Abstract
Secretion of tumor necrosis factor-α (TNF-α) by macrophages plays a predominant role in the development and progression of rheumatoid arthritis. We demonstrate that knockdown of TNF-α expression in systemic macrophages by intraperitoneal (i.p.) administration of chitosan/small interfering RNA (siRNA) nanoparticles in mice downregulates systemic and local inflammation. Chitosan nanoparticles containing an unmodified anti-TNF-α Dicer-substrate siRNA (DsiRNA) mediated TNF-α knockdown (~66%) in primary peritoneal macrophages in vitro. The presence of Cy3-labeled nanoparticles within peritoneal macrophages and specific TNF-α knockdown (~44%) with TNF-α siRNA after i.p. injection supports our therapeutic approach. Downregulation of TNF-α-induced inflammatory responses arrested joint swelling in collagen-induced arthritic (CIA) mice dosed i.p. with anti-TNF-α DsiRNA nanoparticles. The use of 2′-O-Me-modified DsiRNA resulted in the lowest arthritic scores and correlated with reduced type I interferon (IFN) activation in macrophages in vivo compared with unmodified DsiRNA. Histological analysis of joints revealed minimal cartilage destruction and inflammatory cell infiltration in anti-TNF-α-treated mice. The onset of arthritis could be delayed using a prophylactic dosing regime. This work demonstrates nanoparticle-mediated TNF-α knockdown in peritoneal macrophages as a method to reduce both local and systemic inflammation, thereby presenting a novel strategy for arthritis treatment.
Introduction
Rheumatoid arthritis is a chronic inflammatory disease affecting ~1% of the population. Joint destruction associated with this autoimmune condition results from synovial infiltration by helper T cells, and mono-polymorphonuclear leukocytes eliciting local proinflammatory and regulatory cytokine effects,1 modulated predominantly by tumor necrosis factor-α (TNF-α) secreted by systemic-derived macrophages. Intervention of the inflammation cascade in mice with anti-TNF-α,2 CD4+ T-cell receptor,3 and interleukin (IL)-1 (ref. 4) monoclonal antibodies provides the basis for present clinical immunotherapy treatments;5 however, cost and possible auto-immunity to antibodies and side-effects associated with standard methotrexate and corticosteroid combinational therapy necessitates different approaches.
Small interfering RNA (siRNA)-mediated knockdown of proinflammatory cytokines at the messenger RNA (mRNA) level (termed RNA interference),6 offers an alternative therapeutic strategy to overcome inflammatory conditions. In this process, double-stranded RNAs are cleaved by the cellular nuclease Dicer into short 21–22mer fragments referred to as siRNA, which enter a ribonuclear protein complex termed the RNA-induced silencing complex. Guided by the antisense strand of the siRNA, this complex mediates a specific degradation of the corresponding mRNA.7
Direct intra-articular injection of TNF-α-specific siRNA has been reported to reduce joint inflammation in murine collagen-induced arthritis (CIA) and supports the use of siRNA-based therapies.8 Furthermore, systemic delivery of TNF-α-specific siRNA using cationic lipid-based nanoparticles show effective anti-inflammatory effects in arthritic mice.9 Delivery is a key determinant as to whether or not RNAi-based therapeutics will have clinical relevance. In this regard, delivery describes extracellular transport to target cells, intracellular RNA trafficking, and processing. The recruitment of systemic macrophages to local sites and pivotal role during inflammation10,11 makes these an ideal target for RNAi-based TNF-α therapies. We have previously reported knockdown of endogenous genes in primary macrophages using a chitosan/siRNA nanoparticle (or polyplex) system;12 however, systemic delivery of polyplexes by the intravenous route is restricted by serum protein–induced aggregation and clearance to the liver.13,14 As an alternative, i.p. injection allows delivery into a blood-free macrophage-rich environment. This has been exploited as a strategy to silence systemic TNF-α production in mice using liposomal/siRNA formulations for the treatment of murine sepsis15 and investigating antiviral host immunity in herpes simplex virus infection in mice.16 RNA duplexes, which are ~27 nucleotides in length, termed Dicer-substrate siRNA (DsiRNA),17,18 have been shown to exhibit higher silencing activity due to processing by Dicer before RNA-induced silencing complex incorporation. Moreover, incorporation of 2′-O-methyl modified (2′-O-Me) nucleotides,19 recently in Dicer-substrate duplexes20 and asymmetric strand design17 eliminate nonspecific innate immune effects, e.g., type I interferon (IFN), induced by RNA duplexes21 and toll-like receptor (TLR)-rich endosomal localization that is potentiated with nanoparticle delivery.
This work presents a novel treatment for arthritis using polymeric nanoparticles, based on systemic knockdown of TNF-α production for reduction in inflammation at local sites by i.p. targeting of macrophages. Investigation into chitosan/DsiRNA nanoparticle knockdown of TNF-α production in stimulated peritoneal macrophages is followed by determination of TNF-α silencing and type I IFN induction on macrophages harvested after i.p. administration using modified and unmodified DsiRNA formulations. The approach was then evaluated as a therapeutic and prophylactic treatment strategy in a type II collagen murine arthritis model by arthritic scoring and histological joint analysis.
Results
Peritoneal macrophage studies
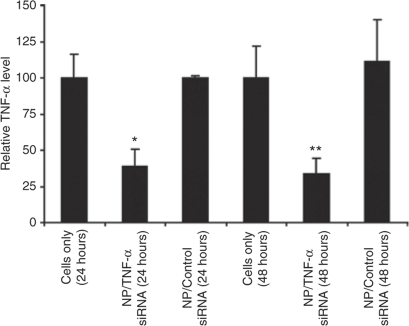
The fundamental requirement for the strategy presented in this work is an ability to silence TNF-α in difficult-to-silence peritoneal macrophages. We have previously demonstrated knockdown of endogenous enhanced green fluorescent protein in peritoneal macrophages harvested from a transgenic enhanced green fluorescent protein mouse with the chitosan-based nanoparticle system.12 Self-assembly-driven nanoparticles formed between 27mer DsiRNA TNF-α or control siRNA and chitosan (N:P 63) ranging from ~350 to 450 nm in size were used for in vitro and in vivo silencing studies. The decrease in TNF-α secretion, detected by enzyme-linked immunosorbent assay, in the supernatants of lipopolysaccharide (LPS)-stimulated peritoneal macrophages was used as the determinant for RNA interference in vitro. We accomplished similar levels of TNF-α knockdown at 24 (61.3%) and 48 hours (66.0%) after transfection with chitosan nanoparticles containing unmodified anti-TNF-α-siRNA (at 50 nmol/l concentration per well) compared to nontreated LPS-stimulated control (Figure 1). A slight increase in TNF-α production was seen in the control siRNA nanoparticles (~11%) after 48 hours.
Figure 1.
Nanoparticle-mediated TNF-α silencing in murine primary macrophages in vitro. Isolated peritoneal macrophages from C57BL/6J mice were transfected with chitosan/TNF-α-specific siRNA or chitosan/TNF-α control for 4 hours (50 nmol/l siRNA/well). The level of TNF-α in the cellular supernantant, measured 5 hours after LPS stimulation 24 or 48 hours after transfection, was used as a measure of TNF-α knockdown (normalized to untreated macrophages (cells only), arbitrarily set to 100). Error bars represent ±SD. Significant differences between NP/TNF-α and NP/Control siRNA are indicated (*P < 0.01; **P < 0.02; two-sided t-test). Data from single experiment with triplicate samples.
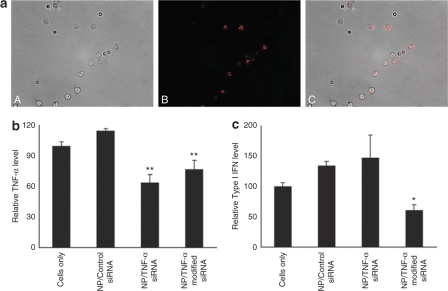
Investigation into the ability of i.p.-administered nanoparticles to deliver siRNA into peritoneal macrophage targets was carried out in female C57BL/6J mice. Nanoparticles containing Cy3-labeled DsiRNA were clearly evident within peritoneal macrophages harvested 4 hours after injection with a 200 μl nanoparticle volume (Figure 2a). Punctate Cy3 fluorescence restricted to cytosolic but not nuclear (DAPI blue stain; data not shown) localization in the majority of cells correlated with previous intracellular studies using this nanoparticle system.12
Figure 2.
Nanoparticle-mediated transfection and TNF-α knockdown in murine peritoneal macrophages in vivo. The ability of chitosan/siRNA nanoparticles to transfect and silence TNF-α production in peritoneal macrophages after i.p. administration. (a) Fluorescence micrograph showing chitosan nanoparticle uptake in macrophages extracted 4 hours after i.p. administration of 200 μl chitosan/Cy3-siRNA nanoparticles (panel A, light image; panel B, Cy3-labeled siRNA (red); panel C, fluorescence overlay of Cy3-labeled siRNA (red) and cells). (b) Macrophage TNF-α production measured by enzyme-linked immunosorbent assay (ELISA) (normalized to untreated LPS-stimulated macrophages (cells only), set to 100; error bars represent ±SD) in LPS-stimulated peritoneal macrophages extracted 2 hours after i.p. administration of 200 μl of chitosan nanoparticles containing ~5 μg of 2′-O-methyl modified anti-TNF-α DsiRNA (NP/TNF-α modified siRNA) or unmodified anti-TNF-α DsiRNA (NP/TNF-α siRNA) or TNF-α Dicer-substrate control siRNA (NP/Control siRNA) in mice (N = 3). Two-sided t-test statistics relative to chitosan/TNFα-control are given (**P < 0.003). (c) ELISA determination of type I IFN expression in macrophages shown in b (normalized to control untreated LPS-stimulated macrophages (cells only), set to 100; error bars represent ± SD). Two-sided t-test statistics for unmodified versus modified siRNA is shown (*P < 0.03).
Nanoparticle-mediated in vivo silencing of peritoneal macrophage TNF-α production was determined with unmodified and 2′-O-Me modified TNF-α DsiRNA at ~5 μg per dose. Two hours after i.p. injection of nanoparticles, the peritoneal macrophages were harvested and incubated for 24–48 hours ex vivo to allow macrophage adherence and RNAi to function. Significant reduction in the TNF-α level in the supernatants of LPS-stimulated peritoneal macrophages was revealed for both unmodified (36% and 44.3%) and modified (23% and 33%) compared to the untreated and control siRNA nanoparticles, respectively (Figure 2b). The relatively low knockdown may be a consequence of sampling freshly recruited nontransfected macrophages or loss of migratory silenced macrophages from the peritoneum during the 2-hour incubation period. This is supported by lower knockdown in peritoneal macrophages isolated 24 hours after nanoparticle incubation (data not shown). The induction of innate immune responses such as type I IFN by the RNA duplexes and nanoparticle-mediated delivery into a TLR environment was also evaluated in the harvested macrophages. Macrophages taken from mice treated with nanoparticles containing modified DsiRNA revealed lower levels of type I IFN in comparison with both control siRNA (54.8% reduction) and unmodified DsiRNA (58.8% reduction) formulations (Figure 2c). Cells treated with the modified DsiRNA formulation also showed reduced amounts of type I IFN in comparison with untreated, which suggests noninduction of innate immunity. The same physicochemical characteristics such as particle size and charge and amount of siRNA in both nanoparticle systems suggest that the reduced type I IFN induction is indeed due to nucleotide modification.
Murine type II CIA treatment
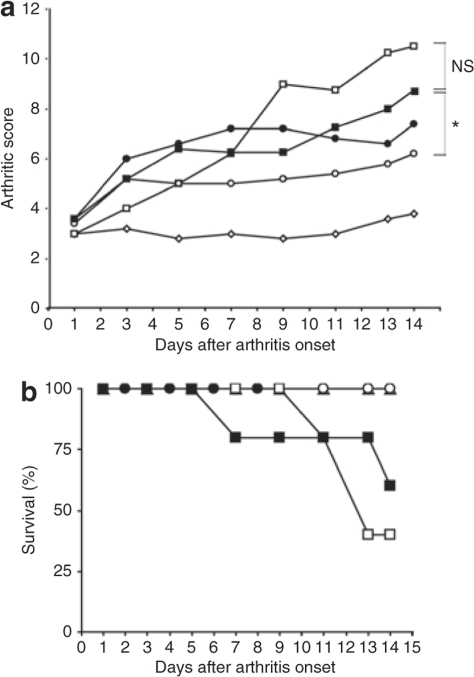
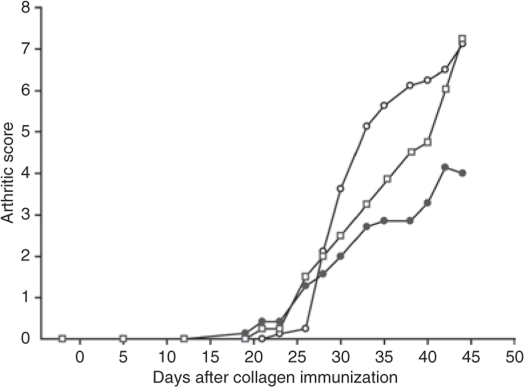
The therapeutic potential of silencing TNF-α production in systemic macrophages with polymeric nanoparticles was investigated in a collagen type II DBA/I arthritic model.22 Onset of arthritis was evident as joint inflammation at ~28 days after collagen immunization and presented on an arthritic scoring scale in Figure 3a (scale: 0 = normal, 1 = mild swelling, 2 = moderate swelling, 3 = severe swelling of entire paw, 4 = maximal inflammation in multiple joints; sum total for all four limbs 0–16). Rapid and aggressive arthritis progression, characteristic of this arthritic model, was observed in animals treated i.p. with sodium acetate buffer (0.2 mol/l, pH 5.5) that resulted in reduced animal numbers over the course of the study because of the termination of animals exhibiting extreme inflammation. The arthritic scores were carried over if mice were killed to counteract the dropout. The animals were dosed five times (days 1, 3, 5, 7, and 9) i.p. with 200 μl of nanoparticle formulations containing 5 μg unmodified or 2.5 μg 2′-O-Me modified TNF-α DsiRNA or control DsiRNA starting at an arthritic score ~3 (day 1 in Figure 3). Accelerated increase in inflammation during the acute arthritic phase at 1–3 days was observed with all DsiRNA formulations compared to the dexamethasone positive control which stayed at ~3 over the dosing period. Arrested development of inflammation was observed with the modified formulation after the second dose (day 3), while the unmodified and control siRNA continued to rise. The trend with the modified siRNA formulation continued during the dosing period showing a slight rise from the initial 3.5 score to 5. After termination of dosing, control siRNA nanoparticles and buffer-treated mice rose with accelerated progression. The reduced arthritic score of the modified anti-TNF-α siRNA nanoparticle–treated mice compared to those with control siRNA nanoparticles was statistically significant (P = 0.028), whereas the difference between control siRNA nanoparticles and buffer treated mice was not. This confirms specific TNF-α silencing effects of the siRNA.
Figure 3.
Therapeutic effects in collagen-induced arthritic (CIA) mice after i.p. administration of chitosan/siRNA nanoparticles. (a) The therapeutic effect of i.p. administration of chitosan/siRNA-TNF-α nanoparticles on the clinical progression of established collagen-induced arthritis. Animals were scored for clinical symptoms using an arthritic scoring method for level of joint inflammation (scale: 0 = normal, 1 = mild swelling, 2 = moderate swelling, 3 = severe swelling of entire paw, 4 = maximal inflammation in multiple joints; sum total for 4 limbs = 16). Day 1 corresponds to onset of arthritis and assignments to treatment groups (N = 5). Animals were dosed i.p. on days 1, 3, 5, 7, and 9 with 200 μl nanoparticles containing either unmodified (closed circles) or 2′-O-Me modified (open circles) anti-TNF-α DsiRNA or control (closed squares) DsiRNA siRNA, or 0.2 mol/l sodium acetate buffer (open squares). Dexamethasone control group were dosed (400 μg/kg) daily (days 1–14) subcutaneously (open diamonds). Kruskal–Wallis one-way analysis of variance on ranks, followed by pairwise multiple comparison procedures (Dunnett's method) for 2′-O-Me modified versus control siRNA is shown (*P = 0.028), NS, non significant (b) The effect of nanoparticle treatment on CIA mouse survival in groups shown in a. Data from single experiment.
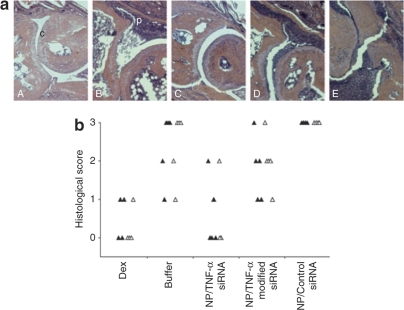
The survival curves of the animals showed a clear distinction between groups treated either with TNF-α DsiRNA nanoparticles (modified and unmodified) and dexamethasone (100% survival) or the control siRNA nanoparticles and buffer (60% and 40% survival, respectively) (Figure 3b). Histological analysis was performed on paws taken from treatment groups (5 days after treatment) and evaluated for joint destruction defined as cartilage and bone erosion, and joint inflammation that measures hyperplasia of the synovial lining layer and cellular infiltration characteristic of this arthritic model. Joint and cartilage integrity was maintained in animals treated with nanoparticles containing unmodified anti-TNF-α DsiRNA (Figure 4c) and exhibited similar effects to the dexamethasone control (Figure 4a). No panus was evident in this group suggesting noninfiltration by inflammatory cells. In comparison, groups treated with chitosan/control siRNA (Figure 4e) and buffer (Figure 4b) exhibited extreme cartilage and bone destruction occupied predominately by mono- and polymorphonuclear leukocytes. Interestingly, mice treated with 2′-O-Me anti-TNF-α DsiRNA showed some degree of cartilage damage and evidence of cellular infiltration (Figure 4d). This trend is confirmed by histological scoring (scale: 0 = normal, 1 = mild, 2 = moderate, 3 = severe, 4 = maximal) for cartilage damage and inflammation shown in Figure 4b; however, control siRNA and buffer showed higher scores than the modified-treated group. It is possible that these abnormalities would improve if higher dosing were used with the modified duplexes.
Figure 4.
Histopathological evaluation of joints from CIA mice dosed i.p. with chitosan/siRNA nanoparticles. (a) Histological sections from nanoparticle-treated mice. Paws were taken 5 days after treatment with dexamethasone (Panel A), 0.2 mol/l sodium acetate buffer (panel B), chitosan nanoparticles containing unmodified (panel C), 2′-O-Me modified (panel D) anti-TNF-α DsiRNA or control siRNA (panel E). Formalin fixed, decalcified, paraffin-embedded, and hematoxylin and eosin–stained 2-μm sections were assessed for cartilage and bone erosion, and cellular infiltration as evidence of joint inflammation. Representative images of the different groups are shown. Intact cartilage (c) and Panus-forming cellular infiltrates (p) are marked in panels A and B. (b) Histological scoring. Histological scores (scale: 0 = normal, 1 = mild, 2 = moderate, 3 = severe, 4 = maximal) used for cartilage damage (closed triangles) and cellular infiltration (open triangles) evaluation are shown for the different groups (N = 5). Dex, Dexamethasone. Counting was performed using a doublet scoring method.
The prophylactic application of TNF-α silencing was investigated in the CIA model (Figure 5). The rational for this experiment was to silence TNF-α production during the inductive phase of arthritis (day 0 to 28 after collagen immunization) to delay the onset of inflammation. Animals were dosed i.p. with either 2′-O-Me TNF-α-DsiRNA or modified control siRNA nanoparticles (5 μg in 200 μl) 2 days before collagen immunization and after immunization on days 1, 5, 9, 13, 17, and 21. From day 26, control siRNA nanoparticles and buffer-treated mice indicated signs of joint inflammation with scores of 1.3 and 1.5 compared to the modified formulation (0.3). Interestingly, 2 days after removal of the mice from 2′-O-Me TNF-α DsiRNA nanoparticle treatment, accelerated progression of joint inflammation resulted (the arthritic score rising from ~0.3 on day 26 to ~5.3 by day 31) compared to the control siRNA nanoparticles (1.5–2.5) and untreated group (1.5–3.2) during the same period.
Figure 5.
Prophylactic effects in CIA mice after i.p. administration of chitosan/siRNA nanoparticles. The effect of i.p. administration of chitosan/siRNA-TNF-α nanoparticles on the onset of collagen-induced arthritis. Animals were scored for clinical symptoms using an arthritic scoring method for level of joint inflammation indicated in Figure 3. Animals (N = 8) were dosed i.p. with 200 μl chitosan nanoparticles containing 5 μg of 2′-O-Me modified (open circles) anti-TNF-α DsiRNA or 2′-O-Me modified control siRNA (closed circles) 2 days before collagen immunization and after immunization on days 1, 5, 9, 13, 17, and 21. Untreated group is shown (open squares). Data from single experiment.
Discussion
This work describes a novel approach for inflammatory reduction in rheumatoid arthritis by chitosan/siRNA nanoparticle–mediated TNF-α silencing in peritoneal macrophages. Rheumatoid arthritis is a systemic inflammatory disease, manifested locally as erosion of the joints. Pathogenesis from early to chronic rheumatoid synovitis involves systemic-derived cellular infiltration and cytokine production that modulate cellular differentiation, recruitment, and activation responsible for cartilage and bone destruction.1 Recruited peripheral macrophages23,24 play a predominant role throughout this process by (i) expression in the joints of cytokines, chemokines (including IL-1, TNF-α, IL-8), and matrix-degrading enzymes and (ii) systemically secreted IL-1 and TNF-α-mediated control of IL-6 expression involved in the acute phase response.11 Reduced inflammation shown in an arthritic rat model by depleting systemic and joint macrophages with chlodronate-loaded liposomes further supports these cells as therapeutic targets.25 Furthermore, the improvement in clinical effects with competitive receptor-based immunotherapies against TNF-α5 provides the rational for the TNF-α target in this study.
A significant TNF-α knockdown was observed in vitro in stimulated primary murine peritoneal macrophages 24 and 48 hours after transfection with chitosan/TNF-α-specific nanoparticles compared to untreated and control siRNA nanoparticles. A slight increase in TNF-α level seen with the control formulation (but not in non-LPS stimulated macrophages) is likely due to unspecific effects or macrophage activation shown to occur in isolated peritoneal macrophages26 and TNF-α stimulation demonstrated in macrophage cell lines with certain soluble chitosan derivatives.27 The effect of TNF-α-specific knockdown, however, seemingly negates this problem.
The ability to target peripheral macrophages after intravenous administration is compromised by the serum instability of polyplexes.13 Our approach is to inject nanoparticles into the peritoneal cavity to allow delivery into a serum-free environment rich in peritoneal macrophages involved in systemic immunity. Activation of IL-1, IL-6, and TNF-α production in peritoneal macrophages during the acute and chronic phase of murine antigen-induced arthritis10,28 implicates these cells. Chitosan nanoparticles were found within labeled macrophages harvested from the peritoneum 4 hours after injection similar to lipid-based i.p. delivery of siRNA.29,30,31 This accumulation exploits the susceptibility of nanoparticles for phagocytic capture by cells of the mononuclear phagocyte system,14 enhanced by carbohydrate recognition by peritoneal macrophages demonstrated i.p. for chitosan-coated nanoparticles.30 TNF-α knockdown was demonstrated in harvested peritoneal macrophages, after i.p. administration of unmodified and 2′-O-Me-modified anti-TNF-α DsiRNA nanoparticles compared to untreated and control siRNA. To our knowledge this is the first direct demonstration of TNF-α knockdown in peritoneal macrophages after i.p. injection. Interestingly, greater knockdown was observed with the unmodified formulation that may reflect an inhibitory effect from the nucleotide modification. The relatively low knockdown efficiency could be due to particle uptake–mediated activation of the macrophages that potentiates macrophage dissemination similar to macrophage clearance shown during inflammation.32 This and possible consequent recruitment of fresh macrophages prior to harvest may result in an underestimation of TNF-α knockdown. Type I IFN levels were elevated in macrophages taken from mice treated with unmodified TNF-α-specific and control siRNA formulations. This may reflect macrophage activation supported by increased TNF-α levels in vivo and in vitro with unmodified control siRNA. Macrophages from mice injected with modified anti-TNF-α DsiRNA-containing nanoparticles, however, exhibited lower type I INF levels suggesting an unspecific siRNA effect of the unmodified duplex. The 27mer duplexes used in this study have been designed to mimic the structural features of precursor microRNA to facilitate processing by Dicer for more potent RNAi.17,31 A nonspecific induction of innate immune inflammatory responses such as type I IFN are associated with extended double-stranded RNA interaction with the TLR-7 potentiated by endosomal delivery of oligonucleotides using nanoparticles. The approach of incorporation of 2′-O-methyl uridine or guanosine nucleotides into the duplex strand as a method to reduce TLR-7 interaction and associated off-target effects19 is supported by our work.
The CIA model used in this work for studying therapeutic and prophylactic intervention is a B- and T-cell dependent arthritis model closely resembling human rheumatoid arthritis.22 Mice administered after arthritic onset with a total dose of 25 μg of nonmodified (TNF-α and control) and 12.5 μg modified siRNA-containing nanoparticles exhibited lower arthritic scores over the 14-day period than mice treated with the control siRNA nanoparticles or buffer. The high joint inflammation, evident by a high arthritic score (~3–4) at the commencement of dosing, ensured that any therapeutic effect is significant in such a highly inflamed state. The rapid disease progression, characteristic of this model, results in a requirement to sacrifice severely inflamed animals and lower survival numbers in control groups. This was the case for some of the animals in the control siRNA and the buffer group that had to be put down before the 15th day. This emphasizes the importance of histological analysis and scoring data presented, in addition to the arthritic score. Surviving animals in the control groups could be less prone to the inflammatory effects of TNF-α. This could explain why two of the animals in the buffer group had a low degree of joint destruction when scoring the histology. In addition, animals killed early in the experiment may have lower scores for joint destruction and inflammation. Mice receiving 2′-O-Me DsiRNA formulations showed lowest arthritic scores, even with less siRNA that correlates with our demonstration of reduced induction of innate immunity in macrophages and emphasizes its importance in anti-inflammatory treatments. In both unmodified and modified TNF-α formulations, a slight rise in arthritic scores was observed on termination of treatment that reflects the transient nature of RNAi. Dexamethasone-treated mice were used as a control to untreated buffer; however, the differing mode of action (nonspecific immunosuppressive) and dosing regime (14× subcutaneous injection) does not allow direct comparison with the siRNA approach. Histological sections of joints taken ~5 days after treatment revealed intact cartilage and bone with no evidence of inflammatory panus formation in the groups receiving dexamethosone and unmodified TNF-α formulation in comparison with control siRNA nanoparticles and buffer control. Some degree of inflammation after treatment of the 2′-O-Me DsiRNA could reflect a combined suppression of TNF-α and type I IFN effect, a notion supported by the recent report of the anti-inflammatory role of dsRNA-mediated type I IFN in murine CIA.33 Delay of arthritic onset was achieved with 2′-O-Me DsiRNA administered before CIA immunization and throughout the inductive phase of arthritis. Rapid elevation of joint inflammation after treatment likely reflects the immunoregulatory effects of TNF-α after suppression similar to inflammatory flares exhibited in patients removed from anti-TNF-α antibody treatment.34 Future studies should extend treatment to see more pronounced differences in arthritic onset offset in light of this bounce effect. Animals treated with control particles do show less inflammation than either the match and untreated, which may reflect anti-inflammatory properties of chitosan35 or nondevelopment of arthritis in some mice that occurs with the CIA model. Immunotherapy-based intervention of the upstream inflammatory modulator TNF-α has been shown as an effective treatment in rheumatoid arthritis;5 however, other macrophage cytokines such as IL-15, 18, and 32 (ref. 1) may be clinically relevant RNAi targets in the future for our nanoparticle approach. In addition, we plan to test possible off-target effects using a second anti-TNF-α DsiRNA having a different sequence. Use of 2′-O-Me RNA blocks unwanted innate immune effects but does not control for gene regulatory events that may arise from the participation of the synthetic RNA duplex with microRNA machinery. Showing similar biological results from an independent sequence will help eliminate this possibility. The i.p. route is used in the treatment of a number of diseases, providing a potential clinically relevant approach for rheumatoid arthritis in humans.
Our work with polymeric nanoparticles and that of others using liposomal carriers15,16 promote the i.p. route for induction of TNF-α-siRNA-mediated systemic anti-inflammatory effects. Other studies have used local (intraarticular)8 and systemic (intravenous)9 administration of 21mer TNF-α-siRNA for arthritis treatment in a murine CIA model. In the study by Schiffelers et al.,8 direct joint injection of naked TNF-α siRNA inhibited joint inflammation; however, electroporation was needed as a method to improve siRNA cellular entry without vector involvement. Khoury et al. have showed impressive therapeutic intervention in inflammation following intravenous administration of a cationic liposomal-based carrier.9 Macrophages were the suggested therapeutic target; however, direct evidence for TNF-α silencing in macrophages in vivo was not demonstrated but serum levels were measured. Predictably, nonsteric particles accumulated in mononuclear phagocyte system organs (termed first-pass effect), presumably within fixed tissue macrophages, e.g., Kupffer cells that might reduce efficiency of the i.v. approach. Our i.p. approach circumvents this drawback and enables direct delivery to pathogenic-relevant macrophages.
This proof-of-concept work is the first demonstration of a novel treatment for arthritis by targeting TNF-α knockdown in systemic macrophages by chitosan nanoparticles. Issues such as administration route, siRNA design, target cell, and induction of the innate immune system addressed in this article are important considerations for development of clinically relevant RNAi-based anti-inflammatory treatments.
Materials and Methods
Chemicals and siRNA. Chitosan (114 kDa, 84% deacetylation) was prepared by Bioneer A/S (Hørsholm, Denmark). TNF-α-specific and control siRNA duplex was supplied by Integrated DNA Technologies (Coralville, USA). TNF-α DsiRNAs contains the sequence: sense, 5′-pGUCUCAGCC UCUUCUCAUUCCUGct-3′, antisense 5′-AGCAGGAAUGAGAAGAGG CUGAGACAU-3′; DsiRNAs negative control (control siRNA) contains the sequence: sense, 5′ pCUUCCUCUCUUUCUCUCCCUUGUga-3′, antisense 5′- UCACAAGGGAGAGAAAGAGAGGAAGGA-3′; and modified 2′-O-Me TNF-α siRNA contains the sequence and modification (m) pattern: sense, 5′- GUCUCAGCCUCUUCUCAUUCCUGct-3′, antisense (m) 5′-AGCAGGAAmUGmAGmAAmGAmGGmCUmGAmGAmCmAmU-3′ 2′-O-Me negative control 5′-pCUUCCUCUCUUUCUCUCCCUUGUga-3′, antisense 5′- UCACAAGGmGAmGAmGAmAAm GAmGAmGGmAAmGmUmU-3′; enhanced green fluorescent protein siRNA containing a fluorescent Cy3-labeled 5′ sense strand was used for cellular uptake studies 5′-Cy3-t*CCUUCCUCUCUUUCUCUCCC UUGUG*a-3′, antisense 5′-Cy3-t*CACAAGGGAGAGAAAGAGAGG AAG*a. RNA bases are uppercase, DNA bases are lower case, mN represents a 2′-O-Me base, “p” represents a 5′-phosphate, and “*” represents a phosphorothioate internucleoside linkage.
Arthrogen-CIA complete Freunds adjuvant and bovine collagen type II immunization grade (IMBII-sol) was purchased from MD BioSciences (St Paul, MN) and Dexamethasone from Scanvet. Goat antimouse TNF-α detection antibody, goat antimouse TNF-α capture antibody, Streptavidin-horseradish peroxidase, and tetramethylbenzidine were purchased from R&D Systems.
Formation of chitosan/siRNA nanoparticles. Chitosan was dissolved in sodium acetate buffer (0.2 mol/l NaAc, pH 4.5) to obtain a 1 mg/ml solution and then adjusted to pH 5.5 for in vivo work. Twenty microliter of siRNA (100 μm) in nuclease-free water was added to 1 ml of filtered chitosan (800 μg/ml) while stirring and left for 1 hour. To calculate specific N:P ratios (defined as the molar ratio of chitosan amino groups/RNA phosphate groups) a mass-per-phosphate of 325 Da was used for RNA and mass-per-charge of chitosan was 167.88 (84% deacetylation). Nanoparticles formed at N:P 63 were used in all experiments.
Photon correlation spectroscopy on chitosan/siRNA nanoparticles. The hydrodynamic size of chitosan/siRNA nanoparticles were determined by photon correlation spectroscopy using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Photon correlation spectroscopy was performed at 25 °C in sodium acetate buffer in triplicate with sampling time and analysis set to automatic.
TNF-α knockdown in murine peritoneal macrophages in vitro. Six- to eight-week-old female C57BL/6J mice were killed by cervical dislocation and injected intraperitoneally (i.p.) with 5 ml of wash medium (PBS supplemented with 5% LPS-free heat-inactivated fetal calf serum (Cambrex, Baltimore, MD) and 4 μl/ml heparin. The abdomen was agitated gently, the peritoneum exposed and breached, and the media removed using a syringe. The suspension was centrifuged (450g for 10 min) and the pellet resuspended in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics. The suspension was plated on a 96-well cell culture plate at 350,000 cells per well in 100 μl. The macrophages were allowed to adhere for 2 hours before media containing nonadherent cells was removed. Fresh preheated media were then added the cells. After 20 hours the media were removed and replaced with serum-free RPMI1640 and chitosan/siRNA nanoparticles added at 50 nmol/l siRNA concentration. After 4 hours, media were removed and replaced with fresh media containing 10% fetal calf serum. After 24 or 48 hours the cells were stimulated with LPS (100 ng/ml). Supernatants were harvested 5 hours later and TNF-α was detected by an enzyme-linked immunosorbent assay.
Transfection and TNF-α knockdown in murine peritoneal macrophages in vivo. Two hundred microliters of chitosan/siRNA nanoparticles (unmodified or 2′-O-Me TNF-α or control DsiRNA or Cy3-labeled siRNA) were injected i.p. into C57BL/6J mice. Peritoneal macrophages were isolated, according to the procedure described earlier, 2 hours after injection and plated in 96-well plates or mounted with DAPI nuclear stain onto glass slides. Uptake of Cy3-labeled siRNA was monitored by a Zeiss semi-confocal epifluorescence microscope. TNF-α levels in the harvested supernatants of LPS-stimulated macrophages were measured 24 hours after injection according to the above-mentioned protocol. IFN-α/β bioactivity was measured by an L929-cell-based bioassay. L929 cells (2 × 104 cells/well in 100 μl) in modified Eagle medium with 5% fetal calf serum were incubated overnight at 37 °C in successive twofold dilutions of samples or murine IFN-α/β as the standard. Subsequently, vesicular stomatitis virus (VSV/V10) was added to the wells, and the cells were incubated for 2–3 days. The dilution mediating 50% protection was defined as 1 U of IFN-μ/β/ml. The specificity was determined by neutralization with two polyclonal rabbit antibodies directed against IFN-α and IFN-β.
Inflammatory downregulation in collagen type II–induced arthritic mice after i.p. administration of chitosan/siRNA nanoparticles. The collagen type II DBA/I arthritic model was used to evaluate the anti-inflammatory potential of chitosan/siRNA formulations. Forty, 6–9 week DBA/I mice were immunized subcutaneously on study day 0 with 0.1 ml of Anthrogen-CIA collagen emulsion prepared according to the manufacturer's protocol. The limbs of the mice were scored for clinical symptoms of arthritis under veterinary supervision at Pipeline Biotech A/S (Trige, Denmark). Between days 28 and 35, mice arthritic scores 1–3, were divided into five groups of five. Mice were i.p. dosed with 200 μl of chitosan/siRNA nanoparticles (5 μg unmodified or 2.5 μg 2′-O-Me TNF-α DsiRNA or 5 μg control siRNA) on days 1, 3, 5, 7, and 9 (where day 1 is onset of arthritis). The abdomen was massaged gently after each administration to ensure uniform distribution throughout the peritoneal cavity. Mice administered subcutaneously on days 1–14 with Dexamethasone were used as a positive control. Animals were assessed for clinical symptoms from day 1 to day 14 using a standard scoring system for evaluation of joint inflammation. Additionally, paw volume was measured using a plethysmometer based on volume displacement. Mice were terminated on day 14 except those showing severe signs of inflammation which were killed earlier. Paws were then fixed in formalin, decalcified, paraffin embedded, and 2-μm sections assessed for evidence of joint inflammation. For prophylactic studies, animals were dosed i.p. with either 2′-O-Me TNF-α DsiRNA or 2′-O-Me negative control DsiRNA nanoparticles (5 μg in 200 μl) 2 days before collagen immunization and after immunization on days 1, 5, 9, 13, 17, and 21. Inflammation was evaluated according to the above protocol.
Groups were compared using Kruskal–Wallis one-way analysis of variance on ranks, followed by pairwise multiple comparison procedures (Dunnett's Method), P < 0.05 was considered significant.
Acknowledgments
We thank Kirsten S Petersen for excellent technical assistance and Carsten Leander Buss for supervision of animal experiments conducted at Pipeline A/S. Our thanks to Christian Holm for help with macrophage antibody labeling and Ulrik Rahbek for fluorescence microscopy. We acknowledge Karina Dalsgaard Sørensen for constructive comments. This work was supported by the Danish Research Council and the EU-FP6 RIGHT program.
REFERENCES
- Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001;45:101–106. doi: 10.1002/1529-0131(200102)45:1<101::AID-ANR90>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M., and , Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Mason LJ, Feldmann M., and , Maini RN. Synergy between anti-CD4 and anti-tumor necrosis factor in the amelioration of established collagen-induced arthritis. Proc Natl Acad Sci USA. 1994;91:2762–2766. doi: 10.1073/pnas.91.7.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RO, Marinova-Mutafchieva L, Feldmann M., and , Maini RN. Evaluation of TNF-α and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-α/anti-CD4 therapy. J Immunol. 2000;165:7240–7245. doi: 10.4049/jimmunol.165.12.7240. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Williams RO., and , Maini RN. Immunotherapy for rheumatoid arthritis. Curr Opin Immunol. 2001;13:611–616. doi: 10.1016/s0952-7915(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Song E, Lee SK., and , Shankar P. Interfering with disease: opportunities and roadblocks to harnessing RNA interference. Trends Mol Med. 2003;9:397–403. doi: 10.1016/S1471-4914(03)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Yalcin A, Weber K., and , Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Schiffelers RM, Xu J, Storm G, Woodle MC., and , Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis Rheum. 2005;52:1314–1318. doi: 10.1002/art.20975. [DOI] [PubMed] [Google Scholar]
- Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C, et al. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor α in experimental arthritis. Arthritis Rheum. 2006;54:1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- Simon J, Surber R, Kleinstauber G, Petrow PK, Henzgen S, Kinne RW.Systemic macrophage activation in locally-induced experimental arthritis J Autoimmun 200117127–136.et al [DOI] [PubMed] [Google Scholar]
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E., and , Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Dash PR, Read ML, Fisher KD, Howard KA, Wolfert M, Oupicky D, et al. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with a multivalent hydrophilic polymer and retargeting through attachment of transferrin. J Biol Chem. 2000;275:3793–3802. doi: 10.1074/jbc.275.6.3793. [DOI] [PubMed] [Google Scholar]
- Howard KA, Dash PR, Read ML, Ward K, Tomkins LM, Nazarova O.Influence of hydrophilicity of cationic polymers on the biophysical properties of polyelectrolyte complexes formed by self-assembly with DNA Biochim Biophys Acta 20001475245–255.et al [DOI] [PubMed] [Google Scholar]
- Sorensen DR, Leirdal M., and , Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Welander PV, Edwards CK, 3rd, van Rooijen N., and , Cantin E. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J Virol. 2007;81:1451–1460. doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME.Functional polarity is introduced by Dicer processing of short substrate RNAs Nucleic Acids Res 2005334140–4156.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Behlke MA, Rose SD, Chang MS, Choi S., and , Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- Judge AD, Bola G, Lee AC., and , Maclachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Collingwood MA, Rose SD, Huang L, Hillier C, Amarzguioui M, Wiiger MT, et al. Chemical modification patterns compatible with high potency Dicer-substrate small interfering RNAs. Oligonucleotides. 2008;18:187–200. doi: 10.1089/oli.2008.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JT, Devosse T, Wang D, Zamanian-Daryoush M, Serbinowski P, Hartmann R, et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat Biotechnol. 2006;24:559–565. doi: 10.1038/nbt1205. [DOI] [PubMed] [Google Scholar]
- Moore AR. Collagen-induced arthritis. Methods Mol Biol. 2003;225:175–179. doi: 10.1385/1-59259-374-7:175. [DOI] [PubMed] [Google Scholar]
- Shahrara S, Proudfoot AE, Woods JM, Ruth JH, Amin MA, Park CC.Amelioration of rat adjuvant-induced arthritis by Met-RANTES Arthritis Rheum 2005521907–1919.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y, Takeno M, Murakami S, Kobayashi M, Kobayashi H, Miura K.Tumor necrosis factor α acceleration of inflammatory responses by down-regulating heme oxygenase 1 in human peripheral monocytes Arthritis Rheum 200756464–475.et al [DOI] [PubMed] [Google Scholar]
- Richards PJ, Williams AS, Goodfellow RM., and , Williams BD. Liposomal clodronate eliminates synovial macrophages, reduces inflammation and ameliorates joint destruction in antigen-induced arthritis. Rheumatology (Oxford) 1999;38:818–825. doi: 10.1093/rheumatology/38.9.818. [DOI] [PubMed] [Google Scholar]
- Mori T, Murakami M, Okumura M, Kadosawa T, Uede T., and , Fujinaga T. Mechanism of macrophage activation by chitin derivatives. J Vet Med Sci. 2005;67:51–56. doi: 10.1292/jvms.67.51. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhao L., and , Yu Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem Biophys Res Commun. 2004;317:414–420. doi: 10.1016/j.bbrc.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Nissler K, Pohlers D, Huckel M, Simon J, Brauer R., and , Kinne RW. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen induced arthritis: influence on macrophage activation. Ann Rheum Dis. 2004;63:1470–1477. doi: 10.1136/ard.2003.013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., and , Sorensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- Ikehara Y, Niwa T, Biao L, Ikehara SK, Ohashi N, Kobayashi T, et al. A carbohydrate recognition-based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicle. Cancer Res. 2006;66:8740–8748. doi: 10.1158/0008-5472.CAN-06-0470. [DOI] [PubMed] [Google Scholar]
- Amarzguioui M, Lundberg P, Cantin E, Hagstrom J, Behlke MA., and , Rossi JJ. Rational design and in vitro and in vivo delivery of Dicer substrate siRNA. Nat Protocol. 2006;1:508–517. doi: 10.1038/nprot.2006.72. [DOI] [PubMed] [Google Scholar]
- Bellingan GJ, Xu P, Cooksley H, Cauldwell H, Shock A, Bottoms S, et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilina A, Dicarlo E., and , Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol. 2007;178:2204–2211. doi: 10.4049/jimmunol.178.4.2204. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Bijl H, et al. Repeated therapy with monoclonal antibody to tumour necrosis factor α (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344:1125–1127. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Yoon HJ, Moon ME, Park HS, Im SY., and , Kim YH. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem Biophys Res Commun. 2007;358:954–959. doi: 10.1016/j.bbrc.2007.05.042. [DOI] [PubMed] [Google Scholar]