Abstract
Targeting tumor-specific gene abnormalities has become an attractive approach in developing therapeutics to treat cancer. Overexpression of Bcl2 and mutations of p53 represent two of the most common molecular defects in tumors. In the nucleus, p53 induces cell cycle arrest, while it interacts with Bcl2 outside of the nucleus to regulate signal pathways involved in apoptosis. To potentiate antitumor activity, we tested a “double target” approach to antitumor therapy by combining H101, a recombinant oncolytic adenovirus that targets the inactive p53 in tumors, with a small interfering RNA (siBCL2) that targets Bcl2. In cell culture, the combined treatment significantly enhanced apoptosis and cytotoxicity as compared with treatment with either H101 or siBCL2 alone. In animals carrying tumor xenographs, combined H101 and siBCL2 treatment significantly inhibited tumor growth and prolonged survival. At the end of the study, all animals in the combined therapy group survived and two of the five animals showed complete eradication of their tumors. Interestingly, siBCL2 treatment increased H101 viral replication in both treated cells and tumor tissues. Simultaneously targeting two tumor-specific gene abnormalities using an oncolytic adenovirus and siRNA potentiates total antitumor activity.
Introduction
Conventional chemotherapeutic agents, though often effective, are highly toxic because of their lack of selectivity for cancer cells. As a result, efforts have focused on developing interventions that can target tumor-specific genes, using techniques that include tumor-selective replicating viruses and siRNA.
The p53 tumor suppressor, the “guardian of the genome”, is the most frequently mutated gene in cancer.1 Loss of function of the wild-type p53 allows cells to evade apoptosis, thereby promoting tumor progression. A clinically validated approach to therapy is to restore p53 function by replacing the mutant gene using a replication-deficient virus that carries the wild-type P53 (Ad5CMV-p53). Restoration of P53 function in tumor cells may induce apoptosis and sensitize cells to cytotoxic killing, thus improving the therapeutic response. Clinical studies have shown that intratumoral injection of Ad5CMV-p53 resulted in wild-type P53 expression in various tumor tissues, leading to selective death of cancer cells (see reviews ref. 2,3,4).
Another virus-based strategy takes advantage of the fact that the replication and production of adenoviral progeny require the cell cycle gatekeeper p53 to be in an inactive status, which is commonly observed in tumors due to mutation or epigenetic silencing of the gene. Adenoviruses infect quiescent cells and induce them to enter the S phase of the cell cycle so that viral DNA replication can proceed. Adenovirus early genes encode proteins that have several functions aimed at activating the S phase of the cell cycle. One viral early gene, E1B, which encodes a 55-kDa protein (E1B 55K), is essential to virus replication. E1B interacts with cellular p53 and inactivates it to allow viral replication. ONYX-015, a mutant adenovirus that lacks the E1B 55K gene, can only replicate and lyse tumor cells that have inactivated P53, sparing normal cells that retain wild-type p53 function.5 Clinical trials in patients with recurrent head and neck cancer, metastatic colorectal cancer, or pancreatic cancer have shown that ONYX-015 is safe and has significant antitumor effect in at least a fraction of the patients, when it is used alone or combined with chemotherapy (review refs 2,6,7,8).
In China, an oncolytic adenovirus called H101 has been clinically approved for the treatment of several malignancies.9 In this virus, both E1B and E3 are deleted, so that it selectively infects and kills tumor cells through viral oncolysis.10 Without E1B to inactivate p53, H101 adenovirus cannot replicate and lyse normal cells where p53 is active. Thus, H101 does not have significant cytopathic effects on normal cells. However, H101 has limited efficacy as a monotherapy in clinical practice. Tumors show great variation in regard to their susceptibility to oncolytic adenoviruses, presumably because E1B has other functions in addition to targeting p53, such as viral RNA export and inhibition of host protein synthesis. Other studies have shown that the replication of this type of oncolytic adenovirus in human tumor cells is actually independent of p53 status.11,12 Thus, in practice, p53-based therapeutic strategies are often combined with conventional cancer therapy to minimize development of therapy resistance.
It is interesting to note that p53 not only functions in the nucleus to induce cell cycle arrest, but also has an “extranuclear” proapoptotic function as it partners with Bcl2,13,14 another well-established tumor target gene. By forming complexes with Bcl2 on the outer mitochondrial membrane, p53 directly disrupts Bcl2/Bax to regulate apoptosis. Bcl2 expression is negatively regulated by p53 and is upregulated in p53-deficient cells.15 On the other hand, Bcl2 phosphorylation blocks this p53 interaction and preserves its antiapoptotic activity.16 Overexpression of antiapoptotic Bcl2 or Bcl-xL abrogates stress signal–mediated mitochondrial p53 accumulation and apoptosis.14 Thus, p53 and Bcl2 are tightly coordinated in balancing “Ying-Yang” pathways of apoptosis. Bcl2 is overexpressed in a wide range of human tumors where it promotes tumor formation by impeding apoptosis.17,18 High levels of Bcl2 expression in tumors is associated with faster time to relapse, shorter survival, and other indications of poor clinical outcome.19 Thus, the concurrence of p53 and Bcl2 genetic defects in tumors may represent a molecular “double-hit” related to tumor growth.
We propose to enhance anticancer efficiency by testing a “double-targeted” therapeutic approach. Suppression of Bcl2 expression by antisense and RNAi compounds inhibited tumor growth and enhanced apoptosis in a variety of animal models.20,21 We have previously used RNAi and methylated oligonucleotides22,23 to knock down Bcl2. Using plasmid vectors expressing short hairpin RNAs (shRNAs), we showed that silencing Bcl2 improved the antitumor effect of the chemotherapeutic agent 5-FU.22 In this communication, we have examined the effect of simultaneously targeting two tumor-related genetic defects: overexpressed Bcl2 and mutant p53. We incubated the H101 oncolytic adenovirus in addition to Bcl2 RNAi with a cervical cancer cell line Hela-S3 that overexpresses Bcl2 but is deficient in p53.24 Preclinical studies demonstrated that Hela-S3 was very sensitive to H101 oncolytic treatment. We found that the this “double target” therapy significantly enhanced anticancer efficiency both in vitro and in vivo, presumably by enhanced viral replication and increased apoptosis in tumors.
Results
Targeted silencing of Bcl2 by synthetic siRNA
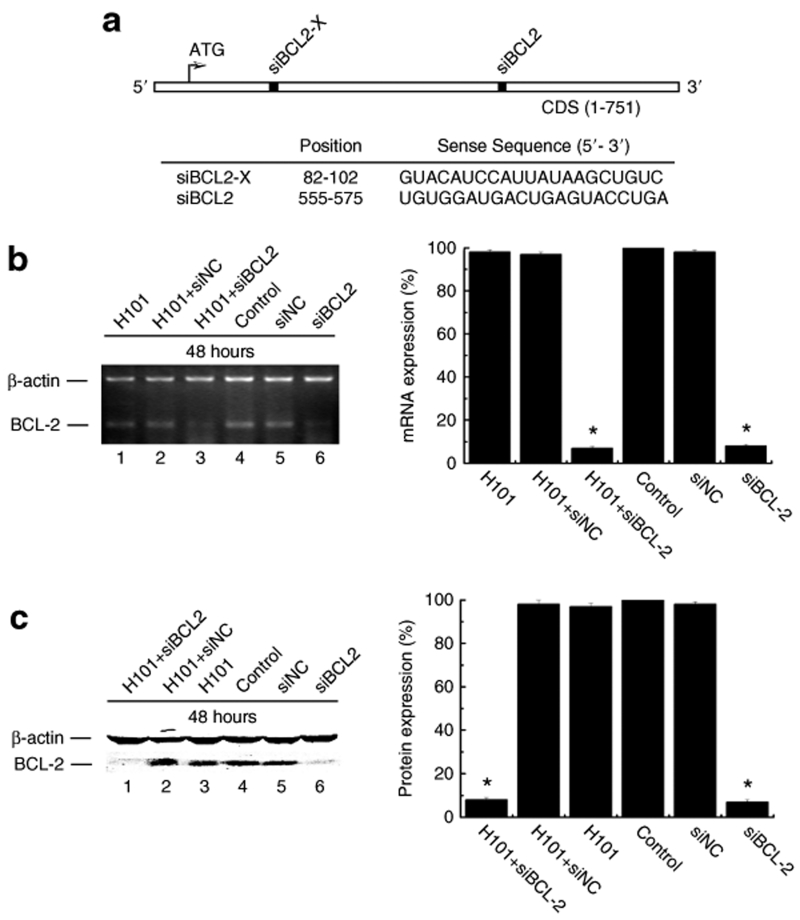
Previously, we silenced Bcl2 transcription using a plasmid DNA vector that delivered two short Bcl2 hairpin RNAs.22 On the basis of our previous data, we synthesized two siRNAs that target two different positions of Bcl2 mRNA as shown in Figure 1a. The first Bcl2 siRNA (siBLC2-X) that targets the 5′ region of the Bcl2 mRNA was completely ineffective in silencing Bcl2 gene expression (data not shown). The second Bcl2 siRNA (siBCL2), however, was found to effectively suppress Bcl2 as determined by semiquantitative PCR (Figure 1b, lane 6) as compared with control siRNA (siNC, lane 5) and control cells (phosphate buffered saline (PBS), lane 4). Bcl2 silencing by siBCL2 was also confirmed at the protein production level using Western blot analysis (Figure 1c, lane 6 versus lanes 5, 4).
Figure 1.
Bcl2 gene knockdown by siRNA. (a) Schematic representation and location of two Bcl2-specific siRNAs used in this study. (b) Semiquantitative RT-PCR analysis of Bcl2 transcripts in Hela-S3 cells. Left panel: PCR gel. Right panel: PCR bands were scanned and normalized over the internal control β-actin. The value of PBS control was set as 100%. (c) Western Blot analysis of Bcl2 protein expression in Hela-S3 cells. All experiments were performed 48 hours after siBLC2 (50 nmol/l) tranfection with or without H101 infection (multiplicity of infection (MOI) = 100). β-actin was used as the internal control for normalization. The value of PBS control was set as 100%.
Oncolytic adenovirus H101 uses an entirely different mechanism to target tumor cells, and therefore we wanted to test whether viral infection would affect siRNA-mediated Bcl2 silencing. Cells were co-treated with siBCL2 and H101. Bcl2 gene expression was examined by both semiquantitative RT-PCR and Western blotting. As expected, viral infection alone or with the control siRNA (siNC) did not significantly inhibit Bcl2 expression. Bcl2 expression was markedly inhibited by siBCL2 or siBCL2 plus H101 (Figure 1b,c). This experiment demonstrates that adenoviral infection does not alter the gene silencing caused by synthetic siRNA in cancer cells.
Enhanced cytotoxicity of Hela-S3 tumor cells by the combined treatment of H101 and Bcl2 siRNA
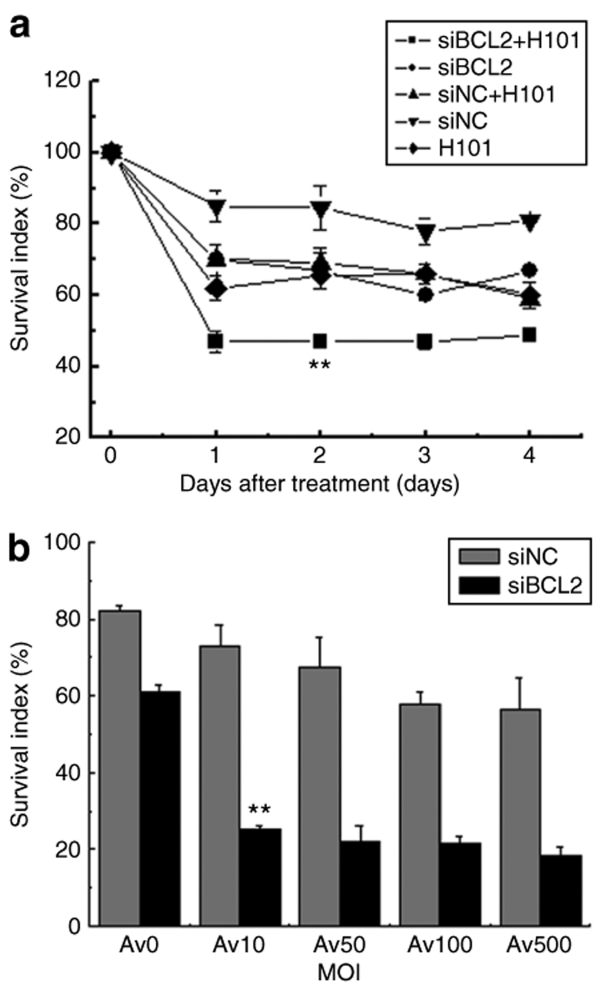
H101 and siBCL2 inhibit tumor cell growth by targeting two different oncoprotein pathways. We were interested in examining whether these two treatments would significantly enhance antitumor effects in tumor cells. As compared with the control group, cells treated with the monotherapy of siBCL2 or H101 showed moderate reduction of cell survival (Figure 2a), starting from the second day following the treatment and maintained thereafter. The control siRNA (siNC) reduced cell growth by 20%. However, in the group treated with combined H101 and siBCL2, cell growth was significantly inhibited, indicating an anticancer augmentation of the combined tumor therapy.
Figure 2.
Growth inhibition of Hela-S3 cells. (a) The combined treatment of siBCL2 and H101. Cell growth was measured by MTT assays at days 1, 2, 3, 4 after co-treatment of siBCL2 (50 nmol/l) and H101 (multiplicity of infection (MOI) = 100). (b) Treatment of siBCL2 with varying doses of H101 adenovirus. Cell growth was measured at day 2 after treatment with 50 nmol/l siBLC2 or siNC. H101 infection was performed at MOIs of 0, 10, 50, 100, and 500. All data are presented as means ± SD. of three independent experiments. P < 0.01: as compared with untreated Hela-S3 cells.
We then examined the effect of using various titers of H101 in siBcl-transfected cells. In cells treated with the control siRNA (siNC), increasing H101 titers showed a slight dose–response inhibition of cell growth (Figure 2b). In cells treated with Bcl2 siRNA (siBCL2) plus H101, however, there was a significant reduction in cell survival, even with a very low titer of the oncolytic adenovirus (multiplicity of infection = 10). Further increases in H101 titers did not dramatically increase cell inhibition. These data suggest that Bcl2 siRNA treatment can reduce the required dose of oncolytic adenovirus H101.
Enhanced apoptosis by combined siBCL2 and H101 treatment
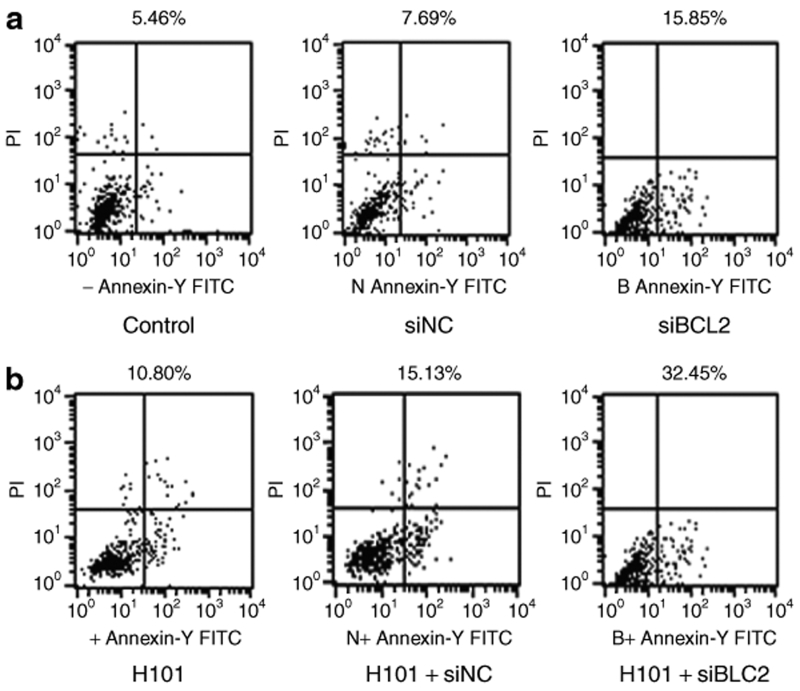
To examine apoptosis, siBCL2 was transfected into Hela-S3 cells using Lipofectamine 2000. Forty-eight hours after transfection, apoptosis were measured using an Annexin V-FITC apoptosis kit and flow cytometric analysis. As seen in Figure 3a, siBCL2 induced apoptosis in 15.9% of Hela-S3 cells as compared with siNC-treated (7.7%) and PBS-treated (5.5%) Hela-S3 cells. Infection of Hela-S3 cells with H101 at a multiplicity of infection of 100 induced cell apoptosis (10.8%).
Figure 3.
Apoptotic activity of in Hela-S3 cells. Apoptosis were measured by flow cytometric analysis at 48 hours after co-treatment of siBCL2 and H101.
Next, we examined whether there was an additive effect on apoptosis when siBCL2 treatment was combined with H101. We found that the degree of apoptosis nearly tripled (32.5%) when both siBCL2 and H101 were administered together (Figure 3b). These data suggest that H101 synergizes with Bcl2 siRNA in the induction of apoptosis.
Bcl2 siRNA enhanced H101 DNA replication
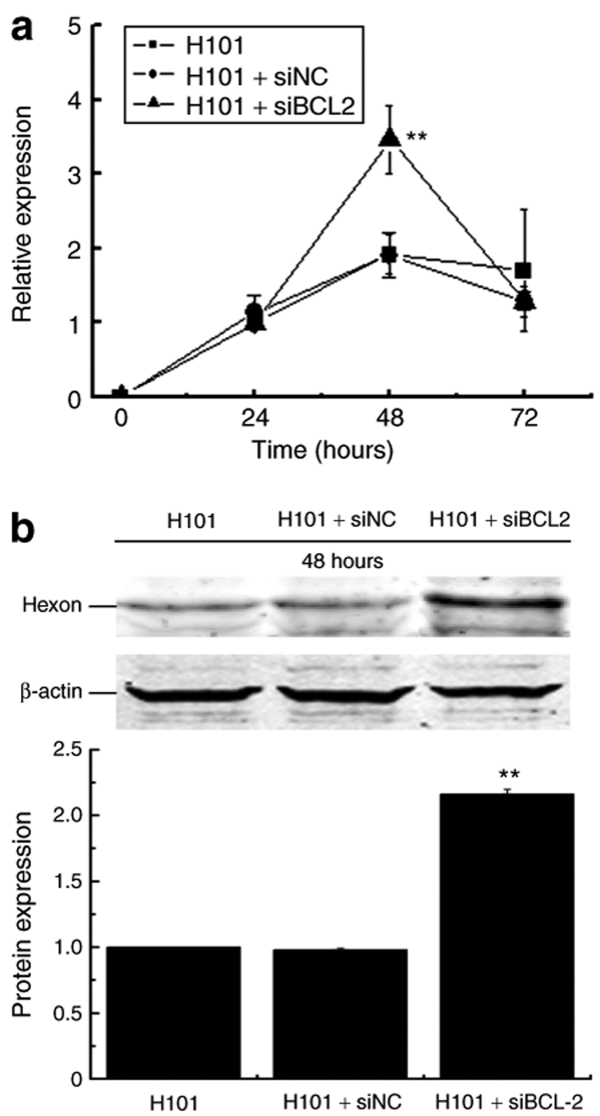
We then tested whether the apoptosis induced by Bcl2 siRNA would interfere with viral replication of H101 and thus reduce the therapeutic effect of the oncolytic adenovirus if the two treatments were used together. To examine adenovirus DNA replication, the expression of the late gene hexon was determined by real-time RT-PCR. We did not observe significant differences in hexon mRNA expression in H101-infected cells in the presence or absence of siNC transfection. However, we found that siBCL2 treatment increased hexon mRNA expression by twofold in H101-infected cells at 48 hours (P < 0.01, Figure 4a). Similarly, western blot analysis also showed an approximately twofold increase in Hexon protein when the combination of siBCL2 and H101 were used (Figure 4b). Taken together, these data indicate that silencing of Bcl2 actually enhanced DNA synthesis of H101, although the specific mechanism is not clear.
Figure 4.
The effect of siBCL2 on viral DNA replication in Hela-S3 cells. (a) Viral DNA replication was determined by real-time RT-PCR quantification of adenoviral late Hexon gene expression at 24, 48, and 72 hours after co-treatment, respectively. For comparison, the mRNA expression of Hexon at 24 hours in H101 group was arbitrarily set as 1, and β-actin was used as the internal control in the calculation. P < 0.01: compared to Hexon mRNA expression of the H101 group. (b) Western Blot analysis of Hexon protein expression at 48 hours. Bar: average band density of quantified Hexon protein after normalization by the internal control β-actin. Protein expression of Hexon in H101 group was arbitrarily set as 1. **P < 0.01: relative to Hexon protein expression in the H101-treated group.
Enhanced cytotoxicity of the cocktail treatment in tumor cells with varied p53 activity
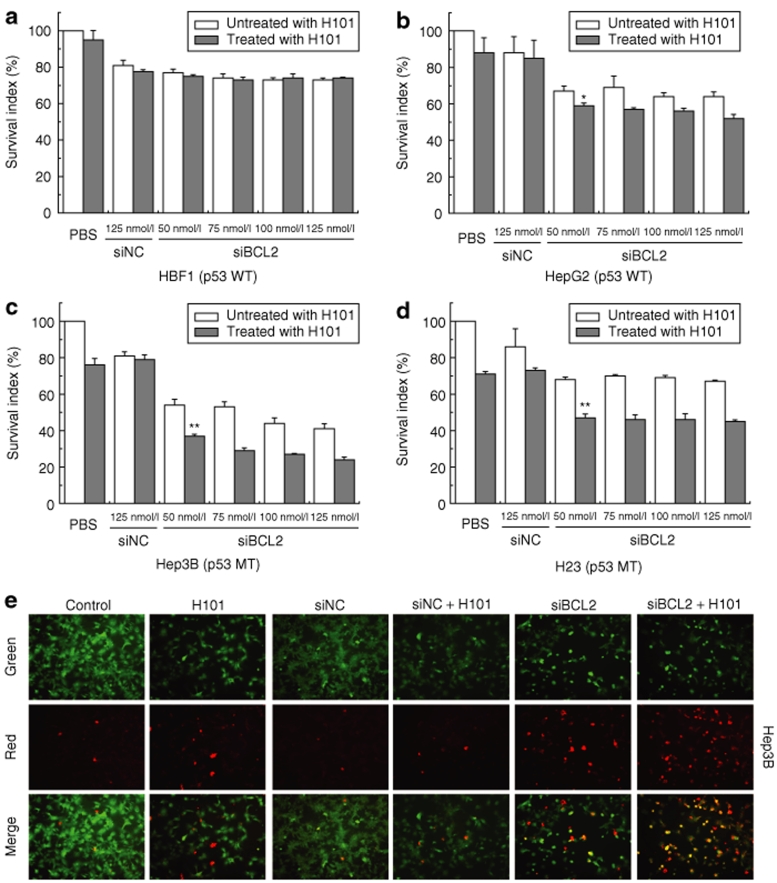
The sensitivity of cancer cells to oncolytic virus is affected by the activity of tumor suppressor p53. We thus tested the combined therapy in four cell lines with varying p53 activity, including HBF1 (normal human fibroblasts), HepG2 (hepatoma cell line with wild-type p53), Hep3B (hepatoma cell line with a p53 deletion25,26), and H23 (non-small cell lung cancer cell line with p53 mutation27). As seen in Figure 5a–d, H101 had minimal effect on cell growth in normal fibroblasts. However, enhanced cytotoxic effects were observed in tumor cells, especially in Hep3B and H23 that bear p53 mutations. Similarly, an enhanced cell apoptosis, as determined by the TUNEL assay, was also observed in p53-negative Hep3B cells that were treated with the H101 and siBCL2 cocktail therapy (Figure 5e).
Figure 5.
Growth inhibition of the combined therapy on human cell lines with varying p53 activity. (a) HBF1, normal human skin-derived fibroblasts; (b) HepG2, p53-wild-type hepatoma cell line; (c) Hep3B, p53-mutated hepatoma cell line; and (d) H23, p53-mutated non-small-cell carcinoma cell line. Cells were exposed to H101 (multiplicity of infection (MOI) = 100) and varying concentrations of siBCL2, and cell growth was measured by MTT assays. All data are presented as means ± SD. of three independent experiments. (e) Cell apoptosis detected by TUNEL assay. Live cells were stained only with 6-CF (green) and necrotic cells with AnnCy3 (red). Apoptotic cells were stained with both AnnCy3 and 6-CF (yellow).
Improved in vivo antitumor activity by the combined treatment of siBCL2 and H101
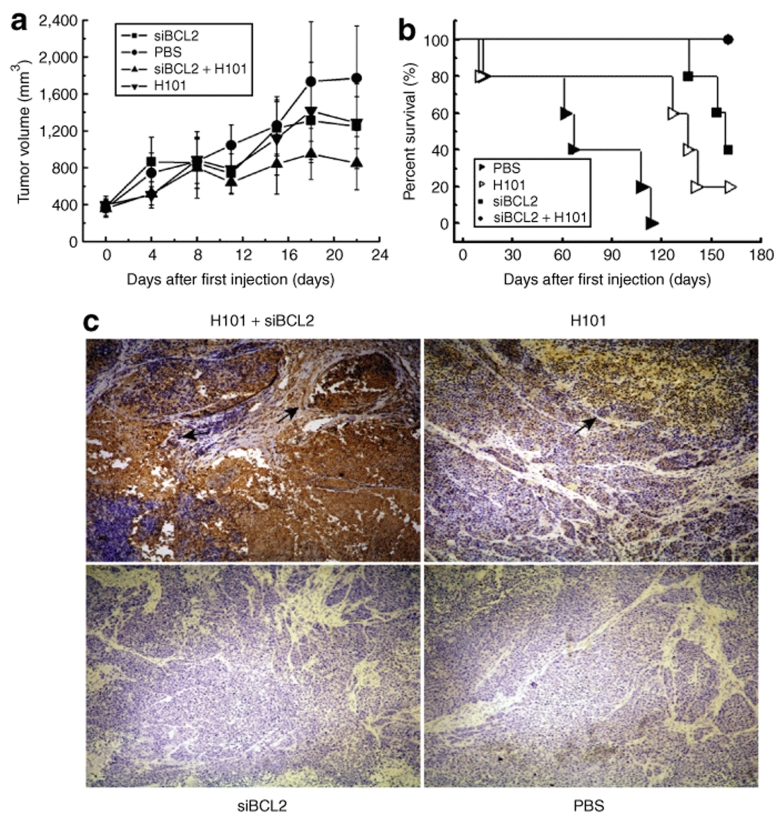
To examine whether the enhanced in vitro cytotoxicity could be translated into in vivo animal testing, Hela-S3 tumor cells were implanted in nude mice. When the volume of the xenografts reached 100–125 mm3, animals were treated by intratumor injection of siBCL2 and H101. As compared with the PBS control group, monotreatment with either oncolytic adenovirus H101 or siBCL2 inhibited tumor growth at a moderate rate (Figure 6a). However, tumor growth was significantly reduced in those animals that received the combined treatment of siBCL2 and H101 group (n = 5, *P < 0.05) in comparison with that in H101, siBCL2, or PBS treatment alone.
Figure 6.
Antitumor activity of the cocktail treatment of siBCL2 and H101 in Hela-S3 xenograft models. (a) Average volume of subcutaneous tumors after treatment with H101 (inverted triangle), H101 plus siBCL2 (triangle), siBCL2 (square), or PBS (circle). Values represent the means ± SD for five animals per group. (b) Percentage of animal survival. Survival was evaluated over a period of 160 days. (c) Viral replication in tumors following the siBCL2 and H101 combined treatment. The tumor spread of adenovirus type 5 particles was determined by immunohistochemical detection using anti-adenoviral particle antibody. Original magnification: ×200.
The long-term therapeutic effect of the combined treatment was examined by measuring animal survival in each treatment group. We observed an increase in survival in the group treated with the combined therapy (Figure 6b). By 160 days following treatment, all animals in the siBCL2/H101 group were alive and relatively healthy. In contrast, only 40% of animals that received siBCL2 monotherapy and only 20% of the animals that received H101 monotherapy were still alive. All of the animals in PBS control group died by day 120. At the end of the study, two of five mice that received siBCL2/H101 combined therapy had complete eradication of the pre-established tumors. These findings demonstrate that the combination of siBCL2 and H101 injection produced markedly improved antitumor outcomes in vivo.
To determine whether the increased production of adenovirus particles by siBCL as observed in vitro also occurred in this in vivo study, tumor tissues were collected and stained for adenovirus type 5 particle. As seen in Figure 6c, more adenovirus particles were present in tumors that were co-treated with siBCL2 and H101 than that in H101 and siBCL2 monotherapy, or PBS control.
Discussion
Malignant tumors are associated with abnormalities in gene expression derived from both genetic and epigenetic lesions, including p53 mutations28 and Bcl2 overexpression.29 Targeting these tumor-specific abnormalities has become an increasingly attractive area in developing molecular antitumor therapies,30 in sharp contrast to traditional therapies that nonspecifically kill tumors as well as normal cells. One typical example is H101, a recombinant E1B, E3-deleted serotype 5 adenoviral drug, that specifically lyses tumor cells by targeting inactivated p53. As H101 adenovirus cannot replicate in normal cells because of the suppressive effect of wild-type p53, the virus should act selectivity on tumor cells with mutated p53 and spare normal cells.9
However, H101 has limited potential to eradicate tumors when used as monotherapy Thus, H101 is often used in combination with traditional modalities, such as chemotherapy. In this communication, we studied the antitumor efficacy of H101 in conjunction with siRNA to Bcl2.
Bcl2 can be silenced by RNA antisense,30,31 DNA-targeted methylation,23 and RNAi.10,21,22,32 To achieve more complete suppression of Bcl2 expression, many studies chose plasmid-expressing shRNA or virus-expressing shRNA to knock down the target gene.33,34 Adenovirus vectors have been widely used as vehicles to deliver shRNA for gene silencing by virtue of their proven clinical safety. However, expression of adenoviral genes, virus-associated RNAI and virus-associated RNAII, resulted in the inhibition of RNAi activity, possibly through competitively interfering with Dicer or RISC activity.35 Moreover, Pan et al. also found that oncolytic adenovirus vectors may not be efficient for long-term siRNA delivery.36
We thus chose to downregulate Bcl2 using chemosynthetic siRNAs. Synthetic Bcl2 siRNA, although vulnerable to degradation when systematically administered, is relatively stable when injected directly into tumors. The H101 oncolytic adenovirus has been approved primarily for tumor topical injection in China. Thus, it would be feasible for the Bcl2 siRNA oligonucleotide to be formulated together with H101 in a single intratumor injection solution, or co-administered locally as separate formulations.
Recently, we demonstrated that Bcl2 siRNA can be efficiently expressed by incorporating siRNA oligonucleotide into the 3′ untranslated region (3′ UTR) of a tumor target gene, like TRAIL (J. Zhang, S. Huang, S. Ge, G. Qian, A.R. Hoffman, J.F. Hu, unpublished data). H101 oncolytic virus, although E1B and E3-deleted, has an intact E1A gene, permitting viral replication. It is therefore possible that Bcl2 siRNA can be incorporated into the 3′ UTR of the E1A of the adenovirus, leading to co-expression of Bcl2 siRNA with the viral replication of H101 in tumors. Using this strategy, we would be able to combine the cocktail therapy as a single therapeutical agent applicable to clinics.
As demonstrated in Figure 1, siBCL2 led to efficient inhibition of Bcl2 mRNA and protein expression. The RNAi activity was not inhibited in the presence of adenovirus H101. The combined action of Bcl2 knockdown and H101 oncolysis effectively inhibited tumor growth, indicating that Bcl2 knockdown can increase the therapeutic efficacy of H101.
In the animal studies, we used consecutive intratumoral injections of siBCL2 to overcome the short half-life of chemosynthetic siRNAs in animal models. The combined approach of H101 with siBCL2 showed superior tumor inhibition and prolonged survival compared with monotherapy with either agent alone. The enhanced antitumor effects of the combined approach in xenograft studies were consistent with the efficacy of co-treatment in vitro.
The precise mechanism of this enhanced activity of oncogene knockdown and viral oncolysis is still unclear. However, we found increased amplification of the adenovirus late gene Hexon, which encodes an adenovirus envelope protein, following Bcl2 knockdown. It is possible that lysis of the tumor cells may release more viral progeny that can infect nearby tumor cells, allowing spread of adenoviral infection. In support with this explanation, we also observed enhanced spread of adenovirus particles in tumors by immunohistochemical analysis (Figure 6b). Thus, enhanced adenovirus oncolysis may partially explain the increased antitumor efficacy in our combined therapeutic approach. Recently, Yoon et al.37 also demonstrated that the chemotherapeutic drug cisplatin significantly enhanced the number of adenovirus particles in tumor tissues. Similarly, Abou El Hassan et al.38 also showed that paclitaxel increased viral gene expression in infected tumors. A similar mechanism may underlie the enhanced viral replication observed in this study.
It is also interesting to note that unlike the in vivo tumor studies, the enhanced viral replication induced by siBCL2 was only observed at 48 hours in the transfected cells (Figure 4a). The pattern of increased replication of H101 oncolytic virus disappeared after a longer period of incubation. The reason for this disparity between in vitro and in vivo studies is not clear, and may reflect the difference in dynamics of cell division and viral replication between in vitro two-dimensional cell culture and three-dimensional tumors. In cell culture, the adenovirus had a peak of replication at 48 hours following cell infection. Thereafter, viral replication slowed, probably as a consequence of cell death. A similar pattern of adenoviral replication was also observed by Abou El Hassan et al. in a non-small-cell lung cancer NCI-H460 cell line.38
Theoretically, the replication of an oncolytic adenovirus should be restricted to tumor cells that are p53 deficient. However, it has been demonstrated that the E1B-deleted adenovirus ONYX-015 also replicated in some p53-positive tumor cells, but not in normal primary cells.39 Recently, Rothmann et al. showed that the oncolytic virus was also able to replicate and kill primary human cells, including normal fibroblasts, keratinocytes, and mammary epithelial cells.11 In this study, we also observed some therapeutic effect of the combined treatment of H101 and siBCL2 in p53-positive HepG2 hepatoma cells (Figure 5b), but this effect was still less effective than that seen in p53-defective Hep3B (Figure 5c) and H23 (Figure 5d) tumor cells. In agreement with Heise et al.,39 we did not observe any cytopathic effects on normal fibroblasts (Figure 5a). Many factors may affect the cytopathic effect of the oncolytic adenovirus, including cell proliferation status before viral infection, cell-to-cell variation in viral response, and the purity and type of oncolytic adenovirus used.
As an apoptotic and/or survival “switch,” Bcl2 plays a key role in the balance between proapoptotic and antiapoptotic factors in the intracellular microenvironment. Overexpression of Bcl2 in tumors critically altered this balance and resulted in permanent survival of tumors. Thus, downregulating Bcl2 may restore this balance and further increase the ability of tumor cells to respond to the apoptotic signal induced by exogenous stimuli. In this article, we observed enhanced apoptosis in combined treatment compared to monotreatments by either siBCL2 or by H101. Adenovirus-expressing proteins, such as E1A, activate the cellular apoptotic pathway.40,41 Induction of H101 viral replication may act as a potential apoptotic trigger inducing tumor apoptosis and synergizing with siBCL2, accounting for enhanced antitumor effects.
In conclusion, this study provides support for the combined use of an oncolytic adenovirus with Bcl2 siRNA as a promising approach in cancer gene therapy, either formulated as a single intratumor injection solution, or co-administered as separate formulations. Alternatively, the oncolytic adenovirus can be further modified by integrating Bcl2 siRNA into the H101 viral genome.
Materials and Methods
Cell culture and recombinant Adenovirus H101. All human tumor cells were purchased from American Type Culture Collection (Manassas, VA), including Hela-S3 (cervical carcinoma), HepG2 (hepatoma), Hep3B (hepatoma), and H23 (non-small-cell lung carcinoma). HBF1 was a normal human fibroblast cell line cultured from human fetal skin.42 Cells were cultured in Dulbecco's modified Eagle's medium (Gibco, CA) supplemented with 10% (vol/vol) fetal calf serum and maintained at 37 °C in a 5% CO2 atmosphere. Recombinant adenovirus H101 was kindly provided as a gift by Shanghai Sunway Biotech (Shanghai, China) and was maintained under conditions recommended by the manufacturer.
Bcl2 siRNA oligonucleotide. Two Bcl2 siRNAs (siBCL2 and siBCL2-X) were designed from the same sequence locations of Bcl2 as described previously.22 Bcl2 siRNA oligonucleotides (siBCL2 and siBCL2-X, Figure 1a) and control siRNA (siNC: 5′ UUCUCCGAACGUGUCACGU 3′) were synthesized and purified by Shanghai Genephrama (Shanghai, China).
Co-treatment of tumor cells with Bcl2 siRNA and oncolytic adenovirus H101. Hela-S3 cells at 30–50% confluence in 6-well plates were transfected with 50 nmol/l siBCL2 or siNC using a Lipofectamine 2000 reagent following the manufacturer's instructions (Invitrogen, Carlsbad, CA). After overnight incubation, cells were infected with H101 at a multiplicity of infection of 100. Control groups included cells that were transfected with siBCL2, siNC, or PBS.
RNA extraction and reverse transcription–PCR analysis. Total RNA was isolated from cells using Trizol reagent (Invitrogen) following the protocol provided by the manufacturer. First-strand cDNA was synthesized with RNA reverse transcriptase as described.43,44 Briefly, 1 µg of total RNA was used, and PCR was carried out in 50 µl reaction containing 5 µl of 10× PCR buffer, 1 µl of 10 mmol/l dNTP Mix, 1 µl of 10 µmol/l each primer, 1.5 U of Taq DNA polymerase, and 2 µl of sample cDNA. After denaturation at 95 °C for 5 minutes, Bcl2 cDNA was amplified by 35 cycling regimen consisting of 95 °C for 30 s, 58 °C for 30 s and 72 °C for 45 s, and subsequently with the extension at 72 °C for 10 minutes. Primers used for amplifying Bcl2 and β-actin mRNA were as follows: Bcl2 sense: 5′-ATGTGTGTGGAGAGCGTCAA-3′, and Bcl2 antisense: 5′-CAGGAGAAATCAA ACAGAGGC-3′; β-actin sense: 5′-CCAAGGCCAACCGCGAGAAGATGAC-3′, and β-actin antisense: 5′-AGGGTACATGGTGGTGCCGCCAGAC-3′. Amplified PCR products of the expected size were quantified by densitometric measurements and normalized to β-actin values.
Quantitative real-time RT-PCR amplification was carried out using QuantiTect SYBR green (Qiagen, Valencia, CA) as previously described.42 Specifically, total RNA was extracted by Trizol reagent (Invitrogen), and cDNA was synthesized with RNA reverse transcriptase. The CT (threshold cycle) value of Hexon was quantitated by Q-PCR in triplicate using an ABI Prism 7900 HT sequence detector (AB Applied Biosciences, CA) following the manufacturer's protocol and was normalized over the CT of the β-actin control.
MTT assay. Cells were seeded at 5,000 cells per well in flat-bottomed 96-well plates. At the end of the incubation time, 20 µl of 5 mg/ml MTT (Sigma, St Louis, MO) in PBS was added to each well. After 4 hours, media were discarded, and cells were lysed with 100 µl dimethylsulfoxide. Cells were incubated for a further 30 minutes at 37 °C with gentle shaking. The optical density was determined with a microplate reader at 570 nm. Absorbance values in the treated groups were normalized to the values of untreated tumor cells to calculate the percentage of survival.
Western blot analysis. Bcl2 and viral Hexon proteins were quantitated by western blotting as previously described.45 Cells were harvested at the indicated time, and proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 12% (wt/vol) polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Membranes were incubated with secondary antibody conjugated to a fluorescent tag; the bands were visualized and quantified by Odyssey infrared Imaging System (LI-COR, Lincoln, NE). The following antibodies were used: Bcl2 (Santa Cruz Biotechnologies, Santa Cruz, CA), Hexon (Abcam, Cambridge, UK), and β-actin (Santa Cruz Biotechnologies).
Apoptotic analysis. Apoptosis was determined by dual staining with annexin-V-fluorescein isothiocyanate and propidium iodide and analyzed by flow cytometry. Cells were prepared according to the manufacturer's instruction provided in the Annexin V-FITC apoptosis kit (BD Biosciences, San Diego, CA). Apoptosis was quantified on a fluorescence-activated cell sorter (Becton Dickinson, Sunnyvale, CA), and data from 10,000 events were collected for further analysis.
Apoptotic cells were also stained with Annexin V-CY3 apoptosis detection kit (Sigma). Briefly, tumor cells were labeled with 6-carboxy fluorescein (6-CF) and Annexin V-Cy3 (AnnCy3) at room temperature and were observed by fluorescence microscopy. Live cells were stained only with 6-CF (green), whereas necrotic cells stained only with AnnCy3 (red). Cells starting the apoptotic process were stained with both AnnCy3 and 6-CF.
Tumor xenograft model in nude mice. Hela-S3 cell tumor xenografts were established by s.c. injection of 5 × 106 cells into the right flank of 4– 6-week-old female athymic nude mice. When tumors reached the required mean tumor volume (100–125 mm3) as determined by the formula volume = length × width2 × 0.5, animals were randomly assigned into four groups. The siBCL2 plus H101 group received intratumoral injections of 10 µg siBCL2 on day 1, 4, 8, 11, 15, and H101 adenovirus at 1 × 108 plague-forming units on day 2, 5, 9, 12, 16; the siBCL2 group received five intratumoral injections of 10 µg siBCL2; H101 adenovirus group received five intratumoral injections of H101; control group mice received five injections with PBS. Non-target control RNAi oligos, including siNC, had been extensively examined in our previous studies22,46,47 and were not included in this animal study. The tumor size was measured by vernier calipers every 4 days. Animal experiments were performed in accordance with institutional guidelines for animal care by Jiao Tong University.
Immunohistochemical analysis. Mice from each group were selected randomly and killed on day 7 after treatment for immunohistochemical staining.48 Tumor samples were taken, fixed in 10% formalin, embedded in paraffin, and 3-µm sections were cut. Tumor sections were incubated at 4 °C overnight with a mouse anti-adenovirus type 5 antibody [M58] (Abcam, Boston, MA) at a dilution of 1:50. Sections were rinsed in PBS-T (0.05% Triton X-100 in PBS), followed by the biotinylated rat anti-mouse secondary antibody at a 1:500 dilution for 1 hour at room temperature. After washing twice with PBS-T, slides were subsequently incubated with streptavidin-horseradish peroxidase (BD Biosciences) and diaminobenzidine substrate to develop the colorimetric reaction. All slides were counterstained with hematoxylin.
Statistical analysis. All experiments were performed in triplicate, and the data were expressed as mean ± SD. The comparative CT method was applied in the quantitative real-time RT-PCR assay according to the delta-delta CT method. The data were analyzed with Student's t-test or by one-way analysis of variance, and results were considered statistically significant at P ≤ 0.05.
Acknowledgments
We thank Tao Li from Stanford University for his helpful discussions of this manuscript and the support by China Scholarship Council. This work was supported by The National Key Program for Basic Research of China (2004CB518804), The Science and Technology Commission of Shanghai (08ZR1412500 and 07JC14034) to G.Q.; The Collaborative Grant for basic and applied research of Basic Medical school and Renji Hospital (ZD0702), The Innovation Program of Shanghai Municipal Education Commission (09ZZ110) to S.G.; and NIH grant (1R43 CA103553-01), The Department of Defense Grant (W81XWH-04-1-0597) to J.F.H.
REFERENCES
- Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC., and , Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- Bouchet BP, de Fromentel CC, Puisieux A., and , Galmarini CM. p53 as a target for anti-cancer drug development. Crit Rev Oncol Hematol. 2006;58:190–207. doi: 10.1016/j.critrevonc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI. INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin Biol Ther. 2006;6:823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- Merritt JA, Roth JA., and , Logothetis CJ. Clinical evaluation of adenoviral-mediated p53 gene transfer: review of INGN 201 studies. Semin Oncol. 2001;28:105–114. doi: 10.1016/s0093-7754(01)90288-x. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Ries S., and , Korn WM. ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus. Br J Cancer. 2002;86:5–11. doi: 10.1038/sj.bjc.6600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman KG. Strategies for therapeutic targeting of the p53 pathway in cancer. Cell Death Differ. 2006;13:921–926. doi: 10.1038/sj.cdd.4401921. [DOI] [PubMed] [Google Scholar]
- Post LE. Selectively replicating adenoviruses for cancer therapy: an update on clinical development. Curr Opin Investig Drugs. 2002;3:1768–1772. [PubMed] [Google Scholar]
- Yu W., and , Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- Kasuya H, Takeda S, Shimoyama S, Shikano T, Nomura N, Kanazumi N, et al. Oncolytic virus therapy—foreword. Curr Cancer Drug Targets. 2007;7:123–125. doi: 10.2174/156800907780058826. [DOI] [PubMed] [Google Scholar]
- Rothmann T, Hengstermann A, Whitaker NJ, Scheffner M., and , zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrum FD., and , Ornelles DA. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A., and , Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- Fuhrken PG, Apostolidis PA, Lindsey S, Miller WM., and , Papoutsakis ET. Tumor suppressor protein p53 regulates megakaryocytic polyploidization and apoptosis. J Biol Chem. 2008;283:15589–15600. doi: 10.1074/jbc.M801923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, Anderson J., and , May WS. Bcl2's flexible loop domain regulates p53 binding and survival. Mol Cell Biol. 2006;26:4421–4434. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D, Nunez G, Milliman C, Schreiber RD., and , Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Chao DT., and , Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- Futami T, Miyagishi M, Seki M., and , Taira K. Induction of apoptosis in HeLa cells with siRNA expression vector targeted against bcl-2. Nucleic Acids Res. 2002. pp. 251–252. [DOI] [PubMed]
- Gibson SA, Pellenz C, Hutchison RE, Davey FR., and , Shillitoe EJ. Induction of apoptosis in oral cancer cells by an anti-bcl-2 ribozyme delivered by an adenovirus vector. Clin Cancer Res. 2000;6:213–222. [PubMed] [Google Scholar]
- Huang SL, Wu Y, Yu H, Zhang P, Zhang XQ, Ying L, et al. Inhibition of Bcl-2 expression by a novel tumor-specific RNA interference system increases chemosensitivity to 5-fluorouracil in Hela cells. Acta Pharmacol Sin. 2006;27:242–248. doi: 10.1111/j.1745-7254.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AR., and , Hu JF. Directing DNA Methylation to Inhibit Gene Expression. Cell Mol Neurobiol. 2006;26:425–438. doi: 10.1007/s10571-006-9057-5. [DOI] [PubMed] [Google Scholar]
- Liang XH, Mungal S, Ayscue A, Meissner JD, Wodnicki P, Hockenbery D, et al. Bcl-2 protooncogene expression in cervical carcinoma cell lines containing inactive p53. J Cell Biochem. 1995;57:509–521. doi: 10.1002/jcb.240570316. [DOI] [PubMed] [Google Scholar]
- Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR., and , Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:1973–1977. doi: 10.1073/pnas.87.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Shaulian E, Littlewood T, Resnitzky D., and , Oren M. Resistance to p53-mediated growth arrest and apoptosis in Hep 3B hepatoma cells. Oncogene. 1997;15:63–70. doi: 10.1038/sj.onc.1201149. [DOI] [PubMed] [Google Scholar]
- Ding H, Duan W, Zhu WG, Ju R, Srinivasan K, Otterson GA, et al. P21 response to DNA damage induced by genistein and etoposide in human lung cancer cells. Biochem Biophys Res Commun. 2003;305:950–956. doi: 10.1016/s0006-291x(03)00873-8. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchenio T, Lozano G, et al. Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci USA. 2002;99:14849–14854. doi: 10.1073/pnas.222406899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- Banerjee D. Genasense (Genta Inc) Curr Opin Investig Drugs. 2001;2:574–580. [PubMed] [Google Scholar]
- Cotter FE. Antisense therapy of hematologic malignancies. Semin Hematol. 1999;36:9–14. [PubMed] [Google Scholar]
- Lima RT, Martins LM, Guimaraes JE, Sambade C., and , Vasconcelos MH. Specific downregulation of bcl-2 and xIAP by RNAi enhances the effects of chemotherapeutic agents in MCF-7 human breast cancer cells. Cancer Gene Ther. 2004;11:309–316. doi: 10.1038/sj.cgt.7700706. [DOI] [PubMed] [Google Scholar]
- Xia H, Mao Q, Paulson HL., and , Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ., and , Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MG, Haasnoot PC, Xu N, Berenjian S, Berkhout B., and , Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Liu B, Liu J, Cai R, Liu X., and , Qian C. Synergistic antitumor activity of XIAP-shRNA and TRAIL expressed by oncolytic adenoviruses in experimental HCC. Acta Oncol. 2007. pp. 1–10. [DOI] [PubMed]
- Yoon AR, Kim JH, Lee YS, Kim H, Yoo JY, Sohn JH, et al. Markedly enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in combination with cisplatin. Hum Gene Ther. 2006;17:379–390. doi: 10.1089/hum.2006.17.379. [DOI] [PubMed] [Google Scholar]
- Abou El Hassan MA, Braam SR., and , Kruyt FA. A real-time RT-PCR assay for the quantitative determination of adenoviral gene expression in tumor cells. J Virol Methods. 2006;133:53–61. doi: 10.1016/j.jviromet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD., and , Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- Roulston A, Marcellus RC., and , Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Chu RL, Post DE, Khuri FR., and , Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin Cancer Res. 2004;10:5299–5312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- Chen HL, Li T, Qiu XW, Wu J, Ling JQ, Sun ZH, et al. Correction of aberrant imprinting of IGF2 in human tumors by nuclear transfer-induced epigenetic reprogramming. EMBO J. 2006;25:5329–5338. doi: 10.1038/sj.emboj.7601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JF, Vu TH., and , Hoffman AR. Genomic deletion of an imprint maintenance element abolishes imprinting of both insulin-like growth factor II and H19. J Biol Chem. 1997;272:20715–20720. doi: 10.1074/jbc.272.33.20715. [DOI] [PubMed] [Google Scholar]
- Hu JF, Vu TH., and , Hoffman AR. Promoter-specific modulation of insulin-like growth factor II genomic imprinting by inhibitors of DNA methylation. J Biol Chem. 1996;271:18253–18262. doi: 10.1074/jbc.271.30.18253. [DOI] [PubMed] [Google Scholar]
- Yao XM, Hu JF, Daniels M, Li T, Yang YW, Li ZH, et al. Epigenetic regulation of the taxol resistance associated gene (TRAG-3) in human tumors. Cancer Genet Cytogenet. 2004;151:1–13. doi: 10.1016/j.cancergencyto.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhao X, Huang S, Jian L, Qian G., and , Ge S. Blocking Notch1 signaling by RNA interference can induce growth inhibition in HeLa cells. Int J Gynecol Cancer. 2007;17:511–516. doi: 10.1111/j.1525-1438.2007.00813.x. [DOI] [PubMed] [Google Scholar]
- Jia RB, Zhang P, Zhou YX, Song X, Liu HY, Wang LZ, et al. VEGF-targeted RNA interference suppresses angiogenesis and tumor growth of retinoblastoma. Ophthalmic Res. 2007;39:108–115. doi: 10.1159/000099247. [DOI] [PubMed] [Google Scholar]
- Yao XM, Hu JF, Daniels M, Shiran H, Zhou XJ, Yien HF, et al. A methylated oligonucleotide inhibits IGF2 expression and enhances survival in a model of hepatocellular carcinoma. J Clin Invest. 2003;111:265–273. doi: 10.1172/JCI15109. [DOI] [PMC free article] [PubMed] [Google Scholar]