Abstract
In a gene therapy clinical trial for hemophilia B, adeno-associated virus 2 (AAV2) capsid–specific CD8+ T cells were previously implicated in the elimination of vector-transduced hepatocytes, resulting in loss of human factor IX (hFIX) transgene expression. To test the hypothesis that expression of AAV2 cap DNA impurities in the AAV2-hFIX vector was the source of epitopes presented on transduced cells, transcription of cap was assessed by quantitative reverse transcription–PCR (Q-RT-PCR) following transduction of target cells with the vector used in the clinical trial. Transcriptional profiling was also performed for residual AmpR, and adenovirus E2A and E4. Although trace amounts of DNA impurities were present in the clinical vector, transcription of these sequences was not detected after transduction of human hepatocytes, nor in mice administered a dose 26-fold above the highest dose administered in the clinical study. Two methods used to minimize encapsidated DNA impurities in the clinical vector were: (i) a vector (cis) production plasmid with a backbone exceeding the packaging limit of AAV; and (ii) a vector purification step that achieved separation of the vector from vector-related impurities (e.g., empty capsids). In conclusion, residual cap expression was undetectable following transduction with AAV2-hFIX clinical vectors. Preformed capsid protein is implicated as the source of epitopes recognized by CD8+ T cells that eliminated vector-transduced cells in the clinical study.
Introduction
Gene transfer vectors based on adeno-associated virus (AAV) have demonstrated significant promise for human gene therapy based on excellent safety and long-term efficacy in animal models, and several clinical studies have been initiated.1,2,3,4 In a recent clinical trial in which recombinant AAV2–expressing human coagulation factor IX (AAV2-hFIX) was administered to the liver and resulted in therapeutic hFIX expression, an AAV capsid–specific CD8+ T-cell response was documented in the setting of loss of transgene expression starting 4 weeks after vector administration.5,6 Mingozzi and colleagues characterized this T-cell response in samples of peripheral blood mononuclear cells obtained from clinical trial subjects, and reported that a population of AAV capsid–specific cytotoxic T lymphocytes (CTLs) expanded following vector administration with kinetics that overlapped with the presumptive elimination of the transduced hepatocytes.6 In these studies, presentation by major histocompatibility complex class I molecules of epitopes derived from the preformed, input capsid protein component of the vector inoculum was proposed as the pathway that resulted in the elimination of the hFIX-expressing cells. The delayed onset of the immune-mediated elimination of hFIX-transduced cells may be partly attributable to slow intracellular degradation of AAV2 capsids.7 An alternative hypothesis, proposed by others to explain the provenance of capsid epitopes presented on the surface of vector-transduced cells, is that AAV cap DNA impurities present in the lot of vector used in the clinical study were expressed.8 Presentation of peptides derived from de novo synthesized capsid protein through classical major histocompatibility complex class I pathways could account for the loss of vector-transduced hepatocytes observed in the clinical study if cap impurities were present in sufficient quantities in the vector lot used and were expressed in the majority of hFIX-expressing cells. Identifying the major source of the AAV capsid–derived epitopes that sensitized vector-transduced hepatocytes to recognition and elimination by CD8+ T cells in the hemophilia B clinical study is important to enable design of effective strategies to address limitations imposed by human host immune responses.
Encapsidated DNA impurities in AAV vector preparations, derived from production plasmids used for transient or stable transfection, and from the producer cell genome, have been reported by several groups.9,10,11,12,13,14,15 Nony and colleagues reported packaged rep-cap sequences in the absence of inverted terminal repeats (ITRs) at levels up to 2% of vector genomes in AAV2 vectors, and implicated a role for a Rep-binding motif (CARE) in generation of this impurity.11 Chadeuf and colleagues reported that encapsidated prokaryotic DNA impurities derived from production plasmids were present at levels ranging from 1.2 to 6.3% in recombinant AAV vectors generated by transfection of HEK293 cells or by helper-virus infection of stable producer cell lines.13 Gao and colleagues reported residual cap at levels ranging from 0.4 to 1.0% in 17 lots of recombinant AAV 2, 7, and 8, and that cap was transcriptionally active.15 Wang and colleagues reported that while cross-presentation of AAV2 capsid protein could activate CTLs, vector-transduced hepatocytes were not targets for capsid-specific CTLs in mice.16 Li and colleagues reported that capsid-specific CTLs eliminated liver and muscle cells that endogenously expressed cap in cell culture and in vivo, but that cells transduced by AAV2 vectors were rarely eliminated by capsid-specific CTLs in a mouse model.17 These findings document the presence and transcriptional potential of cap and other DNA impurities in recombinant AAV preparations, and the inefficiency of preformed capsid protein to sensitize vector-transduced hepatocytes to CTL effector functions in animal models. We tested the hypothesis that expression of AAV cap impurities was the source of epitopes presented on vector-transduced cells that sensitized them to elimination by CTLs in the clinical trial. Vector-manufacturing steps that were used to minimize levels and transcriptional potential of cap and other DNA impurities in the clinical vector are described.
Results
Undetectable transcription of residual cap in the human hepatocyte cell line HHL5
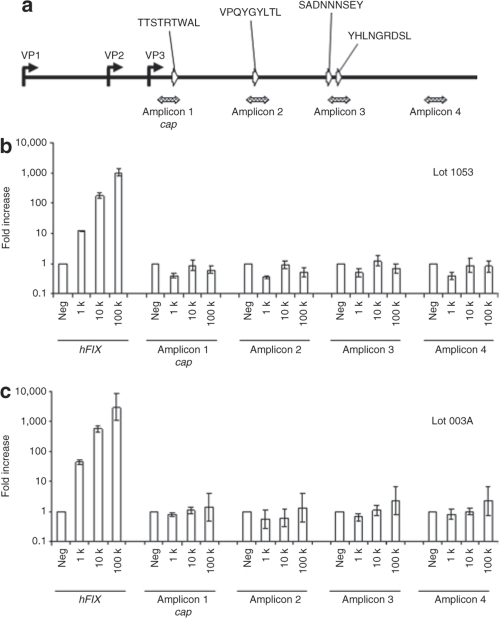
Primer-probe sets corresponding to four independent amplicons distributed from 5′ to 3′ in the VP3 region of the cap sequence were used to quantify residual cap in AAV2-hFIX vectors by quantitative PCR (Q-PCR), and to quantify cap transcripts following transduction of HHL5 cells and mice by Q-RT-PCR (Figure 1a). Amplicons 1–3 overlap with four known immunodominant epitopes: VPQYGYLTL and SADNNNSEY were identified as a CTL target in a human subject treated with AAV-hFIX;5 and TTSTRTWAL and YHLNGRDSL were identified in screening of human peripheral blood mononuclear cell samples.6 Residual cap DNA was detected in AAV2-hFIX Clinical Lot 1053 at a level of 0.00018 (±0.00006) copies per vector genome (cap/vg), an average of the values obtained using the four amplicons: 0.00021 cap/vg (amplicon 1), 0.00016 cap/vg (amplicon 2), 0.00020 cap/vg (amplicon 3), and 0.00017 cap/vg (amplicon 4). Transcription of hFIX in human hepatocyte cell line HHL5 transduced with AAV2-hFIX, Lot 1053 resulted in a dose-dependent increase in hFIX mRNA, ranging from a 14-fold increase relative to the excipient control at a dose of 1,000 vg/cell, to a 920-fold increase at 100,000 vg/cell (Figure 1b). A similar dose–response was observed using AAV2-hFIX reference vector Lot 003A (Figure 1c). In contrast, for both the clinical Lot 1053 (Figure 1b) and reference Lot 003A (Figure 1c), no significant expression of cap was detectable following transduction of HHL5 cells at any dose.
Figure 1.
Transcriptional profiling of residual adeno-associated virus 2 (AAV2) cap in cell culture (HHL5 cells). (a) Schematic overview of the AAV2 cap gene, showing location of the four amplicons (hatched arrows) used for quantitative reverse transcription–PCR (Q-RT-PCR). Locations of cap sequences corresponding to immunodominant epitopes are indicated by open arrows. The human hepatocyte cell line HHL5 was transduced with AAV2-hFIX vector Lot 1053 (b), or Lot 003A (c), at doses of 1 k, 10 k, or 100 k vector genome (vg)/cell. Total RNA was isolated 48 hours after transduction and assessed by Q-RT-PCR. hFIX and cap expression are presented as fold increase relative to excipient-treated cells. Error bars show ±SD of quadruplicate wells. hFIX, human factor IX.
Undetectable transcription of residual DNA impurities in C57Bl/6 mice
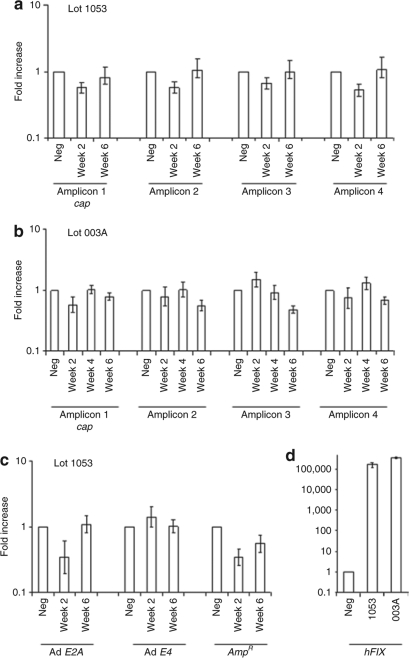
To investigate the transcriptional potential of cap and other DNA impurities in vivo, mice were injected via tail vein with AAV2-hFIX vectors at doses of 1.3 × 1012 vg/mouse (5.2 × 1013 vg/kg) (Lot 1053), or 2.7 × 1012 vg/mouse (1.1 × 1014 vg/kg) (Lot 003A). These doses were 26-fold (Lot 1053) and 56-fold (Lot 003A) higher than the highest dose (2.0 × 1012 vg/kg) that was administered in the hemophilia B clinical trial.5 As measured by Q-RT-PCR, hFIX mRNA levels were 161,550-fold (Lot 1053) and 337,731-fold (Lot 003A) at week 2 following vector administration elevated relative to excipient-treated mice (Figure 2d). Supraphysiological levels of hIX protein were measured in blood samples, 32.5 ± 6.4 µg/ml for Lot 1053 (n = 3) and 70.0 ± 6.0 µg/ml for Lot 003A (n = 3) at week 6 following vector administration. The levels of hFIX mRNA and protein measured after vector administration confirmed efficient transduction by both vectors. Mice injected with Ad-cap at a dose of 4 × 1012 vg/kg resulted in readily detectable cap mRNA at 1 week measured using each of the four amplicons. In contrast, AAV2 cap mRNA levels following administration of AAV2-hFIX, Lot 1053 (Figure 2a), or Lot 003A (Figure 2b) were not significantly different from those measured in excipient-treated animals. The limit of sensitivity of the Q-RT-PCR assay for cap expression, measured as the lowest copy number of cap plasmid DNA spiked into RNA isolated from excipient-treated mouse liver that gave a significant positive signal, was determined to be 20 copies per 500 ng RNA input, corresponding to ~1 copy per 2,500 cells (based on 10 pg total RNA per cell). Transcriptional profiling of mRNA for AmpR, and Ad E2A and E4 was also performed using RNA extracted from the mice that received AAV2-hFIX, Lot 1053, and no transcription of these genes was observed (Figure 2c).
Figure 2.
Transcriptional profiling of residual DNA impurities in C57Bl/6 mice. (a) C57Bl/6 mice injected with AAV2-hFIX Lot 1053 at a dose of 5.6 × 1013 vector genome (vg)/kg. RNA was isolated from livers of three mice harvested at each time point, and subjected to quantitative reverse transcription–PCR (Q-RT-PCR) using AAV2 cap–specific primers and probes. Expression is shown as fold increase relative to excipient (phosphate-buffered saline)-treated cells. (b) C57Bl/6 mice injected with AAV2-hFIX Lot 003A at a dose of 1.1 × 1014 vg/kg were assessed for AAV2 cap expression as described in a. (c) RNA from C57Bl/6 mice described in a was subjected to Q-RT-PCR using AmpR and adenovirus E2A and E4-specific primers and probes. (d) RNA isolated 2 weeks after administration of Lot 1053 or Lot 003A were subjected to Q-RT-PCR using hFIX primers and probes. Error bars show ±SD of triplicate mice. AAV2, adeno-associated virus 2; hFIX, human factor IX.
Hence, using our AAV2-hFIX vectors that contained low amounts of cap impurities, cap expression was undetectable following transduction in a human hepatocyte cell line and after high-dose administration in mice. However, others have reported transcription of residual cap and other DNA impurities following transduction with AAV vectors, and higher levels of residual DNA in AAV vector preparations.11,13,15 These data support that minimizing cap and other DNA impurities is important to reduce the potential for expression of immunogenic sequences. We identified and further characterized the steps of the vector-manufacturing process that were responsible for reducing cap and other encapsidated DNA impurities in our AAV2-hFIX vectors.
Influence of vector plasmid backbone size on levels of DNA impurities
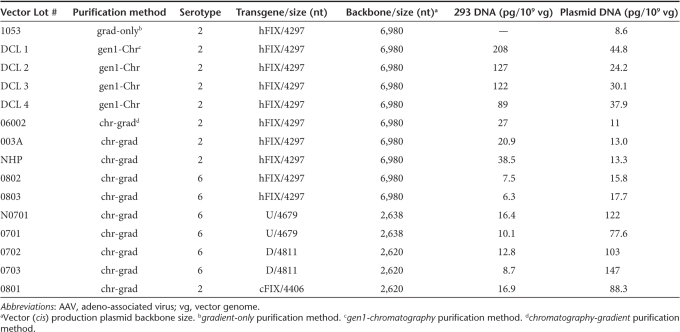
Previous studies reported that encapsidated plasmid DNA impurities are primarily derived from the backbone of the ITR-containing vector (cis) production plasmid.12,13 The influence of vector plasmid backbone size on the amount of residual plasmid DNA impurities in recombinant AAV2 and AAV6 was assessed. Residual plasmid DNA levels were measured by Q-PCR using primers and probes specific for AmpR, a sequence common to all three production plasmids used for vector generation (Table 1). The vectors compared in this experiment were each generated and purified using a common method (chromatography-gradient) that resulted in high vector purity, removal of empty capsids, and which included an efficient nuclease digestion step that removed nonencapsidated nucleic acid impurities.18 The average residual plasmid DNA level measured in five lots generated using a vector plasmid with an oversized (6,980 bp) backbone (Lots 06002, 003A, NHP, 0802, 0803) was 14.2 ± 2.6 pg/109 vg, 7.6-fold lower (P < 0.001) than the average value of 107.6 ± 27.6 pg/109 vg measured in five lots produced with vector plasmids with smaller (2,620 or 2,638 bp) backbones (Lots N0701, 0701, 0702, 0703, 0801). Therefore, the use of the oversized backbone in the vector production plasmid achieved a significant reduction in plasmid-derived DNA impurities.
Table 1.
Levels of DNA impurities in purified AAV vectors
Vector purification methods that minimize encapsidated DNA impurities
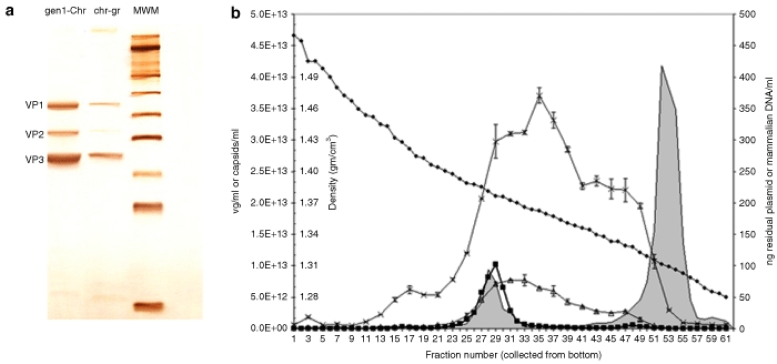
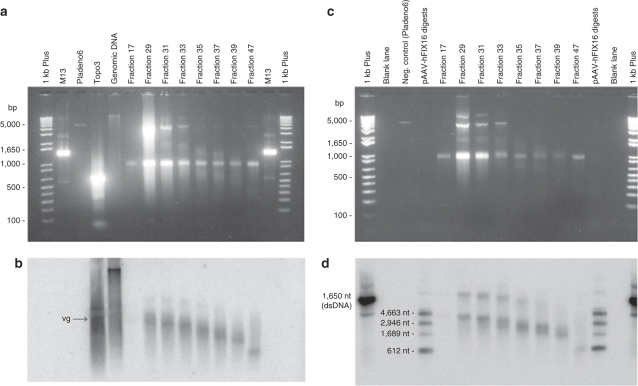
We next evaluated the influence of vector purification on the levels of DNA impurities. The levels of residual plasmid-derived and host-cell DNA impurities in eight AAV2-hFIX lots are provided in Table 1. Clinical AAV2-hFIX vector Lot 1053 was prepared by a double cesium chloride gradient ultracentrifugation method. This method included an incubation step with nuclease (Benzonase) to remove nonencapsidated DNA impurities. The amount of residual plasmid DNA impurity measured in the clinical vector in this study (8.6 pg/109 vg) was not significantly different than the value originally measured before the clinical study (6 pg/109 vg). Four other AAV2-hFIX lots were purified by the gen1-chromatography method (Lots DCL1, DCL2, DCL3, and DCL4), and three additional lots by the chromatography-gradient method (Lots 06002, 003A, and NHP). The AAV2-hFIX vector lots purified by the gen1-chromatography method, a first-generation chromatography method, resulted in the co-purification of empty capsids at levels corresponding to 89–95% of total AAV particles.19 This chromatography-only method, which may be comparable to some scalable AAV vector purification processes, results in vectors that are pure based on protein staining, but which contain vector-related impurities such as empty capsids. In contrast, the vectors purified by the chromatography-gradient method, were essentially empty capsid-free as assessed by spectrophotometry.19 Protein purity as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and silver staining of AAV2-hFIX vectors purified by the gen1-chromatography and chromatography-gradient methods (Figure 3a) showed that while both methods removed non-AAV proteins, the gen1-chromatography method gave more intense capsid protein staining at equal vector genome loading, consistent with the presence of excess capsid protein. The average levels of residual mammalian and plasmid (AmpR) DNA in the four lots purified by the gen1-chromatography method were 136.5 ± 50.6 pg/109 vg and 34.3 ± 9.0 pg/109 vg, respectively. In contrast, the three lots purified by the chromatography-gradient method demonstrated 4.7-fold reduced (P = 0.016) residual mammalian DNA (28.8 ± 8.9 pg/109 vg) and 2.8-fold reduced (P = 0.010) residual plasmid DNA (12.4 ± 1.3 pg/109 vg). To understand how this reduction occurred, an aliquot of vector purified by the gen1-chromatography method, containing 2.5 × 1013 vg and 11-fold excess empty capsids, was subjected to cesium chloride gradient ultracentrifugation, and fractions were then analyzed in detail. As shown in Figure 3b, vector genome–containing particles were detected in fractions 23–33, with the highest concentration in fraction 29 (density 1.38 g/cm3), which coincided with an AAV2 capsid peak detected by enzyme-linked immunosorbent assay (shaded). A larger AAV2 capsid peak detected in fractions 45–57 (densities from 1.30 to 1.33 g/cm3) corresponded to empty capsids. Residual HEK293 and plasmid (AmpR) DNA were primarily distributed in fractions ranging from the vector genome peak to the empty capsid peak (densities from 1.30 to 1.40 g/cm3). The residual plasmid and mammalian DNA were nuclease resistant. The known higher density of DNA (>1.5 g/cm3) when not associated with capsids or other proteins, the absence of detectable noncapsid protein impurities in the gradient starting material, and the observation that the residual DNA impurities detected in the gradient fractions were nuclease-resistant, together support that the DNA impurities were associated and contained within AAV capsids, i.e., encapsidated. Southern blot analysis using a 32P-labeled HEK293 genomic probe revealed that the maximum size of residual mammalian DNA was ~4,300 nt in fraction 29, coincident with the vector genome peak, and decreased progressively in lower density fractions (Figure 4b). Southern blot analysis using an AmpR probe showed that residual plasmid DNA was similarly distributed (Figure 4d). The higher molecular weight bands visible in fractions 29–35 in Figure 4d may correspond to double-stranded DNA resulting from re-annealing of complementary plasmid sequences during sample preparation. The size and density distribution, and nuclease resistance of the DNA impurities in the gradient fractions further support that they are single-stranded DNA impurities contained within AAV2 capsid particles.
Figure 3.
Density gradient fractionation of adeno-associated virus (AAV) particles. (a) AAV2-hFIX vectors [1 × 1010 vector genome (vg)] purified by the gen1-chromatography (gen1-Chr) or chromatography-gradient (chr-gr) methods were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10%) followed by silver staining. (b) AAV2-hFIX vector purified by the gen1-chromatography method was subjected to CsCl density gradient ultracentrifugation. Fractions (0.5 ml) collected from the bottom of the tube were analyzed for AAV capsid protein by enzyme-linked immunosorbent assay (shaded), vector genomes (squares), residual HEK 293 genomic DNA (crossouts), and residual total plasmid DNA (triangles) by quantitative PCR (Q-PCR). The density (diamonds) of individual fractions was determined by refractive index. Error bars show ±SD of triplicate Q-PCR determinations. hFIX, human factor IX.
Figure 4.
Characterization of DNA impurities. (a) Total DNA was isolated from fractions obtained following gradient ultracentrifugation of AAV2-hFIX vector purified by the gen1-chromatography method. Denatured samples were run on an agarose gel containing SYBR Gold. Single-stranded vector genomes migrate at a position approximately corresponding to the 1,000-bp double-stranded DNA marker. M13 is a single-stranded marker (7,250 nucleotides). Plasmid pladeno 6, lacking AmpR, and HEK 293 genomic DNA, are included as negative and positive control, respectively. Topo3 is a fragment amplified from the single-copy human topoisomerase III gene, included as a single-stranded 600-nucleotide marker. (b) Southern blot of the gel shown in a was probed with 32P-labeled HEK293 genomic DNA. The position corresponding to AAV2-hFIX vector genomes (4,297 nt) is indicated by ‘vg'. (c) As described for a, except that M13, topo3, and genomic DNA were not included, and plasmid pAAV-hFIX16 plasmid, containing AmpR, was digested with SbfI and either ClaI, PshAI, or EarI, and fragments pooled, denatured, and included to provide single-stranded DNA size markers for Southern blot analysis. (d) Southern blot of the gel shown in c probed with a 32P-labeled, 612-bp fragment derived from AmpR. AAV, adeno-associated virus; hFIX, human factor IX.
Discussion
In a recent hemophilia B clinical trial, successful hFIX gene expression was achieved following hepatic delivery of AAV2-hFIX, but eventually lost concomitant with a transient, self-limiting, asymptomatic transaminitis, and the appearance in the peripheral blood of a population of capsid-specific CD8+ T cells.5,6 In these reports it was proposed that processing of the preformed, input capsid protein was the source of AAV capsid epitopes presented by the vector-transduced hepatocytes. However, an alternative hypothesis proposed that expression of residual cap DNA, present as an impurity in the AAV2-hFIX vector used for the clinical study, was the source of capsid epitopes.8 In the current study, we assessed transcription of residual AAV2 cap following transduction of cultured human hepatocytes and in vivo in C576Bl/6 mice after administration of the actual AAV2-hFIX vector (Lot 1053) used in the clinical trial, as well as with a reference vector (Lot 003A). These vectors were generated and purified using methods that included specific steps to minimize DNA impurities. The average level of residual cap DNA in clinical Lot 1053 as measured by Q-PCR was 0.00018 cap copies per vector genome (0.018%). In cultured human hepatocyte cell line HHL5, cap expression was not detected following transduction at any dose (to 100,000 vg/cell). In the mouse studies, the dose of clinical vector Lot 1053 used was 5.2 × 1013 vg/kg, 26-fold greater than the highest dose administered in the clinical trial. This dose resulted in a supraphysiological hFIX concentration (32.5 µg/ml, corresponding to 650% normal value in human) in blood samples taken from mice at week 6 after administration, confirming efficient hFIX gene transfer and expression. The reference vector (Lot 003A), available at a higher stock concentration, was administered at a dose of 1.1 × 1014 vg/kg, 56-fold higher than the clinical high dose, and resulted in a blood concentration of 70.0 µg/ml. Quantitative RT-PCR using four independent cap amplicons distributed 5′ to 3′ within the cap sequence, and overlapping with sequences corresponding with known epitopes,5,6 failed to detect cap expression in any of the AAV2-hFIX vector-treated mice. The limit of sensitivity of the Q-RT-PCR assay was determined to be one transcript copy per 2,500 cells. Hence, our data documenting undetectable transcription of cap in cultured human hepatocytes at a high multiplicity of infection, and less than one cap transcript per 2,500 cells in mouse liver following administration of a 26-fold higher dose argue that expression of a cap DNA impurity in the clinical vector following its administration to human subjects was insufficient to account for the loss of hFIX-expressing cells. Instead these data support that input capsid protein was the major source of the capsid epitopes displayed on hFIX-expressing cells in the clinical study. A possible role for trace residual cap expression in the initiation of an immune response cannot be excluded. Initiation of T-cell responses to AAV2 capsid has been described by both classical and cross-presentation pathways.16,17 One conclusion that follows from these observations is that the magnitude of anti-AAV capsid immune-mediated transaminitis that may occur following hepatic administration of recombinant AAV in humans is likely to correlate with the amount of input capsid protein rather than the vector genome dose per se. Hence, AAV vectors prepared for liver-directed human gene transfer should be designed and prepared to reduce the amount of input capsid protein, including removal of empty capsids, to minimize the potential for deleterious immune responses.
We report here that transcription of cap in AAV2 vectors containing low amounts of cap impurities is undetectable. In contrast, others have reported transcription of cap impurities in recombinant AAV.11,15 In comparing our results to these previous reports, one important difference is the relative amounts of DNA impurities in vector preparations. Gao and colleagues reported cap impurities in AAV2, 7, and 8 vectors ranging from 0.4 to 1%,15 and Nony and colleagues reported rep-cap impurities in AAV2 vectors ranging up to 2%,11 values ~20- to 100-fold higher than the residual cap impurity level (0.018%) measured in the AAV2-hFIX vector used in the hemophilia B clinical trial. The report of Li and colleagues describing efficient CTL-mediated lysis of cells endogenously expressing cap following administration of AAV containing a cap transgene in mice17 further supports that the risk of cap expression at immunologically relevant levels depends on its concentration i.e., reducing levels of cap DNA impurities in vector preparations lowers this risk.
Encapsidated, nontransgene DNA impurities produced during the generation of AAV vectors in cell culture are particularly difficult to remove because they closely resemble bone fide vector particles. One type of impurity, helper virus–dependent, replication-competent AAV (rcAAV), is known to be generated at high levels in nonoptimized systems by homologous or nonhomologous recombination between vector ITRs and the rep-cap sequences provided in trans.20,21,22,23 Several strategies have been developed and generally implemented to reduce or eliminate rcAAV in vectors prepared for clinical studies.24 Clinical vector AAV2-hFIX Lot 1053 characterized in this report was prepared using a packaging plasmid in which the P5 promoter was displaced and the TATA box removed. Absence of detectable rcAAV in this vector was confirmed using a sensitive infectious center assay. The mechanism of generation of other heterogeneous encapsidated DNA impurities is not fully understood. Imperfect fidelity of DNA packaging mediated by the helicase function of Rep52/40 that translocates single-stranded DNA genomes into preformed AAV capsids,25 perhaps caused by promiscuous binding by Rep to DNA sequences other than the vector genome, may be one mechanism involved. Concerns about encapsidated DNA impurities, in addition to expression of sequences such as cap that may contribute to unwanted immune responses, include the tumorigenic potential of sequences derived from mammalian cell lines,26 and transfer of prokaryotic sequences such as antibiotic resistance genes.13 Hence, reducing DNA impurities in vectors prepared for clinical studies to the lowest achievable levels is further supported as an important objective.
In the current study, when AAV vectors were generated by transfection of HEK293 cells using vector plasmids with backbones smaller than the AAV packaging limit, nuclease-resistant plasmid DNA impurities ranged from 2.9 to 5.7% in the resulting vectors, consistent with previous reports by others.13 The major source of the encapsidated plasmid DNA was the backbone of ITR-containing vector plasmid. A mechanism for encapsidation of backbone sequences (reverse packaging) involves Rep68-mediated plasmid DNA replication from the terminal resolution site of an AAV plasmid-encoded ITR that proceeds through the ITR junction and into the plasmid backbone.27 We investigated the effect of using a vector plasmid that was modified with a stuffer sequence such that the backbone exceeded the packaging capacity of AAV2, and found that this strategy significantly reduced the levels of encapsidated plasmid DNA impurities (7.6-fold, P < 0.001). For vector serotypes with packaging capacities higher than AAV2,28 the size of the backbone required to prevent reverse packaging is predicted to be correspondingly larger. The importance of effective purification steps to further reduce encapsidated DNA impurities was demonstrated by high-resolution gradient analysis of AAV2 vectors prepared by a first-generation chromatography method that was known to co-purify empty capsids with vectors.19 This chromatography method resulted in AAV particles which, while apparently pure based on the absence of non-AAV proteins, was composed of empty capsids (~90%), vector (~10%), and heterogeneous, encapsidated DNA impurities (~1%), a composition likely representative of the AAV particle populations generated during cell culture. Inclusion of a purification step capable of achieving high-resolution separation of AAV particles, in the current study by a gradient ultracentrifugation purification step, removed the empty capsids, and also reduced encapsidated residual HEK293 DNA by 4.7-fold (P = 0.016) and residual plasmid DNA by 2.8-fold (P = 0.010). Scalable purification methods previously reported to separate AAV vectors from empty capsids29 may similarly reduce encapsidated DNA impurities. Removal of encapsidated DNA impurities of size similar to the actual vector genome remains a fundamental challenge, and may define an irreducible level of this type of impurity.
In conclusion, expression of cap DNA impurities in the AAV2-hFIX vector used in a hemophilia B clinical study, which was prepared by methods that minimized DNA impurities, was not detectable following transduction of cultured human hepatocytes nor after administration to mice using a sensitive Q-RT-PCR assay. These results implicate the preformed, input capsid protein component of the vector inoculum, rather than cap DNA impurities, as the major source of capsid-derived epitopes responsible for sensitizing vector-transduced heptocytes to the immune-mediated clearance observed in the clinical study. Our findings support that methods used to prepare recombinant AAV for liver-directed clinical studies should aim to reduce the amount of capsid protein in the inoculum, for example, by removing empty capsids, and also ensure that DNA impurities are reduced to lowest achievable levels. Transient immune modulation to suppress cytotoxic immune responses until the input vector capsid protein is degraded and cleared may be a useful strategy to achieve long-term gene expression from hepatocytes.
Materials and Methods
AAV generation and purification. Vector generation was performed by helper virus–free transfection of HEK293 cells using three plasmids30 with modifications.18 The clinical AAV2-hFIX vector, Lot 1053, was purified by double CsCl gradient ultracentrifugation18 (gradient-only method). Fourteen additional AAV vector lots were produced and analyzed in this study. Eight AAV2 and two AAV6 vector lots expressing human coagulation factor IX (AAV-hFIX) were generated using a vector plasmid containing a 6,980 bp backbone exceeding the ~4,700 nt packaging limit of AAV.31 One AAV2 and four AAV6 vector lots were generated using vector plasmids with backbones of 2,620 or 2,638 bp. Four lots of AAV2-hFIX (Lots DCL1, DCL2, DCL3, DCL4) were purified by a first-generation chromatography method (gen1-chromatography method) previously reported to result in co-purification of AAV2 empty capsids corresponding to 89–95% of total purified AAV2 particles.19 The vector prepared by the gen1-chromatography method was compared in this study because it provides an example of a recombinant AAV product prepared by a method that efficiently removes non-AAV capsid protein impurities, but co-purifies empty capsids. Three lots of AAV2-hFIX (Lots 06002, 003A, and NHP) and one lot of AAV2-canineFIX (Lot 0801) were purified by cation-exchange chromatography using Poros 50HS (GE Healthcare, Piscataway, NJ) combined with a single CsCl gradient ultracentrifugation step18 (chromatography-gradient method). Six lots of recombinant AAV6, including two of AAV2-hFIX (Lots 0802 and 0803), and four of AAV6 expressing other transgenes (Lots N0701, 0701, 0702, and 0703) were purified by a modification of chromatography-gradient method in which anion-exchange chromatography (Poros 50HQ) was substituted for the cation-exchange chromatography step. The chromatography-gradient method was compared in this study because it provides an example of recombinant AAV prepared by a method that efficiently removes non-AAV capsid protein impurities as well as empty capsids.
Quantification of vector genomes and residual DNA impurities by Q-PCR. For AAV-hFIX vector genomes quantification, Q-PCR was performed as described previously,19 using primer and probes specific for hIX sequence as follows: forward primer, 5′-CCACTGTGTTGAAACT GGTGTTAAA-3′; reverse primer, 5′-GCTCTGTATGTTCTGTCTCCTC AAT-3′; probe, 5′-6FAM-TTCACCTGCGACAACTG-NFQ-3′. Other transgenes were similarly quantified by Q-PCR using specific primers and probes. Residual plasmid DNA was quantified by Q-PCR using primers and probes specific for the ampicillin resistance gene (AmpR) common to all plasmids used for rAAV vector generation: forward primer, 5′-CGCG CCACATAGCAGAACTT-3′; reverse primer, 5′-CGCCCCGAAGAACG TTT-3′; probe, 5′-VIC-AAAAGTGCTCATCATTG-NFQ-3′. Residual capsid DNA was measured using the primers and probes described in the transcriptional profiling. Linearized plasmid DNA standards were used for all assays. Residual HEK293 host-cell DNA was quantified using TaqMan Gene Expression Assay for 18S rRNA (Applied Biosystems, Foster City, CA), with EcoRI-digested genomic DNA used as a standard.
Nuclease resistance of residual DNA. To assess nuclease resistance of DNA impurities, 100 µl of AAV2-hFIX, purified either by the gen1-chromatography or chromatography-gradient methods, were digested with 500 U of Benzonase (Calbiochem, San Diego, CA) in 500 µl reactions. Control reactions were spiked with 85 ng of purified genomic DNA, and additional controls were not treated with Benzonase. After 60 minutes, reactions were quenched by adding EDTA to 10 mmol/l, then samples were subject to DNA quantification by Q-PCR.
Transcriptional profiling of residual DNA impurities in HHL5 cells and C57Bl/6 mice. The human hepatocyte cell line HHL5 was a gift from Dr Arvind Patel.32 Cells were seeded at a density of 2 × 105 cells per well in 6-well plates. One day after seeding, AAV2-hFIX clinical Lot 1053 or reference Lot 003A was added at doses of 1,000, 10,000, or 100,000 vg/cell. Etoposide (Sigma-Aldrich, St. Louis, MO) was added to 10 µmol/l final concentration to enhance transduction. Cells were harvested 3 days later, and total RNA was purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). Purified total RNA was quantified by spectrophotometry and analyzed immediately by Q-RT-PCR, or stored at −80 °C before analysis.
C57Bl/6 mice were injected with rAAV vector via tail vein. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of The Children's Hospital of Philadelphia. Six mice were injected via the tail vein with the clinical AAV-hFIX vector Lot 1053 for a total dose of 1.3 × 1012 vg/mouse (5.2 × 1013 vg/kg), and nine additional mice were injected with the reference AAV-hFIX vector Lot 003A for a total dose of 2.7 × 1012 vg/mouse (1.1 × 1014 vg/kg). The injection volume was 900 µl for all AAV injections. The clinical vector was previously administered to two subjects during a clinical trial for hemophilia B.5 A control group of three mice was injected with AAV excipient [phosphate-buffered saline supplemented with 0.001% Pluronic F68 (BASF, Ludwigshafen, Germany)] alone as a negative control for the Q-RT-PCR. An additional group of three mice was injected with 1 × 1011 vg per mouse of Ad-cap vector, an adenovirus expressing AAV2 cap.33 At 2, 4 (Lot 003A only), and 6 weeks following vector administration, mice were euthanized and the entire liver (~1.2 g) was excised. One lobe (~0.4 g) was placed into RNA stabilizing reagent (Qiagen) and stored at 4 °C until use. The remainder of the liver was immediately frozen and stored at −80 °C. The isolated lobe was removed from the RNA stabilization reagent and homogenized. Total RNA was purified from the homogenized tissue using the RNeasy Mini Kit (Qiagen) and quantified by spectrophotometry. Purified RNA was analyzed immediately by Q-RT-PCR, or stored at −80 °C before analysis. The limit of sensitivity of the Q-RT-PCR assay was determined to be 20 copies per 500 ng RNA, determined by spiking known amounts of plasmid DNA containing AAV2 cap into 500 ng of RNA (corresponding to ~50,000 cells) purified from the liver of excipient-treated mice (performed in triplicate). Levels of hFIX protein in blood samples taken from mice immediately before euthanization were evaluated by enzyme-linked immunosorbent assay.34
Total RNA purified from HHL5 cells and C57Bl/6 mouse livers was subjected to Q-RT-PCR using a model 7500 Real Time PCR System, and the One-Step RT-PCR Mix (Applied Biosystems, Foster City, CA) (200 ng RNA per reaction, in quadruplicate). As a control, the TaqMan Gene Expression Assay for 18S rRNA (Applied Biosystems) was used. RNA input was normalized against 18S rRNA and expressed as fold-change compared to the excipient-treated control. Primer-probe sets used to quantify hFIX and residual AmpR mRNA were the same as those used for AAV-hFIX genome concentration and residual AmpR DNA quantification, respectively (described earlier). To assess residual AAV cap expression, four Q-PCR primer-probe sets were used (Figure 1a), three corresponding to previously described AAV2 cap epitopes5,6: TTSTRTWAL (VP1 241–249) (Amplicon 1); VPQYGYLTL (VP1 371–379) (Amplicon 2); SADNNNSEY (VP1 491–499) and YHLNGRDSL (VP1 507–515) (Amplicon 3), and one corresponding to the 3′-end of the cap gene (Amplicon 4). Amplicon 1: forward primer 5′-CATGGTGCCACAGTATGGATACC-3′; reverse primer, 5′-AAGAGCGTCCTACTGCCTGACT-3′; probe, 5′-6FAM-CACCCTGA ACAACG-NFQ-3′. Amplicon 2: forward primer 5′-TCGGGAAATTGG CATTGC-3′; reverse primer, 5′-GTGCTGGTGGTGATGACTCTGT-3′; probe, 5′-6FAM- TTCCACATGGATGGGC-NFQ-3′. Amplicon 3: forward primer 5′-CGCCAGCAGCGAGTATCAA-3′; reverse primer, 5′-TCCAG TCCACGAGTATTCACTGTT-3′; probe, 5′-6FAM-ACATCTGCGGATA ACAA-NFQ-3′. Amplicon 4: forward primer 5′-CACCTTCAGTGCGGC AAAGT-3′; reverse primer, 5′-CTGACCTGTCCCGTGGAGTAC-3′; probe, 5′-6FAM-TGCTTCCTTCATCACAC-NFQ-3′.
To assess residual adenovirus helper plasmid DNA expression, two primer-probe sets were used: Ad E2A: forward primer, 5′-TTGCTGAAAC CCACCATTTG-3′; reverse primer, 5′-TCGTGGACAGCGAGGAAGA-3′; probe, 5′-6FAM-CGCCACATCTTCTCT-NFQ-3′, and Ad E4: forward primer, 5′-TCGGCGCACTCCGTACA-3′; reverse primer, 5′-CGCGGGT CTCTGTCTCAAAA-3′; probe, 5′-6FAM-TAGGGATCGCCTACCTC-NFQ-3′.
Gradient fractionation and analysis of Lot DCL1. A portion of Lot DCL1, purified by the gen1-chromatography method, was supplemented with CsCl to a final density of 1.35 g/cm3, loaded into a single ultracentrifugation tube, and subjected to isopycnic ultracentrifugation. After centrifugation, 0.5-ml fractions were collected from the bottom of the tube. All fractions were measured by refractometry to determine density. Every second fraction was assessed for AAV2 capsid protein by enzyme-linked immunosorbent assay,19 and for hFIX vector genomes, residual plasmid DNA (AmpR), and HEK293 cell DNA by Q-PCR. For Southern blotting, DNA was isolated from selected fractions by organic extraction and ethanol precipitation. Samples were adjusted to 50 mmol/l NaCl, denatured, and then placed on ice until loading on a 1% agarose gel containing SYBR Gold (Molecular Probes, Eugene, OR). DNA was transferred to a nylon membrane (Schleicher and Schuell, Dassel, Germany) and then probed with 32P-labeled, EcoRI-digested, genomic DNA purified from HEK 293 cells, or a 32P-labeled, 612-bp PCR fragment from the ampicillin resistance gene (AmpR) which was amplified using primers 5′-TGCTATGTGGCGCGGTATTA-3′ and 5′-CAGCGATCTGTCTATTTCGTTCA-3′. Probes were labeled using a random priming kit (Stratagene, La Jolla, CA), and blots were visualized using a PhosphorImager.
Acknowledgments
We thank Christopher Connolly, Jesse Isaacs, Amy Parker, and Susannah Patarroyo-White for their expert technical assistance; Glenn Pierce, Michael Lochrie, and Jennifer Wellman McDonnell for their helpful discussion, and Gregory M Podsakoff for critical scientific review of the manuscript. This work was support by The Children's Hospital of Philadelphia, The Howard Hughes Medical Institute, and Avigen Inc.
REFERENCES
- Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Warrington KH., and , Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- Fiandaca M, Forsayeth J., and , Bankiewicz K. Current status of gene therapy trials for Parkinson's disease. Exp Neurol. 2008;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's Congenital Amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko J, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z., and , Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotypes of adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Recombinant DNA Advisory Committee, AAV Symposium Immune responses to adeno-associated virus (AAV) vectors 2007 < http://www4.od.nih.gov/oba/RAC/meeting.html >.
- Allen JM, Debeklak DJ, Reynolds TC., and , Miller AD. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J Virol. 1997;71:6816–6822. doi: 10.1128/jvi.71.9.6816-6822.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Qing K, Ponnazhagan S., and , Srivastava A. Adeno-associated virus type 2 DNA replication in vivo: mutation analyses of the D sequence in viral inverted terminal repeats. J Virol. 1997;71:3077–3082. doi: 10.1128/jvi.71.4.3077-3082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nony P, Chadeuf G, Tessier J, Moullier P., and , Salvetti A. Evidence for packaging of rep-cap sequences into adeno-associated virus (AAV) type 2 capsids in the absence of inverted terminal repeats: a model for generation of rep-positive AAV particles. J Virol. 2003;77:776–781. doi: 10.1128/JVI.77.1.776-781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Wright JF, Qu G, Patarroyo-White S, Parker A., and , Sommer JM. Packaging of host cell and plasmid DNA into recombinant adeno-associated virus particles produced by triple transfection. Mol Ther. 2003;7:S348. [Google Scholar]
- Chadeuf G, Ciron C, Moullier P., and , Salvetti A. Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol Ther. 2005;12:744–753. doi: 10.1016/j.ymthe.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Report from the CHMP Gene Therapy Expert Group Meeting European Medicines Agency 2005 2005. EMEA/CHMP/183989/2004 . < www.emea.europa.eu/pdfs/human/genetherapy/18398904en.pdf >.
- Gao G, Wang Q, Wang L, Vandenberghe L, Lock M, Grant R, et al. Inadvertent gene transfer of co-packaged Rep and Cap sequences during the production of AAV vector and its potential impact on vector performance. Mol Ther. 2008;16:S105. [Google Scholar]
- Wang L, Figueredo J, Calcedo R, Lin J., and , Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T-cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18:185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- Li C, Hirch M, Asokan A, Zeithaml B, Ma H, Kafri T, et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol. 2007;81:7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF, Le T, Prado J, Bahr-Davidson J, Smith PH, Zhen Z, et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol Ther. 2005;12:171–178. doi: 10.1016/j.ymthe.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Sommer JM, Smith PH, Parthasarathy S, Isaacs J, Vijay S, Kieran J, et al. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol Ther. 2003;7:122–128. doi: 10.1016/s1525-0016(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Shang LS., and , Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;61:3096–3101. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, et al. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther. 1998;9:695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]
- Wang XS, Khuntirat B, Qing K, Ponnazhagan S, Kube DM, Zhou S, et al. Characterization of wild-type adeno-associated virus type 2-like particles generated during recombinant viral vector production and strategies for their elimination. J Virol. 1998;72:5472–5480. doi: 10.1128/jvi.72.7.5472-5480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15:840–848. doi: 10.1038/gt.2008.65. [DOI] [PubMed] [Google Scholar]
- King JA, Dubielzig R, Grimm D., and , Kleinschmidt JA. DNA helicase-mediated packaging of adeno-associated virus type 2 genomes into preformed capsids. EMBO J. 2001;20:3282–3291. doi: 10.1093/emboj/20.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for Proprietary Medicinal Products Position statement on the use of tumorigenic cells of human origin for the production of biological and biotechnological medicinal products 2001. The European Agency for the Evaluation of Medicinal Products
- Ward P, Urcelay E, Kotin R, Safer B., and , Berns KI. Adeno-associated virus DNA replication in vitro: activation by a maltose binding protein/Re68 fusion protein. J Virol. 1994;68:6029–6037. doi: 10.1128/jvi.68.9.6029-6037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G, Bahr-Davidson J, Prado J, Tai A, Cataniag F, McDonnell J, et al. Separation of adeno-associated virus type 2 empty particles from genome-containing vector by anion-exchange column chromatography. J Virol Methods. 2007;140:183–192. doi: 10.1016/j.jviromet.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Berns KI., and , Parrish CR.Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, et al. Parvoviridae Fields Virology 2007Lippincott Williams & Wilkins: Philadelphia, PA; 2437–2477.2nd edn [Google Scholar]
- Clayton RF, Rinaldi A, Kandyba EE, Edward M, Willberg C, Klenerman P, et al. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int. 2005;25:389–402. doi: 10.1111/j.1478-3231.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Mingozzi F, Hui DJ, Chen H, Colosi P, Ertl HC, et al. Identification of mouse AAV capsid-specific CD8+ T-cell epitopes. Mol Ther. 2005;12:1023–1033. doi: 10.1016/j.ymthe.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]