Abstract
Recombinant adeno-associated virus (rAAV)-mediated gene transfer is an attractive approach to the treatment of Duchenne muscular dystrophy (DMD). We investigated the muscle transduction profiles and immune responses associated with the administration of rAAV2 and rAAV8 in normal and canine X-linked muscular dystrophy in Japan (CXMDJ) dogs. rAAV2 or rAAV8 encoding the lacZ gene was injected into the skeletal muscles of normal dogs. Two weeks after the injection, we detected a larger number of β-galactosidase-positive fibers in rAAV8-transduced canine skeletal muscle than in rAAV2-transduced muscle. Although immunohistochemical analysis using anti-CD4 and anti-CD8 antibodies revealed less T-cell response to rAAV8 than to rAAV2, β-galactosidase expression in rAAV8-injected muscle lasted for <4 weeks with intramuscular transduction. Canine bone marrow-derived dendritic cells (DCs) were activated by both rAAV2 and rAAV8, implying that innate immunity might be involved in both cases. Intravenous administration of rAAV8-lacZ into the hind limb in normal dogs and rAAV8-microdystrophin into the hind limb in CXMDJ dogs resulted in improved transgene expression in the skeletal muscles lasting over a period of 8 weeks, but with a declining trend. The limb perfusion transduction protocol with adequate immune modulation would further enhance the rAAV8-mediated transduction strategy and lead to therapeutic benefits in DMD gene therapy.
Introduction
Duchenne muscular dystrophy (DMD) is an inherited disorder causing progressive deterioration of skeletal and cardiac muscles because of mutations in the dystrophin gene. No effective treatment has been established despite the development of various novel therapeutic strategies including pharmacologic and gene therapies. Dystrophin-deficient mdx mice and dystrophin-utrophin double-knockout mice are the animal models most widely used to evaluate therapeutic efficacy, although the symptoms of mdx mice are not comparable to those of human DMD patients. Dystrophin-deficient canine X-linked muscular dystrophy was found in a golden retriever,1,2 and we have established a Beagle-based model of canine X-linked muscular dystrophy in Japan (CXMDJ) dogs.3 The clinical and pathological characteristics of the dystrophic dogs are more similar to those of DMD patients than murine models.3
The recombinant adeno-associated virus (rAAV) can be used for delivering genes to muscle fibers. Several serotypes of rAAV exhibit a tropism for striated muscles.4,5 Intramuscular or intravenous administration of rAAV carrying the microdystrophin gene was reported to restore specific muscle force and extend the lifespan in dystrophic mice.6,7 In contrast to the success of transgene delivery in mice, rAAV2 or rAAV6 delivery to canine striated muscles without immunosuppression resulted in insufficient transgene expression, and rAAV evoked strong immune responses.8,9 An assay of interferon-γ released from murine and canine splenocytes suggested that the immune responses against rAAV and transgene products in mice and in dogs are dissimilar.8 Uptake of rAAV2 by human dendritic cells (DCs) and T-cell activation in response to the AAV2 capsid have been reported,10 indicating that DCs play key roles in the immune response against rAAV-mediated transduction. On the other hand, other serotypes, including rAAV8, that are capable of whole-body skeletal muscle expression after intravenous administration,4,5induce less T-cell activation.11 We hypothesized that the level of activation of canine DCs by rAAV8 might be lower than that achieved by rAAV2. However, the transduction profile and immune response in the rAAV8-injected dog skeletal muscle have not been elucidated.
In this study, we chose to use intramuscular injections under ultrasonographic guidance so as to minimize the inflammatory reaction caused by incisional intramuscular injection.8 In addition, intravascular delivery was performed as a form of limb perfusion, in an attempt to bypass the immune activation of DCs in the injected muscle.12 We investigated the transgene expression and host immune response to two distinct serotypes of rAAV in normal and dystrophic dogs after direct intramuscular injection and after limb perfusion.
Results
Extensive expression of β-galactosidase in rAAV8-transduced muscles in wild-type dogs
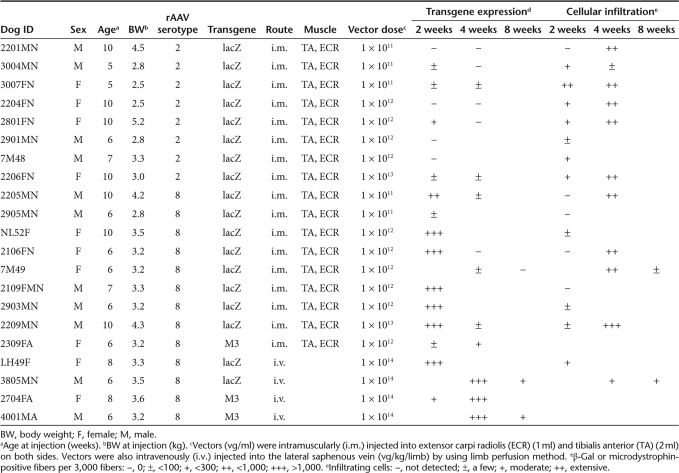
We administered nonincisional intramuscular injections under ultrasonographic guidance so as to minimize injury. With incisional injection, the ordinary method of intramuscular viral administration in dogs,8 the skin is opened to identify the individual muscles. This may enhance the immune reaction by recruiting inflammatory cells for wound healing. After nonincisional injection of rAAV2-lacZ, faint β-galactosidase (β-gal) expression was detected, whereas lymphocyte infiltration still occurred (Supplementary Figure S1). To investigate the transduction efficiency of rAAV8 in canine skeletal muscle, normal dogs were transduced with rAAV-lacZ serotypes 2 and 8 (Table 1). Prominent expression of β-gal was observed in the rAAV8-lacZ-injected muscles, whereas the rAAV2-lacZ-injected muscles showed minimal transgene expression (Figure 1). While β-gal expression in the rAAV8-injected muscle was correlated with the viral dose, β-gal expression in the rAAV2-injected muscle was not augmented with viral dose escalation. However, rAAV8-lacZ-injected muscles, which showed extensive β-gal expression at 2 weeks, also exhibited reduced expression at 4 weeks after the injection, thereby suggesting that the transgene product had immunogenicity (Supplementary Figure S2).
Table 1.
Summary of gene transduction experiments
Figure 1.
Canine skeletal muscles stained for β-galactosidase. Two milliliters of rAAV2-lacZ or rAAV8-lacZ (1 × 1011–1013 vg/ml) were injected intramuscularly into the tibialis anterior (TA) muscle of normal dogs (n = 16) under ultrasonographic guidance. The muscles were biopsied 2 weeks after the injection. Upper: rAAV2-lacZ-injected TA muscles, Lower: rAAV8-lacZ-injected TA muscles. Bar = 200 µm.
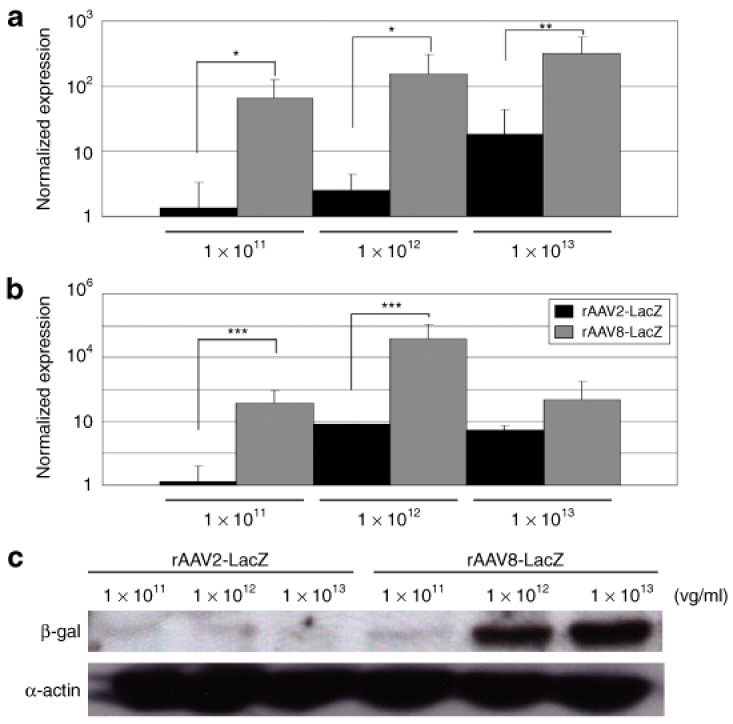
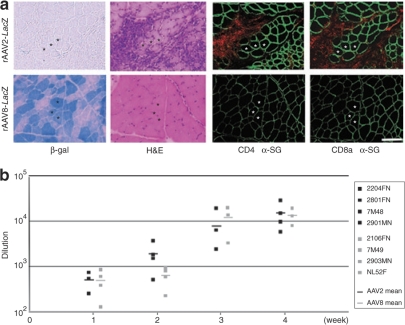
To evaluate the difference in transduction efficiency between rAAV2 and rAAV8 at 2 weeks after the injection, relative quantifications of the vector genome and mRNA were performed. The result demonstrated higher transduction rates in the rAAV8- injected muscles as increasing amounts of the vector were administered (Figure 2a,b). The amount of protein expression was also well correlated with that of transgenic DNA (Figure 2c, Supplementary Table S1). Immunohistochemical analysis revealed that the rAAV2-injected muscles showed much more infiltration of CD4+ and CD8+ T lymphocytes in the endomysial space than the rAAV8-injected muscles did (Figure 3a). mRNA levels of TGF-β1 and IL-6 (representative markers of inflammation) in the rAAV-injected muscles were standardized with the β-gal expression. rAAV2-injected muscles had higher TGF-β1 and IL-6 expression than rAAV8-transduced muscles (Supplementary Figure S3). We also examined humoral immune responses against the rAAV particles in the sera of rAAV-injected dogs. The levels of serum IgG in reaction to rAAV2 or rAAV8 gradually increased with time in both serotypes (Figure 3b). These results suggest that cellular and humoral immune responses are elicited in both rAAV2- and rAAV8-transduced muscles.
Figure 2.
Quantification of viral vector genome, mRNA, and transgene expression. (a) Relative quantification of genomic PCR for rAAV2-lacZ-injected muscle (black bars) or rAAV8-lacZ-injected muscle (gray bars). DNA samples were extracted from the TA muscles. *P < 0.05. **P < 0.01. Error bars represent 2 SD. (b) Relative quantification showed more extensive β-gal mRNA expression caused by rAAV8-lacZ (gray bars) as compared to that caused by rAAV2-lacZ (black bars). 18S rRNA was used for an internal control. ***P < 0.05. Error bars represent 2 SD. (c) Western blots of β-gal protein (120 kDa) and α-actin (42 kDa); the β-gal signal was normalized to α-actin for comparison.
Figure 3.
Immune response to rAAV. (a) Lymphocyte infiltration after rAAV transduction. Muscles were biopsied 2 weeks after rAAV2- or rAAV8-lacZ injection (2 × 1012 vg/muscle). Serial cross-sections were stained with β-gal and H&E, and were immunohistochemically stained with antibodies against canine CD4, CD8a (Alexa 568, red), and α-sarcoglycan (α-SG, Alexa 488, green). Upper: rAAV2-lacZ-injected TA muscle; lower: rAAV8-lacZ-injected TA muscle. Bar = 100 µm. (b) Humoral immune responses to rAAV capsid in dogs. Serum was collected weekly from rAAV2- or rAAV8-lacZ-injected dogs and analyzed for the presence of IgG antibody against the rAAV2 or rAAV8 capsid. The data represent dilution rates with 50% reactivity of anti-rAAV2 (black boxes) and anti-rAAV8 (gray boxes) capsid antibodies. The mean reconstitution values are shown as straight lines. Each symbol represents an individual dog that was injected with rAAV at 2 × 1012 vg/muscle.
Bone marrow-derived DC reactions to rAAV2 and rAAV8
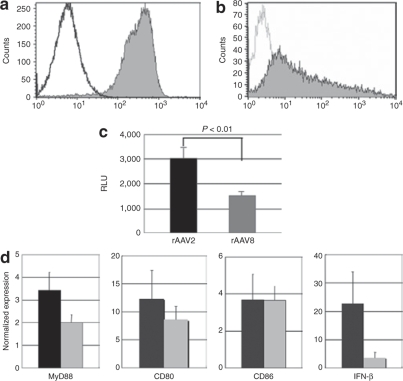
We next cultured bone marrow-derived DCs to investigate their response to rAAV injection in dogs. Flow cytometric analyses of these cells at 7 days of culture revealed marked expressions of CD11c and MHC class II molecules on the surface (Figure 4a,b). The DCs were cultured with the rAAV-luciferase of either serotype 2 or 8 for 48 hours to evaluate transduction efficiency, or cultured with rAAV-lacZ for 4 hours to investigate kinetic changes in mRNA. The luciferase assay showed that the transduction efficiency of rAAV2-luciferase in DCs was approximately two times that of rAAV8-luciferase (Figure 4c). mRNA levels of MyD88 and costimulating factors, such as CD80, CD86, and type I interferon (interferon-β, IFN-β) were elevated in both conditions (Figure 4d), suggesting that rAAV8 also induces a considerable degree of innate immune response in dog skeletal muscles. Although rAAV2-transduced DCs showed higher IFN-β expression than rAAV8-transduced DCs, the differences between the effects of rAAV2 and rAAV8 on the mRNA levels of MyD88, CD80, CD86, and IFN-β were not statistically significant.
Figure 4.
Responses of dendritic cells (DCs) to rAAV in dogs. Bone marrow-derived DCs were obtained from the humerus bones of dogs and cultured in RPMI (10% FCS, p/s) for 7 days with canine GM-CSF and IL-4. (a) Flow cytometric analysis of cell surface molecules on day 7. The cells were stained with PE-conjugated CD11c antibody and isotype control. (b) DCs were stained with FITC-conjugated MHC Class II antibody and isotype control. (c) DCs were transduced with rAAV-luciferase (1 × 106 vg/cell) for 48 hours. To analyze luciferase expression relating to the use of rAAV2 or rAAV8, relative light unit (RLU) ratios were measured. *P < 0.01. Error bars represent s.e.m., n = 8. (d) DCs were transduced with 1 × 106 vg/cell of rAAV2 (black bars) or rAAV8-lacZ (gray bars) for 4 hours, and mRNA levels of MyD88, CD80, CD86, and IFN-β were analyzed. Untransduced cells were used as a control to demonstrate the relative value of expression. The results are representative of two independent experiments. Error bars represent s.e.m., n = 3.
Successful microdystrophin gene transfer with rAAV8 into dystrophic dogs
Dystrophin expression in normal skeletal muscle is localized on the sarcolemma, whereas it is totally absent in CXMDJ dogs (Supplementary Figure S4a,b). Microdystrophin expression in the rAAV8-injected skeletal muscle of CXMDJ dogs was maintained, even in the absence of any immunosuppressive therapy, for at least 4 weeks after the injection (Table 1). Previously, we had shown that microdystrophin expression of ca 20% was sufficient to achieve functional recovery in mdx mice6. However, the amount of the expression in intramuscularly injected muscles seemed to be insufficient to produce the expected functional recovery (Supplementary Figure S4c).
For more efficient gene delivery by rAAV8, we tried a limb perfusion method in the hind limb through the lateral saphenous vein, in an attempt to prevent muscle damage due to direct injection and to bypass immune activation through DCs in the injected muscle. We had observed highly efficient β-gal expression in nearly all the muscles of the distal hind limb at 2 weeks after a single injection (Table 1, Figure 5a). We then injected rAAV8-M3 into the hind limbs of CXMDJ dogs, using the same method (Table 1). The induction of microdystrophin expression in the muscle at 4 weeks after intravascular injection was more efficient and free of noticeable immune response as compared to intramuscularly injected muscle (Figure 5b, Supplementary Figure S4d). These results suggest that the intravascular method is superior to the intramuscular method of administration. Although microdystrophin expression persisted at 8 weeks after injection of rAAV8-M3, the number of microdystrophin-positive cells at this time point was lower than in the muscles that were sampled at 4 weeks after injection. It is clear, therefore, that long-term microdystrophin expression can be obtained by the limb perfusion method, but that the expression does not last at the same level over a period of weeks. The same phenomenon was observed in rAAV8-lacZ-transduced muscles (Supplementary Figure S5).
Figure 5.
rAAV8-mediated muscle transduction using the limb perfusion method. (a) Transduction of normal dog with rAAV8-lacZ, using the limb perfusion method. Muscles were biopsied 2 weeks after the injection and stained with β-gal and H&E. TA, tibialis anterior, EDL, extensor digitorum longus. Bar = 200 µm. (b) Transduction of canine X-linked muscular dystrophy in Japan (CXMDJ) dog with rAAV8-M3. Muscles of CXMDJ dogs were biopsied 4 and 8 weeks after limb perfusion with rAAV8-M3. Samples were immunohistochemically stained with anti-dystrophin antibody (dys2, NCL). Left: nontreated CXMDJ muscle. Middle and right: muscles injected with rAAV-M3 using limb perfusion method, examined at 4 or 8 weeks after the transduction. Bar = 100 µm.
Discussion
In this article, we present evidence that the transfer of rAAV8-lacZ to canine skeletal muscles produces higher transgene expression with less lymphocyte proliferation than rAAV2-lacZ does, at 2 weeks after injection. Given the advantages of rAAV8, the administration of rAAV8-M3 by limb perfusion produced extensive transgene expression in the distal limb muscles of CXMDJ dogs without obvious immune responses for as long as 8 weeks after injection. However, transgene expression in the rAAV8-transduced muscles attenuated in the absence of an immunosuppressive regimen over the course of observation. In addition, humoral immune responses were elicited by both rAAV2 and rAAV8. mRNA levels of MyD88 and costimulating factors such as CD80, CD86, and type I interferon (interferon-β) were elevated in both rAAV2- and rAAV8-transduced DCs in vitro.
In our previous study, we had demonstrated extensive lymphocyte-mediated immune responses to rAAV2-lacZ after direct intramuscular injection into dogs, in contrast to the reported successful delivery of the same viral construct into mouse skeletal muscle.8 The fact that the promoter-deleted rAAV2 caused fewer cytotoxic cellular responses suggested that the massive destruction of transduced muscle cells might be the result of cellular immunity against the transgene product. In this study, there was extensive expression of β-gal in rAAV8-lacZ-injected canine muscles even in the absence of any immunosuppressive treatments (Figure 1), while the rAAV2-lacZ-injected muscles showed minimal β-gal expression with considerable inflammatory infiltration. If the transgene product were the main inducer of immune responses, lymphocyte activation would be correlated with transduction efficiency; however, this is not the case based on our results relating to the vector genome, mRNA expression level, and protein delivered through either rAAV2 or rAAV8 (Figure 2). These data suggested that the rAAV particle is associated with potent immunogenicity. Besides, β-gal expression disappeared 4 weeks after injection in the rAAV8-injected muscle as in the rAAV2-transduced muscles (Supplementary Figure S2). To investigate whether AAV itself has immunogenicity properties, we further characterized the immune responses caused by rAAV2 or rAAV8.
Immunohistochemical analysis revealed that the rAAV2-injected muscles showed higher rates of infiltration of CD4+ and CD8+ T lymphocytes in the endomysium than rAAV8-injected muscles did (Figure 3a). Considering the stringent immunogenicity of lacZ gene expression, we normalized the activity of TGF-β1 and IL-6 by lacZ expression to exclude the effect of transgene products (Supplementary Figure S3a). The total activity of TGF-β1 and IL-6 in the rAAV8-injected muscles was higher than that in rAAV2-injected muscles (Supplementary Figure S3b). As a result, rAAV2 induced a stronger cellular immune response than rAAV8 did. To investigate the humoral immune response, we quantitated neutralizing antibodies against rAAV particles in the sera of rAAV-injected dogs (Figure 3b). Antibodies against AAV2 and AAV8 capsids were below the detectable level before the injection and were elevated with time after the injection. Because the dogs were bred in a specific pathogen-free facility and not vaccinated, we assume that the elevation of antibody levels was not caused by anamnestic reaction.
Recently, Li et al.10 reported that the AAV2 capsid can induce a cellular immune response through MHC class I antigen presentation with a cross-presentation pathway, and the effects of rAAV2 on human DCs have been described.10,13 In contrast, other serotypes such as rAAV8 induced less T-cell activation.11,14 Plasmacytoid DCs are critically important in innate immunity because of their unsurpassed ability to present adenoviral antigens to T-cells for the generation of primary cellular and humoral immune responses.15,16,17 The response of DCs against rAAV in dogs was yet to be elucidated. We prepared bone marrow-derived DCs to investigate rAAV-mediated transduction of DCs. The difference between rAAV2 and rAAV8 in respect of the transduction rate of DCs in vitro was no greater than the difference in distinct β-gal expressions in vivo (Figure 2,4c). Quantitative analysis of mRNA of the transduced DCs by RT-PCR revealed that both rAAV2 and rAAV8 upregulated the expression of costimulating factors, with no significant difference between mRNA levels in rAAV2- and rAAV8-transduced cells. Therefore, both rAAV2 and rAAV8 may activate innate immunity in the context of extensive muscle transduction. Whereas AAV capsids cause immune response, transgene products may play adjuvant roles in the immunity to the AAV capsids.18
rAAV8 encoding the human microdystrophin gene was also intramuscularly injected into the skeletal muscles of CXMDJ dogs. rAAV8-mediated gene expression without any immunosuppression was confirmed over a period of 8 weeks after the injection, whereas there was much less transduction with the use of rAAV2 (data not shown). rAAV8-mediated transduction was also expected to provide effective intravenous delivery.12 In this context, the venous system is an attractive route for limb perfusion administration because it is a direct channel to multiple muscles of the limb. Moreover, veins are easier to access through the skin and there is less potential for muscle damage during injection. By using the limb perfusion method, we could reach nearly all the muscles of the lower limb, held transiently isolated by a tourniquet around the thigh. Limb perfusion administration could possibly have the potential to bypass the DC recognition caused by intramuscular injection. We intravenously injected rAAV8-lacZ into the hind limbs of normal dogs and rAAV8-M3 into the hind limbs of CXMDJ dogs, and obtained more extensive expression of β-gal or microdystrophin than by intramuscular injection. Interestingly, the inflammatory response was not significant in the intravenously injected muscles, although no immune suppression was attempted. We think that one reason rAAV8-M3 resulted in better expression than rAAV8-lacZ is that the immunogenicity of M3 is lower than that of lacZ. Although microdystrophin expression was lower at 8 weeks after the transduction with the limb perfusion, cellular infiltration was not significant.
In the future, systemic delivery of rAAV8-microdystrophin could ameliorate the symptoms of DMD patients. Even though portal vein injection of rAAV2-FIX into hemophilia B dogs produced long-term expression, a clinical study failed to demonstrate long-term expression in humans.19,20 In advance of future clinical trials, several studies are required to confirm safety. Sequential peripheral blood monitoring showed no severe adverse events, including liver dysfunction, during 8 weeks (data not shown). We are now developing a systemic delivery strategy with a muscle-specific promoter. It is also necessary to improve vector constructs or regulate immune reaction against transgene products. Recently, Wang et al. reported sustained AAV6-mediated human microdystrophin expression in dystrophic dogs for 30 weeks, using combined immunosuppressive therapy of Ciclosporin, Mycophenolate Mofetil, and anti-thymocyte globulin.9 In this study with rAAV8-M3, we confirmed effective transduction into dog skeletal muscle for 4 weeks without immunosuppressive therapy. However, considering the fact that not only rAAV2 but also rAAV8 induced activation of DCs in vitro, immunological modulation would be required for sufficient long-term expression. A novel protocol with systemic or localized immunosuppression using immunosuppressive drugs or local immunosuppression with an IFN-α or -β blockade could help avoid host immune reaction.
In summary, we achieved successful rAAV8-mediated muscle transduction in wild-type dogs as well as in dystrophic dogs by using the limb perfusion method of administration. Also, by manipulating bone marrow-derived DCs, we observed the probable contribution of antigen-presenting cells to the immune response against rAAV8-mediated gene therapy. Although the cellular responses against rAAV8 were not significant in vivo, this DC activation may possibly be involved in limiting long-term transduction when the limb perfusion method is used. The limb perfusion transduction protocol with improved AAV constructs or immune modulation would further enhance rAAV8-mediated transduction strategy and lead to therapeutic benefits.
Materials and Methods
Animals. Five- to ten-week-old male and female wild-type dogs obtained from the Beagle-based CXMDJ breeding colony at the National Center of Neurology and Psychiatry (Tokyo, Japan) were used for the lacZ gene transduction.3 Six- to eight-week-old CXMDJ dogs were used for microdystrophin gene transduction. All the animals were cared for and treated in accordance with the guidelines approved by the Ethics Committee for Treatment of Laboratory Animals at National Center of Neurology and Psychiatry, where the three fundamental principles of replacement, reduction, and refinement are also considered. Dogs were not vaccinated to avoid the immune responses to vaccination.
Construction of proviral plasmid and recombinant AAV vector production. The AAV2 vector proviral plasmids harboring the lacZ or luciferase gene with a CMV promoter and SV40 late-gene polyadenylation sequence were propagated.8 As a therapeutic gene for DMD, the human microdystrophin gene, M3, was used under the control of the CMV promoter and a bovine growth hormone polyadenylation sequence.21 The vector genome was packaged into the AAV2 capsid or pseudotyped AAV8 capsid in HEK293 cells. A large-scale cell culture method with an active gassing system was used for transfection.22 The vector production process involved triple transfection of a proviral plasmid, an AAV helper plasmid pAAV-RC (Stratagene, La Jolla, CA) or p5E18-VD2/8, and an adenovirus helper plasmid pHelper (Stratagene).21 All the viral particles were purified by CsCl gradient centrifugation. The viral titers were determined by quantitative PCR using SYBR-green detection of PCR products in real time with the MyiQ single-color detection system (Bio-Rad, Hercules, CA) and the following primer sets: for AAV-lacZ, lacZ-Q60: forward primer 5′-TTATCAGCCGGAAAACCTACCG-3′, and reverse primer 5′-AGCCAGTTTACCCGCTCTGCTA-3′; for AAV-microdystrophin: forward primer 5′-CCAAAAGAAAAAGGATCCACAA-3′, and reverse primer 5′-TTCCAAATCAAACCAAGAGTCA-3′; and for AAV-luciferase: forward primer 5′-GATACGCTGCTTTAATGCCTTT-3′, and reverse primer 5′-GTTGCGTCAGCAAACACAGT-3′.
Direct administration of rAAVs into normal and dystrophic skeletal muscle. Experimental dogs (n = 16) were sedated with isoflurane by mask inhalation and intubated. Anesthesia was maintained with 2–4% isoflurane. Two milliliters of rAAV2-lacZ or rAAV8-lacZ (1 × 1011–1013 vg/ml) were injected intramuscularly into the tibialis anterior muscles and 1 ml into the extensor carpi radialis muscles of the normal dogs under ultrasonographic guidance. rAAV8-M3 (1 × 1012 vg/ml) was intramuscularly injected at a volume of 2 ml into the tibialis anterior muscles and 1 ml into the extensor carpi radialis muscles of a CXMDJ dog.
Intravenous delivery of rAAVs into the limb veins of dogs. Intravenous injection was administered as described elsewhere.12 Briefly, a blood pressure cuff was applied just above the knee of an anesthetized normal dog. A 24-gauge intravenous catheter was inserted into the lateral saphenous vein, connected to a three-way stopcock, and flushed with saline. With the blood pressure cuff inflated to over 300 mm Hg, saline (2.6 ml/kg) containing papaverine (0.44 mg/kg, Sigma-Aldrich, St Louis, MO) and heparin (16 U/kg) was injected by hand over 10 seconds. The three-way stopcock was connected to a syringe containing rAAV8-lacZ (1 × 1014 vg/kg, 3.8 ml/kg). The syringe was placed in a PHD 2000 syringe pump (Harvard Apparatus, Edenbridge, UK). Five minutes after the papaverine/heparin injection, the rAAV8-lacZ was injected at a rate of 0.6 ml/second. Two minutes after the rAAV injection, the blood pressure cuff was released and the catheter was removed. The CXMDJ dogs were injected with rAAV8-M3 using the same method.
Sampling of transduced muscles. Either the muscles of the transduced dogs were biopsied or the animals were killed at 2, 4, and 8 weeks after the injection. We sampled tibialis anterior and extensor carpi radialis muscles on both sides in the intramusculary transduced dog. In the case of the limb perfusion study, tibialis anterior or extensor digitorum longus muscle of the injected side of the leg was sampled. For biopsy and necropsy, the individual muscle was cropped tendon-to-tendon, divided into several pieces, and immediately frozen in liquid nitrogen-cooled isopentane. Two to eight blocks were sampled from the transduced muscle. We analyzed at least 30 sections from the blocks to observe the general representation.
Histological analysis. Transverse cryosections (10 µm) from the rAAV-lacZ-injected muscles were stained with hematoxylin and eosin or 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside.23 Eight-micrometer-thick cryosections from the rAAV-M3-injected muscles were immunohistochemically stained as described.24 Briefly, the cryosections were fixed by immersion in cold acetone at −20 °C. Fixed frozen sections were blocked in 5% goat serum in phosphate-buffered saline at room temperature and incubated with mouse monoclonal anti-dystrophin C-terminal antibody (NCL-dys2, Novocastra, Newcastle upon Tyne, UK). The signal was visualized with an Alexa 568-conjugated anti-mouse IgG. Fluorescent signals were observed using a confocal laser scanning microscope (Leica TCS SP, Leica, Heidelberg, Germany). Immunohistochemical analyses were performed with mouse monoclonal antibodies against canine CD4 (CA13.1E4, Serotec, Oxford, UK), canine CD8a (CA9. JD3, Serotec), and double-stained with rabbit polyclonal antibody against α-sarcoglycan.25 The signal was visualized with an Alexa 568-conjugated anti-mouse IgG, and 488-conjugated anti-rabbit IgG.
Detection of AAV genomes. Total DNA was extracted from muscle cryosections. Cryosections were homogenized using a Multi-beads shocker (Yasui Kikai, Osaka, Japan), and extracted using a Wizard SV Genomic DNA purification system (Promega, Madison, WI). The rAAV genome was detected by relative quantitative PCR using SYBR-green detection of PCR products in real time with a primer set of lacZ-Q60. For an internal control, forward primer, 5′-GAACACGCGTTAATAAGGCAATCA-3′, and reverse primer, 5′-CTGACATTCATCGCATCTTTGACA-3′, directed to an ultra-conserved region, were used.26
Real-time RT-PCR. Total RNA was isolated from cryosections using a Multi-beads shocker (Yasui Kikai), and RNeasy Fibrous Tissue Mini kit (Qiagen, Hilden, Germany), and first-strand cDNA was synthesized using a QuantiTect Reverse Transcription kit (Qiagen). mRNA was detected using primer sets of lacZ-Q60, forward primer 5′-TGATGGCTA CTGCTTTCCCTAC-3′ and reverse primer 5′-GAGATTTTGCCGA GGATGTACT-3′ for IL-6, and forward primer 5′-CAAGGATCTGGGC TGGAAGTGGA-3′ and reverse primer, 5′-CCAGGACCTTGCTGTA CTGCGGT-3′ for TGF-β1. For an internal control, a primer set of 18S rRNA (Ambion, Foster City, CA) was used.
Western blot analysis. Muscle cryosections were homogenized with four volumes of sample buffer (10% SDS, 70 mmol/l Tris-HCl, 10 mmol/l EDTA, and 5% β-mercaptoethanol). The samples were boiled for 5 minutes and centrifuged at 14,500 rpm for 15 minutes. Protein samples (30 µg per lane) were electrophoresed on a 7.5% polyacrylamide gel (Bio-Rad). The membranes were incubated with a 1:1,000 dilution of the primary antibody for detecting 120 kDa lacZ protein (rabbit anti-β-galactosidase IgG fraction, Molecular Probes, Eugene, OR) or 42 kDa α-actin (mouse anti α-sarcomeric actin IgM, Sigma-Aldrich). Anti-rabbit IgG peroxidase F(ab′) (GE Healthcare, Buckinghamshire, UK), or peroxidase-conjugated donkey anti-mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA) was used for ECL immunodetection (GE Healthcare). Quantification of LacZ protein was performed using a specialized software (ImageJ, US National Institutes of Health, Bethesda, MD).
ELISA for anti-canine AAV IgG. A microtiter plate (MS-8596F, Sumitomo Bakelite, Tokyo, Japan) was precoated with promoter-deleted rAAV2 or rAAV8 (2 × 109 genomes/well) and blocked with a blocking buffer (Block Ace, DS Pharma Biomedical, Osaka, Japan). The plate was incubated for 2 hours at room temperature with the sera from rAAV-transduced dogs, followed by a 1:5,000 dilution of peroxidase-conjugated rabbit anti-dog IgG (Sigma-Aldrich) for 1 hour. Color was visualized using a peroxidase substrate system (TMBZ, ML-1120T, Sumitomo Bakelite). Reactivity was detected at a wave-length of 450 nm with a reference at 570 nm, using an APPLISKAN Multimode Reader (Thermo Fisher Scientific, East Greenbush, NY).
Bone marrow aspiration and preparation of DCs. After the dogs were anesthetized with thiopental and isoflurane, ~0.5 ml of bone marrow was obtained from each humerus by aspiration with a syringe containing 2 ml of 16 mmol/l EDTA-2Na PBS. Bone marrow-derived DCs were generated as described.15 Mononuclear cells were isolated by density centrifugation using Histopaque-1077 (Sigma-Aldrich). Cells were suspended in RPMI-1640 culture medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (MP Biomedicals, Aurora, OH) and 1% penicillin-streptomycin (Sigma-Aldrich), and cultured at 37 °C in a humidified 5% CO2-containing atmosphere. Recombinant canine GM-CSF (25 ng/ml, R&D Systems, Minneapolis, MN) and canine IL-4 (12.5 ng/ml, R&D Systems) were added to the culture medium. On days 3 and 5 of the culture, 60% of the medium volume was changed. On day 7 of the culture, loosely adherent cells were collected and used for fluorescence-activated cell analysis. A FACS Vantage system (Becton Dickinson, Franklin Lakes, NJ) was used for flow cytometry event collection. For the purpose of examining the infectious rate of rAAV, cells were cultured for 48 hours with rAAV2- or 8-luciferase. The luciferase activity of rAAV2- or rAAV8-luciferase co-cultured cells was estimated using an APPLISKAN Multimode Reader (Thermo Fisher Scientific). Total RNA was isolated using an RNeasy Fibrous Tissue Mini kit (Qiagen), and QuantiTect Reverse Transcription kit (Qiagen). mRNA of cytokines were analyzed using the primer set, forward primer 5′-GAGGAGATGGGCTTCGAGTA-3′ and reverse primer 5′-GTTCCACCAACACGTCGTC-3′ for MyD88; forward primer 5′-GCATCATCCAGGTGAACAAG-3′ and reverse primer 5′-AAGTCAGCAAAGGTGCGATT-3′ for CD80; forward primer 5′-AGGTTACCCAGAACCCAAGG-3′ and reverse primer, 5′-TTGC AGGACACAGAAGATGC-3′ for CD86; and forward primer 5′-ATT GCCTCAAGGACAGGATAAA-3′ and reverse primer 5′-TTGACG TCCTCCAGGATTATCT-3′ for IFN-β. mRNA levels of MyD88, CD80, CD86, and IFN-β in DCs were normalized with a house keeping gene, 18s rRNA. The mRNA levels in the transduced cells were presented as ratios relative to the sample obtained from the untransduced DCs.
Statistical analysis. Statistical significance was determined on the basis of an unpaired, two-tailed Student's t-test using specialized software (Statview; SAS Institute, Cary, NC). A P value of <0.05 was considered significant.
Supplementary MaterialFigure S1. Histological findings with incisional and nonincisional injection under ultrasonographic guidance.Figure S2. β-gal expression 4 weeks after injection.Figure S3. Levels of mRNA were investigated using rAAV-injected muscles.Figure S4. Intramuscular injection of rAAV8-M3 into CXMDJFigure S5. Long-term β-gal expression using limb perfusion injection.Table S1. Protein expression analyzed with ImageJ.
Supplementary Material
Histological findings with incisional and nonincisional injection under ultrasonographic guidance. Serial cross-sections were stained with β-gal and H&E. TA muscles were biopsied 2 weeks after rAAV2-lacZ injection (2 × 1012 v.g./2 ml). Upper: rAAV2-lacZ-injected muscle with skin incision. Lower: rAAV2-lacZ-injected muscle with intramuscular injection under ultrasonographic guidance, scale bar, 100 μm.
β-gal expression 4 weeks after injection. TA muscles were biopsied 4 weeks after rAAV2 or 8-lacZ injection (2 × 1012 v.g./2 ml). Serial cross-sections were stained with β-gal and H&E. Upper: rAAV2-lacZ-injected muscle. Lower: rAAV8-lacZ-injected muscle. Scale bar, 200 μm.
Levels of mRNA were investigated using rAAV-injected muscles. (a) mRNA levels of TGF-β1 or IL-6 were standardized by those of lacZ. rAAV2-injected muscle (black bar) showed higher expression than rAAV8-injected muscle (gray bar) in both cases. The difference was not significant. (b) Total activity of TGF-β1 or IL-6 normalized by 18s. Black bar showed mRNA levels of rAAV2-injected muscle, and gray bar showed those of rAAV8-injected muscle.
Intramuscular injection of rAAV8-M3 into CXMDJ Muscles sampled at 4 weeks after transduction were immunohistochemically stained with antibodies against dystrophin C-terminal. (a) Normal dog muscle, (b) CXMDJ muscle, (c) intramuscularly-injected muscle (2×1012 v.g./2 ml/muscle), and (d) intravascularly injected-muscle (1×1014 vg/kg), scale bar, 100 μm.
Long-term β-gal expression using limb perfusion injection. rAAV8-lacZ was injected into normal dogs using limb perfusion method. Muscles were biopsied 4 or 8 weeks after injection. Serial cross-sections were stained with β-gal.
Protein expression analyzed with ImageJ.
Acknowledgments
We thank James M. Wilson for providing p5E18-VD2/8. We also thank Akinori Nakamura, Hiroyuki Nakai, Yuko Nitahara-Kasahara, and Toshimasa Aranami for technical advice and helpful discussions; Kazue Kinoshita for AAV preparation; Ryoko Nakagawa for technical assistance; Satoru Masuda for FACS analysis; and Hideki Kita, Shinichi Ichikawa, and other staff members of JAC Co. for their care of the dogs. This work is supported by Grants-in-Aid for Scientific Research on Nervous and Mental Disorders and Health Sciences Research Grants for Research on Human Genome and Gene Therapy from the Ministry of Health, Labor and Welfare of Japan, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
REFERENCES
- Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R., and , Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- Shimatsu Y, Yoshimura M, Yuasa K, Urasawa N, Tomohiro M, Nakura M, et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy in Japan, CXMDJ. Acta Myol. 2005;24:145–154. [PubMed] [Google Scholar]
- Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y., and , Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y, et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther. 2004;10:821–828. doi: 10.1016/j.ymthe.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Li C, Hirsch M, Asokan A, Zeithaml B, Ma H, Kafri T, et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol. 2007;81:7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL, et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10:386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao G., and , Wilson J. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J Virol. 2000;74:8003–8010. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SW, Hensley SE, Tatsis N, Lasaro MO., and , Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8 T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isotani M, Katsuma K, Tamura K, Yamada M, Yagihara H, Azakami D, et al. Efficient generation of canine bone marrow-derived dendritic cells. J Vet Med Sci. 2006;68:809–814. doi: 10.1292/jvms.68.809. [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang X., and , Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., and , Wang FS. Plasmacytoid dendritic cells act as the most competent cell type in linking antiviral innate and adaptive immune responses. Cell Mol Immunol. 2005;2:411–417. [PubMed] [Google Scholar]
- Mahadevan M, Liu Y, You C, Luo R, You H, Mehta JL, et al. Generation of robust cytotoxic T lymphocytes against prostate specific antigen by transduction of dendritic cells using protein and recombinant adeno-associated virus. Cancer Immunol Immunother. 2007;56:1615–1624. doi: 10.1007/s00262-007-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Yuasa K, Yoshimura M, Yokota T, Ikemoto T, Suzuki M, et al. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem Biophys Res Commun. 2002;293:1265–1272. doi: 10.1016/S0006-291X(02)00362-5. [DOI] [PubMed] [Google Scholar]
- Okada T, Nomoto T, Yoshioka T, Nonaka–Sarukawa M, Ito T, Ogura T, et al. Large-scale production of recombinant viruses by use of a large culture vessel with active gassing. Hum Gene Ther. 2005;16:1212–1218. doi: 10.1089/hum.2005.16.1212. [DOI] [PubMed] [Google Scholar]
- Ishii A, Hagiwara Y, Saito Y, Yamamoto K, Yuasa K, Sato Y, et al. Effective adenovirus-mediated gene expression in adult murine skeletal muscle. Muscle Nerve. 1999;22:592–599. doi: 10.1002/(sici)1097-4598(199905)22:5<592::aid-mus7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J, et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- Araishi K, Sasaoka T, Imamura M, Noguchi S, Hama H, Wakabayashi E, et al. Loss of the sarcoglycan complex and sarcospan leads to muscular dystrophy in beta-sarcoglycan-deficient mice. Hum Mol Genet. 1999;8:1589–1598. doi: 10.1093/hmg/8.9.1589. [DOI] [PubMed] [Google Scholar]
- Sandelin A, Bailey P, Bruce S, Engstrom PG, Klos JM, Wasserman WW, et al. Arrays of ultraconserved non-coding regions span the loci of key developmental genes in vertebrate genomes. BMC Genomics. 2004;5:99. doi: 10.1186/1471-2164-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological findings with incisional and nonincisional injection under ultrasonographic guidance. Serial cross-sections were stained with β-gal and H&E. TA muscles were biopsied 2 weeks after rAAV2-lacZ injection (2 × 1012 v.g./2 ml). Upper: rAAV2-lacZ-injected muscle with skin incision. Lower: rAAV2-lacZ-injected muscle with intramuscular injection under ultrasonographic guidance, scale bar, 100 μm.
β-gal expression 4 weeks after injection. TA muscles were biopsied 4 weeks after rAAV2 or 8-lacZ injection (2 × 1012 v.g./2 ml). Serial cross-sections were stained with β-gal and H&E. Upper: rAAV2-lacZ-injected muscle. Lower: rAAV8-lacZ-injected muscle. Scale bar, 200 μm.
Levels of mRNA were investigated using rAAV-injected muscles. (a) mRNA levels of TGF-β1 or IL-6 were standardized by those of lacZ. rAAV2-injected muscle (black bar) showed higher expression than rAAV8-injected muscle (gray bar) in both cases. The difference was not significant. (b) Total activity of TGF-β1 or IL-6 normalized by 18s. Black bar showed mRNA levels of rAAV2-injected muscle, and gray bar showed those of rAAV8-injected muscle.
Intramuscular injection of rAAV8-M3 into CXMDJ Muscles sampled at 4 weeks after transduction were immunohistochemically stained with antibodies against dystrophin C-terminal. (a) Normal dog muscle, (b) CXMDJ muscle, (c) intramuscularly-injected muscle (2×1012 v.g./2 ml/muscle), and (d) intravascularly injected-muscle (1×1014 vg/kg), scale bar, 100 μm.
Long-term β-gal expression using limb perfusion injection. rAAV8-lacZ was injected into normal dogs using limb perfusion method. Muscles were biopsied 4 or 8 weeks after injection. Serial cross-sections were stained with β-gal.
Protein expression analyzed with ImageJ.