Abstract
Bone marrow–derived multipotent mesenchymal stroma cells (MSCs) have emerged as cellular vectors for gene therapy of solid cancers. We implanted enhanced green fluorescent protein–expressing rat MSCs directly into rat malignant gliomas to address their migratory capacity, phenotype, and effects on tumor neovascularization and animal survival. A single intratumoral injection of MSCs infiltrated the majority of invasive glioma extensions (72 ± 14%) and a substantial fraction of distant tumor microsatellites (32 ± 6%). MSC migration was highly specific for tumor tissue. Grafted MSCs integrated into tumor vessel walls and expressed pericyte markers α-smooth muscle actin, neuron-glia 2, and platelet-derived growth factor receptor-β but not endothelial cell markers. The pericyte marker expression profile and perivascular location of grafted MSCs indicate that these cells act as pericytes within tumors. MSC grafting did not influence tumor microvessel density or survival of tumor-bearing animals. The antiangiogenic drug Sunitinib markedly reduced the numbers of grafted MSCs migrating within tumors. We found no MSCs within gliomas following intravenous (i.v.) injections. Thus, MSCs should be administered by intratumoral implantations rather than by i.v. injections. Intratumorally grafted pericyte-like MSCs might represent a particularly well-suited vector system for delivering molecules to affect tumor angiogenesis and for targeting cancer stem cells within the perivascular niche.
Introduction
Solid tumors are dependent of neovascularization to grow beyond a certain size.1 Several antiangiogenic drugs have therefore been developed to target the tumor vasculature.2 The tumor vasculature consists of endothelial cells which form the inner lining of vessels, and pericytes (perivascular cells) which provide structural support to endothelial cells. Pericytes also play an important role for tumor vessel function and survival by providing, e.g., vascular endothelial growth factor (VEGF) to endothelial cells.3,4 It has been suggested that bone marrow–derived endothelial cells and pericytes are recruited to contribute to the neovasculature of tumors,4,5,6,7,8 although recent data question the contribution of bone marrow circulating endothelial precursors to tumor vascular endothelium.9 Impaired recruitment of endothelial cells from the bone marrow leads to inhibition of tumor angiogenesis and decreased tumor growth.6 Depletion of pericytes causes hyperdilation of tumor vessels and increased apoptosis of tumor cell endothelium.4 Therapeutic targeting of tumor vasculature may thus involve targeting of both endothelial cells and pericytes.10
Glioblastoma multiformae (GBM) is a highly malignant brain tumor which displays extensive neovascularization. The growth pattern of GBM is characterized by tumor extensions and tumor microsatellites growing far away from the main tumor mass into the normal brain. Conventional therapy is not curative and the shortcomings of GBM treatment have, in part, been attributed to the failure to target the infiltrating tumor cells.11 Antiangiogenic treatment has been explored in malignant gliomas.12 Systemic administration of angiogenic inhibitors as well as local delivery of antiangiogenic factors by means of viral-vector mediated gene delivery and encapsulated producer cells have been utilized. Although these modes of delivery have shown some promising results against gliomas there are serious shortcomings, such as short half-life of systemically administered inhibitors, host immune response to viral vectors and inefficient intratumoral spread of viral vectors and encapsulated cells.12
Neural, mesenchymal, and endothelial stem or progenitor cells have, because of their tumor-tropic migratory capacity, emerged as promising delivery vehicles of antitumoral substances in therapy of malignant tumors, including GBM.13,14,15,16,17 Culture-derived mesenchymal stroma cells (MSCs), also called mesenchymal stem cells or marrow stroma cells, possess tropism for experimental tumors, including gliomas, following intraarterial and intracranial injections.16,17 Moreover, MSCs genetically modified to produce antitumoral substances have been shown to prolong survival in experimental brain tumor therapy.16,17 Because of the infiltrative growth pattern of GBM, the success of MSCs as delivery vehicles in human GBM therapy will likely depend on their capacity to migrate to invasive tumor extensions and to distant tumor microsatellites. A quantitative assessment of the capacity and specificity of intratumorally grafted MSCs to infiltrate tumor tissue is highly warranted. Furthermore, their phenotype and relation to tumor microvessels need to be clarified.
In this study, we grafted bone marrow–derived rat MSCs into established orthotopic N29 and N32 rat malignant brain tumors. These glioma models are syngeneic when implanted in Fischer344 rats and exhibit characteristics of human high-grade malignant brain tumors. From a clinical perspective, grafting of MSCs into tumor remnants following surgical tumor resection is a potentially efficient route of delivery of therapeutic transgenes. MSCs can be isolated from the bone marrow, expanded in culture, and genetically modified to produce antitumoral substances before grafting. The aims of this study were to explore the capacity of intratumorally grafted and intravenously (i.v.) injected MSCs to migrate to invasive tumor cells, and to investigate the association between MSCs and tumor vasculature as well as the phenotype of MSCs in brain tumors.
Results
MSCs characteristics in vitro
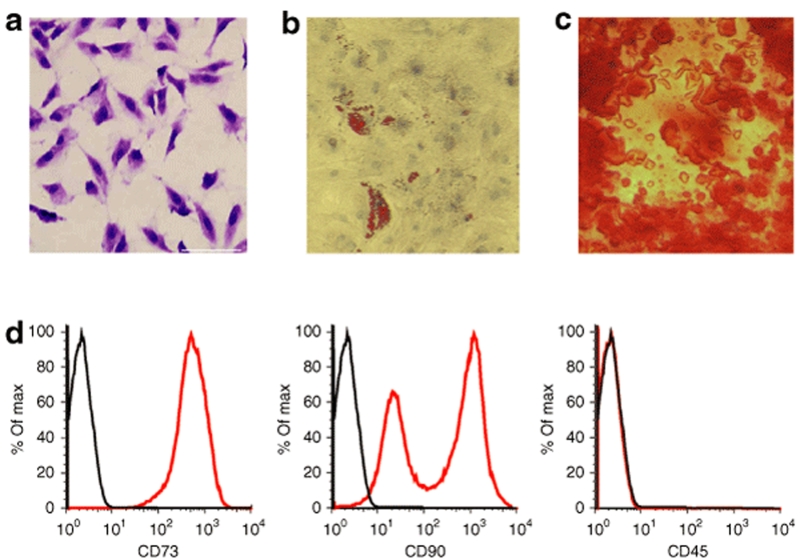
Following seeding in mesenchymal culture medium, typical fibroblastoid colonies could be detected after a few days rapidly forming a monolayer of adherent cells. MSC cultures contained a homogenous population of spindle-shaped cells (Figure 1a). To assay the differentiation potential of the MSCs, second passage cells were grown to near confluency and assayed for differentiation into osteoblasts or adipocytes. When MSC cultures were incubated in the adipogenic medium, lipid vacuoles could be clearly detected by Oil-red-O staining (Figure 1b). When cells were grown in the osteogenic medium, a change in cell morphology was found from spindle shaped to cuboidal shaped and calcium mineral deposits could be observed (Figure 1c). MSCs grown in control medium, on the other hand, showed no signs of differentiation.
Figure 1.
In vitro characteristics of bone marrow–derived rat multipotent mesenchymal stroma cells (MSCs). (a) Spindle-shaped morphology of MSCs generated from adult bone marrow. Cultures were stained with crystal violet. (b) Differentiation capacity of MSCs into adipocytes and (c) osteoblasts was assessed for passage 2 cells. (b) Lipid vacuoles were stained with Oil-red-O. (c) Osteogenic differentiation was detected by staining with Alizarin Red. Bar = 60 µm. (d) Flow cytometric analysis of MSCs using antibodies against CD73 (left panel), CD90 (middle panel), and the hematopoietic marker CD45 (right panel).
Flow cytometric analysis revealed that MSCs were positive for the typical mesenchymal markers CD73 and CD90 but negative for the hematopoietic marker CD45. While CD73 expression was homogenous in all MSCs, CD90 was heterogeneously expressed in one high (60% of total cells)- and one low (40%)-expressing subpopulation (Figure 1d).
Enhanced green fluorescent protein expression of MSCs
To visualize the MSCs following grafting in vivo, we genetically modified the cells to express enhanced green fluorescent protein (eGFP). MSCs were transduced with a Moloney leukemia–based retroviral vector, which has the characteristic of infecting dividing cells. Flow cytometry revealed that >98% of MSCs expressed eGFP in vitro.
MSC migration to tumor extensions and tumor microsatellites
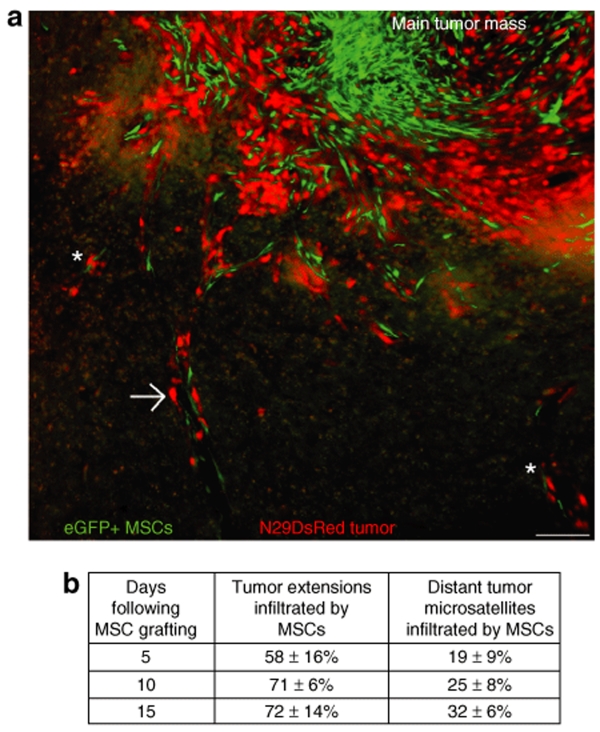
The growth pattern of the malignant N29 tumor resembles the invasive growth pattern of human GBM, which means that the tumor grows with infiltrative tumor extensions reaching far into the normal brain and with distant tumor microsatellites separated from the main tumor mass. The numbers of tumor extensions and distant tumor microsatellites were 7.2 ± 1.4 and 4.6 ± 1.0 per section on day 15 following tumor cell inoculation, 9.9 ± 0.7 and 9.4 ± 0.6 on day 20, and 10.1 ± 1.2 and 12.7 ± 2.2 on day 25. We estimated the capacity of intratumorally grafted eGFP+ MSCs to migrate to tumor extensions and to distant tumor microsatellites. The majority of tumor extensions contained at least one, but most often several, eGFP+ MSCs and MSCs were also found in a substantial fraction of distant tumor microsatellites 5, 10, and 15 days following MSC grafting (Figure 2a,b). The capacity of MSCs to migrate to distant tumor extensions and microsatellites was persistent in parallel with the infiltrative growth of the N29 tumor (Figure 2b). Only minimal numbers of intratumorally grafted MSCs were found in normal brain parenchyma adjacent to tumor (Figure 2a). The numbers of grafted eGFP+ MSCs were, 15 days following MSC grafting, 0.5 ± 0.1 per section in normal brain tissue adjacent to tumor, 41.5 ± 5.0 in tumor extensions (P < 0.05, compared to MSCs numbers in normal brain tissue) and 7.1 ± 2.7 in tumor microsatellites (P < 0.05).
Figure 2.
eGFP+ MSCs grafted into established N29DsRed tumor migrate along infiltrative tumor extensions and to distant microsatellites. The invasive growth pattern of the N29 tumor resembles that of glioblastoma multiformae. (a) eGFP+ MSCs (green) are mainly located within the main tumor mass but several mesenchymal stroma cells (MSCs) are also found along tumor extensions (arrow) and at distant tumor microsatellites (asterisks) infiltrating into the normal brain parenchyma. Only minimal numbers of grafted eGFP+ MSCs can be found in normal brain tissue adjacent to tumor. Bar = 100 µm. (b) Number of tumor extensions and distant tumor microsatellites infiltrated by eGFP+ MSCs at various time points following MSC grafting into established N29DsRed tumor. Data are shown as mean ± SEM, n = 4–5 animals for each time point. eGFP, enhanced green fluorescent protein.
Fluorescent in situ hybridization
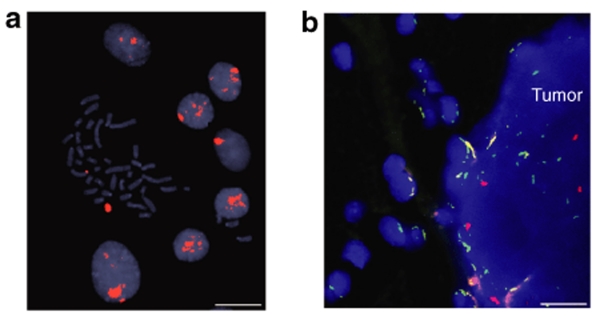
In addition to analysis of eGFP+ cells, fluorescent in situ hybridization (FISH) was used to independently assess MSC migration in vivo. MSCs, derived from a male rat, were grafted into female animals, carrying the N29wt tumor developed in a female animal. MSC migration pattern was analyzed on day 15 following intratumoral grafting. FISH for the Y chromosome (red signal) was performed to specifically label MSCs (in vitro, Figure 3a). eGFP expression of the MSCs was lost in this experiment because of heavy enzymatic pretreatment of the tissue sections. However, cells carrying the Y chromosome were clearly visualized, locating within the N29wt tumor. No Y chromosome–positive cells were seen in the normal brain. FISH for rat chromosome 12 (green signal) was performed as positive control (Figure 3b).
Figure 3.
Whole-chromosome fluorescent in situ hybridization (FISH) painting for the Y chromosome in mesenchymal stroma cells (MSCs) derived from a male rat. (a) A metaphase spread with a Y chromosome (large red signal) and several interphase nuclei with a corresponding signal pattern. (b) Tissue section showing parts of normal brain parenchyma and N29 tumor. Male-derived MSCs are grafted into N29 tumors (female-derived tumor) established in female hosts (n = 4). Cell nuclei are depicted by 4′,6-diamidino-2-phenylindole staining (blue). The intratumoral localization of male-derived MSCs is confirmed by Y chromosome signals (red). Enhanced green fluorescent protein expression was lost because of heavy enzymatic pretreatment. No Y carrying cells can be seen in the normal brain. FISH for chromosome 12 (green) is used as positive control. Bar = 10 µm in a and 15 µm in b.
Grafted MSCs migrate to tumor endothelium and do not express endothelial markers or affect tumor microvessel density
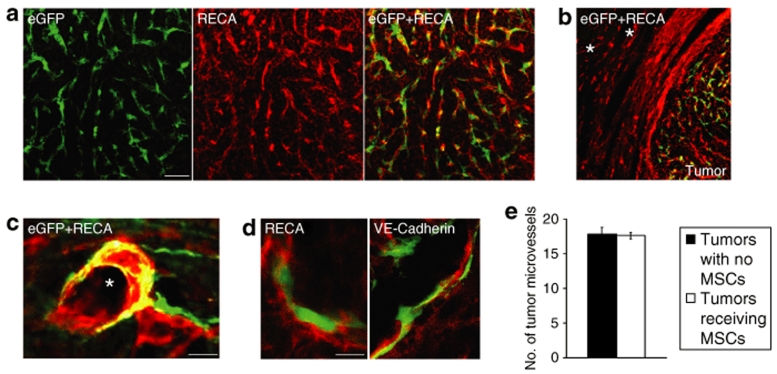
Colabeling of eGFP and rat endothelial cell antigen (RECA) was performed to assess the association between grafted MSCs and tumor endothelium. There was a strong tropism of grafted MSCs to RECA+ tumor vessels (Figure 4a), but not to RECA+ blood vessels in normal brain tissue adjacent to tumor (Figure 4b). Quantitative analysis revealed that 84 ± 4% of the migratory MSCs closely associated to RECA+ tumor vessels and a significant fraction of MSCs also integrated into the vessel wall (Figure 4a). Highly vascularized tumor extensions attracted higher numbers of MSCs compared to tumor extensions with a lower vascular density. Migratory MSCs, clearly separated from the core of the graft, could be found closely attached to major tumor vessels (Figure 4c), whereas minimal numbers of MSCs were seen in the surrounding nonvascularized tumor area.
Figure 4.
Grafted eGFP+ MSCs closely associate to tumor endothelium and do not express endothelial cell markers. (a) eGFP+ MSCs (green) 8 days following grafting into established N32wt brain tumor. Tumor endothelium is delineated by rat endothelial cell antigen (RECA; red). The majority of mesenchymal stroma cells (MSCs) are closely associated to tumor endothelium. (b) Intratumorally grafted MSCs migrate along RECA+ tumor vessels and do not associate with RECA+ blood vessels in normal brain tissue adjacent to tumor. Asterisks indicate major blood vessels in normal brain. (c) Grafted eGFP+ MSCs closely attached to a major RECA+ tumor vessel. Asterisk indicates tumor vessel lumen. (d) Confocal microscopy analysis was used to determine coexpression of grafted eGFP+ MSCs with endothelial markers RECA or VE-Cadherin within tumors. Grafted eGFP+ MSCs attached to tumor endothelium (RECA and VE-Cadherin, red) and without coexpression of RECA or VE-Cadherin. Bar = 60 µm in a, 100 µm in b, 20 µm in c, and 10 µm in d. (e) Quantification of RECA+ tumor microvessels revealed no difference in tumor microvessel density in tumors receiving eGFP+ MSCs compared to tumors with no MSCs. Data are shown as mean ± SEM, n = 4 in each group. eGFP, enhanced green fluorescent protein; VE-Cadherin, vascular endothelial-Cadherin.
We determined whether MSCs themselves are differentiating into endothelial cells and directly contribute to tumor endothelium. There was no MSC expression of the endothelial cell markers RECA or vascular endothelial-Cadherin (VE-Cadherin) in vitro (data not shown). Confocal microscopy analysis was used to determine coexpression of grafted eGFP+ MSCs, integrated to tumor vessels, with RECA or VE-Cadherin. We found no evidence of grafted eGFP+ MSCs that coexpressed RECA or VE-Cadherin (Figure 4d). We therefore conclude that MSCs are nonendothelial cells which become closely associated and integrate to tumor neovasculature following intratumoral grafting.
To investigate the effect of MSC grafting on tumor microvessel density, we quantified the numbers of RECA+ tumor vessels in tumors receiving eGFP+ MSCs and in tumors with no eGFP+ MSCs. Results indicate that there was no difference in tumor microvessel density between the two groups (17.8 ± 0.9 for tumors with no MSCs and 17.6 ± 0.5 for tumors with MSCs, Figure 4e).
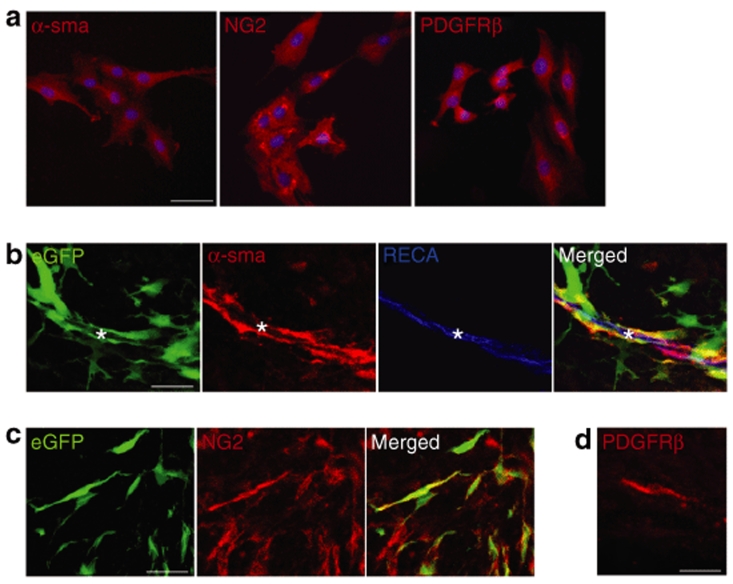
MSCs express pericyte markers in vitro and in vivo
In light of the tropism and close association of grafted MSCs to tumor vasculature, we analyzed the MSC expression pattern of perivascular cell (pericyte) markers α-smooth muscle actin (α-sma),4,18 neuron-glia 2 (NG2),19,20 and platelet-derived growth factor receptor-β (PDGF-receptor-β).4,21 In vitro, the absolute majority (>99%) of MSCs expressed pericyte markers α-sma, NG2, and PDGF-receptor-β (Figure 5a). A high fraction of eGFP+ MSCs grafted into established tumors continued to express pericyte markers α-sma (~60%, Figure 5b) and NG2 (~80%, Figure 5c). We found PDGF receptor-β–expressing cells with migratory morphology within tumors receiving MSCs; however eGFP expression was lost in the tissue processing and we were therefore unable to colocalize eGFP with PDGF-receptor-β in vivo (Figure 5d). Thus, MSCs utilized in our experiments display a pericyte-like phenotype in vitro and in vivo.
Figure 5.
Mesenchymal stroma cells (MSCs) express pericyte markers in vitro and in vivo. (a) The absolute majority (>99%) of MSCs express pericyte markers α-smooth muscle actin (α-sma), neuron-glia 2 (NG2) and platelet-derived growth factor receptor-β (PDGF-receptor-β) in vitro. Cells are counted-stained with 4′,6-diamidino-2-phenylindole nuclear staining (blue). (b) eGFP+ MSCs grafted into established tumors continue to express pericyte marker α-sma (red) and localize closely to a tumor vessel [rat endothelial cell antigen (RECA), blue)]. Other eGFP+ MSCs display no or very weak α-sma expression. Asterisks indicate tumor vessel lumen. (c) eGFP+ MSCs expressing NG2 within tumor. (d) Fresh-frozen tissue section showing a representative PDGF receptor-β–expressing migratory cell with typical MSC morphology found within tumors containing grafted MSCs. The expression of enhanced green fluorescent protein (eGFP) was lost in tissue processing. Bar = 50 µm in a and 30 µm in b–d.
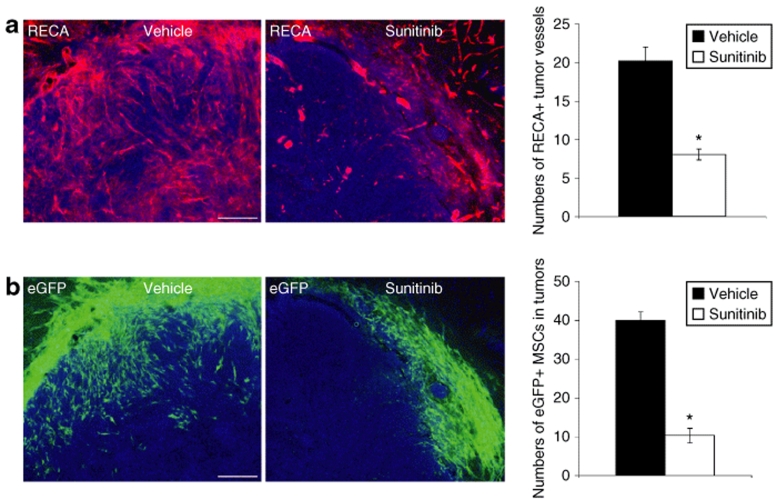
Sunitinib treatment inhibits MSC migration into tumors
N32wt glioma-bearing animals were treated with either vehicle or the antiangiogenic drug Sunitinib which inhibits multiple tyrosine kinase receptors including PDGF-receptor-α and -β, VEGF receptor-1 and -2, KIT (stem cell factor receptor), and Fms-like tyrosine kinase-3 receptor.22 The numbers of tumor microvessels and grafted MSCs within tumors were quantified 4 days following MSC grafting. As expected, the numbers of tumor microvessels decreased in animals receiving Sunitinib treatment. The numbers of RECA+ microvessels within tumors were, after vehicle treatment, 20 ± 1.8, compared to 8 ± 0.7 following treatment with Sunitinib (Figure 6a, P < 0.05, unpaired t-test). We counted the numbers of eGFP+ MSCs that migrated from the injection site, within or outside tumor, to tumor periphery. Treatment with Sunitinib effectively reduced the numbers of eGFP+ MSCs migrating to tumor periphery compared to the numbers of migrating MSCs following administration of vehicle. The numbers of eGFP+ MSCs within tumor periphery were, after vehicle treatment, 40 ± 2.2, compared to 10 ± 1.9 following treatment with Sunitinib (Figure 6b, P < 0.05, unpaired t-test). High numbers of grafted MSCs were found migrating into the tumor core in vehicle-treated animals while grafted MSCs were found mainly in the outer tumor periphery in Sunitinib-treated animals.
Figure 6.
The antiangiogenic drug Sunitinib decreases tumor vascularization and the numbers of grafted mesenchymal stroma cells (MSCs) migrating to the periphery of N32 gliomas. (a) Tumor microvessels [rat endothelial cell antigen (RECA), red] in N32wt tumors (Hoechst nuclear staining, blue) following administration of either vehicle or Sunitinib. Treatment with Sunitinib significantly decreased the numbers of RECA+ tumor microvessels. (b) High numbers of grafted eGFP+ MSCs (green) were found migrating from the injection site to tumor periphery (Hoechst, blue) following administration of vehicle. Treatment with Sunitinib effectively reduced the numbers of grafted MSCs found within tumor periphery. Data are presented as mean ± SEM. *P < 0.05, n = 4 in each group. Bar = 170 µm in a and b. eGFP, enhanced green fluorescent protein.
Grafted MSCs do not prolong survival of tumor-bearing animals
To investigate the effect of MSC grafting on survival of glioma-bearing animals, we grafted MSCs intratumorally on day 2 into N29 or N32 tumor–bearing animals. Animals were killed when symptoms of tumor growth emerged. There was no significant difference in survival of tumor-bearing animals that received eGFP+ MSCs intratumorally compared to animals that received control medium. The mean survival for N32 tumor–bearing animals receiving MSCs was 24 (range: 21–27) days and for tumor-bearing animals receiving control medium 23 (range: 21–25) days. N29 tumor–bearing animals that received MSCs survived 44 (range: 32–56) days and animals that received control medium 49 (range: 40–54) days.
No evidence of MSC homing to established gliomas following i.v. injections
N29 or N32 glioma cells were inoculated intracerebrally and eGFP+ MSCs were administered systemically by i.v. injections 14 days later. Animals were killed 2 and 7 days following MSC injections. At both time points, we found vascularized tumors in the striatum of all animals but careful microscopic analysis of serial sections revealed no evidence of eGFP+ MSCs within tumors. Neither did we detect NG2 or α-sma expressing cells with spindle-shaped morphology within tumors.
Discussion
The principal findings of this study are that bone marrow–derived MSCs, grafted into malignant brain tumors, associate closely and integrate to tumor vessels and express typical pericyte markers in vitro as well as in vivo within tumors. MSCs, identified by their adherent growth, marker expression profile, and mesenchymal differentiation capacity, have been used as cellular delivery vehicles in various types of cancers, including melanoma, breast cancer, Kaposi's sarcoma, lung cancer, and brain tumors.16,17,23,24,25,26 Our findings suggest that the cells used in these previous studies might associate to tumor vessels and possibly act like pericytes within tumors. Furthermore, we demonstrate by quantitative analysis that intratumorally grafted bone marrow–derived MSCs migrate effectively to infiltrative tumor extensions and display capacity to migrate to distantly located tumor microsatellites. MSC grafting did not affect tumor microvessel density or survival of glioma-bearing animals. Our findings indicate that tumor angiogenic signaling factors, and possibly tumor vessels per se, are important in the recruitment of grafted MSCs within tumors. However, we found no evidence of MSC homing to established gliomas following i.v. injections.
Tumor neovasculature is critical for tumor growth1 and the finding that grafted MSCs associates to and integrates into tumor vessels indicates that these cells may act as very potent vehicles for delivery of antiangiogenic substances to vascularized tumors. Antiangiogenic therapy of gliomas and of brain metastases have previously shown that tumors might adopt to decreased vessel density and hypoxia by increased migration along preexisting microvessels and by increased perivascular satellite formation, a phenomenon known as vascular co-option.27,28 This needs to recognized when designing antiangiogenic trials for gliomas but it is possible that MSCs robust capacity to track tumor extensions and microsatellites might overcome this therapeutic challenge.
Human MSCs can be found in human glioma xenografts in immunocomprised mice following intracarotid injections.16 We pursued to investigate whether i.v. injected rat MSCs home to established syngeneic orthotopic gliomas and we found no evidence of i.v. injected MSCs within tumors following single injections. Another study reported tropism of i.v. injected human MSCs to melanomas in immunocompromised mice.26 MSC homing efficiency to subcutaneous tumors was, however, very low because five MSC injections during a 20-day period resulted in homing to only a fraction of melanoma-bearing animals. Furthermore, melanoma lung metastasis were established by i.v. melanoma cell inoculation, and i.v. administered MSCs were found randomly distributed in both the lung parenchyma and tumor at 1 day after injection.26 This suggests a lack of tumor-specific homing of i.v. injected MSCs in the melanoma lung metastasis model. Although we performed systematic analysis of serial sections, we cannot exclude the possibility that very low numbers of MSCs home to glioma vasculature following i.v. injections. In contrast, a single intratumoral injection of MSCs resulted in infiltration of the majority of the invasive tumor extensions and to a significant fraction of distantly located tumor microsatellites in the invasive N29 glioma model. Thus, our results clearly show that the MSC cellular vector system in glioma therapy should be administered by intratumoral implantation rather than by i.v. injections.
Using two independent methods, retroviral eGFP labeling of MSCs and FISH to track male‐derived MSCs grafted into female hosts, we showed that MSC migration was largely restricted to tumor and not to normal brain parenchyma. The tumor-specific migratory capacity of MSCs makes these cells promising cellular vehicles for delivery of cytotoxic substances that specifically can kill tumor cells without substantially damaging normal brain tissue. Moreover, our finding that grafted MSCs intermingled with tumor endothelial cells and tumor pericytes implies that an approach to locally target both endothelial cells and pericytes within tumors is possible. Combinatorial targeting of both tumor endothelium and tumor pericytes by systemic administration of VEGF receptor and PDGF receptor tyrosine kinase inhibitors indeed diminish tumor vascularization more than any of the respective inhibitors individually.10 The therapeutic implication of the perivascular tropism of grafted MSCs within tumors is further strengthened by recent findings by Calabrese et al. demonstrating that cancer stem cells within brain tumors are maintained within a perivascular niche. This study suggests that tumor endothelial cells secrete factors that maintain brain cancer stem cells in a stem cell–like state.29 Grafted MSCs are therefore strategically well located to deliver substances that can either directly attack cancer stem cells within the perivascular niche, or indirectly by eliminating tumor endothelial cells necessary for cancer stem cell growth. In cancer immunotherapy, blood-borne immune cells, e.g., cytotoxic T cells and natural killer cells, cross the blood–brain barrier to reach the tumor. MSCs, genetically modified to produce proinflammatory substances such as interleukin-2, interferon-β, and interleukin-23, can decrease tumor growth and prolong survival of tumor-bearing animals.16,17,30 The close association of grafted MSCs to tumor vessels found in the present study may thus explain their capacity to attract blood-borne immune cells to tumors.
Our findings indicate that grafted MSCs utilize tumor vessels as their migratory route. The tumor-specific migratory behavior of MSCs has previously shown to be associated to glioma-produced angiogenic cytokines. In vitro assays have revealed that specifically VEGF-A, interleukin-8, transforming growth factor-β1, and neurotrophin-3, all involved in tumor angiogenesis, mediate recruitment of MSCs to gliomas.31,32 Findings from our study suggest that tumor angiogenic signaling factors regulate MSC migration intratumorally in vivo. Although we cannot exclude decreased MSC survival following Sunitinib treatment, the MSC migratory pattern was radically changed by Sunitinib, thus suggesting that tumor vascularization is important for substantial migration of grafted MSCs within tumors. The pericyte-like phenotype of MSCs indicates that endothelial interactions and specifically PDGF-β, sphingosine-1-phosphate, and angiopoietin-1 may mediate MSC recruitment to tumors as these factors contribute to recruit pericytes to tumor vessels.33,34
We could not detect an effect of grafted MSCs on tumor microvessel density. This is in contrast to previous findings showing that systemic administration of human MSCs can contribute to angiogenesis in the pathological brain.35 No effect on survival of tumor-bearing animals was seen in the present study. However, the pericytic phenotype of MSCs gives an indication of their potential functions within tumors. Normal pericytes are essential for endothelial cell survival and function and tumor pericytes may regulate tumor vessel stability, maintenance, and function.33,36 Recent findings suggest that pericyte maturation within tumors contributes to vascular normalization which in turn enhances the influx of immune effector cells leading to decreased tumor growth.37 It would be interesting to see whether grafted pericyte-like MSCs can contribute to normalization of tumor vasculature and in this way facilitate immunotherapy of the tumor. In addition, the role of MSCs and pericytes in tumor cell metastasis has recently been addressed. Xian et al. demonstrated that pericytes within tumors limit tumor cell metastasis via neural cell adhesion molecule–dependent normalization of tumor vasculature.38 On the other hand, Karnoub et al. showed that MSCs, when mixed with breast cancer cells, can increase the metastatic potency of the cancer via CCL5 signaling.39 Further studies are needed to clarify the role of grafted pericyte-like MSCs in glioma cell invasion and metastasis.
Future studies should also aim at optimizing the infiltrative capacity of grafted MSCs because not all tumor extensions and microsatellites were infiltrated by MSCs. Grafting of MSCs genetically modified to overexpress receptors crucial for tumor-specific migration,40 and identification of potential MSC subpopulations more prone to migrate, could in combination with multiple MSC injections, improve the MSC tumor infiltration.
In brief, our results indicate that bone marrow–derived MSCs act as pericyte-like cells following intratumoral grafting, efficiently migrating to and integrating into the tumor neovasculature to which they likely are recruited by factors involved in neoangiogenesis.
Materials and Methods
Tumor cell lines and culture. The rat glioma cell lines N29 and N32 were originally induced by transplacental injection of ethyl-N-nitrosourea to pregnant Fischer344 rats.41 Tumor cell culturing was performed as previously described (see Supplementary Materials and Methods).42
Flow cytometry. MSCs were detached using Trypsin/0.5% EDTA (GIBCO, Invitrogen, Carlsbad, CA) and washed in phosphate-buffered saline plus 1% bovine serum albumin. Primary antibodies were mouse anti-rat CD45 (1:25; BD Bioscience, Franklin Lakes, NJ), mouse anti-rat CD73 (1:400; BD Bioscience), and mouse anti-rat CD90 (1:400; BD Bioscience). Cells were blocked in 5% normal goat serum in phosphate-buffered saline and stained with secondary antibody goat anti-mouse-IgG F(ab′)2 APC (Jackson Immunoresearch Laboratories, West Grove, PA). Secondary antibody alone served as control for unspecific binding. Samples were measured on a FACSCalibur flow cytometer with CellQuest software (BD Bioscience) and data were analyzed using FlowJo software (Tree star, Ashland, OR).
Animal procedure and experimental design. Adult male and female Fischer 344 rats (8–9 weeks old) from Scanbur, Stockholm, Sweden, were used. Animal procedures were approved by the Ethical Committee for Use of Laboratory Animals at Lund University, Sweden. Animals were anesthetized with isoflurane (2.5% in O2, Forene; Abbott Scandinavia AB, Solna, Sweden) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). The following coordinates were used for tumor cell inoculation and stem cell grafting (relative to bregma): anterior–posterior: +1.7, medial–lateral: −2.5, and dorso–ventral: −5.0. Tooth bar was set at −3.3 mm. Tumor cells were inoculated at 1 µl/min, using a 10-µl Hamilton syringe. eGFP+ MSCs (2.5 × 106 cells) were grafted at 0.5 µl/min, using a 10-µl Hamilton syringe with a glass micropipette attached to the needle tip. After cell inoculation, the micropipette was kept in place for 5 minutes before being slowly retracted. The above-mentioned parameters were used in all experiments unless otherwise mentioned.
To study MSC migration along tumor extensions and to distant tumor microsatellites, 3,000 N29DsRed tumor cells were inoculated on day 1 into the striatum of adult rats (n = 15). eGFP+ MSCs were grafted on day 10 and the animals were killed on day 5, 10, and 15 following MSC grafting (n = 4–5 animals for each group). For FISH, eGFP+ MSCs were grafted intratumorally into N29wt tumor–bearing female rats (n = 4) using the above-mentioned parameters.
Animals (n = 4 in each group) were treated p.o. once daily for 6 days with either vehicle solution (0.5% Polysorbate80; Sigma, St. Louis, MO, and 10% polyethylene glycol; Sigma, dissolved in water, pH adjusted to 3.5) or Sunitinib (50 mg/kg, kindly provided by Pfizer, New York, NY) dissolved in vehicle solution. Three thousand N32wt glioma cells were inoculated on day 1 and treatment with either vehicle or Sunitinib started on day 12. On day 14, eGFP+ MSCs were grafted into tumors and animals were killed on day 18. Animals were continuously observed for signs of illness.
We performed two survival studies to assess the effect of grafted MSCs on tumor growth. Three thousand N32 tumor cells were inoculated on day 1 and eGFP+ MSCs (n = 8) or control medium (n = 8) was inoculated intratumorally on day 2. In a similar experiment utilizing the invasive N29 tumor model, 5,000 N29 tumor cells were inoculated on day 1 and eGFP+ MSCs (n = 10) or medium (n = 10) was stereotactically injected intratumorally on the following day. Animals were continuously observed and immediately killed when symptoms of tumor growth (e.g., reduced motor activity, reduced washing, weight loss) were seen.
MSCs were administered i.v. to glioma-bearing animals. N29 tumor cells (5,000 cells/5 µl) or N32 tumor cells (3,000 cells/5 µl) were inoculated intracerebrally as previously described and eGFP+ MSCs (2 × 106 cells in 500 µl) were administered during 5 minutes via the tail vein 14 days later. Animals were killed either 2 or 7 days following MSC injections (n = 5 animals for each tumor type and time point).
Immunohistochemistry. Processing of brains and immunohistochemistry on tissue sections were performed as previously described.42 Alternatively, for PDGF-receptor-β immunohistochemistry, brains were removed and placed for 2 minutes in isopentane at a temperature of −50 °C. Brains were kept in −80 °C until sectioned (6 µm) on a freezing microtome. Fresh-frozen tissue sections were mounted onto glass slides and fixated with Acetone for 10 minutes. Primary antibodies were chicken anti-GFP (1:1,500; Chemicon, Temecula, CA), mouse anti-rat RECA (1:100; Serotec, Oxford, UK), mouse anti-VE-Cadherin (1:150; Abcam, Cambridge, UK), mouse anti-NG2 (1:500; Chemicon), rabbit anti-α-sma (1:400; Abcam), and rabbit anti-PDGF-receptor-β (1:100; Abcam). The following secondary antibodies were used: Alexa488 goat anti-chicken (1:500; Molecular Probes, Eugene, OR), Alexa594 goat anti-mouse (Molecular Probes), Alexa594 goat anti-rabbit (Molecular Probes), and Cy5 goat anti-mouse (Jackson Immunoresearch Laboratories). Free-floating sections were mounted onto glass slides. All glass slides were cover-slipped with 1,4-diazabicyclo[2.2.2]octane mounting medium.
FISH. FISH was performed on 25-µm thick tissue sections pretreated with 1 mg/ml proteinase K in phosphate-buffered saline for 1 hour, followed by 20 mg/ml pepsin in 0.01 mol/l HCl for 1 hour. After protease treatment, sections were washed in buffered saline, dehydrated in 70–85–96% ethanol, and codenatured for 10 minutes with the rat Y/12 Probe (Y Cy3; 12 FITC, CA-1631; Cambio, Cambridge, UK) for the rat Y chromosome and rat chromosome 12. Hybridization was performed overnight at 37 °C. Stringency washing was performed in 0.2× saline-sodium citrate for 5 minutes and sections were counterstained by 4′,6-diamidino-2-phenylindole.
Microscopic analysis. Sections were examined with an Olympus BX60 epifluorescense microscope (Olympus, Tokyo, Japan) using ×20 objective or by confocal laser scanning microscopy (Leica Microsystems, Mannheim, Germany).
The capacity of grafted eGFP+ MSCs to migrate within invasive N29DsRed tumors was estimated. A tumor extension was defined as an arm of the tumor, coherent with the main tumor mass, infiltratively growing into the normal brain parenchyma. A distant tumor microsatellite was defined as a cluster of tumor cells, clearly separated from the main tumor mass. Systematic histological analysis of tumor area of interest was performed to determine whether clusters of cells were cross-sections of tumor extensions or microsatellites. The number of tumor extensions and distant tumor microsatellites containing at least one eGFP+ MSC were counted and divided by the total number of tumor extensions and distant tumor microsatellites, respectively. For each animal, one section containing dorsal tumor periphery, one section containing tumor center, and one section containing ventral tumor periphery were quantified. However, 3–10 sections, depending on the size of the microsatellite, were analyzed (to distinguish cross-sections of tumor extensions from microsatellites) for every section that was quantified. Four to five animals at each time point were analyzed.
To assess eGFP+ cells in close association with tumor blood vessels, the number of eGFP+ cells located within the distance of one cell body from tumor vessels (delineated by RECA) were counted within a 0.2 mm × 0.2 mm grid, using a ×20 objective, and divided by the total number of eGFP+ cells within the area. Five animals from day 8 to 12 following MSC grafting were included and three sections per animal were quantified.
Tumor microvessel density was assessed by quantification of the numbers of RECA+ tumor vessels within a 0.2 mm × 0.2 mm grid, using a ×20 objective. The numbers of RECA+ tumor vessels were counted in tumors with high numbers of migratory eGFP+ MSC and in tumors with no eGFP+ MSCs. Four animals from each group were chosen and five fields of each tumor were analyzed.
In the Sunitinib experiment, tumor microvessel density was assessed by quantification of the numbers of RECA+ tumor vessels within a 0.2 mm × 0.2 mm grid, using a ×20 objective. The numbers of eGFP+ MSCs, found migrating from the injection site within or outside tumor to tumor periphery, were counted within a 0.2 mm × 0.2 mm grid, using a ×40 objective. Four fields of each tumor were analyzed.
Statistical analysis. Student's unpaired t-test was used for comparison between groups. Data are presented as means ± SEM and considered significant at P < 0.05. Survival curves were compared using a log rank test. Survival is presented as mean (range).
Supplementary MaterialMaterials and Methods.
Supplementary Material
Acknowledgments
We thank Leif G. Salford (Department of Neurosurgery, Lund University) and Olle Lindvall (Lund Strategic Research Center for Stem Cell Biology and Cell Therapy) for kindly providing laboratory facilities, Catarina Blennow, Susanne Strömblad, and Petra Bergman (Department of Neurosurgery, Lund University) for excellent technical assistance and Henrik Semb (Lund Strategic Research Center for Stem Cell Biology and Cell Therapy) for excellent comments on the manuscript. This work was supported by the Hans and Märit Rausing Charitable Fund, the Swedish Childhood Cancer Foundation, the Crafoord, Segerfalk, Elsa Schmitz, Magnus Bergvall and Lund University Hospital Foundations, the Gunnar Nilsson Cancer Foundation and the Royal Physiographic Society in Lund. The Lund Stem Cell Center is supported by a Center of Excellence grant in Life Sciences from the Swedish Foundation for Strategic Research.
REFERENCES
- Hanahan D., and , Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Neri D., and , Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D., and , Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z., and , Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL., and , Tse VC. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- Spring H, Schuler T, Arnold B, Hammerling GJ., and , Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E., and , Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkanen KJ., and , Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- Jansen M, de Witt Hamer PC, Witmer AN, Troost D., and , van Noorden CJ. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res Brain Res Rev. 2004;45:143–163. doi: 10.1016/j.brainresrev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- Moore XL, Lu J, Sun L, Zhu CJ, Tan P., and , Wong MC. Endothelial progenitor cells' “homing” specificity to brain tumors. Gene Ther. 2004;11:811–818. doi: 10.1038/sj.gt.3302151. [DOI] [PubMed] [Google Scholar]
- Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, et al. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E., and , Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S., and , Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990;136:1393–1405. [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P., and , Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Sakamoto KM. Su-11248 Sugen. Curr Opin Investig Drugs. 2004;5:1329–1339. [PubMed] [Google Scholar]
- Elzaouk L, Moelling K., and , Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006;15:865–874. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ, Hertens E., and , Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ., and , Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Kusters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ, et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002;62:341–345. [PubMed] [Google Scholar]
- Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Yuan X, Hu J, Belladonna ML, Black KL., and , Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- Schichor C, Birnbaum T, Etminan N, Schnell O, Grau S, Miebach S, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P., and , Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., and , Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, et al. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Sato H, Kuwashima N, Sakaida T, Hatano M, Dusak JE, Fellows-Mayle WK, et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12:757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- Siesjö P, Visse E, Lindvall M, Salford L., and , Sjögren HO. Immunization with mutagen-treated (tum-) cells causes rejection of nonimmunogenic rat glioma isografts. Cancer Immunol Immunother. 1993;37:67–74. doi: 10.1007/BF01516944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexell D, Gunnarsson S, Nordquist J., and , Bengzon J. Characterization of the subventricular zone neurogenic response to rat malignant brain tumors. Neuroscience. 2007;147:824–832. doi: 10.1016/j.neuroscience.2007.04.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.