Abstract
Kupffer cells are the resident macrophage population of the liver and have previously been implicated in the pathogenesis of hepatic ischemia–reperfusion injury (IRI). Kupffer cells are the major site of expression of hepatic heme oxygenase-1 (HO-1), which has been shown to have anti-inflammatory actions and to protect animals and cells from oxidative injury. Kupffer cells and circulating monocytes were selectively ablated using liposomal clodronate (LC) in the CD11b DTR mouse before induction of hepatic ischemia. Kupffer cell depletion resulted in loss of HO-1 expression and increased susceptibility to hepatic IRI, whereas ablation of circulating monocytes did not affect IRI phenotype. Targeted deletion of HO-1 rendered mice highly susceptible to hepatic IRI. In vivo, HO-1 deletion resulted in pro-inflammatory Kupffer cell differentiation characterized by enhanced Ly6c and MARCO (macrophage receptor with collagenous structure) expression as well as decreased F4/80 expression, mirrored by an expansion in immature circulating monocytes. In vitro, HO-1 inhibition throughout macrophage differentiation led to increased cell numbers, and pro-inflammatory Ly6c+ CD11c− F4/80− phenotype. These data support a critical role for tissue-resident macrophages in homeostasis following ischemic injury, and a co-dependence of HO-1 expression and tissue-resident macrophage differentiation.
Introduction
As key mediators of immune responses following hepatic injury, Kupffer cells have been implicated in the pathogenesis of ischemia–reperfusion injury (IRI). Antigen presenting cells (APCs) are at the heart of the pathway of immune activation described by the “danger model.”1 When activated by danger associated molecular patterns (DAMPs) released by injured parenchymal cells, APCs secrete pro-inflammatory cytokines, activate T cells, and initiate neutrophil chemotaxis.2 “Classical” activation is characterized by secretion of pro-inflammatory cytokines including IL-1β and TNF-α, while “alternate” activation is characterized by secretion of anti-inflammatory IL-10. Analogous to “classical” and “alternate” modes of macrophage activation, macrophage differentiation has been shown to proceed broadly down two divergent pathways. One population, termed “inflammatory monocytes” express CCR2 and are attracted to acutely inflamed sites by MIP-1 signaling: these cells also express the antigen Ly6c. The second group, termed “resident” monocytes, express CX3CR1, patrolling, and re-populating resting tissues.3 These cells also express the cell surface marker CD11c.4
Modulation of immune activation at any stage has been shown to protect animals from IRI. Interruption of DAMPs,5 Toll-like receptors (TLRs),6 pro-inflammatory cytokines,7 T-cell receptors,8 co-stimulatory pathways,9 or T cells10 have all been shown to ameliorate IRI. Experimental modulation of Kupffer cell function has been attempted using gadolinium chloride and glycine. Although the mechanisms through which these reagents have their protective effects are incompletely characterized, both have been shown to suppress TNF-α secretion11 and to ameliorate IRI. This has been taken to imply that Kupffer cells have a broadly harmful effect following ischemic injury.
Heme oxygenase-1 (HO-1) catalyzes the degradation of heme into biliverdin, free iron, and carbon monoxide. In the liver, it is expressed principally by Kupffer cells.12 HO-1 induction protects cells and animals from IRI by modulation of every stage of the immune activation pathway. It confers direct cytoprotection13 which may limit production of DAMPs, mediates the protected phenotype arising from interruption of TLR signaling,14 switches macrophages in vitro from “classical” to “alternate” activation,15 modulates T-cell activation, and enhances immunological tolerance.16
In light of the anti-inflammatory role of HO-1, we hypothesized that inhibition or deletion of this enzyme might drive macrophage differentiation down an inflammatory pathway. We demonstrate in vivo and in vitro that HO-1 is required for differentiation of “resident” macrophages, which promote homeostasis, while suppressing expansion of inflammatory monocyte populations. By selectively depleting macrophage populations using liposomal clodronate (LC)17 and diphtheria toxin in the CD11bDTR mouse,18 we show that Kupffer cells and not circulating monocytes are required for hepatocellular survival of IRI. We suggest that the effect of HO-1 upon macrophage differentiation is a potential avenue of investigation for the amelioration of injury in the wide range of human diseases caused by ischemia. Since HO-1 has been shown to modulate the immunopathological contribution to a wide range of disorders, our findings concerning its effect upon macrophage differentiation may have broad application.
Results
Kupffer cells but not circulating monocytes are essential for hepatic protection from ischemic insults
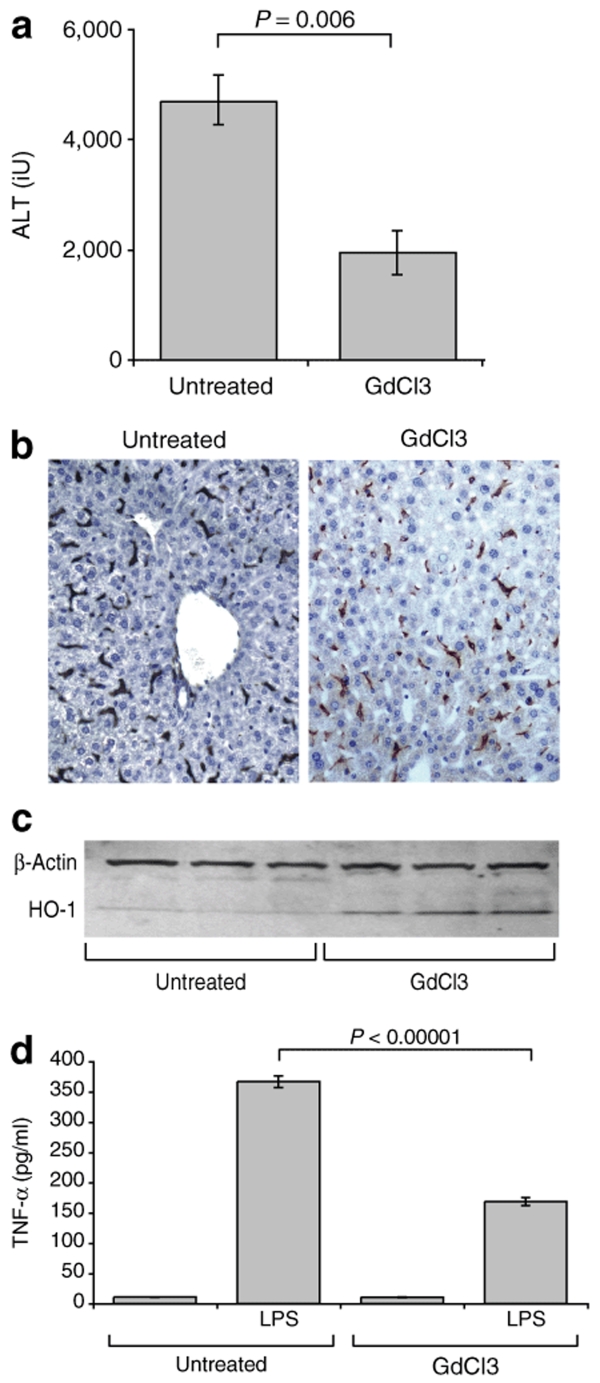
First, to verify our model against others in the published literature, we treated animals with GdCl3 before 50′ hepatic ischemic insults and found them to be protected from injury (GdCl3-treated alanine aminotransferase (ALT) 1929 ± 414 IU, untreated ALT4467 ± 532 P = 0.006) (Figure 1a). Immunohistochemistry for F4/80 demonstrated no significant anatomical ablation of Kupffer cells (Figure 1b). Western blotting of liver lysates from GdCl3-treated animals demonstrated HO-1 induction (Figure 1c), and pretreatment of bone marrow–derived monocytes (BMDMs) with GdCl3 before LPS stimulus resulted in decreased TNF-α secretion (Figure 1d). Thus, our findings were broadly in line with published data using this reagent, with the added observation that HO-1 was induced by GdCl3 without altering Kupffer cell number or spatial distribution. In light of these findings and previously published literature19,20 it is likely that GdCl3 induces a functional change in macrophages, which accounts for its protective effect against ischemia.
Figure 1.
The effect of gadolinium chloride. (a) Wild-type animals treated with GdCl 20 mg/kg IP were protected from 50 minute hepatic ischemic insults compared with vehicle treated controls (n = 4 per group). (b) Immunohistochemistry demonstrated no significant loss of F4/80+ Kupffer cells following GdCl administration. (c) When taken from animals 24 hours after GdCl treatment, liver lysates demonstrated HO-1 induction on western blotting. (d) Pretreatment of BMDMs with GdCl prior to LPS stimulus decreased TNF-α secretion.
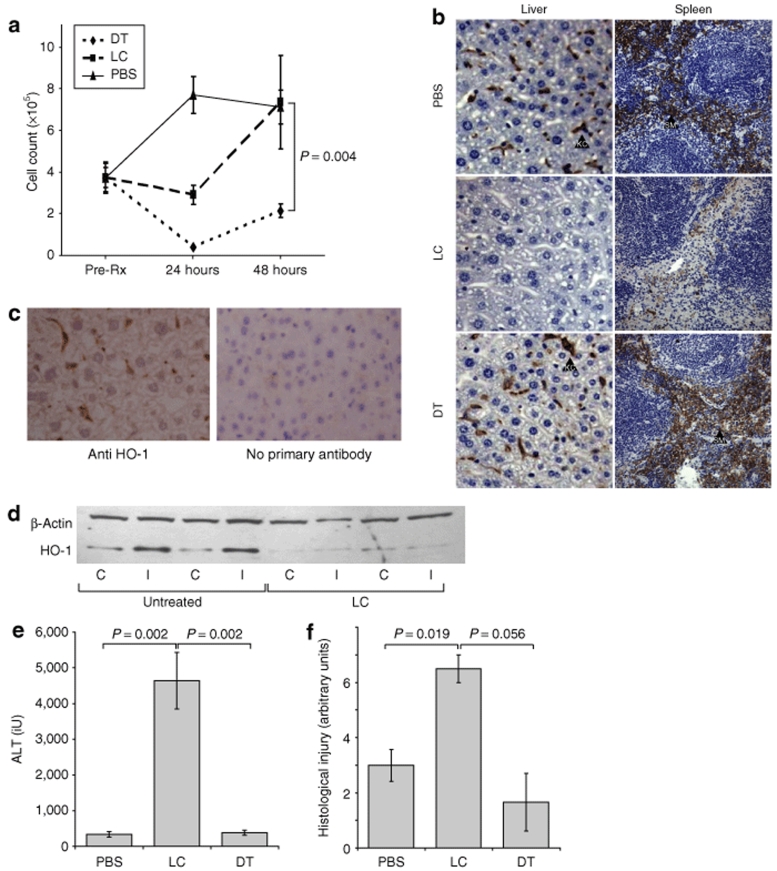
As an alternative manipulation of macrophage populations, we used the CD11bDTR mouse to selectively ablate tissue-resident and circulating macrophage populations before ischemia–reperfusion insults. As previously described,18 treatment of CD11bDTR mice with diphtheria toxin (DT) 25 ng/g i.p. resulted in loss of circulating monocytes (DT 2.1 × 105 monocytes/ml, PBS 7.2 × 105 monocytes/ml P = 0.004) (Figure 2a) but sparing of Kupffer cells and splenic macrophages (Figure 2b). Administration of LC 100 µl i.v.17 resulted in complete loss of Kupffer cells from the liver, and substantial depletion of splenic macrophages (Figure 2b). Whereas the systemic stress caused by serial blood sampling led to a progressive monocytosis in PBS-treated mice, this was suppressed at 24 hours in LC-treated mice with recovery at 48 hours after injection (Figure 2a). In keeping with immunohistochemical findings that HO-1 expression is principally within Kupffer cells (Figure 2c), Western blotting demonstrated that Kupffer cell ablation arising from LC treatment led to complete loss of hepatic HO-1 expression, even in the ischemic liver lobe in which HO-1 is normally potently induced (Figure 2d).
Figure 2.
Selective depletion of monocyte/macrophage populations. CD11bDTR mice received DT to deplete circulating monocytes or LC to deplete Kupffer cells prior to left lobar hepatic ischemic insults (n = 4 per group). (a) Circulating monocytes were counted using flow cytometry. Whereas on serial sampling from BS treated mice showed a progressive monocytosis, this was transiently suppressed by LC, although monocyte counts recovered in these animals at 48 hours. DT treatment led to complete depletion of circulating monocytes at 24 hours. (b) Immunohistochemistry for F4/80 demonstrated complete ablation of Kupffer cells (KC) and significant depletion of splenic macrophages (SM) following LC but not DT administration. (c) In keeping with the finding that HO-1 expression is concentrated within Kupffer cells, (d) western blotting showed that Kupffer cell ablation led to complete loss of hepatic HO-1 expression from both right nonischemic (control, C) and left ischemic (I) lobes in which HO-1 is normally potently induced. Twenty-four hours following PBS, LC, or DT administration, CD11bDTR animals were subjected to hepatic ischemia reperfusion insults. Kupffer cell but not circulating monocyte ablation led to susceptibility to ischemia reperfusion injury in terms of (e) ALT release and (f) histological injury score.
Twenty-four hours following DT, LC, or PBS treatments, mice were subjected to 40-minute left hepatic ischemia–reperfusion insults. Following injury, circulating monocyte depleted and PBS-treated animals were not significantly injured in terms of ALT release or histological injury (PBS ALT 339 ± 78 IU, Hist inj 3 ± 0.6 arbitrary units, DT ALT 384 ± 67 IU, Hist inj 1.7 ± 1 arbitrary units) whereas Kupffer cell–depleted animals were highly susceptible to ischemia (ALT 4640 ± 790, P = 0.002, Hist inj 6.5 ± 0.5 arbitrary units P = 0.019) (Figure 2e,f). These data imply a homeostatic role for Kupffer cells but not circulating monocytes following hepatic ischemic injury. The focus of HO-1 expression within Kupffer cells and its loss following Kupffer cell ablation suggests a key role for this enzyme in the protection afforded by Kupffer cells.
HO-1 expression is essential for protection from hepatic ischemic insults
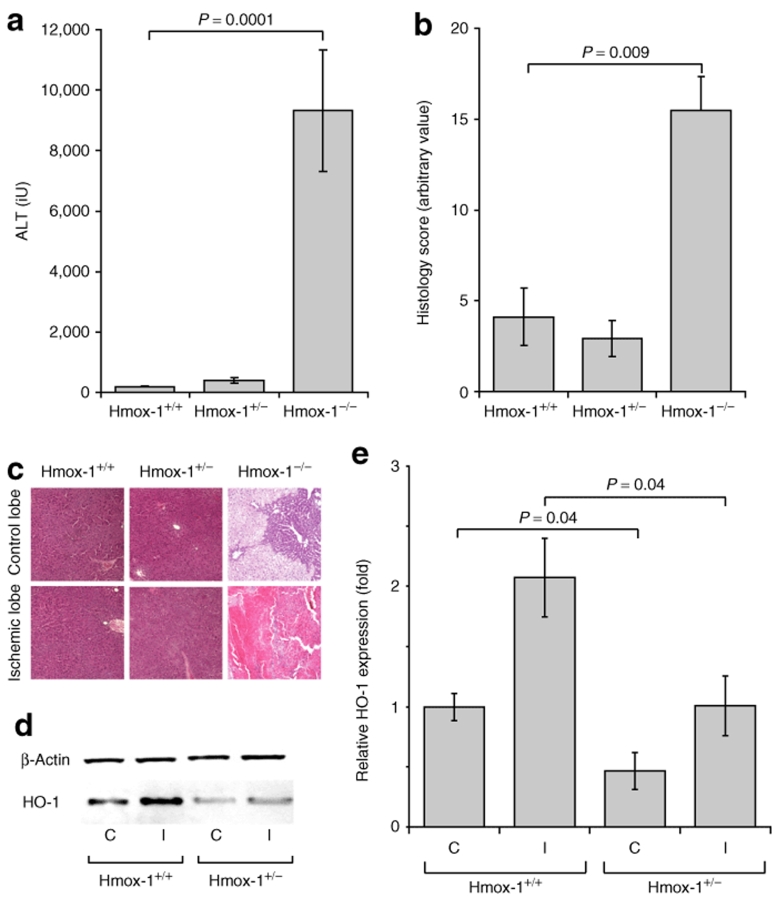
To examine the influence of heme oxygenase in protection from liver IRI we studied response in wild-type, heterozygous and homozygous Hmox-1 deleted mice. In accordance with previously published pharmacological inhibitor studies, Hmox-1−/− mice were more susceptible to hepatic ischemic insults than Hmox-1+/− and +/+ animals in terms of ALT release and histological injury score (Figure 3a,b). Following ischemia, relative HO-1 expression in the nonischemic and ischemic lobes of Hmox-1+/− animals was approximately half that of wild-type animals (Figure 3c,d) (nonischemic Hmox-1+/+ 1 ± 0.11x, Hmox-1+/− 0.47 ± 0.16x, P = 0.04; ischemic Hmox-1+/+ 2.1 ± 0.32x, Hmox-1+/− 1.0 ± 0.25x P = 0.04). No HO-1 expression was seen in Hmox-1−/− animals in control or ischemic lobes (data not shown).
Figure 3.
The effect of Hmox-1 gene deletion. Hmox-1−/−, +/− and +/+ animals were subjected to 40 minute left lobar hepatic ischemic insults (n = 6 per group). Hmox-1−/− but not Hmox-1+/− animals were susceptible to hepatic IRI in terms of (a) ALT release and (b, c) histological injury score. (d, e) Relative HO-1 expression in the control (C) and ischemic (I) lobes of Hmox-1+/− mice was approximately half that of wild-type animals.
Tissue-resident macrophage differentiation is HO-1 dependent. Hmox-1−/− Kupffer cells have inflammatory immunophenotype
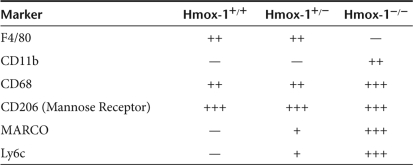
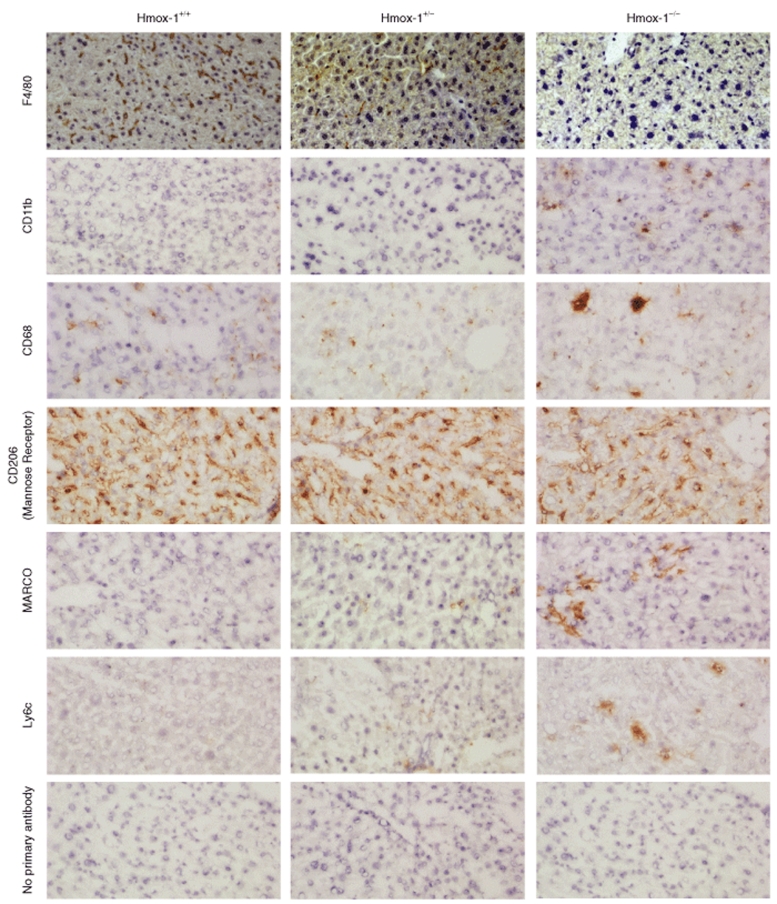
Kupffer cells in resting Hmox-1+/+, +/−, and −/− mice were phenotyped for F4/80, CD11b, CD68 (macrosialin), CD206 (mannose receptor), MARCO (macrophage receptor with collagenous structure), and Ly6c. Immunohistochemical findings are summarized in Table 1. CD68 staining was highly abnormal in Hmox-1−/− livers, revealing dysmorphic, multinucleate cells (Figure 4).
Table 1.
Cell surface marker expression by genotype
Figure 4.
The effect of Hmox-1 copy number on Kupffer cell phenotype. Staining for F4/80, CD11b, CD68, CD206 (mannose receptor), MARCO, and Ly6c demonstrated that Hmox-1−/− Kupffer cell F4/80 expression is decreased, and MARCO and Ly6c expression is increased.
These data suggest that HO-1 may be required for normal “resident” macrophage/monocyte differentiation. Hmox-1−/− Kupffer cells had a distinctly inflammatory phenotype characterized by expression of Ly6c and MARCO (Figure 4). Ly6c, a component of the Gr1 epitope is associated with inflammatory monocytes.4 MARCO is associated with “classical” macrophage activation, being upregulated following LPS stimulus.21,22 CD68 has also been associated with macrophage activation.23,24 F4/80, which is associated with modulation of adaptive immune responses,25 was absent from Hmox-1−/− Kupffer cells (Figure 4).
Hmox-1−/− animals have larger numbers of Immature circulating monocytes
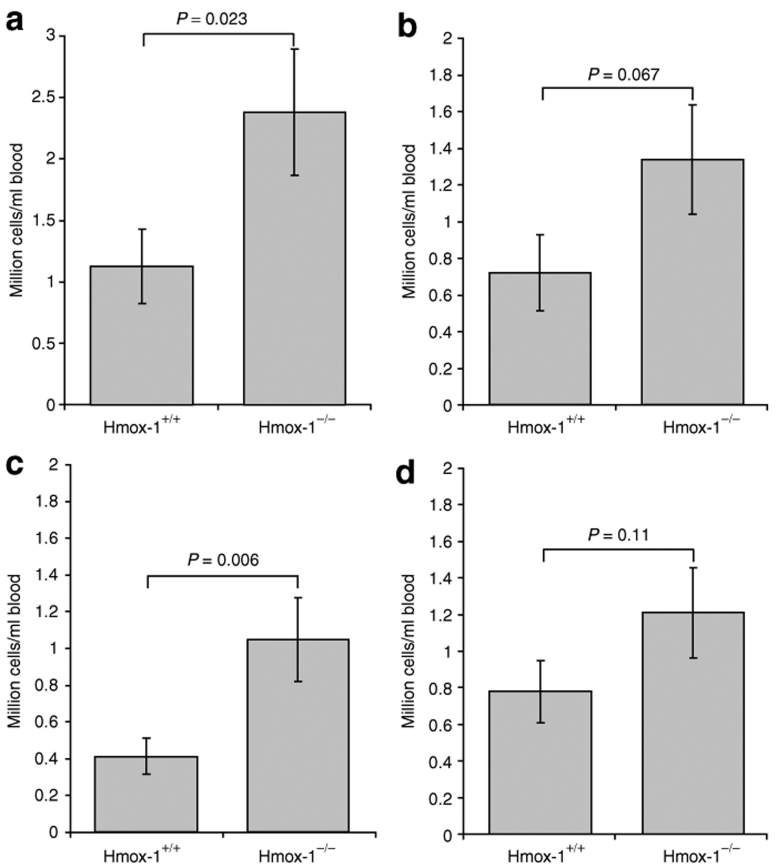
Blood samples from Hmox-1−/− and Hmox-1+/+ mice were compared on flow cytometry with respect to monocyte (Figure 5a–c) and neutrophil counts (Figure 5d). Hmox-1−/− mice had 2.1 times more monocytes than wild-type controls (P = 0.02). This was made up of a large immature Gr1hi group of which there was a 2.5-fold increase compared with Hmox-1+/+ animals (P = 0.06). An increase was also seen in Gr1lo monocytes although this did not achieve significance (P = 0.067). There was a trend toward more circulating neutrophils (1.6-fold increase compared with Hmox-1+/+ animals) but this did not achieve statistical significance (P = 0.11).
Figure 5.
Monocyte and neutrophil counts in Hmox-1+/+ and Hmox-1−/− animals. (a) Monocyte counts in resting Hmox-1−/− animals were increased by 2.1-fold. This arose from a 2.5-fold expansion in immature Gr1hi monocytes and there was also a trend toward increased numbers of (b) Gr1lo monocytes, (c) although this did not achieve statistical significance. There was a trend toward increased numbers of neutrophils which again did not achieve statistical significance.
These findings are consistent with the inflammatory state previously observed in Hmox-1−/− mice26 and the pro-inflammatory phenotype we have observed in Hmox-1−/− Kupffer cells.
HO-1-inhibited BMDMs have inflammatory phenotype in vitro
In view of the progressive tissue iron overload, iron deficiency anemia, and chronic immune activation previously reported in Hmox-1−/− animals,26,27 it was important to confirm the role of HO-1 in macrophage maturation in vitro in the absence of pro-inflammatory stimuli. Wild-type, Hmox-1+/−, and Hmox-1−/− bone marrow–derived hemopoeitic progenitors were matured for 6 days into monocytes (BMDMs) in L929 conditioned medium. Wild-type cells were cultured throughout their maturation in the presence or absence of the highly specific HO-1 inhibitor Chromium Mesoporphyrin IX (CrMP).28
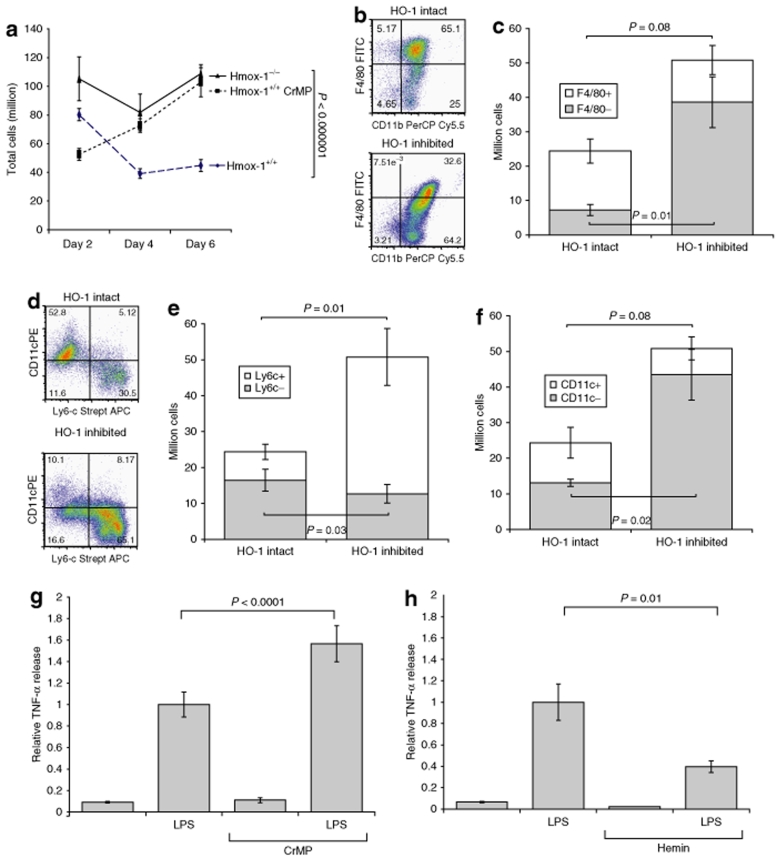
HO-1 inhibition and deletion led to expansion of cell numbers (Figure 6a) as well as pleomorphism in terms of differences in size and granularity (not shown) compared with the homogenous population of cells derived from HO-1 intact mice.
Figure 6.
The effect of HO-1 function upon macrophage differentiation in vitro. Suspension cultures of Hmox-1+/+, +/−, and −/− bone marrow–derived stem cells were grown in M-CSF conditioned medium for 6 days. Wild-type cells were grown in the presence or absence of the highly specific HO-1 inhibitor CrMP throughout their maturation. Hmox-1−/− and wild-type HO-1 inhibited BMDMs were considerably (a) more numerous than control wild-type BMDMs. Flow cytometry demonstrated development of a large F4/80− population in (b, c) HO-1 inhibited cultures. (d) Dot plots indicated that Ly6c and CD11c markers were seen on mutually exclusive populations. Calculation of cell numbers demonstrated that HO-1 inhibition led to expansion of a large pro-inflammatory (e) Ly6c+, (f) CD11c− population. LPS stimulus of BMDMs in vitro led to increased TNF-α production in conditions of (g) HO-1 inhibition and (h) decreased TNF-α secretion when HO-1 was induced.
In common with our in vivo observations, HO-1 inhibition in vitro resulted in loss of F4/80 expression. In HO-1 intact cultures, 71 ± 1% of cells were F4/80+ whereas HO-1 inhibition reduced this proportion to 23.5 ± 4.6% (P = 0.002). Calculation of cell number indicated a trend toward a smaller absolute number of F4/80+ cells (untreated 17.1 ± 3.5 million cells, HO-1 inhibited 12.2 ± 4.2 million cells, P = 0.08), and expansion of a very large F4/80− population in HO-1 inhibited cultures (accounting for their increased cell numbers) (HO-1 intact 7.2 ± 1.6 million cells, HO-1 inhibited 38.6 ± 7.4 million cells, P = 0.01) (Figure 6b,c).
Flow cytometry dot plots indicated that “inflammatory” Ly6c+ and “resident” CD11c+ subsets were mutually exclusive indicating divergent differentiation (Figure 6d). As we saw in our in vivo investigations, the inflammatory monocyte marker Ly6c was upregulated in HO-1-inhibited BMDMs. In HO-1 intact cultures, 31.3 ± 2.2% of cells were Ly6c+ whereas in HO-1-inhibited cultures, 74.6 ± 1.8% cells were Ly6c+ (P = 0.0003). Calculation of absolute cell number indicated that the number of Ly6c+/Ly6c− cells in HO-1 intact cultures was 13.1 ± 1/ 16.5 ± 3 million cells, compared with 43.5 ± 7.1/12.7 ± 2.6 million cells in the HO-1-inhibited group, P = 0.02/P = 0.03 (Figure 6e). The opposite was true of the resident macrophage marker CD11c which was much more frequent in HO-1 intact cultures (41.1 ± 9%) compared with HO-1-inhibited cultures (12.3 ± 4%), giving absolute cell counts of CD11c+/CD11c− in HO-1 intact cultures of 11.3 ± 4.3/13.1 ± 1 million cells compared with 7.4 ± 4.3/43.5 ± 7.1 million cells in HO-1-inhibited cultures P = 0.08/P = 0.02 (Figure 6f).
HO-1 inhibition by CrMP (5 µmol/l for 1 hour) increased TNF-α production (1.57 ± 0.17 times untreated, P < 0.00001) (Figure 6g) whereas HO-1 induction by hemin (50 µmol/l overnight) reduced TNF-α production following LPS stimulus (0.4 ± 0.05 times untreated, P = 0.01) (Figure 6h).
These in vitro data confirm our in vivo observation that HO-1 activity is required for “resident” patterns of macrophage differentiation, and suppression of “inflammatory” macrophage populations. We also confirm in primary culture the previously published finding15 that HO-1 induction reduces inflammatory cytokine production by macrophages. Together, these data indicate that HO-1 has an anti-inflammatory role which extends from macrophage differentiation right through to modulation of pro-inflammatory cytokine release. In the context of ischemia–reperfusion insults, resident macrophages appear critical for the survival of surrounding parenchymal cells.
Discussion
In this article, we have shown that Kupffer cells are essential for hepatic survival of ischemia–reperfusion insults, that hepatic HO-1 expression is Kupffer cell dependent, and conversely that macrophage differentiation critically depends upon HO-1 expression.
Numerous studies have employed gadolinium chloride and glycine to modulate Kupffer cell activity, and have shown these drugs to protect animals from hepatic ischemia.19,29,30,31,32,33 Such data give the impression that Kupffer cells have a harmful effect following ischemia. We found that GdCl3 induced HO-1 and, as shown before in rats,31 reduced pro-inflammatory cytokine release. The effect of GdCl3 is thus somewhat complex and incompletely elucidated: as such it provides limited information regarding the physiological role of macrophage populations during ischemic insults.
To unpick the relative importance of circulating monocytes and Kupffer cells during ischemia–reperfusion insults, we selectively ablated each population in CD11bDTR mice using diphtheria toxin or LC, respectively. Kupffer cell but not circulating monocyte ablation led to HO-1 deletion from the liver, and susceptibility to hepatic ischemic insults. Taken together with data showing the importance of TNF-α in causing IRI,7 and our own data showing HO-1 induction and reduction in TNF-α production from GdCl3-treated macrophages, these data suggest that the differentiation state of tissue-resident macrophages is critical to the survival of surrounding parenchymal cells following ischemia. Tissue-resident macrophages could have their protective effect by modulation of immune responses to ischemically injured tissue,34 by modulation of apoptosis induced directly by TNF-α35 or by release of carbon monoxide acting as a diffusible paracrine protective factor.36
As the immune system contributes so heavily to IRI, the site of hepatic HO-1 expression is the Kupffer cell, and previously published data has shown such profound effects of HO-1 upon macrophage cytokine profiles,15,37 we hypothesized that differences in APC differentiation might account for the susceptible phenotype of Hmox-1−/− mice. Indeed, Hmox-1−/− but not Hmox-1+/+ or +/− Kupffer cells expressed Ly6c which is found on inflammatory monocytes4 and MARCO, which is associated with macrophage activation.21,22 Hmox-1−/− Kupffer cells also expressed less F4/80, which has been implicated in modulation of adaptive immunity.25 Finally, HO-1 induction decreased, and HO-1 inhibition increased, TNF-α secretion following LPS stimulus. These in vivo findings were confirmed in vitro, with huge expansion of inflammatory monocyte subsets in the absence of HO-1. In contrast with other investigators,38 we did not find Hmox-1+/− animals to be susceptible to ischemic injury, a difference we attribute to subtle differences between IRI models developed in different laboratories. Although hmox-1+/− animals expressed less HO-1 protein than wild types, their lack of susceptibility to ischemic injury correlates with their Kupffer cell phenotype which we found to be very similar to wild-type animals.
This work clarifies the role of macrophages in responses to ischemia–reperfusion insults and the effect of HO-1 upon macrophage differentiation. The finding that HO-1 drives macrophage differentiation down an “anti-inflammatory” pathway provides potential insights into the mechanism of ischemic preconditioning which results in HO-1 induction and subsequent protection from injury. On the basis of data presented herein, it could be hypothesized that HO-1 induction during preconditioning might lead to alterations in the behavior of tissue-resident macrophages in response to ischemic insults and thus confer protection.
The ability of HO-1 to manipulate macrophage phenotype could be harnessed for therapeutic gain by infusion of exogenous “anti-inflammatory” macrophage populations, or by using agents such as heme arginate39 or curcumin40 to induce HO-1 in the patient to protect from ischemic injury.
The ability of HO-1 to dictate macrophage phenotype adds a further dimension to its apparently great therapeutic potential, offering new avenues for treatment of a range of common and deadly diseases with their pathophysiology rooted in IRI.
Materials and Methods
Hmox-1−/−and Hmox-1+/− animals. All procedures and animal care were approved by institutional ethics committees and were in accordance with UK Home Office licensing regulations. C57/Bl6J animals with targeted deletion of the Hmox-1 by neomycin resistance gene insertion26 were supplied by A Agarwal (Birmingham, Alabama), rederived and bred by heterozygote to heterozygote mating in accordance with local regulations.
Operative procedure. Operated animals underwent clamping of the portal pedicle (hepatic artery and portal vein) supplying the left hepatic under isoflurane anesthesia, with core temperature maintained at 36 °C throughout. Preliminary experiments demonstrated optimal ischemic times of 40 minutes for the detection of susceptible phenotypes, and 50 minutes for demonstration of protection from ischemic injury.41 Twenty fours hours after operation, animals were culled when blood and tissues were collected.
Experimental groups. Preliminary experiments demonstrated that the sham effect of surgery was negligible. Wild-type, Hmox-1+/−, and Hmox-1−/− mice underwent 40 minutes of ischemia (n = 6 per group). Two groups of C57Bl6J animals underwent 50 minutes of ischemia following injection of 20 mg/kg GdCl3 (Sigma-Aldrich, Poole, UK) or vehicle (n = 6 per group). Three groups (four per group) of CD11bDTR (SVB background) mice received 100 µl PBS (control), 25 ng/g DT, or 100 µl LC17 (courtesy of Nico van Rooijen, www.clodronateliposomes.org) 24 hours before 40′ left hepatic lobar IRI.
ALT measurement. At 24 hours after ischemic insult, blood was taken via terminal cardiac puncture. ALT was measured on Olympus AU2700 (Olympus Optical, Watford, UK) utilizing methodology recommended by the International Federation of Clinical Chemistry.
Histological injury scoring. Each animal was scored for necrosis and cytoplasmic injury on standardized scales by an experienced liver pathologist (C.O.B.) who was blinded to the experimental group and conditions. The two scores were added to give a single index of liver injury (maximum score 8). Degree of severity for the two parameters was determined as follows: for necrosis, 1 = increased single cell death (apoptosis, necrosis) without confluent necrosis; 2 = microfoci of confluent necrosis in a minority of lobules, not encircling the full circumference of the central vein; 3 = confluent necrosis in a majority of lobules, or confluent necrosis around the full thickness of the central vein >4 hepatocyte layers deep; 4 = panlobular necrosis. For cytoplasmic injury, 1 = sparse and minor non-specific changes (minor microvesicular change), 2 = confluent perivenular hepatocyte cytoplasmic injury in a small minority of lobules (<25%), 3 = perivenular hepatocyte injury in 25–50% lobules, 4 = diffuse severe cytoplasmic injury in a majority (>50%) of lobules.
Western blot. Western blotting was performed as described previously.12 HO-1 was detected using rabbit polyclonal anti HO-1 (Stressgen SPA-896; Nventa, San Diego, CA) diluted 1:5,000 in 1%BSA TBS 0.05% Tween. Loading control was Abcam mouse anti β-Actin (ab6276, Abcam, Cambridge, UK) diluted 1:10,000 in 1% BSA TBS 0.05% Tween. All antibodies were used overnight at 4 °C.
Immunohistochemistry. Paraffin-fixed tissue sections underwent antigen retrieval by boiling in citrate buffer before goat serum, peroxide, avidin/biotin blocking (Vector SP2001, Vector UK, Peterborough, UK) staining for HO-1 (Stressgen SPA 896). Secondary antibody was Dako goat anti-rabbit biotinylated (E0432, Dako UK, Cambridgeshire). Finally, tertiary reagent (Vectastain elite pk-6100) was used before DAB color reagent and hematoxylin counterstain. For F4/80, antigen retrieval was with Proteinase-K (Dako S3020), primary antibody was rat anti-mouse F4/80 (Serotec MCA497GA, Serotec, Oxford, UK), and secondary antibody was rabbit anti-rat biotinylated (Dako E0468). Immunohistochemistry for CD11b, CD68, Ly6c, CD206, and MARCO was performed on frozen sections. Primary antibodies were CD11b (Serotec MCA711G), CD68 (Serotec MCA1957GA), Ly6c (Serotec MCA2389GA), CD206 (Serotec MCA2235GA), MARCO (Serotec MCA1849). Blocking, secondary, and tertiary steps were as for F4/80.
Preparation of bone marrow–derived monocytes. Femurs and tibias from 8–10-week-old Hmox-1+/+, +/−, and −/− mice were harvested and grown as described elsewhere.18 At the point of marrow aspiration, cells from the femurs of the same animal were placed into paired cultures with the highly specific HO-1 inhibitor chromium mesoporphyrin IX (CrMP, Frontier Scientific, Logan, Utah) or vehicle (PBS).
Flow cytometry. BMDMs were stained with biotinylated anti-mouse Gr1 (eBioscience RB6-8D5, eBioscience, San Diego, CA), PE-conjugated anti F4/80 (eBioscience clone BM8), PerCP-Cy5.5-labeled anti-CD11b (eBioscience M1/70) and FITC-conjugated anti-CD68 FITC (Acris SM1550F, Acris Antibodies GmbH, Germany), following permeabilization with Cytoperm plus (BD 554715; Becton Dickinson, Franklin Lakes, NJ), or with biotinylated anti-mouse Ly6c (cat. no. 557359, clone AL21; Becton Dickinson, Oxford, UK; Pharmingen, Oxford, UK), FITC-conjugated anti-F4/80 (Serotec, Oxford, UK, cat no. MCA497F, clone A3-1), PE-conjugated CD11c (clone N418, cat no. 12-0114-83, eBioscience), PerCP-Cy5.5-labeled anti-CD11b (BD Pharmingen cat. no. 550993, clone M1/70). Experiments were conducted on cells from paired, age-, and sex-matched wild type, Hmox-1+/− and −/− animals at 7 days of maturation, four animals per group. Samples were analyzed on a BD FACScalibur flow cytometer and analyzed using Flow-Jo software (Tree Star, Ashland, OR).
Macrophage TNF-α production studies. Day 7 BMDMs were plated at a density of 1 × 105 cells per well in 24 well plates, 1 day before stimulus. Before the addition of LPS 500 ng/ml, cells were preincubated with medium containing CrMP (5 µmol/l) or plain medium for 1 hour. Supernatants were collected after 2 hours and immediately frozen at −20 °C until ELISA analysis. ELISA for mouse TNF-α was performed using a “Duoset” kit obtained from R&D systems (Minneapolis, MN) according to the manufacturer's instructions. Experiments were repeated three to five times with cells derived from separate animals.
Statistical analysis. Datasets were prepared using Microsoft Excel. Statistical analysis was performed using Minitab 13 for Windows XP. Comparison of means was performed using unpaired two-tailed, parametric t tests (in vivo data, in vitro “between plate” data) or paired two-tailed, parametric t tests (in vitro, same plate data). P values of ≤0.05 were taken as significant. Data are presented as mean ± SEM.
Acknowledgments
Liposomal clodronate was a kind gift of Dr Nico van Rooijens, www.clodronateliposomes.org. Breeding pairs of Hmox-1−/− animals were a kind gift of Dr Anupam Agarwal, UAB, Birmingham, Alabama. We are grateful to Miguel Soares, Instituto Gulbenkian de Cienca, Oeiras, Portugal; Professor Siamon Gordon, University of Oxford; and Professor Sir John Savill, University of Edinburgh for constructive advice during the development of this manuscript.
REFERENCES
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S., and , Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B, Shen XD, Tsuchihashi SI, Gao F, Araujo JA, Busuttil RW, et al. Viral Interleukin-10 Gene Transfer Prevents Liver Ischemia-Reperfusion Injury: Toll-Like Receptor-4 and Heme Oxygenase-1 Signaling in Innate and Adaptive Immunity. Hum Gene Ther. 2007;18:355–366. doi: 10.1089/hum.2007.181. [DOI] [PubMed] [Google Scholar]
- Teoh N, Field J, Sutton J., and , Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412–421. doi: 10.1002/hep.20035. [DOI] [PubMed] [Google Scholar]
- Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L., and , Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 2006;69:233–238. doi: 10.1038/sj.ki.5000038. [DOI] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- Huang Y, Rabb H., and , Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck R, Wardi J, Aeed H, Avni Y, Shirin H, Avinoach I, et al. Glycine modulates cytokine secretion, inhibits hepatic damage and improves survival in a model of endotoxemia in mice. Liver Int. 2003;23:276–282. doi: 10.1034/j.1600-0676.2003.00839.x. [DOI] [PubMed] [Google Scholar]
- Patel A, van de Poll MC, Greve JW, Buurman WA, Fearon KC, McNally SJ, et al. Early stress protein gene expression in a human model of ischemic preconditioning. Transplantation. 2004;78:1479–1487. doi: 10.1097/01.tp.0000144182.27897.1e. [DOI] [PubMed] [Google Scholar]
- Vile GF, Basu-Modak S, Waltner C., and , Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, et al. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, Shao J, et al. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 t-regulatory cells, interleukin-10, and membrane-bound transforming growth factor-β1. Am J Pathol. 2007;171:1904–1914. doi: 10.2353/ajpath.2007.070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen N., and , Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–1243. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejima K, Arai M, Ikeda H, Tomiya T, Yanase M, Inoue Y, et al. Ischemic preconditioning protects hepatocytes via reactive oxygen species derived from Kupffer cells in rats. Gastroenterology. 2004;127:1488–1496. doi: 10.1053/j.gastro.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Tian Y, Jochum W, Georgiev P, Moritz W, Graf R., and , Clavien P-A. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci USA. 2006;103:4598–4603. doi: 10.1073/pnas.0600499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefowski S, Arredouani M, Sulahian T., and , Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–8041. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- van der Laan LJ, Döpp EA, Haworth R, Pikkarainen T, Kangas M, Elomaa O, et al. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J Immunol. 1999;162:939–947. [PubMed] [Google Scholar]
- Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Wang SY, Tai GX, Zhang PY, Mu DP, Zhang XJ., and , Liu ZH. Inhibitory effect of activin A on activation of lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Cytokine. 2008;42:85–91. doi: 10.1016/j.cyto.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Lin HH, Faunce DE, Stacey M, Terajewicz A, Nakamura T, Zhang-Hoover J, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med. 2005;201:1615–1625. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD., and , Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold SP, Adams E, Graca L., and , Waldmann H. Serial analysis of gene expression provides new insights into regulatory T cells. Semin Immunol. 2003;15:209–214. doi: 10.1016/s1044-5323(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Appleton SD, Chretien ML, McLaughlin BE, Vreman HJ, Stevenson DK, Brien JF, et al. Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab Dispos. 1999;27:1214–1219. [PubMed] [Google Scholar]
- Hardonk MJ, Dijkhuis FW, Hulstaert CE., and , Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- Kamei T, Callery MP., and , Flye MW. Kupffer cell blockade prevents induction of portal venous tolerance in rat cardiac allograft transplantation. J Surg Res. 1990;48:393–396. doi: 10.1016/0022-4804(90)90001-i. [DOI] [PubMed] [Google Scholar]
- Lee CM, Yeoh GC., and , Olynyk JK. Differential effects of gadolinium chloride on Kupffer cells in vivo and in vitro. Int J Biochem Cell Biol. 2004;36:481–488. doi: 10.1016/j.biocel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Tian Y, Jochum W, Georgiev P, Moritz W, Graf R., and , Clavien PA. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci USA. 2006;103:4598–4603. doi: 10.1073/pnas.0600499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Shi JH, Tang Z, Wu Y., and , Chen S. Protective effects of glycine pretreatment on brain-death donor liver. Hepatobiliary Pancreat Dis Int. 2005;4:37–40. [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM., and , Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amersi F, Shen X-D, Anselmo D, Melinek J, Iyer S, Southard DJ, et al. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- Chauveau C, Rémy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi S-i, Livhits M, Zhai Y, Busuttil RW, Araujo JA., and , Kupiec-Weglinski JW. Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. J Immunol. 2006;177:4749–4757. doi: 10.4049/jimmunol.177.7.4749. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takahashi T, Maeshima K, Shimizu H, Toda Y, Morimatsu H, et al. Heme arginate pretreatment attenuates pulmonary NF-kappaB and AP-1 activation induced by hemorrhagic shock via heme oxygenase-1 induction. Med Chem. 2006;2:271–274. doi: 10.2174/157340606776930781. [DOI] [PubMed] [Google Scholar]
- McNally SJ, Harrison EM, Ross JA, Garden OJ., and , Wigmore SJ. Curcumin induces heme oxygenase-1 in hepatocytes and is protective in simulated cold preservation and warm reperfusion injury. Transplantation. 2006;81:623–626. doi: 10.1097/01.tp.0000184635.62570.13. [DOI] [PubMed] [Google Scholar]
- Devey L, Festing MF., and , Wigmore SJ. Effect of temperature control upon a mouse model of partial hepatic ischaemia/reperfusion injury. Lab Anim. 2008;42:12–18. doi: 10.1258/la.2007.06009e. [DOI] [PubMed] [Google Scholar]