526–2533. doi: 10.1210/endo-128-5-2526. [DOI] [PubMed] [Google Scholar]
- 6.Balkan B, Dunning BE. Muscarinic stimulation maintains in vivo insulin secretion in response to glucose after prolonged hyperglycemia. Am J Physiol. 1995;268:R475–R479. doi: 10.1152/ajpregu.1995.268.2.R475. [DOI] [PubMed] [Google Scholar]
- 7.N’Guyen JM, Magnan C, Laury MC, et al. Involvement of the autonomic nervous system in the in vivo memory to glucose of pancreatic B cell in rats. J Clin Invest. 1994;94:1456–1462. doi: 10.1172/JCI117483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teff KL, Townsend RR. Prolonged mild hyperglycemia induces vagally mediaed compensatory increase in C-peptide secretion in humans. J Clin Endo Metab. 2004;89:5606–5613. doi: 10.1210/jc.2003-032094. [DOI] [PubMed] [Google Scholar]
- 9.Gil KM, Skeie B, Kvetan V, Askanazi J, Friedman MI. Parenteral nutrition and oral intake: effect of glucose and fat infusions. J Parenter Enteral Nutr. 1991;15:426–432. doi: 10.1177/0148607191015004426. [DOI] [PubMed] [Google Scholar]
- 10.Petrova M, Townsend RR, Teff K. Prolonged (48-h) modest hyperinsulinemia decreases nocturnal heart rate variability and attenuates the nocturnal decline in blood pressure. J Clin Endo Met. 2006;91:851–859. doi: 10.1210/jc.2005-1752. [DOI] [PubMed] [Google Scholar]
- 11.Zawalich WS, Zawalich KC. Induction of memory in rat pancreatic islets by tolbutamide. Dependence on ambient glucose level, calcium and phosphoinositide hydrolysis. Diabetes. 1988;37:816–823. doi: 10.2337/diab.37.6.816. [DOI] [PubMed] [Google Scholar]
- 12.Teff KL, Townsend RR. Early phase insulin infusion and muscarinic blockade in obese and lean subjects. Am J Physiol. 1999;277:R198–R208. doi: 10.1152/ajpregu.1999.277.1.R198. [DOI] [PubMed] [Google Scholar]
- 13.Sive AA, Vinik AI, van Tonder SV. Pancreatic polypeptide (PP) responses to oral and intravenous glucose in man. Am J Gastroenterology. 1979;71:183–185. [PubMed] [Google Scholar]
- 14.Wallace T, Levy J, Matthews D. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 15.Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H. Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects. Diabetes. 1996;45:1364–1366. doi: 10.2337/diab.45.10.1364. [DOI] [PubMed] [Google Scholar]
- 16.Saad M, Riad-Gabriel M, Khan A, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endo Metab. 1998;83:453–459. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 17.Lihn A, Pedersen S, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 18.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;Suppl 1:S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 19.Kantartzis K, Fritsche A, Tschritter O, et al. The association between plasma adiponectin and insulin sensitivity in humans depends on obesity. Obes Res. 2005;13:1683–1691. doi: 10.1038/oby.2005.206. [DOI] [PubMed] [Google Scholar]
- 20.Calles-Escandon J, Robbins DC. Loss of early phase of insulin release in humans impairs glucose tolerance and blunts thermic effect of glucose. Diabetes. 1987;36:1167–1172. doi: 10.2337/diab.36.10.1167. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SE, Montgomery B, Howell W, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endo Metab. 2001;86:5824–5829. doi: 10.1210/jcem.86.12.8105. [DOI] [PubMed] [Google Scholar]
- 22.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanism and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 23.D’Alessio DA, Kieffer TJ, Taborsky GJ, Havel PJ. Activation of the parasympathetic nervous system is necessary for normal meal-induced insulin secretion in rhesus macaques. J Clin Endo Metab. 2001;86:1253–1259. doi: 10.1210/jcem.86.3.7367. [DOI] [PubMed] [Google Scholar]
- 24.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 25.Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis:studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Ann Rev Nutr. 1998;18:331–351. doi: 10.1146/annurev.nutr.18.1.331. [DOI] [PubMed] [Google Scholar]
- 26.Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson PA. Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Ann Intern Med. 1999;131:27–31. doi: 10.7326/0003-4819-131-1-199907060-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kriketos AD, Sam W, Schubert T, Maclean E, Campbell LV. Postprandial triglycerides in response to high fat: role of dietary carbohydrate. Eur J Clin Invest. 2003;33:383–389. doi: 10.1046/j.1365-2362.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- 28.Banks WA, Coon AB, Robinson SM, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 29.Mayer J. Glucostatic mechanism of the regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 30.Campfield LA, Smith FJ. Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol Rev. 2003;83:25–58. doi: 10.1152/physrev.00019.2002. [DOI] [PubMed] [Google Scholar]
- 31.Woods SC, Stein LJ, McKay LD, Porte D., Jr Suppression of food intake by intravenous nutrients and insulin in the baboon. Am J Physiol. 1984;247:R393–R401. doi: 10.1152/ajpregu.1984.247.2.R393. [DOI] [PubMed] [Google Scholar]
- 32.Chapman IM, Goble EA, Wittert GA, Morley JE, Horowitz M. Effect of intravenous glucose and euglycemic insulin infusions on short-term appetite and food intake. Am J Physiol. 1998;274:R596–R603. doi: 10.1152/ajpregu.1998.274.3.R596. [DOI] [PubMed] [Google Scholar]
- 33.Woo R, Kissileff HR, Pi-Sunyer FX. Elevated postprandial insulin levels do not induce satiety in normal-weight humans. Am J Physiol. 1984;247:R745–R749. doi: 10.1152/ajpregu.1984.247.4.R745. [DOI] [PubMed] [Google Scholar]
- 34.Porte D, Jr, Woods SC. Regulation of food intake and body weight by insulin. Diabetologia. 1981;20:Suppl:274–Suppl:280. [PubMed] [Google Scholar]
- 35.Schwartz M. Brain pathways controlling food intake and body weight. Exp Biol Med (Maywood) 2001;226:978–981. doi: 10.1177/153537020122601103. [DOI] [PubMed] [Google Scholar]
- 36.Keim N, Stern J, Havel P. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801. doi: 10.1093/ajcn/68.4.794. [DOI] [PubMed] [Google Scholar]
- 37.Heini AF, Lara-Castro C, Kirk KA, Considine RV, Caro JF, Weinsier RL. Association of leptin and hunger-satiety ratings in obese women. Int J Obes Relat Metab Disord. 1998;22:1084–1087. doi: 10.1038/sj.ijo.0800731. [DOI] [PubMed] [Google Scholar]
- 38.Blom WAM, Mars M, Hendriks HFJ, de Groot CPGM, Stafleu A, Kok FJ, de Graaf C. Fasting ghrelin does not predict food intake after short-term energy restriction. Obesity. 2006;14:838–846. doi: 10.1038/oby.2006.97. [DOI] [PubMed] [Google Scholar]
- 39.Romon M, Lebel P, Velly C, Marecaux N, Fruchart JC, Dallongeville J. Leptin response to carbohydrate or fat meal and association with subsequent satiety and energy intake. Am J Physiol. 1999;277:E855–E861. doi: 10.1152/ajpendo.1999.277.5.E855. [DOI] [PubMed] [Google Scholar]