Abstract
We have previously demonstrated safety of G207, a doubly mutated (deletion of both γ134.5 loci, insertional inactivation of UL39) herpes simplex virus (HSV) for patients stereotactically inoculated in enhancing portions of recurrent malignant gliomas. We have now determined safety of two inoculations of G207, before and after tumor resection. Inclusion criteria were histologically proven recurrent malignant glioma, Karnofsky score ≥70, and ability to resect the tumor without ventricular system breach. Patients received two doses of G207 totaling 1.15 × 109 plaque-forming units with 13% of this total injected via a catheter placed stereotactically in the tumor. Two or five days later, tumor was resected en bloc with catheter in place. The balance of G207 dose was injected into brain surrounding the resection cavity. Six patients with recurrent glioblastoma multiforme were enrolled. Two days after the second G207 inoculation, one patient experienced transient fever, delirium, and hemiparesis, which entirely resolved on high-dose dexamethasone. No patient developed HSV encephalitis or required treatment with acyclovir. Radiographic and neuropathologic evidence suggestive of antitumor activity is reported. Evidence of viral replication was demonstrated. G207 appears safe for multiple dose delivery, including direct inoculation into the brain surrounding tumor resection cavity.
Introduction
Malignant gliomas represent the most common primary malignant brain tumor and almost universally result in death despite surgery, radiotherapy, and chemotherapy. Patients with glioblastoma multiforme (i.e., World Health Organization Grade IV astrocytoma), have a median survival of 12–15 months from initial diagnosis and 4–9 months after recurrence.1,2,3,4 Patients with anaplastic astrocytomas (AA), i.e., World Health Organization Grade III lesions, have a median survival of 36–40 months after initial diagnosis and 12–18 months after recurrence.5,6 Traditional management of these lesions includes biopsy and/or tumor resection with subsequent external beam radiotherapy. Treatment doses are typically ~60 Gy. Recently, the addition of temozolomide to the treatment regimen has been shown to modestly increase median survival to nearly 15 months; however, patients with active O6-Methylguanine DNA methyltransferase function remain completely resistant to this therapy.7,8
Because of the lack of success of standard treatments, efforts to improve survival have included various biologic therapies including monoclonal antibodies, immunotherapy, and gene therapy. We have developed the use of genetically engineered herpes simplex virus type 1 (HSV-1) for the experimental treatment of malignant glioma. We have previously demonstrated that an engineered HSV-1 propagates in and kills nervous system tumor cells with little to no evidence of viral encephalitis.9 In contrast, wild-type HSV-1 causes hemorrhagic, necrotizing encephalitis that is associated with significant mortality and morbidity in spite of antiviral therapy. G207, a conditionally replicating HSV-1 was developed as a potential agent for the treatment of malignant glioma and a phase I study of direct tumor inoculation of up to 3 × 109 plaque-forming units (pfu) in humans demonstrated safety.10 G207 contains deletions in both loci of the γ134.5 gene, as well as a disabling lacZ insertion in UL39, the gene that encodes the large subunit of the viral ribonucleotide reductase (infected cell protein 6).11 These mutations result in an oncolytic HSV that does not cause encephalitis.10,11,12,13 Despite these mutations, a significant antitumor effect is noted in a wide variety of in vitro and in vivo models of human and murine gliomas11 and in several nonglioma tumor models.14,15,16 The safety of G207 was demonstrated in a dose-escalating phase I trial involving 21 patients with recurrent glioma in which the highest dose that could be physically administered directly into the enhancing portions of the gliomas (3 × 109 pfu/ ml) was not the maximally tolerated dose.10 The present phase Ib study was designed to (i) determine the safety of direct inoculation of this genetically engineered HSV-1 into the brain surrounding the tumor; (ii) determine the safety of two inoculations of G207 within 1 week; (iii) examine inoculated tumor to determine evidence of HSV replication, and (iv) determine the degree of early immune response to HSV in the tumors of these patients.
Results
Patient characteristics
The trial was a single-site, open-label protocol conducted at the University of Alabama at Birmingham from January 2002 to August 2003. Seven patients were recruited into the study, of whom six were treated. All six inoculated patients completed the study, and no patient was lost to follow-up. Of the six subjects, two were male and four were female. The mean age was 54.0 years (±10 years) and the median age was 54.5 years (range 39–65 years). All six subjects had an initial histologically confirmed diagnosis of glioblastoma multiforme. Diagnosis preceded G207 treatment by a mean of 18 months (median 9; range 6–40 months). Table 1 summarizes the demographic data. Two patients, 102 and 104, were consented but did not undergo inoculation as scheduled. One underwent stereotactic biopsy but was found to have only radiation necrosis on frozen section, despite multiple biopsies within the mass. Findings were subsequently confirmed on paraffin sections. Three months later, however, evidence of progression by imaging in initial patient 102 resulted in our reconsenting of the patient (reassigned as 108). A second stereotactic biopsy demonstrated evidence of recurrent tumor, allowing inoculation as planned. The other patient (104) was excluded because a second biopsy again revealed only radiation-induced changes. A protocol deviation was believed to have occurred in patient 105 with possible inadvertent transgression of the ventricle by the inoculation needle (see later text). Each subject received the assigned dose of G207 at both the intratumoral inoculation, and the inoculation into residual tumor tissue following tumor excision. G207 was administered and documented by the surgeon and support staff.
Table 1.
Demographic data and primary clinical information
Toxicity
There were no dose-limiting toxicities in the trial; thus, no de-escalation occurred. Because of limitations in GMP production of G207, further dose escalation was not possible in this trial. Thus, while a maximally tolerated dose was not reached, the maximal achievable dose, 1.15 × 109 pfu, was tolerated when administered in the two doses, including inoculation into the tumor-infiltrated brain surrounding the tumor resection cavity. The trial was designed so that no patients underwent additional inoculations of G207 after their initial two inoculations.
All six subjects experienced at least one adverse event (AE), the most common being headache (83%), nausea (83%), hemiparesis (67%), anxiety (67%), and elevated γ-glutamyl transferase (67%). In total, 121 AEs were reported. Most were mild (26 of 121; 21%) or moderate (59; 49%) in severity. Some AEs were severe but not serious, and 28 met one of the “serious” criteria. All subjects experienced serious AEs, most of which were due to their underlying disease. Table 2 highlights the serious AEs. The National Cancer Institute Common Terminology Criteria for Adverse Events17 and protocol severity scales were used along with the event's likelihood of being related to treatment.
Table 2.
Serious adverse events
Most AEs were not related to G207 administration: only 16 AEs (13%), in five of six subjects, were considered to be possibly, probably or definitely related to G207 administration. No subjects discontinued study participation because of AEs that were presumed related to G207 administration and there were no discontinuations because of clinically significant laboratory abnormalities. G207 was not detected in the samples tested for the presence of HSV (saliva, urine, conjunctiva, and serum). None of the subjects showed magnetic resonance imaging (MRI), laboratory, or pathologic evidence of inflammatory changes or other findings to suggest encephalitis. No apparent evidence of long-term toxicity of G207 administration was demonstrable.
Of the six subjects, two died within 4 months of administration of G207. The most severe adverse reaction that occurred in this cohort of patients was the result of an inadvertent protocol deviation. Patient 105 underwent a right frontal craniotomy on day 2 after stereotactic inoculation of the first dose of G207 with uneventful resection of the tumor. Because of the known proximity of the tumor to the ventricle, an intraoperative ultrasound was performed, because the possibility of intraoperative brain shift rendered the data provided by the neuronavigation device less reliable. The ultrasound appeared to indicate adequate distance between the edge of the resection cavity and the ventricle to permit injection of G207, and the second dose was administered without incident. The patient initially recovered from anesthesia neurologically intact; however, ~36 hours after operation mental status changes and a temperature to 39.7 °C developed. Subsequently, the patient became unresponsive and developed a dense left hemiparesis. Treatment consisted of intravenous phenytoin and electroencephalogram monitoring was instituted, which was unremarkable. Computerized tomography scan and subsequent MRI found no changes indicative of encephalitis. However on coronal images (with contrast) the MRI suggested a possible needle track entering the frontal horn of the ipsilateral lateral ventricle. The patient was started on high-dose intravenous dexamethasone and a lumbar puncture was performed. The opening pressure was 35 cm H2O. The white blood cell count was 220 (lymphocytes 29% and monocytes 26%) with protein and glucose values of 122 and 62 mg/dl, respectively. PCR analyses detected evidence of HSV DNA using two sets of primers, DNA polymerase and glycoprotein B (gB), as well as a primer set for lacZ. Promptly, the patient's clinical condition markedly improved with no other therapies as evidenced by defervescence, and a near normal mental status and motor examination. The patient's neurologic status quickly improved to normal, and dexamethasone was tapered without recurrence of symptoms. While acyclovir administration was considered, the rapid and complete improvement with dexamethasone resulted in a decision not to use antiviral medication. Follow-up imaging continued to show no convincing evidence of encephalitis, although small areas of blood–brain barrier breakdown were seen in the region of some needle track passes. The cerebrospinal fluid cultures ultimately grew HSV as well. This constellation of findings led to the conclusion that an inadvertent protocol deviation had occurred with inoculation of G207 into the ventricle. While these events were not thought to be a dose-limiting toxicity, the protocol was amended to further decrease the possibility of inadvertent ventricular inoculation of virus. The highest temperature recorded in the other patients was 38.7 °C (patient 108) that developed 72 hours after tumor resection and was associated with transient pyrexia and increased left-sided weakness, persisting ~36 hours. These findings resolved spontaneously and he was discharged home. Only one subject underwent postmortem brain evaluation (patient 101). For this patient, all PCR analyses or culture for the tissues obtained at autopsy (brain, liver, spleen, and blood) were negative for the presence of HSV.
Progression and survival
No determination regarding efficacy can be made from this small trial of six patients, none of whom received retreatment after the first 5 days of the protocol. Nonetheless, no subject had a complete response (complete disappearance of all enhancing tumor on consecutive MRI scans) or a partial response (at least a 50% decrease in contrast enhancing tumor volume at any time after G207 injection). The median time to progression by MRI, using the criteria established by the protocol, was 3 months. Since tumors were resected, the McDonald criteria cannot be strictly applied to determine progression because of the expected appearance of non-neoplastic rim enhancement on immediate postoperative imaging. In cases of complete/near complete resection of the pretreatment enhancing abnormality, progression was defined as the development of nodular enhancement, consistent with tumor recurrence, and determined by the Principal Investigator. Patient 107, interestingly, never showed MRI changes consistent with progression at the site of G207 inoculation and resection. Eventual death was related to a regional, noncontiguous failure/recurrence in the contralateral hemisphere (Figure 1).
Figure 1.
Sequential images of patient 107 after treatment. (a,b) Enhancement present 4 months after resection and G207 treatment. Enhancing infiltrative tumor was left at the time of resection because of positive stimulation with subcortical mapping done intraoperatively. (c,d) Residual enhancement 12 months post-G207 treatment is decreased in the area of inoculation (red arrow), but new enhancement has appeared in the contralateral hemisphere (blue arrow). This new enhancement in the left hemisphere progressed 14 months post-G207 treatment (e,f). On stereotactic biopsy, this left hemispheric lesion was found to represent glioblastoma multiforme. T2 images (data not shown) showed the development of marked T2 changes throughout the left hemisphere at this time.
Median survival was determined to be 23 months from initial diagnosis and 6.6 months from G207 administration (range; 2–20.75 months).
Neurological status
Karnofsky performance status (KPS), serial patient assessments, and scrutiny of adverse medical events were used to follow the neurologic status of our cohort (see Materials and Methods). All six patients had a screening Karnofsky status ≥70. Most patients maintained relatively stable scores throughout the first 3 months of follow-up. Half of the patients were noted to have improved KPS at a minimum of one time point after inoculation. Table 3 details the summary statistics of the KPS measured at each clinic visit. Patient 105, as previously described, had a significant AE that caused a precipitous decline in her KPS. She quickly recovered, however, to her prior level. Except for patient 105, sentinel events were few in our cohort. Patients 103 and 107 had no AEs attributable to G207. Patient 101 had a possible seizure that may have been related. Patient 106 experienced somnolence, intermittent seizure activity, and lateralizing signs that may have been related as well. Patient 107 developed left upper extremity weakness consistent with an ulnar neuropathy, also documented by electromyography and nerve conduction velocities, and underwent ulnar decompression without significant improvement. Later she developed a hemiparesis associated with tumor progression. Patient 108 had transient pyrexia and increased hemiparesis that spontaneously resolved and may have been related. Overall, the neurologic status of our cohort was relatively stable.
Table 3.
Karnofsky performance scores
Laboratory evaluation and immune status
Hematologic changes were serially evaluated for each subject: at screening, day 3, and day 28 (see Supplementary Table S1). Baseline lymphocyte counts for five of six patients were within normal range (0.85–4.10 × 109/l). The lymphocyte count for patient 107 decreased to 0.67 × 109/l. The patients almost uniformly demonstrated a decrease in total lymphocyte count at day 3; four of six patients had low absolute lymphocyte counts. At day 28, three of the six continued to have low absolute lymphocyte counts.
As often seen in patients with recurrent malignant glioma, low CD4 and CD8 counts were present in some patients both pre- and post-G207. Two patients (103, 108) had a significant reduction in CD4 (103, 108) and CD8 (108) cells in the first week after tumor resection. The results of the CD4 and CD8 counts for all patients are shown in Supplementary Table S2.
Delayed hypersensitivity skin tests were utilized to evaluate T-cell responsiveness to a candida antigen (see Supplementary Table S3). Only one patient (103) had evidence of an incomplete response (5 × 5 mm2 erythema) to 0.1 ml of a 1:500 dilution but no evidence of induration.
Tumor specimens obtained from patient 101 pre- and post-G207 treatment were evaluated for presence of infiltrating lymphocytes and macrophages by immunohistochemical (IHC) analysis. As shown in Figure 2, only minimal immune cell infiltrates could be detected in tissue sections before G207 inoculation. However, tumor sections of tissue from the same patient 2 days following G207 treatment revealed significant positive staining with anti-CD3 (specific for mature T lymphocytes and a natural killer cell subset), anti-CD8 (specific for cytotoxic T lymphocytes), and anti-HAM56 staining (specific for monocytes, macrophages, and microglia). B-cell infiltrates (using an anti-CD20 antibody) were also detected but were much less evident. Table 4 summarizes the immune infiltrate IHC scores.
Figure 2.
Immunohistochemical staining of patient 101 tumor tissue pre- and post-G207 treatment. Sections from paraffin blocks of tumor tissue obtained from patient 101 pre- and post-G207 treatment were evaluated for presence of tumor-infiltrating lymphocytes and macrophages. Monoclonal antibodies used for detection were CD3 (for detection of infiltrating T cells), CD8 (for detection of cytotoxic/suppressor T cells), CD20 (B-cell infiltrates), and HAM56 (for detection of monocyte/macrophage populations). Samples were stained as described in Materials and Methods. Original magnification ×40.
Table 4.
Patient immune infiltrate IHC scores
Antibody determinations
Three patients were seronegative for HSV-1 antibody at screening (patients 105, 106, and 108), all of whom subsequently seroconverted, as determined by enzyme-linked immunosorbent assay. The results of HSV-1 and -2 antibody determinations are shown in Supplementary Table S4. Two of the six patients had a stable antibody titer (patients101 and 107) and three patients had a 2- to 11-fold increase (patients 103, 105, and 106) of antibody titers during the study. Peak antibody titers were documented at visits on days 9 and 28.
Detection of HSV in peripheral samples
Two patients had HSV-1 isolated from saliva at some point in the study (Supplementary Table S5). All other culture assessments, as well as PCR determinations, for these two patients (i.e., patient 101 at day of resection, 48 and 72 hours after resection, 4 days after resection, day 28 and month 2; patient 107 at 4 days after resection, day 28, months 2 and 3) were negative. As described in the Materials and Methods, PCR for polymerase (pol) and glycoprotein B (gB) was performed on all samples. Because these primers do not differentiate G207 from wild-type HSV-1, if the screening PCR was positive, repeat assessments were performed to determine the presence of the lacZ insertion gene (indicating presence of G207). Both patients with positive HSV-1 by original PCR were noted to have negative PCR for the lacZ insertion gene. These findings indicated that the HSV-1 recovered from the saliva was wild-type HSV-1 and did not originate from the G207 inoculation. All other patients had negative PCR and culture examinations of saliva, urine, conjunctiva, and serum.
Evaluation of tumor specimens
Detection of HSV in tumor specimens. All tumor samples tested negative for the presence of wild-type HSV and of G207 by culture, except for a single segment from subject 107, which was culture positive for G207. PCR analysis confirmed infection of the tumor tissues with G207 in all samples (A, B, and C) of all patients. Viral replication was noted in the last three patients. The results of the PCR analysis for intratumoral G207 infection and replication appear in Table 5.
Table 5.
Analysis for intratumoral G207 replication (PCR) following resection
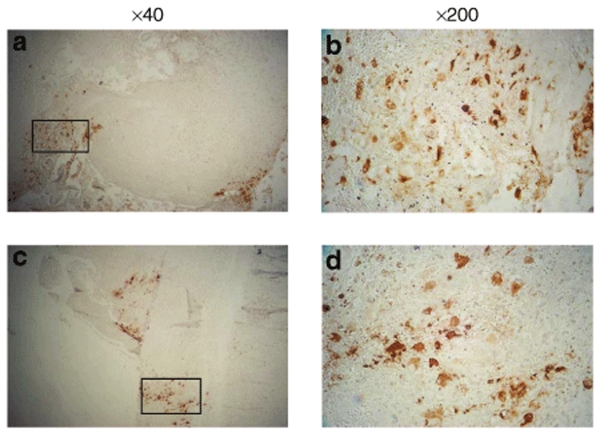
Immunohistochemistry of tumor tissues. To demonstrate expression of late viral genes, IHC staining was performed using an antibody specific for glycoprotein C (gC), a γ2, or true late gene, in that its transcription occurs only after initiation of viral DNA synthesis. Expression of gC was observed in tumor tissues from the final two patients, who had their tumors resected 5 days (instead of 2 days) after inoculation of G207 into the tumor. Cytoplasmic staining, indicative of viral infection, was seen in all patients except subject 106. Stronger staining was observed in the patients with tumor resection after 5 days (patients 107 and 108). The results for all patients are shown in Supplementary Table S6. Figure 3 demonstrates HSV-1 gC antibody staining in patients 107 and 108.
Figure 3.
Immunohistochemical staining for HSV-1 gC (late gene) expression, using a mouse monoclonal antibody specific for gC, was performed in tumors resected from patients 107 (top panels) and 108 (bottom panels) 5 days following G207 injection. Tumor sections are shown at low magnification (×40, a,c) and high magnification (×200, b,d) for each patient tissue. b and d are high magnification of regions indicated in a and c by the rectangular box.
Discussion
We report the results of the first North American human trial of a G207, a conditionally replicating HSV, directly inoculated into human brain using a multiple-dose regimen separated by 2 or 5 days. We demonstrated that doses of 1 × 109 pfu can be inoculated safely into brain surrounding a glioma resection cavity without the development of significant AEs or toxicity. No patient developed MRI, laboratory, or pathological evidence of encephalitis. Three of the six subjects (50%) demonstrated an improved Karnofsky performance score following G207 inoculation. One month after G207 injection all patients were alive with stable disease. The median time to progression was 3 months. The median survival was 23 months from date of diagnosis and 6.6 months from the time of G207 inoculation. No patient received chemotherapy following inoculation of G207, which would suggest that any decrease in tumor progression was most likely secondary to G207 administration. The deaths of the six study patients were attributed to disease progression. One patient developed transient hyperthermia and decreased responsiveness probably related to G207 which resolved within 12 hours on steroid therapy. No subjects needed to be treated with antiviral therapy (e.g., acyclovir). None of the complications or deaths was definitively attributable to G207 administration into the tumor or brain tissue next to the resection cavity.
As expected, our cohort did not have a normal immunological profile, as has been previously well established in patients harboring recurrent malignant glioma. Consistent with previous studies, the laboratory data reveal no unexpected results or trends.18 Patients were unable to mount delayed-type antigen recall skin testing. Only one subject had even a partial response to such testing, and this was to a single antigen. Despite the fact that this subject had a significant decrease in CD4 and CD8 counts shortly after inoculation with G207, the lymphocyte count at 3 months was higher in this patient than in the remainder of the cohort. Nearly all immunocompetent adults will react to one or more of the antigens tested with erythema and induration at 48 hours. The general lack of response in our cohort is attributable to their underlying disease as well as treatment effect (previous radiation and/or chemotherapy, and concurrent steroid usage).
The HSV-1 antibody status of our small cohort was slightly lower, and the HSV-2 antibody status was slightly higher than that of the general population.19,20,21 Despite their immunocompromised state, HSV-1 seroconversion did occur in three patients. We and others have hypothesized that the antitumor effect of oncolytic HSV seen in preclinical models represents a balance between a favorable, antitumor immune response and a detrimental, antiviral immune response. This hypothesis is supported by preclinical studies in which both mononuclear and inflammatory infiltrates have been detected following virus injection.22,23 The findings in the current trial are consistent with this hypothesis, in that there was IHC evidence of significant lymphocyte infiltration into tumor following G207 administration. Whether this infiltrate represents an antitumor or antiviral response, or a combination thereof, is not determined by these data. Patient 107, who exhibited the longest survival, had the least change in lymphocyte infiltration (as determined by the IHC scores for pre- and post-G207 administration). This was accompanied by the greatest replication of G207 (see following text). Thus, the lack of lymphocytic infiltration may have allowed an increase of the spread of the virus throughout the tumor.
Though HSV was isolated by culture from the tumor of only one patient (patient 107), PCR analysis demonstrated the presence of HSV DNA in tumor samples from all patients. Of note, patient 107 had the lengthiest time to progression and longest survival. The increased viral load demonstrated in tumor samples from this patient may explain this antitumor effect.
Additionally, HSV pol RNA was detected following reverse transcriptase–PCR analyses of tumor lysates, indicative of G207 infection and replication following intratumoral inoculation. Moreover, the patient with the highest replication of G207 (1.4 × 105 copies HSV pol RNA/mg tissue) had the longest survival (20.75 months from therapy). The detection of significant gC by IHC in this patient further supports viral replication, because expression of this late gene is not initiated until after the onset of viral DNA synthesis. No patient demonstrated systemic spread of G207 clinically, as evidenced by lack of detectable viral DNA by PCR of serum, conjunctival swabs, and saliva. This is not surprising, given G207's known decreased virulence.
The impact of this study on the design of future studies using oncolytic HSV vectors needs to be carefully considered. First, although viral replication did occur in some of our patients, it was not uniformly seen. Whether this is related to timing of tumor harvest, the tumor genotype itself, or the host immune response elicited following G207 administration, is not clear at this time. It is evident, however, that measures to increase the efficacy of viral replication need to be considered. We have undertaken a trial of G207 plus a single fraction of radiation administered 24 hours after treatment in patients with recurrent malignant glioma, based on our preclinical studies demonstrating an increase in viral oncolysis when radiation is administered after treatment.24,25,26 Pending the results of this trial, an upfront study of patients with newly diagnosed malignant glioma has been designed which would utilize the benefit of post-HSV radiation as well as the increase in replication produced by temozolomide.27 Finally, a virus that expresses IL-12, a cytokine that is known to produce antitumor immune responses as well as antiangiogenic effects (without causing a decrease in replication in in vivo preclinical studies), is being advanced for potential clinical use pending the results of the prior studies.
The results of this study need to be considered in the context of our knowledge of malignant brain tumors. Malignant glioma was chosen for study because of its resistance to traditional therapeutics and almost always results in death. Ninety percent of gliomas recur locally, within a few centimeters of their resection margins, and systemic spread is exceedingly rare.28,29 As a result, these tumors are excellent targets for experimental therapy with a conditionally replicating virus such as G207 HSV in that it replicates in and destroys tumor cells, while sparing normal nervous tissue. All of the patients in our trial had recurrent glioblastoma multiforme, harboring the worst prognosis of these tumors.
HSV-1 is a DNA virus that has been extensively researched in regards to its genome. Approximately 90% of the adult population has been exposed to the virus resulting in acquired antibodies.24 Although its wild-type form is quite neurovirulent, a variety of mutations can be introduced which decrease this effect. Two copies of the neurovirulence gene, γ134.5, are present in the wild-type virus. Both loci can be deleted while allowing the virus to maintain its antitumor effects.30 Additionally, the enzyme ribonucleotide reductase is required for replication. Viruses lacking functional ribonucleotide reductase can still replicate in rapidly dividing cells by presumably using the cellular homolog. These viruses cannot replicate in and lyse quiescent cells such as neurons and other cells comprising most of normal adult brain.31,32 The use of engineered HSV-1 with an intact thymidine kinase gene (UL23) for tumor therapy has the added advantage of efficacious antiviral therapy for life-threatening disease (e.g., acyclovir). As the G207 HSV retains native thymidine kinase, available antiviral therapy can be administered.
Both copies of the diploid γ134.5 gene are deleted in G207, causing significant attenuation in neurovirulence. In addition, a lacZ insertion disables the large subunit of the viral ribonucleotide reductase.11 As a result of this insertional mutation, G207 loses its ability to replicate in nondividing cells, and is rendered more sensitive to antiviral medications than wild-type HSV-1.11 G207 has been investigated in a variety of rodent and primate models. Oncolytic effects and improved survival have been noted in a variety of immunocompromised and immunocompetent mouse models of glioma. At least a portion of G207's antitumor effects is attributable to the direct oncolytic effect of the virus; and results of other investigations reveal that an antitumor immune response can be elicited by the virus in animal models.15,33
Sensitive mouse strains (BALB/c, A/J strain) and highly susceptible primates (Aotus nancymae) have undergone intracerebral inoculation of G207 with excellent safety results.11,12,13 As a result of these preclinical studies, G207 was examined in a phase I study of patients with recurrent malignant glioma and no encephalitis was produced by doses up to 3 × 109 pfu.10
Oncolytic virus inoculation into the brain has been previously reported.18,34 Although previous inoculation of HSV1716 into the brain surrounding glioma resection has been reported under a single-dose regimen, the highest dose administered in that trial was 1 × 105 pfu.18 In comparison, the initial dose in our two-dose trial was 1.5 × 108 pfu, infused into the enhancing portions of the tumor through a catheter that was left in place, followed in 2 or 5 days by free-hand injections of a total of 1 × 109 pfu, inoculated into multiple sites in the brain surrounding the resection cavity. Though multiple-dose regimens have been utilized in retrovirus studies,35,36 this is the first example of such an approach using a conditionally replicating HSV. Our study suggests that G207 can be safely inoculated into human brain surrounding tumor resection cavities at doses of 1 × 109 pfu. Further studies with G207 and other genetically engineered HSV in the treatment of human glioma are warranted.
Materials and Methods
Trial design. This protocol and its amendments were approved by the Institutional Review Board of the University of Alabama at Birmingham (F05041106) and reviewed by the Recombinant DNA Advisory Committee of the National Institutes of Health and the Food and Drug Administration. The combined results of the NG1-003 and NG1-004 investigations, which comprise a 3-month study and its 9-month follow-up, are reported.
Inclusion and exclusion criteria. The inclusion and exclusion criteria for the study are provided in the Supplementary Materials and Methods. Patients underwent screening with a history, physical examination, chemistry profile, complete blood count, urinalysis, HSV-1 and HSV-2 antibody titers, HSV-1 cultures of saliva, urine, conjunctival secretions and blood, electrocardiogram, chest radiograph, and volumetric MRI. Screening volumetric MRI included axial imaging: fast spin echo, FLAIR, and T1 (pre- and postgadolinium). T1-weighted images in the coronal and sagittal planes were also included. The image matrix was 256 × 192 and the field of view was 200 mm. Scan thickness was 10 mm with interscan spacing of 2 mm. Laboratory studies were performed by Mayo Central Laboratories (Rochester, MN) unless otherwise specified. One of the enrolled patients was originally deemed a screening failure, however, was later re-enrolled due to demonstrated recurrence of glioblastoma multiforme (seen on second biopsy; only radiation necrosis was found on multiple biopsy specimens at original screen) and ultimately underwent inoculation with G207.
Safety was assessed throughout by evaluating toxicities (rated using the classification developed by the National Institutes of Health17), laboratory testing, and neurologic status. Efficacy was evaluated by progression of disease (clinical and imaging), immunohistochemistry to monitor viral replication and infection, and PCR to detect evidence of G207 in resected tumor. The time to progression was defined as the time at which there was clinical evidence of progression, increased steroid dose dependence, or clinically relevant increase in enhancement on imaging (as determined by senior author).
Virus preparation. Production of G207 following current Good Manufacturing Practices was performed under contract by BioReliance (Rockville, MD) using a process developed at MediGene. Production details are provided in the Supplementary Materials and Methods. Immediately before surgery, the cryovial with virus was removed from storage and thawed by rubbing gently between gloved hands. An aliquot was diluted with sterile saline for injection (USP) to the concentration appropriate for each dose cohort, loaded aseptically in sterile syringes that were transported to the operating room in an insulated container with ice packs. All handling of the virus and materials potentially contaminated with virus was conducted in accordance with Biosafety Level 2 precautions.
Initial biopsy and stereotactic inoculation. On the morning of the biopsy and initial inoculation, the patients underwent Cosman-Roberts-Wells stereotactic head frame application under local anesthesia, followed by a contrast-enhanced computerized tomography scan and target localization. Patients were then taken to the operating room and a stereotactic biopsy was performed. The initial target was chosen by the neurosurgeon, with the goal of injecting virus in the largest enhancing (likely active tumor) region and avoiding inoculation of central nonenhancing (likely necrotic) regions of tumor. Following confirmation of recurrent tumor by frozen section, a standard external ventricular catheter was stereotactically placed into the largest area of enhancement. This catheter had a solid tip with 16 openings in a circumferential pattern beginning 1 cm from the tip and extending proximally for 2 cm. Trajectory of the catheter placement was undertaken to avoid ventricular entry. Once placed, the catheter was attached to a 3-ml syringe via a luer-lock connector, and 1 ml of G207 inoculum, containing 1.5 × 108 pfu of virus, was slowly infused over a 10-minute period to minimize the possibility of reflux. The catheter was clamped, the syringe replaced and an additional 0.8 ml of sterile saline, calculated to clear the ventricular catheter, was then infused over 2 minutes. Two vascular clips were placed across the catheter at the level of the skull to prevent reflux, and the catheter was divided immediately distal to this, followed by routine scalp closure.
Patients were observed in the hospital for a minimum of 2 days, and a MRI scan was obtained before discharge. Patients 107 and 108 were discharged home between their biopsy and eventual resection (5 days later). The other patients remained in the hospital until their resection 2 days later. Encephalitis was considered if a fever >38 °C was present for >48 hours, deterioration in neurological status occurred, or there was progressive hemorrhage and/or swelling inside or around the inoculated enhancing tissue.
En bloc resection and virus administration. Tumor resection was performed via craniotomy 2 or 5 days following the initial inoculation. These were carried out either awake or under general anesthesia based on whether the attending neurosurgeon felt awake motor or speech mapping was necessary. Neuronavigation was utilized in all six craniotomies. Tumor was resected en bloc with meticulous hemostasis and transferred to a sterile side table for separate processing as described in the following text. Immediately after resection, and before closure of the dura, the second dose of G207 (1 × 109 pfu) was administered by multiple injections into the resected tumor bed, using approximately equal volumes (6 ml total). Each inoculation was administered ~1 cm apart and delivered into the resected tumor bed over 1 minute and at a 1 cm depth (28G, ½-inch needle fitted to a 1-cc tuberculin syringe), where possible, and avoiding penetration of the ventricular system. In the event penetration did occur, the procedure was aborted, with no further virus injection. Inoculation was aided by intraoperative navigation devices and intraoperative ultrasound. The size and orientation of the resection bed determined the number of inoculations and the amount inoculated per injection site. After each inoculation, the needle was left in place for 2 minutes and then slowly removed. To minimize inoculum seepage, each needle entry point was covered with a 1 cm2 of absorbable hemostatic agent. G207 administration was to be interrupted if at any time during administration the subject developed symptoms of decreased cerebral or cardiovascular perfusion, if there were signs of allergic reaction or anaphylaxis, or for any AE that, in the investigator's opinion, warranted interruption. This did not occur during this trial.
Resected tissue processing. The en bloc resected tumor still containing the silastic injection catheter was immediately placed on sterile gauze, weighed, and photographed. Tumors ranged from 7.72 to 17.64 g, for a mean of 12.3 g. The proximal and distal tips of the catheter were visualized and the tumor was aseptically sliced, perpendicular to the catheter, into at least three portions encompassing the portion of the catheter containing the 16 outlet holes. A separate sterile cryostat blade was used to produce each section. The distal slice was designated “A,” the next more proximal “B” and the third “C.” Each slice was quartered, providing a wedge with the smallest edge in proximity to the catheter and each quarter was weighed. Quarter 1 was submitted in neutral-buffered formalin for routine histopathologic examination and HSV immunohistology to Pathology Associates International Frederick, MD. Quarter 2 was submitted snap-frozen in liquid nitrogen to MediGene Ag for PCR for HSV polymerase and β-galactosidase. Quarter 3 was submitted fresh in serumless Dulbecco's modified Eagle's medium/F12 medium for HSV culture. Quarter 4 was bisected from inner to outer borders to provide snap-frozen and FFPE portions for archive storage. Any remaining tumor tissue was submitted to the Neurosurgery Brain Tumor Tissue Facility for tumor cell culture and snap-frozen storage as tumor chunks.
Post-treatment evaluation. Patient assessments were made, including physical examination, HSV antibody titer, saliva, urine, conjunctival secretions, and blood HSV cultures as well as MRI preoperatively and at 24 hours, 72 hours, 7 days, 1 month, 2 months, 3 months, 6 months, 9 months and 1 year following inoculation. Neurological examination included Karnofsky grading and neurological assessment. Patients had sera and saliva, urine, and conjunctival secretions cultured at each follow-up visit for evidence of HSV shedding. Follow-up MRIs were classified as complete response, partial response, stable disease, or progressive disease. Development of ring enhancement alone in the resection cavity was not considered progression unless a nodular area of enhancement developed, felt to represent true progression, rather than simply postsurgical changes. This decision was made by the principal investigator. Patients who showed signs of clinical or MRI disease progression were declared a treatment failure and released to pursue additional therapy. Even after disease progression, patients were included in clinical, MRI, and autopsy follow-up whenever possible.
HSV culture and PCR. One milliliter of serum, saliva, urine, and centrifugally clarified transport media from conjunctival swabs was used for virus isolation and PCR assays. Of this, 0.2 ml was inoculated into duplicate wells of 24-well TC plates containing Vero and A549 cells. Plates were incubated in a CO2 incubator (37 °C, 95% humidity) and examined by inverted microscope daily for cytopathic effect for 7 days. As little as 10 pfu of G207 can be detected in Vero cell culture. Positive cultures were passaged in Vero cells, and infected cell supernates were collected for identity verification by PCR. Positive cultures were serotyped using a MicroTrak HSV-1/HSV-2 Culture Identification/typing Test (Trinity Biotech, Bray, Ireland).
DNA was extracted from a separate 0.2-ml aliquot using the QIAamp DNA Blood Mini Kit and QIAvac 24 Vacuum manifold (Qiagen, Valencia, CA; cat. no. 51106). Initial screening of specimens was performed using two primer sets which amplify glycoprotein B and DNA polymerase genes of HSV.
Tumor slices (from Quarter 3) were homogenized in serumless medium, subjected to brief centrifugation, and tenfold serial dilutions of homogenate supernates were plated onto Vero or A549 cells (American Type Culture Collection, Manassas, VA). Cells were observed daily for cytopathic effect, and number of plaque-forming units was determined 4 days following plating. Cells without cytopathic effect at 4 days were monitored an additional 3 days. Additionally, the tumor samples (Quarter 2) that had been snap-frozen in the operating room were analyzed by quantitative PCR (Q-PCR) for the detection of wild-type HSV DNA and RNA. These PCR analyses were conducted at MediGene using one HSV-specific primer set, which amplified HSV polymerase sequences. The primer sequences were: J0925F (forward) 5′-TGG GTG TCC GGC AGA ATA A-3′ and J0994R (reverse) 5′-AGC AGA TCC GCG TCT TTA CGT-3′. The run cycle for standard real-time PCR was: 50 °C for 2 minutes, 95 °C for 10 minutes, for a total of 40 cycles.
Hematology laboratory studies. All screening and monitoring hematology studies were performed by the Mayo Central Laboratory for Clinical Trials, Rochester, MN. Blood was collected and shipped overnight according to their specifications using their vacutainers and shipping containers.
Histologic methods for tumor histology. Five-micron thick sections (hematoxylin and eosin-stained) of formalin-fixed, paraffin-embedded tissue were microscopically examined. When appropriate, IHC studies (streptavidin–biotin peroxidase complex method) were employed for diagnostic purposes and utilized antibodies directed against the following antigens: glial fibrillary acidic protein (Dako, Carpinteria, CA; polyclonal), Ki-67 (MIB-1 subclone; Ventana, Tucson, AZ) and HSV (Ventana, Tucson, AZ; polyclonal against both HSV-1 and HSV-2). All six tumors were evaluated by a neuropathologist well versed in trials involving viral vector therapy. Additional immunohistochemistry was conducted by Pathology Associates International (PAI, Frederick, MD) on tumor samples from each subject for detection of HSV virus replication and infection. Two antibodies were used in parallel: anti-β-galactosidase, which recognizes 116-kd Escherichia coli β-galactosidase protein, and anti-VP-5 (anti-NC-1), which detects HSV-1 virus capsid protein. As the G207 virus contains a lacZ insertion, the anti-β-galactosidase antibody permits distinction from other endogenous herpes viruses.
Immunohistochemistry staining for tumor-infiltrating lymphocytes and macrophages was performed at University of Alabama using sections cut from the same paraffin blocks prepared by PAI. Before adding primary antibody, samples were deparaffinized in xylene, and rehydrated gradually in ethanol, and treated with Citra Antigen Retrieval solution (Biogenex, San Ramon, CA). After hydrogen peroxide treatment, samples were treated with FC Receptor Block and Background Buster block (Innovex Biosciences, Richmond, CA) to eliminate nonspecific staining. The primary mouse monoclonal antibodies used were CONFIRM anti-CD3 (cat. no. 790-2921, Ventana Medical Systems, Tucson, AZ), anti-CD8 (cat. no. CMA023), anti-CD20 (cat. no. CMA301), and anti-macrophage (HAM56; cat. no. CMA343), all from Cell Marque (Rocklin, CA). The mouse monoclonal antibody specific for glycoprotein C was a kind gift from Dr Bernard Roizman (University of Chicago). The biotinylated antimouse secondary antibody, and streptavidin–horseradish peroxidase conjugate were both obtained from Biogenex and the color development using Turbo DAB (3,3′-diaminobenzidine tetrahydrochloride, Innovex Biosciences).
Supplementary MaterialTable S1. Individual subject hematology changes.Table S2. CD4 and CD8 counts.Table S3. DTH skin testing.Table S4. HSV antibody detection (ELISA).Table S5. Positive results for the PCR analysis.Table S6. Study flow chart.Materials and Methods.
Supplementary Material
Individual subject hematology changes.
CD4 and CD8 counts.
DTH skin testing.
HSV antibody detection (ELISA).
Positive results for the PCR analysis.
Study flow chart.
Acknowledgments
We thank Jennifer Coleman for excellent technical assistance with the immunohistochemical analyses of the patient tumor specimens. We also thank Bernard Roizman (University of Chicago) for his generous gift of gC monoclonal antibody. The trial was sponsored by MediGene AG. J.M.M., G.Y.G., J.N.P., W.W., R.J.W., and S.G. were supported in part by the National Cancer Institute grants P01 CA71933 (R.J.W., J.M.M., G.Y.G., and S.G.) and SPORE P50 CA097247 (G.Y.G., J.M.M., W.W., and S.G.).
REFERENCES
- Chang SM, Butowski NA, Sneed PK., and , Garner IV. Standard treatment and experimental targeted drug therapy for recurrent glioblastoma multiforme. Neurosurg Focus. 2006;20:E4. [PubMed] [Google Scholar]
- Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M., and , Schiff D.Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series Neurosurgery 19994517, 22–22.discussion [DOI] [PubMed] [Google Scholar]
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Yang SH, Kim MK, Lee TK, Lee KS, Jeun SS, Park CK, et al. Temozolomide chemotherapy in patients with recurrent malignant gliomas. J Korean Med Sci. 2006;21:739–744. doi: 10.3346/jkms.2006.21.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain MC., and , Kormanik P. Salvage chemotherapy with taxol for recurrent anaplastic astrocytomas. J Neurooncol. 1999;43:71–78. doi: 10.1023/a:1006277631745. [DOI] [PubMed] [Google Scholar]
- Prados MD, Gutin PH, Phillips TL, Wara WM, Larson DA, Sneed PK, et al. Highly anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989. Int J Radiat Oncol Biol Phys. 1992;23:3–8. doi: 10.1016/0360-3016(92)90537-r. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Martuza RL, Malick A, Markert JM, Ruffner KL., and , Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD., and , Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Hunter WD, Martuza RL, Feigenbaum F, Todo T, Mineta T, Yazaki T, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan P, Hunter WD, Martuza RL., and , Rabkin SD. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation in mice. J Virol. 2000;74:3832–3841. doi: 10.1128/jvi.74.8.3832-3841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Martuza RL, Kojima H., and , Rabkin SD. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- Todo T, Rabkin SD, Sundaresan P, Wu A, Meehan KR, Herscowitz HB, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- Yazaki T, Manz HJ, Rabkin SD., and , Martuza RL. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752–4756. [PubMed] [Google Scholar]
- National Cancer Institute . National Institutes of Health, National Cancer Institute; 2003. Common Terminology Criteria for Adverse Events, version 3.0. [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Bunzli D, Wietlisbach V, Barazzoni F, Sahli R., and , Meylan PR. Seroepidemiology of herpes simplex virus type 1 and 2 in Western and Southern Switzerland in adults aged 25-74 in 1992-93: a population-based study. BMC Infect Dis. 2004;4:10. doi: 10.1186/1471-2334-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashido M, Kawana T, Matsunaga Y., and , Inouye S. Changes in prevalence of herpes simplex virus type 1 and 2 antibodies from 1973 to 1993 in the rural districts of Japan. Microbiol Immunol. 1999;43:177–180. doi: 10.1111/j.1348-0421.1999.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Rabenau HF, Buxbaum S, Preiser W, Weber B., and , Doerr HW. Seroprevalence of herpes simplex virus types 1 and type 2 in the Frankfurt am Main area, Germany. Med Microbiol Immunol. 2002;190:153–160. doi: 10.1007/s00430-001-0102-1. [DOI] [PubMed] [Google Scholar]
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ., and , Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- Bradley JD, Kataoka Y, Advani S, Chung SM, Arani RB, Gillespie GY, et al. Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–1522. [PubMed] [Google Scholar]
- Markert JM, Gillespie GY, Weichselbaum RR, Roizman B., and , Whitley RJ. Genetically engineered HSV in the treatment of glioma: a review. Rev Med Virol. 2000;10:17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Aghi M, Rabkin S., and , Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- Choucair AK, Levin VA, Gutin PH, Davis RL, Silver P, Edwards MS, et al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg. 1986;65:654–658. doi: 10.3171/jns.1986.65.5.0654. [DOI] [PubMed] [Google Scholar]
- Pasquier B, Pasquier D, N'Golet A, Panh MH., and , Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980;45:112–125. doi: 10.1002/1097-0142(19800101)45:1<112::aid-cncr2820450121>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Markert JM, Malick A, Coen DM., and , Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Cobbs C., and , Markert JM. Gene Therapy of Glioma: A Review. Perspect Neurol Surg. 1999;10:1–20. [Google Scholar]
- Mineta T, Markert JM, Takamiya Y, Coen DM, Rabkin SD., and , Martuza RL. CNS tumor therapy by attenuated herpes simplex viruses. Gene Ther. 1994;1 suppl. 1:S78. [PubMed] [Google Scholar]
- Toda M, Rabkin SD, Kojima H., and , Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Harsh GR, Deisboeck TS, Louis DN, Hilton J, Colvin M, Silver JS, et al. Thymidine kinase activation of ganciclovir in recurrent malignant gliomas: a gene-marking and neuropathological study. J Neurosurg. 2000;92:804–811. doi: 10.3171/jns.2000.92.5.0804. [DOI] [PubMed] [Google Scholar]
- Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual subject hematology changes.
CD4 and CD8 counts.
DTH skin testing.
HSV antibody detection (ELISA).
Positive results for the PCR analysis.
Study flow chart.