Abstract
Peripheral nerve injury occasionally causes chronic neuropathic pain with hyperalgesia and allodynia. However, its treatment is difficult. Here, we used a chronic constriction injury (CCI) model in rats to investigate the effects on experimental neuropathic pain of the human hepatocyte growth factor (HGF) gene delivered into the nervous system by retrograde axonal transport following its repeated intramuscular transfer, using liposomes containing the hemagglutinating virus of Japan (HVJ). CCI (control) rats exhibited marked mechanical allodynia and thermal hyperalgesia, and decreased blood flow in sciatic nerve and hind paw. All these changes were significantly reversed by HGF gene transfer. In the sciatic nerve in HGF-treated rats, the size-frequency distributions for myelinated and unmyelinated axons each showed a rightward shift, the number of myelinated axons >5 µm in diameter was significantly increased, and the mean diameter of unmyelinated axons was significantly increased (versus CCI rats). Levels of P2X3, P2X4, and P2Y1 receptor mRNAs, and of interleukin-6 (IL-6) and activating transcription factor 3 (ATF3) mRNAs, were elevated in the ipsilateral dorsal root ganglia and/or sciatic nerve by CCI, and these levels were decreased by HGF gene transfer. These results may point toward a potential new treatment strategy for chronic neuropathic pain in this model.
Introduction
Peripheral nerve injury is relatively common and sometimes results in chronic neuropathic pain with hyperalgesia and allodynia. Treatment of neuropathic pain remains a major clinical challenge because of our poor understanding of the underlying mechanisms. Analgesics, especially opioids, are relatively effective, but their use is limited by patient tolerance.1 Several new pharmacological agents—such as peripheral nerve sodium-channel blockers,2 nuclear factor-κB decoy,3 neurotrophin-3,4 and cannabinoid agonists5—can be used effectively to reverse these symptoms in animal models. However, there are major problems in delivering these agents into the nervous system: (i) the serum half-life of the recombinant protein may be short and (ii) the blood–brain barrier limits access to the central nervous system.
Gene transfer is a novel means of expressing identified transgenes—including those for γ-aminobutyric acid,6,7 glutamic acid decarboxylase,8 µ-opioid receptor,9 and neurotrophic factors10,11—within targeted locations in the nervous system. In the past, many studies were performed using adenoviral vectors, but such vectors can have deleterious side effects, although recently improvements have been made as regards such side effects.12,13 Five years ago, we achieved successful gene transfer into the nervous system via retrograde axonal transport from muscle injected with nonviral hemagglutinating virus of Japan (HVJ)-liposomes containing plasmids encoded with luciferase or β-galactosidase.14 In the present study, rats with a chronic constriction injury (CCI) were investigated to see what effects might be induced via retrograde axonal transport from muscle injected with nonviral HVJ liposomes containing plasmids encoded with the sequence for human hepatocyte growth factor (HGF).
It is well known that HGF is a powerful angiogenetic factor,15,16 and that it exhibits strong neurotrophic activity toward motorneurons both in vitro and in vivo.17,18 However, no report has focused on the possible effect of HGF on neuropathic pain, which is mediated by sensory neurons. To investigate the hypothesis that delivery and continuous expression of HGF within the nervous system would improve neuropathic pain, we measured (i) mRNA levels for P2X and P2Y receptors, and mRNA levels for interleukin-6 (IL-6) and activating transcription factor 3 (ATF3); (ii) the threshold for withdrawal of the hind paw in response to non-noxious mechanical stimuli; and (iii) the thermal threshold in response to noxious heat applied to the plantar surface of the hind paw. We also made histological measurements using light- and electron-micrographs of the sciatic nerve. P2X and P2Y receptors, which are activated by extracellular ATP, were studied because they may act as nociceptive purinoceptors on sensory nerve terminals (see DISCUSSION). Furthermore, it is well known that IL-6 acts as a neuropoietic cytokine after nerve injury19 and that ATF3 plays fundamental roles in nerve survival and regeneration.20,21
Results
In the experimental group, HVJ liposomes containing 100 µg human HGF plasmid DNA were injected percutaneously into the right hindlimb at 1, 2, and 3 weeks after the CCI operation.
Analysis of human HGF protein, and endogenous rat HGF mRNA and protein
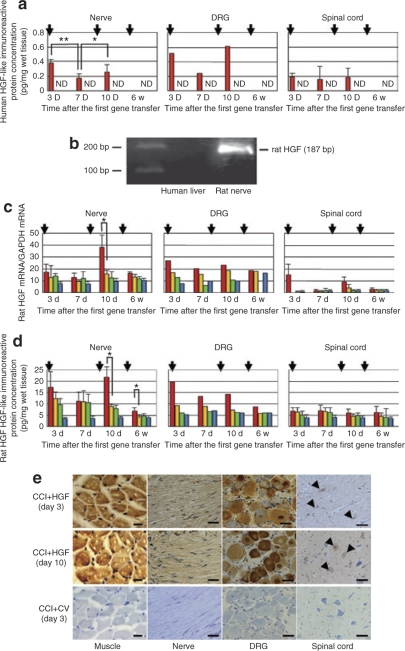
In the CCI+HGF gene-transfer (CCI+HGF) group, human HGF-like immunoreactive protein could be detected in the ipsilateral sciatic nerve, ipsilateral dorsal root ganglion (DRG), and spinal cord on days 3 and 7 after the first gene transfer, and on day 3 after the second gene transfer, but not in week 3 after the third gene transfer (=“6w” in Figure 1). In the nerve and DRG, expression was high on day 3, declined on day 7, only to resurge on day 3 after the second gene transfer, but this pattern was not seen in spinal cord. Human HGF-like immunoreactive protein was detected neither in the CCI+control vector-transfer (CCI+CV) group nor in the non-CCI group (Figure 1a). By reverse transcriptase (RT)-PCR, rat HGF mRNA was detected at 187 bp in the rat RNA extracted from rat nerve, but not in the human RNA extracted from human liver. Therefore, the specific primers we used against rat HGF mRNA did not cross-react with human HGF mRNA (Figure 1b). Expression of rat HGF mRNA was apparently higher in the CCI+HGF group than in the CCI+CV, CCI, and non-CCI groups in the nerve and DRG at each defined time point, the difference being significant for the nerve on day 10 (P < 0.05; Figure 1c). However, it should be noted that measurement of rat HGF mRNA in the DRG was not done in week 6. Likewise, rat HGF-like immunoreactive protein was apparently higher in the CCI+HGF group than in the CCI+CV, CCI, and non-CCI groups, the difference being significant for the nerve both on day 10 and in week 6 (each, P < 0.05; Figure 1d). By immunohistochemistry on day 3 after the first and second gene transfers, human HGF was detected in the ipsilateral muscle (i.e., the tissue into which the injection had been given), sciatic nerve, DRG, and spinal cord in the CCI+HGF group, but not in any of these tissues in the CCI+CV group (Figure 1e).
Figure 1.
Analysis of human hepatocyte growth factor (HGF)-like immunoreactive protein, and endogenous rat HGF mRNA and protein. (a) Expression of human HGF-like immunoreactive protein was detected in ipsilateral sciatic nerve, dorsal root ganglia (DRG), and spinal cord at the 3-day, 7-day, and 10-day time points in the CCI+HGF (HGF gene-transfer after chronic constriction injury (CCI)) group (red bars), but not in the CCI+CV (control-vector gene-transfer after CCI) group (yellow bars) nor in the CCI (green bars) or non-CCI (aged-matched non-CCI controls) (blue bars) groups. (b) Reverse transcriptase-PCR for rat HGF mRNA in rat ipsilateral sciatic nerve and human liver. Expression of rat HGF mRNA was detected in rat nerve, but not in human liver. (c) Expression of endogenous rat HGF mRNA in the ipsilateral sciatic nerve and DRG in four groups (CCI+HGF (red bars), CCI+CV (yellow bars), CCI (green bars), and non-CCI (blue bars) groups). (d) Expression of endogenous rat HGF-like immunoreactive protein in the ipsilateral sciatic nerve and DRG in four groups (CCI+HGF (red bars), CCI+CV (yellow bars), CCI (green bars), and non-CCI (blue bars) groups). (e) Immunohistochemistry for human HGF. Human HGF-like immunoreactive protein was detected in the ipsilateral muscle, sciatic nerve, DRG, and spinal cord dorsal horn neurons (arrowheads) in the CCI+HGF group on day 3 after both the first and second gene transfers, but not in any of these tissues in the CCI+CV group. ND, not detected; arrows (in a, c, and d), gene transfer; bar = 20 µm; *P < 0.05; **P < 0.01; (n = 6 for each group); #not done (DRG at 6 weeks in c).
Behavioral studies
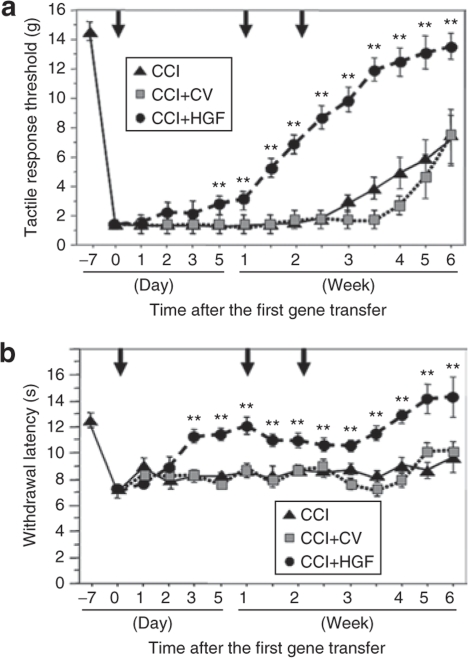
Our time-course study of changes in mechanical and thermal thresholds revealed that on day 0 after the first gene transfer, hind paw withdrawal thresholds were significantly decreased compared to the day before the operation (i.e., 7 days before the first gene transfer). Subsequently, both mechanical and thermal thresholds became significantly greater in the CCI+HGF group than in the CCI+CV and CCI groups (each P value: P < 0.01) on day 5 and day 3, respectively, after the first gene transfer (Figure 2). In the CCI+HGF group, the mechanical threshold thereafter increased with time. In the case of the thermal threshold, the value recorded on day 3 after the first gene transfer for the CCI+HGF group did not differ significantly from that recorded on the last preoperative day for the non-CCI group. In the CCI group, the mechanical and thermal thresholds remained at low levels until 4 weeks and 7 weeks (the end of the study), respectively, after the CCI operation.
Figure 2.
Behavioral studies. (a) Time course of changes in the tactile-response threshold in the CCI (chronic constriction injury) group (black triangle), CCI+HGF (HGF gene-transfer after chronic constriction injury (CCI)) group (black circle), and CCI+CV (control-vector gene-transfer after CCI) group (gray square). (b) Time course of changes in the withdrawal latency in the above three groups. Arrows, gene transfer; **P < 0.01 versus CCI and CCI+CV groups (n = 16 for each group).
Laser Doppler flux and thermographic studies
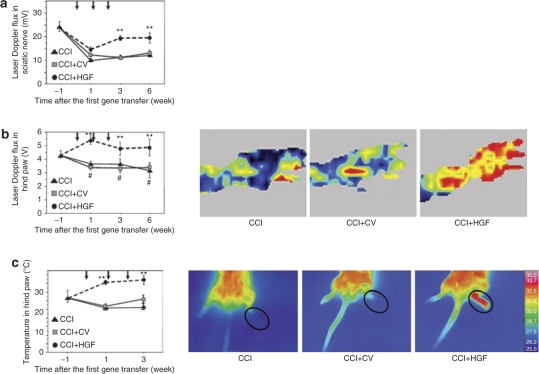
In the CCI+HGF, CCI+CV, and CCI groups, whole-nerve laser Doppler flux displayed significant reduction 2 weeks after the CCI operation (1 week after the first gene transfer) compared to the last preoperative day (i.e., 1 week before the first gene transfer) (each group: P < 0.01; Figure 3a). However, it was significantly higher in the CCI+HGF group than in the CCI+CV and CCI groups at both 3 and 6 weeks after the first gene transfer (each P value: P < 0.01). Laser Doppler flux in the hind paw was significantly higher in the CCI+HGF group than in the CCI+CV and CCI groups at 1, 3, and 6 weeks after the first gene transfer (each P value: P < 0.01; Figure 3b). Moreover, in the CCI+HGF group laser Doppler flux was significantly higher at 1 week than on the last preoperative day (P < 0.05). The skin temperature of the hind paw was significantly higher in the CCI+HGF group than in the CCI+CV and CCI groups at 1 and 3 weeks (each P value: P < 0.01) (Figure 3c).
Figure 3.
Laser Doppler flux and thermographic studies. (a) Time course of changes in laser Doppler flux in the sciatic nerve of the CCI (chronic constriction injury) group (black triangle), CCI+HGF (HGF gene-transfer after chronic constriction injury (CCI)) group (black circle), and CCI+CV (control-vector gene-transfer after CCI) group (gray square). (b) Time course of changes in laser Doppler flux in the hind paw, together with fluxmetry scanning photographs taken at 3 weeks after the first gene transfer in the above three groups. (c) Time course of changes in the temperature of the hind paw (circled in accompanying temperature photographs taken at 1 week after the first gene transfer) in the above three groups. Arrows, gene transfer; **P < 0.01 versus CCI and CCI+CV groups (n = 6 for each group). #P < 0.05 versus preoperative value (“week −1”).
Histological studies of the sciatic nerve
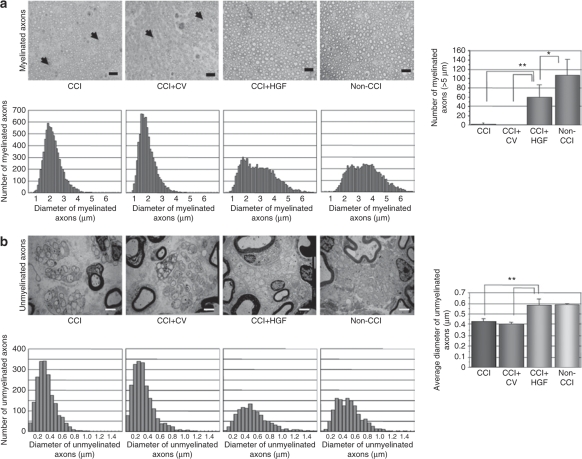
In semi-thin transverse sections of the sciatic nerve obtained 3 weeks after the first gene transfer from the four groups (CCI+HGF, CCI+CV, CCI, and non-CCI groups), myelinated and unmyelinated axons were found to coexist in each group (Figure 4). Wallerian degeneration of myelinated fibers was occasionally found in the CCI and CCI+CV groups, but inflammatory cell infiltration was not found. In the size-frequency distributions for myelinated axons, (i) the non-CCI group showed evidence of two (small) peaks, (ii) the CCI and CCI+CV groups each showed only one (high) peak, and (iii) that for the CCI+HGF group was skewed to the right (versus those for the CCI+CV and CCI groups). In the number of myelinated axons with a diameter >5 µm (thought to be Aβ-fibers), the CCI+HGF group was intermediate between the non-CCI group and the CCI+CV and CCI groups (CCI+HGF group versus non-CCI group, P < 0.05; CCI+HGF group versus CCI+CV or CCI group, P < 0.01 in each case) (Figure 4a). In the size-frequency distributions for unmyelinated axons, (i) the non-CCI, CCI, CCI+CV, and CCI+HGF groups each showed one peak and (ii) the CCI+HGF group tended to be skewed to the right (compared to the CCI and CCI+CV groups). In their size-frequency distribution profiles, there was a closer resemblance between the CCI+HGF and non-CCI groups than between the CCI+HGF group and the CCI and CCI+CV groups. The mean diameter of the unmyelinated axons was significantly greater for the CCI+HGF group than for the CCI or CCI+CV group (each case: P < 0.01), but there was no significant difference between the CCI+HGF group and the non-CCI group (Figure 4b).
Figure 4.
Histological studies of the sciatic nerve. (a) Semi-thin transverse sections, size-frequency distributions for myelinated axons, and number of large myelinated axons (>5 µm) observed in a 0.0604 mm2 area of the sciatic nerve at 3 weeks after the first gene transfer in the CCI (chronic constriction injury), CCI+CV (control-vector gene-transfer after CCI), CCI+HGF (HGF gene-transfer after chronic constriction injury (CCI)), and non-CCI groups. Degeneration of wallerian-type fibers (arrowheads) was sometimes observed in the CCI and CCI+CV groups. Bar = 20 µm; *P < 0.05; **P < 0.01 (n = 6 for each group). (b) Representative electron microscopic sections, size-frequency distributions, and mean diameter of unmyelinated axons in the sciatic nerve at 3 weeks after the first gene transfer. Bar = 2 µm; **P < 0.01 (n = 6 for each group).
RT-PCR studies of P2X2, P2X3, P2X4, P2Y1 receptors, and of ATF3 and IL-6
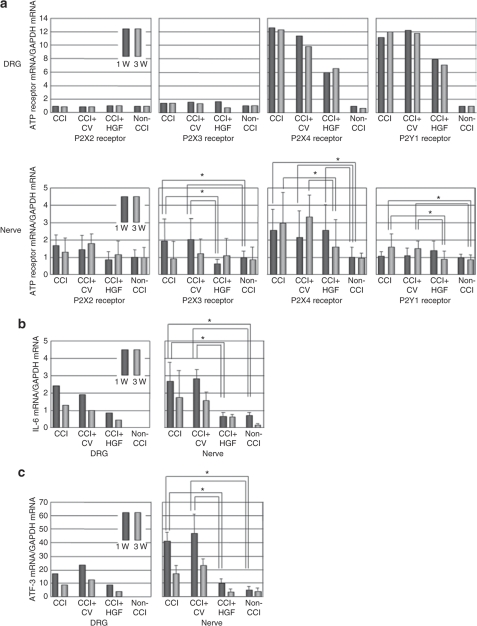
We detected mRNA expressions for P2X2, P2X3, P2X4, and P2Y1 receptors in the ipsilateral DRG and sciatic nerve samples obtained at time points equivalent to weeks 1 and 3 after the first gene transfer in all four groups (CCI+HGF, CCI+CV, CCI, and non-CCI groups) (Figure 5a). Expressions of IL-6 and ATF3 mRNAs were detected in both tissues in three groups (CCI+HGF, CCI+CV, and CCI groups), but in the non-CCI group they were not detected in the normal DRG (Figure 5b,c).
Figure 5.
RT-PCR studies in dorsal root ganglia (DRG) and sciatic nerve. (a) P2X2, P2X3, P2X4, and P2Y1 receptors (n = 6 for each group). (b) Interleukin-6 (IL-6) (n = 5 for each group). (c) Activating transcription factor 3 (ATF3) (n = 5 for each group). Observations were made at 1 and 3 weeks after the first gene transfer in the CCI (chronic constriction injury), CCI+CV (control-vector gene-transfer after CCI), CCI+HGF (HGF gene-transfer after CCI), and non-CCI (aged-matched non-CCI) groups. *P < 0.05.
When the mRNA levels in DRG were measured using all specimens collected at a given defined time point, the ratio of P2X4 receptor mRNA to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and the ratio of P2Y1 receptor mRNA to GAPDH mRNA were found to be approximately ten times greater in the CCI and CCI+CV groups than in the non-CCI group. In terms of these ratios, the CCI+HGF group was intermediate between the non-CCI group and the CCI and CCI+CV groups at both 1 and 3 weeks after the first gene transfer. No significant difference was found in the ratio of P2X2 receptor mRNA to GAPDH mRNA or in the ratio of P2X3 receptor mRNA to GAPDH mRNA at either 1 or 3 weeks after the first gene transfer among the four groups. The ratio of IL-6 mRNA to GAPDH mRNA and that of ATF3 mRNA to GAPDH mRNA in DRG were each found to be greater in the CCI and CCI+CV groups than in the non-CCI group. In terms of these two ratios, the CCI+HGF group was intermediate between the non-CCI group and the CCI and CCI+CV groups at both 1 and 3 weeks after the first gene transfer.
In the case of the ipsilateral sciatic nerve, the ratio of P2X3 receptor mRNA to GAPDH mRNA (i) showed a significant elevation in both the CCI and CCI+CV groups at a time point equivalent to week 1 (versus the CCI+HGF and non-CCI groups; each P value: P < 0.05), but (ii) showed no significant difference between the CCI+HGF group and the non-CCI group at 1 week, while (iii) by week 3, the CCI and CCI+CV groups were not significantly different from the non-CCI group. The ratio of P2X4 receptor mRNA to GAPDH mRNA (i) showed a significant elevation in the CCI, CCI+CV, and CCI+HGF groups at 1 week compared to the non-CCI group (each P value: P < 0.05) and (ii) remained elevated in the CCI and CCI+CV groups at 3 weeks, but in the CCI+HGF group had returned to the level shown by the non-CCI group. In the case of the ratio of P2Y1 receptor mRNA to GAPDH mRNA (i) no significant difference was found among the CCI, CCI+CV, CCI+HGF, and non-CCI groups at 1 week and (ii) the CCI and CCI+CV groups each showed a significant elevation (versus the non-CCI group) at 3 weeks, but the CCI+HGF group was then not different from the non-CCI group. In the case of the ratio of P2X2 receptor mRNA to GAPDH mRNA, no significant difference was found among the CCI, CCI+CV, CCI+HGF, and non-CCI groups at either 1 or 3 weeks. The ratio of IL-6 mRNA to GAPDH mRNA and that of ATF3 mRNA to GAPDH mRNA (i) showed a significant elevation in both the CCI and CCI+CV groups at a time point equivalent to week 1 (versus the CCI+HGF and non-CCI groups; each P value: P < 0.05), but (ii) showed no significant difference between the CCI+HGF group and the non-CCI group at 1 week, while (iii) by week 3, the CCI and CCI+CV groups were not significantly different from the non-CCI group.
Discussion
HGF is a good candidate to be one of the factors facilitating nerve regeneration because of its powerful angiogenetic and neurotrophic actions.17,18 In this study, we examined whether delivery and continuous expression of HGF within the nervous system by means of nonviral HVJ liposome-mediated gene transfer can improve phenomena related to neuropathic pain in CCI rats. First, we demonstrated that in CCI rats, mechanical and thermal thresholds were significantly increased on day 5 and day 3, respectively, after the first HGF gene transfer. Second, laser Doppler flux study of the sciatic nerve showed that values that were reduced in CCI rats were significantly improved after HGF gene transfer. Third, the effects of CCI on the size-frequency distributions of both myelinated and unmyelinated axons were significantly attenuated after HGF gene transfer. Fourth, in the ipsilateral DRG and sciatic nerve, the elevated levels of P2X3, P2X4, and P2Y1 receptor mRNAs, and of IL-6 and ATF3 mRNAs induced by CCI showed partial or complete recovery after HGF gene transfer. Collectively, these results may point toward a potential new therapeutic strategy for the treatment of chronic neuropathic pain in this CCI rat model.
In our preliminary study, we examined the feasibility of achieving gene transfer by giving percutaneous intramuscular injections of nonviral HVJ liposomes containing the human HGF gene into the muscle of the right hindlimb. In that study, expression of human HGF-like immunoreactive protein was found in the ipsilateral sciatic nerve, but neither in the ipsilateral skin nor in the contralateral sciatic nerve (data not shown), suggesting that this method successfully transferred the gene only into the ipsilateral nervous system via retrograde axonal transport. On the basis of the present study, three major favorable points can be made. First, we demonstrated that human HGF-like immunoreactive protein was expressed in the ipsilateral DRG and sciatic nerve after such gene transfer, and that there was a marked boosting effect on the production of endogenous rat HGF-like immunoreactive protein. In fact, in the DRG and sciatic nerve at 3 days after the first and second HGF gene transfers, the levels of rat HGF mRNA and protein were increased 1.2- to 2.5-fold versus those in CCI+CV rats, despite the low-level expression of human HGF-like immunoreactive protein in these tissues. The same phenomenon has been reported in experimental studies of potential treatments for liver cirrhosis17 and skin wounds.18 The authors of those papers suggested that the human HGF gene may serve as a positive regulator of the production, secretion, and/or post-translational modification of rat HGF. In the present study, the endogenous productions of rat HGF mRNA and rat HGF-like immunoreactive protein tended to be enhanced in both CCI-CV and CCI rats versus the non-CCI controls, although statistical significance was not established. These apparent increases in the production of rat HGF mRNA and rat HGF-like immunoreactive protein may have been consequences of the sciatic nerve injury (viz., CCI). This notion may be supported by the recent finding of Munesima et al. that the expressions of endogenous rat HGF protein and rat HGF mRNA were increased after spinal cord injury.22 The second favorable point is that this nonviral gene transfer method should be a useful tool for gene transfer into the nervous system because it is associated with little or no immunogenicity or toxicity.14 That said, it should be mentioned that high transfer efficiency into the nervous system can also be achieved using viral vectors (such as the Herpes simplex virus), and the deleterious side effects once associated with viral vectors have been improved in recent years.12,13 The third point in favor of the present method of gene transfer into the nervous system is that retrograde axonal transport was achieved simply by giving intramuscular injections percutaneously, demonstrating its practical feasibility. Clearly, repeated gene transfer without the need for any surgical procedure at all (as in the present method) is more desirable as a way of achieving stable transfer efficiency into the nervous system.
CCI of the rat sciatic nerve has been widely used as a model of the mechanical allodynia and thermal hyperalgesia associated with neuropathic pain. Such CCI rats show significant reductions in withdrawal latencies to both innocuous mechanical stimuli and noxious heat.23 In the present study, the CCI rats maintained low thresholds to innocuous mechanical stimuli for at least 3 weeks, which is in agreement with a previous report.11 Moreover, a fall in the withdrawal latency to noxious heat developed within 1 week after the CCI operation and persisted for at least 6 weeks (the end of the study). Repeated HGF gene transfer successfully reversed the development of this mechanical allodynia and thermal hyperalgesia. In nerve ligation-induced neuropathic pain, mechanical allodynia is predominantly mediated by myelinated afferents, whereas thermal nociception is mainly transmitted through unmyelinated c-fiber afferents.24 By examining size-frequency distributions, we found that the decreases in myelinated and unmyelinated axons found in CCI rats were reversed by repeated HGF gene transfer. Moreover, the levels of P2X4 and P2Y1 receptor mRNAs in the ipsilateral DRG and the levels of P2X3, P2X4, and P2Y1 receptor mRNAs in the ipsilateral sciatic nerve were found to be elevated in our CCI model, and these raised levels were all decreased following repeated HGF gene transfer. It is known that the P2X3 receptor is expressed by unmyelinated small-diameter (c-fiber) sensory fibers, and that it mediates thermal hyperalgesia, whereas P2X2/3 heteromeric and P2Y1 receptors are expressed by myelinated intermediate-diameter (Aδ-fiber) and large-diameter (Aβ-fiber) sensory fibers, respectively, and they mediate mechanical allodynia.25,26,27,28,29,30 Mechanical allodynia also requires activation of the P2X4 receptor, and nerve injury–induced pain hypersensitivity depends upon ongoing signaling via the P2X4 receptor, which is most likely activated by ATP released from primary sensory terminals.31 In addition, we found that the levels of IL-6 and ATF3 mRNAs in the ipsilateral DRG and sciatic nerve were elevated in our CCI model, and these raised levels showed decreases following repeated HGF gene transfer. Therefore, the improvements we observed in mechanical and thermal thresholds may be supported both by the morphological changes we detected and by molecular changes in the nociceptive purinoceptors on sensory nerve terminals.
It is well known that HGF is a potent angiogenic growth factor both in vitro and in vivo.32,33 In the present study, we observed that HGF gene transfer led to increases in whole-nerve blood flow and in the blood flow and skin temperature of the hind paw. These findings support the idea that HGF has a potent angiogenic action. In a CCI model, Myers et al. demonstrated (i) endoneurial edema in the subperineurial region, perivascular spaces, and between nerve fibers that was prominent at 7 days after the CCI operation and persisted for 23 days and (ii) that nerve blood flow between the ligatures was reduced to 58% of the control value at 7 days.34 They suggested that the hyperesthesia observed in this model was closely related to the reduction in nerve blood flow. This raises the possibility that one of the explanations for the effects of repeated HGF gene transfer on neuropathic pain is an improvement in nerve blood flow.
In conclusion, we have demonstrated that transfer of the human HGF gene into the nervous system via repeated intramuscular injection of nonviral HGF-HVJ liposomes is an efficient way of preventing or limiting sensory nerve degeneration in a rat model of neuropathic pain. This is the first report of successful human HGF liposome-mediated transfer in a rat model of neuropathic pain.
Materials and Methods
Overall, 288 male Wistar rats, ~6-weeks old and weighing 150–170 g, were assigned to this study. They were housed in a temperature-controlled room with a 12-hour light–dark cycle. This experimental study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and the protocol was approved by the Committee on the Ethics of Animal Experiments of the National Defense Medical College.
Neuropathic pain behavior was assessed using a CCI model, as previously described by Bennett and Xie.23 Briefly, male rats were anesthetized with sodium pentobarbital, and the right common sciatic nerve, after exposure at the mid-thigh level, was loosely tied with four chromic catgut ties, spaced ~1 mm apart. By this means, the nerve was slightly constricted, so that the circulation through the epineural vasculature was not interrupted. At 1 week after the CCI operation, HVJ liposomes, containing either 100 µg human HGF plasmid DNA or 100 µg pcDNA 3.1 (–) (as a control vector), were carefully injected percutaneously into the proximal one-third of the tibialis cranialis of the right hindlimb over a few minutes via a 27-gauge needle. This injection was repeated at 2 and 3 weeks after the CCI operation.
Construction of plasmid DNA and preparation of the HVJ liposome-complex vector. To produce an HGF expression vector, human HGF cDNA (2.2 kb) was inserted between the NotI sites of a pcDNA 3.1 (–) vector.
Preparation of the HVJ liposome-complex vector. The HVJ liposome-complex vector was prepared as described previously.35 Briefly, phosphatidylserine, phosphatidylcholine, and cholesterol were mixed in tetrahydrofuran. The lipid mixture was deposited on the sides of a flask by removal of the solvent in a rotary evaporator. The high mobility group (HMG)-1, 2-mixture was mixed with plasmid DNA, and the mixture was then added to the dried lipid. The liposome-DNA- HMG-1, 2-mixture complex suspension was mixed with inactivated HVJ (Z strain). Free HVJ was removed from the HVJ liposomes by sucrose-gradient centrifugation.
Analysis of HGF protein expression. The expression of human HGF protein and endogenous rat HGF protein (strictly speaking, HGF-like immunoreactive protein) in each tissue was measured using enzyme-linked immunosorbent assays on days 3 and 7 after the first transfer, on day 3 after the second gene transfer, and at 6 weeks after the first transfer (to evaluate the effectiveness of repeated gene delivery). Specimens were homogenized using a previously described method, and the human HGF enzyme-linked immunosorbent assay system specifically detected human HGF, not endogenous rat HGF, whereas the rat HGF enzyme-linked immunosorbent assay system specifically detected endogenous rat HGF, not human HGF.18 Measurements involving DRG (L5) were performed using six specimens collected at each defined time point due to the small volume of DRG tissue obtained from a given individual.
Analysis of the distribution of human HGF expression (immunohistochemistry). At 3 days after the first and second transfers, intracardiac perfusion was carried out with PBS containing 4% paraformaldehyde. Specimens were harvested, and indirect immunohistochemistry was assessed using frozen sections, using mouse monoclonal antibody against human HGF (1:5; Institute of Immunology, Tokyo, Japan).14 This monoclonal antibody specifically detected human HGF, not rat HGF. For the negative control, the incubation step with the primary antibody was omitted.
Behavioral studies. Behavior after a CCI operation has been described by Bennett and Xie23 Although Wall et al. reported that rats with a sciatic nerve transection exhibited autotomy,36 none of our rats displayed major autotomy. Mechanical allodynia and thermal hyperalgesia were measured at 15 defined time points (see Figure 2; n = 16 for each group). Mechanical allodynia was determined by quantifying the withdrawal threshold of the right hind paw (ipsilateral to the site of CCI) in response to Von Frey hairs.37 Von Frey hairs were applied perpendicularly to the plantar surface of the hind paw, sufficient force being used to bend the filament. Brisk withdrawal or paw flinching was taken as a positive response. Thermal hyperalgesia was measured using a Hargreaves apparatus (Plantar Test; Ugo Basile, Italy).38 A radiant heat stimulus was applied to the midplantar area of the hind paw ipsilateral to the CCI site, and the time between initial heat and paw withdrawal was recorded. Each testing for mechanical allodynia and thermal hyperalgesia was repeated five times, and the mean value was used.
Laser Doppler flux studies of the sciatic nerve. Sciatic nerve blood flow was assessed at a site 15 mm distal to the most distal part of the CCI lesion using a laser Doppler flowmeter (ALF 21R; Advance, Tokyo, Japan). At four defined time points (weeks −1, +1, +3, and +6 relative to the first gene transfer), the probe tip was placed just above the sciatic nerve in the mid-thigh region under anesthesia. Consecutive measurements in arbitrary flow units were made, and the median of these values was used to represent blood flow in each tissue.
Laser Doppler flux and thermographic studies of the hind paw. Blood flow in the hind paw was measured using a laser Doppler flowmeter (PIM II: Perimed AB, Järfälli, Sweden) at four defined time points (weeks −1, +1, +3, and +6 relative to the first gene transfer). Low or no perfusion is displayed as dark blue, whereas the highest perfusion is displayed as white. These laser images were quantitatively converted into a histogram for the scanned area. Thermography was performed using a TH9100 ML camera (NEC Sanei, Tokyo, Japan) in a given group at three defined time points (−1, +1, and +3 weeks relative to the first gene transfer).
Histological studies of the sciatic nerve. At 3 weeks after first HGF gene therapy, specimens were taken from the sciatic nerve at 15 mm distal to the most distal part of the CCI lesion. The number of myelinated axons in each rat was determined in an area 0.0604 mm2. The diameters of the myelinated and unmyelinated axons in each rat were measured in at least 1,000 or 180 axons, respectively.
Total RNA extraction and semiquantitative RT-PCR for rat HGF mRNA, for P2X2, P2X3, P2X4, and P2Y1 receptor mRNAs, and for IL-6 and ATF3 mRNAs. The mRNA expressions for the rat HGF, for P2X2, P2X3, P2X4, P2Y1 receptors, and for IL-6 and ATF3 (i) at sites distal to the region of constriction in the ipsilateral sciatic nerve and (ii) in the ipsilateral DRG (L5) were examined by semiquantitative RT-PCR. Total RNA was isolated using ISOGEN (Nippon Gene, Tokyo, Japan), and RT-PCR was performed using an amplification reagent kit (TaqMan EZRT-PCR kit; Applied Biosystems, Alameda, CA) by using several primers. The following primers were synthesized using an automated DNA synthesizer: P2X2, P2X3, P2X4, and P2Y1 receptors, rat HGF, IL-6, ATF3, and GAPDH. Sequence information for all the PCR primers and TaqMan probes used is listed in Table 1.
Table 1.
Primer sequences, TaqMan probe sequences, and thermocycle conditions used for RT-PCR
Statistical analysis. Values for each parameter are expressed as means ± SEM (mean alone for pooled DRG tissues). Fisher's protected least-significant-difference test was applied to the data when significant F-ratios were obtained in an analysis of variance, and significance was set at P < 0.05 (Stat View 5.0, SAS Institute, Cary, NC). The statistical significance of the difference between curves representing repeated measures of behavioral function (allodynia or hyperalgesia) was assessed using a repeated measures analysis (SPSS, Chicago, IL).
Acknowledgments
This study was supported by a grant for special research from National Defense Medical College. We thank S. Tominaga, Y. Ichiki, T. Oguma, H. Kasamatsu, and G. Hammond-Tooke for their excellent technical assistance and R. Timms for correcting the English version of the manuscript.
REFERENCES
- Ossipov MH, Lopez Y, Nichols ML, Bian D., and , Porreca F. Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci Lett. 1995;199:83–86. doi: 10.1016/0304-3940(95)12026-z. [DOI] [PubMed] [Google Scholar]
- Brochu RM, Dick IE, Tarpley JW, McGowan E, Gunner D, Herrington J, et al. Block of peripheral nerve sodium channels selectively inhibits features of neuropathic pain in rats. Mol Pharmacol. 2006;69:823–832. doi: 10.1124/mol.105.018127. [DOI] [PubMed] [Google Scholar]
- Inoue G, Ochiai N, Ohtori S, Nakagawa K, Gemba T, Doya H, et al. Injection of nuclear factor-κB decoy into the sciatic nerve suppresses mechanical allodynia and thermal hyperalgesia in a rat inflammatory pain model. Spine. 2006;31:2904–2908. doi: 10.1097/01.brs.0000248424.46652.67. [DOI] [PubMed] [Google Scholar]
- Wilson-Gerwing TD., and , Verge VM. Neurotrophin-3 attenuates galanin expression in the chronic constriction injury model of neuropathic pain. Neuroscience. 2006;141:2075–2085. doi: 10.1016/j.neuroscience.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Liu C., and , Walker JM. Effects of a cannabinoid agonist on spinal nociceptive neurons in a rodent model of neuropathic pain. J Neurophysiol. 2006;96:2984–2994. doi: 10.1152/jn.00498.2006. [DOI] [PubMed] [Google Scholar]
- Hwang JH., and , Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- Malan TP, Mata HP., and , Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC., and , Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol. 2005;57:914–918. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Gu Y, Xu GY, Wu P, Li GW., and , Huang LY. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: a strategy to increase opioid antinociception. Proc Natl Acad Sci USA. 2003;100:6204–6209. doi: 10.1073/pnas.0930324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC., and , Fink DJ. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Mol Ther. 2003;8:367–375. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Blits B, Ruitenberg MJ, Verhaagen J., and , Oudega M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002;9:1387–1395. doi: 10.1038/sj.gt.3301814. [DOI] [PubMed] [Google Scholar]
- Mueller C., and , Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;19:681–689. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Bangari DS., and , Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Nakanishi K, Nemoto K, Morishita R, Kaneda Y, Uenoyama M, et al. Efficient gene transfer from innervated muscle into rat peripheral and central nervous systems using a nonviral HVJ (Hemagglutinating Virus of Japan)-liposome method. J Neurochem. 2003;85:810–815. doi: 10.1046/j.1471-4159.2003.01730.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A, Kaise N., and , Nishino T. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987;224:311–316. doi: 10.1016/0014-5793(87)80475-1. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Morishita R, Nakagami H, Yoshimura S, Hara A, Matsumoto K, et al. Gene therapy for preventing neuronal death using hepatocyte growth factor: in vivo gene transfer of HGF to subarachnoid space prevents delayed neuronal death in gerbil hippocampal CA1 neurons. Gene Ther. 2001;8:1167–1173. doi: 10.1038/sj.gt.3301498. [DOI] [PubMed] [Google Scholar]
- Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Uenoyama M, Tomita N, Morishita R, Kaneda Y, Ogihara T, et al. Gene transfer of human hepatocyte growth factor into rat skin wounds mediated by liposomes coated with the sendai virus (Hemagglutinating Virus of Japan) Am J Pathol. 2002;161:1761–1772. doi: 10.1016/S0002-9440(10)64453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatters SJ, Fox AJ., and , Dickenson AH. Nerve injury alters the effects of interleukin-6 on nociceptive transmission in peripheral afferents. Eur J Pharmacol. 2004;484:183–191. doi: 10.1016/j.ejphar.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Kim HY, Park CK, Cho IH, Jung SJ, Kim JS., and , Oh SB. Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain. 2008;9:280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Hu P, Bembrick AL, Keay KA., and , McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Sato N, Sata M, Wakayama K, Ogihara T., and , Morishita R. Expression of hepatocyte growth factor and c-Met after spinal cord injury in rats. Brain Res. 2007;1151:188–194. doi: 10.1016/j.brainres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Bennett GJ., and , Xie Y-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Bian D, Malan TP Jr, Lai J., and , Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, et al. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T., and , Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S., and , Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J Neurosci. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Ueno S, Koizumi S, Ueda H, Iwanaga T, et al. Downregulation of P2X3 receptor-dependent sensory functions in A/J inbred mouse strain. Eur J Neurosci. 2002;15:1444–1450. doi: 10.1046/j.1460-9568.2002.01982.x. [DOI] [PubMed] [Google Scholar]
- Nakamura F., and , Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci USA. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, et al. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000;7:417–427. doi: 10.1038/sj.gt.3301104. [DOI] [PubMed] [Google Scholar]
- Koike H, Morishita R, Iguchi S, Aoki M, Matsumoto K, Nakamura T, et al. Enhanced angiogenesis and improvement of neuropathy by cotransfection of human hepatocyte growth factor and prostacyclin synthase gene. FASEB J. 2003;17:779–781. doi: 10.1096/fj.02-0754fje. [DOI] [PubMed] [Google Scholar]
- Myers RR, Yamamoto T, Yaksh TL., and , Powell HC. The role of focal nerve ischemia and Wallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology. 1993;78:308–316. doi: 10.1097/00000542-199302000-00015. [DOI] [PubMed] [Google Scholar]
- Kaneda Y, Morishita R., and , Dzau VJ.Prevention of restenosis by gene therapy Ann NY Acad Sci 1997811299, 308–308.discussion [DOI] [PubMed] [Google Scholar]
- Wall PD, Bery J., and , Saade N. Effects of lesions to rat spinal cord lamina I cell projection pathways on reactions to acute and chronic noxious stimuli. Pain. 1988;35:327–339. doi: 10.1016/0304-3959(88)90142-X. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM., and , Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C., and , Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]