Abstract
Oncolytic viruses delivered directly into the circulation face many hazards that impede their localization to, and infection of, metastatic tumors. Such barriers to systemic delivery could be overcome if couriers, which confer both protection, and tumor localization, to their viral cargoes, could be found. Several preclincal studies have shown that viruses can be loaded into, or onto, different types of cells without losing the biological activity of either virus or cell carrier. Importantly, such loading can significantly protect the viruses from immune-mediated virus-neutralizing activities, including antiviral antibody. Moreover, an impressive portfolio of cellular vehicles, which have some degree of tropism for tumor cells themselves, or for the biological properties associated with the tumor stroma, is already available. Therefore, it will soon be possible to initiate clinical protocols to test the hypopthesis that cell-mediated delivery can permit efficient shipping of oncolytic viruses from the loading bay (the production laboratory) directly to the tumor in immune-competent patients with metastatic disease.
The Need for a New Player in Oncolytic Virotherapy
In Aristophanes' play The Birds, Pisthetairos (Trustyfriend) and Euelpides (Goodhope) become fed up with life on Earth and enlist the help of the birds to build a perfect city in the clouds, which they call Cloud Cuckoo Land.1 Some years ago at a lab retreat, we were discussing strategies for our first clinical trial for delivery of oncolytic vectors to patients with systemic metastases. The best of these amounted to nothing much more sophisticated than simple intravenous injection of vector stocks into the bloodstream. Two Clinical Fellows at that meeting, although keen to initiate the trial, made it brutally clear that, unless we could come up with something better for the longer term, we too would be better off consulting the birds.
In retrospect, this viewpoint has turned out to be overly pessimistic and several trials of intravenous delivery of oncolytic viruses have now been successfully completed with some surprisingly encouraging indicators of success2 and reviewed by Liu and Kirn.3 These trials support data from various preclinical models in which intravenously administered virus has successfully treated tumors.4 In addition, it is now becoming clear that certain viruses, such as vaccinia,5 have evolved efficient mechanisms for spread in the bloodstream and may be more suited for systemic delivery protocols than others.
The development of replication-competent oncolytic viruses was driven by the hypothesis that successful delivery of even a small dose of virus to the tumor would be sufficient to seed a spreading infection of the entire tumor, thereby dispensing with the need for highly efficient delivery systems. Unfortunately, in many cases, even direct intratumoral injection of oncolytic agents struggle to clear established tumors, due to a variety of immune, biochemical, and physical barriers to the spread of virus and progressive oncolysis.6,7 It remains to be seen whether it will be consistently possible to inject sufficient quantities of virus into the bloodstream for enough infectious particles to survive, find tumors, extravasate, and infect large numbers of tumor cells in patients in whom a largely intact immune system still persists, the function of which is specifically to recognize, remove, and neutralize invading pathogens.
On a paradoxically more positive note, viruses and bacteria do gain access to the circulation and can, under certain circumstances, spread systemically. Therefore, if we have to treat anything more than simple local disease with oncolytic viruses, we need to understand how our designer viruses can mimic the infectious strategies of more malevolent, naturally occurring infectious agents.
The problem we face is simple. How is it possible to dispatch a therapeutic oncolytic virus from the tip of a needle into the heart of a tumor, with an intact immune system in between? The solutions are similar to the logistical issues faced by FedEx, United Parcel Service, and all other shipping companies. Essentially, it comes down to packaging, protection, distribution, and delivery. When a virus accesses the bloodstream, it faces a host of hostile opponents. These include circulating and static scavenger cells in the blood or reticuloendothelial organs. In addition, a high concentration of exquisitely evolved immune molecules (complement, antibodies) are on constant patrol ready to neutralize pathogens before they can infect cells in which they might cause real damage. Therefore, pathogens that successfully evade this extensive surveillance network must do so using subtle mechanisms, which we would do well to understand and emulate. In this way, we may be able to establish our own distribution system for delivery of oncolytic viruses to disseminated metastases.
Cell Carriers: A Natural Paradigm
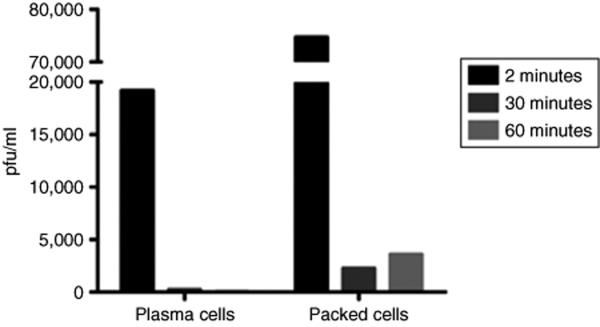
Viruses that enter the circulation do not exist as free-floating particles for longer than a few minutes at most.4,8 Many particles are rapidly complexed by complement, antibodies, or phagocytosing immune scavenger cells. However, efficient immune clearance/neutralization accounts for only one component of the short half-life of most viruses in the circulation. A second component is provided by rapid sequestration without, necessarily, concomitant destruction. Thus, within 2 minutes of intravenous injection of the vesicular stomatitis virus (VSV), an oncolytic virus, into an immune-competent mouse, the majority of detectable virus is associated with cells, as opposed to free in the serum (Figure 1). Within 30 minutes of injection, the only virus that can be detected is associated with cells (Figure 1). A major component of that sequestration is likely to be by cells well equipped to clear viral particles terminally from the circulation. However, a subset is also likely to be opportunistic adhesion to, or infection of, cells that just happened to be in the wrong place at the wrong time (at least from the host's point of view). Importantly, this rapid opportunistic sequestration (packaging) of virus leads directly to its protection from the cells and molecules, which constantly patrol the circulation ready to pounce. Those newly hijacked cells will subsequently go about their normal business and speed the stowaway virus to cells/tissues/organs far removed from the initial site of access of the virus into the circulation. If the virus is simply hanging on to the carrier cell surface,9,10,11 it may then become available to infect cells at distant sites upon simple dissociation. For example, human immunodeficiency virus (HIV) binds to passing dendritic cells (DCs) whose natural destination are the lymph nodes.12,13,14,15,16 Once there, HIV particles can be passed on to CD4+ T cells—which conveniently enough for the virus, happen to be its target cell population of choice for infection.12,14 Alternatively, the virus might directly infect a passing cell and undergo a full replicative cycle within it. In this case, timing of viral replication, combined with distribution of the carrier, will determine where new virus is produced. The virus will then be presented with a whole new set of opportunities for infection of susceptible target cells, well removed from the initial site of access to a hostile circulation.
Figure 1.
Intravenously injected virus does not exist as cell-free particles. We injected 5 × 108 pfu of VSV intravenously into C57Bl/6 immunocompetent mice. At 2, 30, and 60 minutes after injection, we sampled either cell-free plasma or the packed cell fraction from harvested blood for infectious virus. Within 2 minutes, most of the detectable virus was in the packed cell fraction. By 30 minutes postinjection, only virus associated with cells was detectable. These data suggest that viral particles associate rapidly with cells in the circulation which subsequently either neutralize the virus or, in some cases, act as carriers for distribution around the body.
Cell carriers for cancer—a new virus shipping company
Packaging, protection, distribution, and delivery of viruses by endogenous circulating cells play a significant role in the patterns of natural infections in the presence of a hostile neutralizing environment. By mimicking, and refining, this natural precedent, a new concept for delivery of oncolytic viruses has been developed to target therapeutic vectors to metastatic disease throughout the body.9,17,18,19,20,21,22,23,24 This approach offers the potential to package the oncolytic virus, protect it from would-be scavengers, distribute it through the body, and deliver it precisely to the tumor's door. The virus, in turn, offers the potential, if correctly delivered, to destroy the targeted tumor.9,18,19,20,21,22,23,24
Packaging/protection
In order to protect the cargo during shipping, it must first be appropriately packaged. Viruses can either be loaded onto or into cell carriers. Either way, the kinetics with which the virus interacts with the carrier cell must be compatible with the kinetics of trafficking of the carrier in vivo to the tumor. Just as it is critical that the package does not leak, or burst open, before it reaches its destination, it is equally important that it can discharge its contents at the correct time and place when finally delivered.
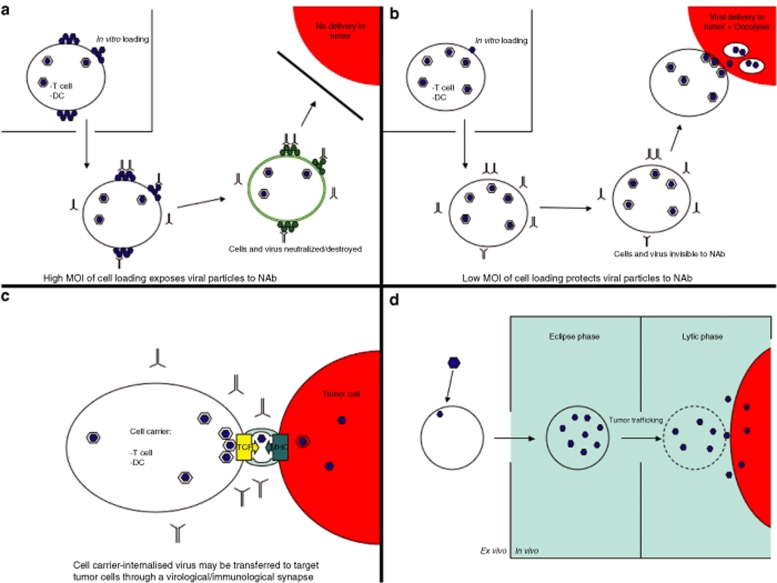
On top or underneath. When we loaded T cells with retroviral particles in vitro, we observed minimal levels of infection. However, large numbers of particles adhered to the surface of the T cells as seen by electron microscopy.23 This was consistent with reports that retroviruses adhere to the surface of cells, even in the absence of viable receptors for the envelope.8,25 We exploited this phenomenon to deliver what we believed to be surface-adhered retroviral vectors to tumors through the agency of tumor antigen–specific T cells9,11 (Figure 2a). T-cell accumulation in tumors peaked within 48–72 hours of adoptive T-cell transfer, which corresponded with our ability to detect transfer of adhered virus to tumor cells in culture up to 96 hours following loading. That these in vitro and in vivo kinetics were sufficiently compatible was confirmed by the fact that T cells loaded with retroviral vectors expressing either the HSVtk (herpes simplex virus thymidine kinase) suicide gene, interleukin-12, or chemokine ligand-21 could effect significantly better therapy than the T cells, or intravenous virus, alone.9,11,26
Figure 2.
Mechanisms by which cell carriers can package and protect oncolytic viruses. (a) High MOI, antibody sensitive, viral loading. If viral particles are loaded at a relatively high density onto the cell carrier, many particles are likely to adhere to the cell surface, in addition to any particles that are internalized as part of the infection process. When these in vitro loaded cells are injected in vivo, the surface exposed virus is visible, and highly sensitive, to neutralizing factors in the circulation, including NAbs with specificity against the loaded virus. Therefore, in recipients who are preimmune to the virus, not even heavily loaded cell carriers can protect the packaged viruses. (b) Low MOI, antibody-insensitive, viral loading. When virus is loaded onto cell carriers at a low MOI, most of the virus is internalized, either as part of the productive infection pathway or into cell compartments which allow for recycling of virus for further infection (see text for details). When injected in vivo, the absence of virus on the cell surface prevents the neutralization of either virus or carrier cells and the carriers can deliver virus effectively to the tumor site. (c) Cell-to-cell transmission through the virological synapse. Several lines of experimental evidence (see text) suggest that some viruses can be internalized into cells and, without being degraded, can be recycled for infectious transmission to recipient cells. This transfer is mediated through a virological synapse and protects the loaded virus from neutralization. Our own studies suggest that, in T cells, the virological synapse may co-opt the immunological synapse between the TCR and the target antigen-bearing MHC complex on the tumor cell. This may be a method by which virus can pass between immune and tumor cells in a protected environment, which is not neutralized by antiviral neutralizing antibody and which may allow virus targeting to tumors with the specificity that is inherent in T cell–target cell recognition. (d) Intracellular replication both amplifies and protects input virus. If the cell carrier is highly permissive to the oncolytic virus, low MOI of infection ex vivo can lead to a period in which the virus is fully internalized and undergoes its replicative cycle. During this eclipse phase, in vivo injection will allow the cells to circulate without being highly visible to NAb. Once the cells have trafficked to the tumor site, the lytic/release phase of the viral life cycle provides a plentiful supply of virus for infection of tumor cells at the local site of delivery. DC, dendritic cell; MHC, major histocompatibility complex; MOI, multiplicity of infection; NAb, neutralizing antibody; TCR, T-cell receptor.
This “On the Top” method of packaging viral particles on the surface of cell carriers (Figure 2a) is reminiscent of how HIV is captured in vivo by DC using C-type lectin-related receptors on the cell surface. The DCs do not become infected, migrate to the lymph nodes and then hand the virus onto CD4+ T cells.12,14
We subsequently observed that transfer of preloaded retroviral particles can proceed through intracellular perforin-containing, cytotoxic granules released from the T cell upon its activation by tumor antigen at the tumor.10 These findings suggested that (i) viral delivery from T cells may occur via routes additional to the surface loading/dissociation concept; and (ii) retroviral particles may exploit a functional immunological synapse for cell–cell transmission.10
We also extended T-cell delivery to oncolytic viruses including VSV19,22,23 and reovirus.18 Once again, we observed very low productive infection of primary murine T cells (either antigen specific or antigen nonspecific).19,22,23 However, T cells preincubated with virus readily transferred infectious particles to cocultured tumor cells in vitro for several days after loading. Virus-loaded T cells were also effective in vivo at reducing established tumor burdens. Based on these and other20 data, we suggested that adhesion of viruses to the surface of immune cells can both package an oncolytic payload and protect it from antiviral neutralizing factors in the circulation (see below).
However, several diverse observations have made us rethink this “packaging by surface adhesion” model. For example, packaging VSV on antigen-specific T cells effectively protected these viruses from neutralization in vivo in mice with high levels of neutralizing antibody (NAb) against the virus19,22—but by a complex mechanism. Whereas T cells loaded at high multiplicity of infection (MOI) of VSV-reduced tumor burden in mice with no anti-VSV NAb, efficacy was lost in mice preimmunized against the virus.19,22 In contrast, antitumor efficacy was retained in mice with high levels of anti-VSV NAb when the T cells were loaded at low MOI of VSV. From in vitro experiments, our early results suggest that there exists a pool of VSV which is (i) internalized into the T cells, (ii) neither infects nor is degraded by the T cell, and (iii) remains available for recycling to the cell surface and subsequent release for infection of tumor cells. This model (Figure 2b) is consistent with our findings that T cells activated by recognition of their cognate antigen on a target tumor cell can deliver retroviral particles via cytotoxic granules derived from an intracellular compartment.10 It may also help to explain why low MOI loading of virus onto T cells permits escape from antibody neutralization. Thus, these loading conditions may allow access of the virus to the internal, recyclable compartment at relatively high frequency. At higher MOI, this pathway may become saturated leaving a high concentration of virus on the surface exposed, and sensitive, to NAb. Finally, other groups have demonstrated that viruses derived from an intracellular pool can readily pass between cells through cell–cell contacts, by mechanisms similar to exploitation of the immunological synapse that we observed with T cell–mediated transfer of murine leukemia virus particles.10 Thus, a so-called virological synapse has been described, which facilitates the spread of both HIV27,28,29 and human T-lymphotropic virus type-1 (refs. 30,31) via specialized sites of immune cell-to-cell contact. The virological synapse allows virus transmission without the need for cell-free virus to be released, thereby protecting the cell associated virions from the dangers of NAb, or other serum-neutralizing agents32,33 (Figure 2c). Our observations of antibody protected-, T cell–mediated transmission of retro-/oncolytic vectors may reflect a subset of this virological synapse. Moreover, they may also reveal a coincidence between the virological and immunological synapse, which is formed between an antigen-specific T cell and its target cell.10,19,23
Pan et al. have also reported a population of intracellularly captured HIV-derived particles, which are protected from neutralization by antibody or protease.34 However, distinct from the intracellular route of virus transmission represented by the virological synapse, these infectious genomes are transferred between cells by release in exosome-like structures associated with tetraspanin proteins and multivesicular bodies.35
In summary, cells can package infectious viral particles on their surface by both nonspecific25 and specific interactions9,10 (Figure 2a). They can also direct viral particles/genomes into intracellular compartments separate from conventional trafficking pathways. Fully infectious genomes can subsequently be passed on to neighboring cells, protected from NAb, protease degradation, or complement inactivation (Figure 2b). They are either passed through cell-to-cell contact mechanisms involving virological and/or immunological synapses, or through release of exosome-like structures associated with multivesicular bodies (Figure 2c). As both T cells and DCs can package/protect several types of viruses (including retrovirus,9,10,11 Newcastle disease virus,36 VSV and reovirus18,19,22,23), these newly characterized mechanisms of cell-to-cell transmission are probably quite extensive. If true, they offer great opportunities to use cell carriers to deliver oncolytic viruses to tumors, both in the context of highly neutralizing immune environments and without the need for replication in the cell carrier. The use of specific immune cells also offers the potential that the specificity of cell-based delivery of oncolytic viruses could be determined at the level of very highly specific molecular interactions—such as recognition of T-cell receptors with major histocompatibility complex–tumor antigen complexes (for T cell–mediated delivery) or other components of the immunological synapse.
Inside. Loading virus onto the carrier cell surface, or sequestering it into cellular compartments for later recycling, excludes the possibility of viral amplification between packaging and delivery. In fact, using this approach, the amount of virus delivered to the tumor is likely to be significantly less than is loaded onto the cells in vitro. Although this loss of virus should, in theory, be compensated by the use of replicating oncolytic viruses, it is still desirable to get as much virus to the tumor as possible.
Alternatively, cells can be used as both carriers and amplifiers (Figure 2d). Hence, if a cell carrier supports active replication of the virus payload, it should be possible to deliver significantly more virus to the tumor than was loaded in vitro. Once again, it is critical to ensure compatibility between the kinetics of cell trafficking and viral replication within the carrier cells. If the carrier and the virus are not suitably matched in these respects, the package may burst open, or start to shed its contents, before it reaches its destination. In addition, at least the early stages of viral replication must be “immune invisible” so that the carrier cells do not reveal themselves to immune surveillance mechanisms prematurely. Equally, the consequences of viral replication must not materially affect the natural properties of the cell carrier, upon which tumor trafficking is predicated.
Using cells to carry and amplify oncolytic viruses is attractive because it is rare for any shipping company to deliver more than was actually packed. However, superficially at least, this strategy seems to present an intrinsic contradiction. How is it possible to use normal cells to amplify an oncolytic virus, which supposedly has replicative preference for tumor cells? One clever way to circumvent this apparent ”Have the Cake and Eat It Too” problem is simply to use transformed cells as the carriers, as cancer cells should produce maximal yields of oncolytic virus during the eclipse phase between in vitro infection and in vivo tumor trafficking21,37 (Figure 2d). Indeed, carcinoma cells infected with VSV effectively delivered virus to lung metastases in immune-competent mice and protected the virus from neutralization. There are also theoretical advantages to use transformed cells in the distribution and delivery departments—no one knows where tumor cells go better than the tumor cells themselves. However, health and safety considerations must also be addressed when contemplating the administration of tumor cells to patients, as discussed below.
It has also been possible to select partnerships between normal cell carriers and oncolytic viruses, which combine both viral packaging and amplification. For example, cytokine-induced killer (CIK) cells have been used to carry tumor-selective vaccinia viruses and to incorporate a significant viral burst size between initial infection and tumor accumulation.38,39,40 Viral replication had minimal effects upon the intratumoral localization of CIK cells, along with their burgeoning viral payloads, although just how well CIK cells protect vaccinia viruses in a preimmune environment is still somewhat unclear. In a variation of the intracellular replication model, Ong et al. showed that primary human T cells can be infected by replication-competent measles virus and can pass virus to tumor cells predominantly through cell-to-cell fusion.20 The lymphocyte carriers protected virus from NAb but only in a dose dependent fashion—possibly because of rapid viral antigen expression on the T cells during transit in vivo.
Overall, a strategy in which more viruses are produced at the tumor from the cell carrier than was initially loaded has a certain “something for nothing” appeal to it (Figure 2d). There are clearly examples where the cell can be matched to the virus payload without catastrophic loss of carrier viability or trafficking. However, premature virus expression/replication might lead to either direct (cell shutdown or viral lysis), or indirect (immune attack and clearance) destruction of the cell carrier. In addition, viral replication within almost any cell is likely to affect its normal physiology—such as circulating, trafficking, and effector functions. Therefore, when considering the carriage of toxic cargoes such as replication-competent oncolytic viruses, careful packaging will be mandatory.
Distribution/delivery
When a pathogen penetrates into the circulation and survives by hitching a ride on a passing cell, it becomes hostage to the circulatory fate of that cell. So, whatever protection a cell carrier confers upon a prepackaged oncolytic virus, it will count for nothing if the cell never gets the virus close enough to deliver its “kiss of death” to the tumor cells.
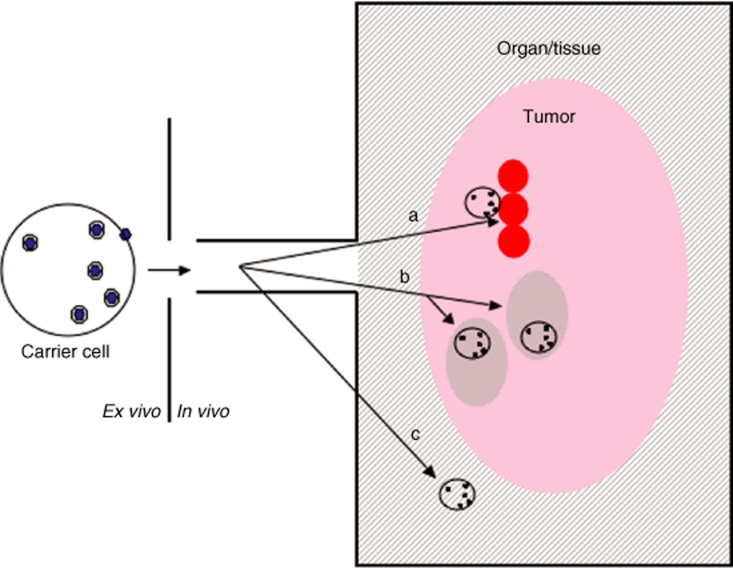
The perfect cell carrier would recognize a very highly specific “postcode” associated exclusively with tumor cells and with no other cell type, or location, in the body. The cell would not get sequestered by any other organ/tissue, thereby preventing both wasteful loss of vector and toxicity caused by release of replicating vector at nontumor sites. Unfortunately, as yet, neither the perfect cell carrier nor the perfect tumor-specific postcode has been identified. However, many different cell types have been shown to have efficacy as carriers of viral vectors to tumors9,18,20,21,22,23,39,41 target postcodes associated with (i) tumor cells directly; (ii) biological properties associated with tumor, as opposed to most normal tissues/organs; or (iii) the anatomical location of the tumor, as opposed to the tumor itself (Figure 3).
Figure 3.
Levels of tumor targeting by cell carriers. (a) Direct targeting to tumor cells. If the cell carrier recognizes a determinant, which is expressed directly on/by the tumor cell, it can deliver the loaded virus directly to the doorstep of the tumor cells. An example of this is the recognition of tumor-associated antigens by antigen-specific T cells. In such cases, the cell carrier (T cells) must traverse endothelial cell barriers at the tumor site, penetrate through any tumor-associated stroma and access the tumor cell directly. (b) Targeting tumor-associated properties. Several cell types do not recognize tumor cells per se but rather the microenvironment created by them. Examples include the homing of macrophages to tumor-associated hypoxia, trafficking of mesenchymal stem cells to areas of tumor-associated cytokine production, or the recruitment of endothelial cells to tumor-associated angiogenesis. In these cases, the carriers will deposit the viruses in “neighborhoods” within the tumor, with the hope that they will be able to diffuse the short distances required for productive infection of the tumor cells themselves. (c) Targeting tumor locations. Certain cell carriers may have no tumor-associated specificity at all. Instead, they may demonstrate intrinsic trafficking to specific organs or tissues. If tumor is known to reside within these specific locations, the cell carriers can be used to deliver their payloads into the territories within which tumor will be found. An example is the use of normal, nonspecific T cells or other immune cells, which naturally home to lymphoid organs. We have shown that targeting oncolytic viruses to lymph nodes using peripheral blood lymphocytes, or dendritic cells, preloaded with oncolytic viruses can effectively purge these organs of tumor cells (see text).
Cell carriers targeting tumor cells. We have used antigen-specific T cells for delivery of viral vectors to tumors expressing the cognate antigen for the T cells.9,11,19,23 At least in theory, specificity of virus delivery is, therefore, mandated by presence of the target antigen, and the very high levels of specificity of the T-cell receptor/major histocompatibility complex antigen interaction. High levels of accumulation of antigen-specific T cells into metastatic tumors have been reported following adoptive transfer in clinical trials from highly expert centers, supporting development of this concept for clinical translation.42,43,44,45,46 The use of a cell carrier with direct antitumor effector function itself is also attractive.9,10,19,23,26,39,47,48 This might allow at least additive, if not synergistic, therapeutic effects to be achieved by combining T-cell therapy with the benefits of oncolytic therapy.19,23 Even in those instances where the adoptively transferred T cells lose effector function in vivo,46 combining adoptive T-cell therapy with carriage of oncolytic viruses offers an excellent opportunity to improve both modalities.6,19 However, raising T-cell populations against highly tumor-specific antigens from patients is still arduous, expensive, and problematic.49,50 This currently restricts the possibilities for clinical translation to a few specialized centers with expertise in the preparation of these T-cell populations from patients.49
A further reality of adoptive T-cell transfer therapy for cancer is that genuinely tumor-specific antigens are rare. Hence, T cells that are generally used also recognize antigens expressed on at least a subset of normal cells. This can lead to autoimmune toxicities as a result of recognition/killing by the T cells43,51,52—even in the absence of carriage of viruses by the T cells. In addition, even transgenic T cells, which are highly specific for antigens expressed exclusively on experimental tumors, localize to organs such as the lung, spleen, and liver (where no antigen is expressed) as well as the tumor.45 Further release of a virus at such sites might exacerbate toxicities, although the supposed oncolytic specificity of the virus would be an important additional safety feature here.
Other cells, which target a tumor cell–specific postcode, have also been used as carriers of oncolytic virus. Most notable amongst these are the CIK cells used to deliver vaccinia viruses to tumors.39 CIK cells have the theoretical advantage over antigen-specific T cells in that they are easier to prepare from patients.53,54 These cells do not need to be isolated from tumors, or expanded in vitro with antigen-specific stimuli.54,55 Conversely, the exact nature of the tumor-specific postcode(s) that they recognize is still not entirely clear55 and it is likely that they are less genuinely specific for tumor than a pure, tumor antigen–specific T cell.
Cell carriers targeting tumor-associated properties. Antigen-specific T cells or CIK cells will bring the virus directly to the doorstep of the tumor cells. In contrast, other cell carriers, which target the neighborhood of the tumor, rather than the cells themselves, can be used (Figure 3b). In this case, specificity of targeting is to an environmental property that the tumor creates and which is significantly different from that which exists in most normal tissues. For example, tumors are associated with several different disruptions of normal tissue homeostasis. They are highly angiogenic, hypoxic, metabolically active, often heavily immunosuppressive, or vascularly chaotic. These properties are usually associated with the tumor stroma. As such, they all provide potential tumor-selective, if not specific, postcodes for cell-based targeting (Figure 3b).
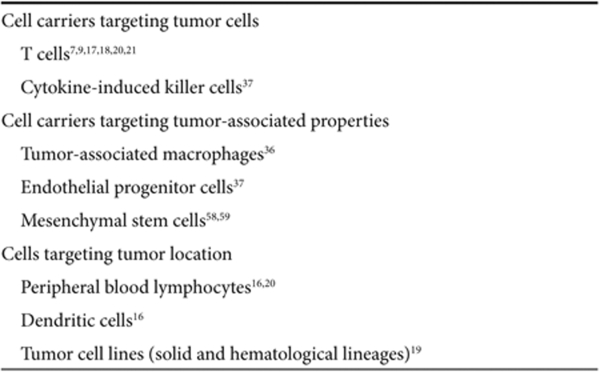
Detailed characterization of the tumor stroma56 provides a rich source of candidate cells, which could, in theory, be pressed into action as viral carriers (Table 1). For example, tumors are classically associated with high levels of hypoxia to which tumor-associated macrophages are recruited in large numbers.57,58 As a result, macrophages have been used in adoptive transfer protocols to target tumor with some success.41 It has also been possible to exploit the highly angiogenic nature of tumor growth as a homing beacon for adoptively transferred endothelial progenitor cells.59,60 Similarly, mesenchymal stem cells (MSCs) have a propensity for accumulation within tumor stroma based on the tumor-associated expression of a variety of inflammatory chemokines. This tumor tropism has been exploited using MSCs transduced with therapeutic vectors, which have proved effective following adoptive transfer into tumor-bearing mice.61,62
Table 1.
Examples of carriers for systemic deliver of viral vectors
Cell carriers, which target the tumor stroma, turn what are usually considered as the therapy-thwarting strengths of the tumor against itself (angiogenesis, hypoxia). Conversely, there may be a reluctance to “feed the fire” by adoptively transferring cells into patients that might contribute to the tumorigenic phenotype.63,64,65,66 However, sending cells to penetrate deep into the tumor stroma may have real benefits. By seeding the tumor stroma with genetically modified cells of our choice, it may be possible to turn tumor collaborators into “sleeper” units of undercover, subversive tumor infiltrators. Adoptively transferred endothelial progenitor cells, tumor-associated macrophages or MSC may even be allowed to infiltrate the tumor stroma and to proliferate to some extent with time. These “first wave” cells may not carry directly cytotoxic viruses or genes but could be stably transduced to express cytokine/chemokine genes. Expression of these proteins could be the “flares” that illuminate the location of the tumor to secondary waves of adoptively transferred cells such as CIK or T cells loaded with the full artillery of oncolytic viruses.
Overall, cells that target the tumor-associated stroma by sensing disturbances in normal tissue homeostasis form the majority of the carrier cells used so far (Table 1). A potential problem with this class of carrier remains that the virus is not necessarily delivered right to the tumor cells. This may then necessitate virus release at some distance from tumor cells, which will form the substrate for replication. Nonetheless, depositing the virus into the neighborhood of the target cells using stromal targeting cells will still significantly shorten the odds in favor of achieving productive tumor infection.
Cell carriers targeting tumor location—regional targeting. An even less specific approach uses cells that traffic to specific tissues/organs or sites where tumor is known to be present (Table 1; Figure 3c). In such cases, the package is delivered to an area rather than the neighborhood or the doorstep. We used this approach with naive peripheral blood lymphocytes (PBLs) and DCs as the cell carriers, which naturally circulate and pass through lymphoid organs such as the lymph nodes and spleen. Thus, we demonstrated that both PBLs18,22 and mature DCs18 could deliver oncolytic viruses (VSV or reovirus) to lymphoid organs in which micrometastatic disease was known to be present. Naive T cells loaded with VSV partially purged both lymph nodes and spleens of B16 metastases relatively soon after adoptive T-cell transfer (2–3 days). This therapeutic purging resulted from release of the virus in the lymphoid organs and direct infection of metastatic foci. An added bonus of this approach was that oncolysis of tumor cells in the lymph node turns out to be significantly more effective at priming tumor antigen–specific T-cell responses than is VSV-mediated oncolysis of subcutaneous tumors in the periphery.22 As a result, the partial purging of metastases seen at day 3 after T-cell transfer was converted into a much more comprehensive tumor clearance at later time points (10 days post-transfer of virus-loaded T cells). This slower developing purging was mediated by tumor-specific T cells.22 Similar effects were obtained with DC-mediated delivery of reovirus to lymph nodes and spleens harboring metastatic disease.18 Interestingly, a direct comparison between PBLs and DCs showed little difference in their ability to purge disease through priming of T-cell responses.18 However, mature DCs are able to protect loaded reovirus much more effectively from anti-reovirus NAb than the PBLs. This allowed DCs to be loaded at higher MOI of infection in vitro and still retain tumor-purging capabilities even in mice preimmune to reovirus.18
The attraction of this “regional” cell-targeting approach (Figure 3c) is that cells with highly specific phenotypes do not need to be isolated from the patient. Simple and widely used protocols for the preparation of patient PBLs, or DCs, already exist. Moreover, these cells traffic in patients with reproducible and predictable distribution patterns. This contrasts with predicting the trafficking patterns of cells (be they tumor-associated macrophages, endothelial progenitor cells, MSC, CIK cells, or antigen-specific T cells), which need to be cultured in experimental conditions ex vivo.
A variation on “regional” targeting mimics the in vivo distribution pattern of tumor cells by using tumor cells themselves as carriers.21,37,67 The rationale of such an approach is attractive—few cells will traffic in vivo to sites of tumor cell distribution as faithfully as more tumor cells. This approach relies, however, on very close compatibility between the carrier tumor cells and the tumor type being chased in vivo. This is because the cell surface molecules associated with tumor cell circulation, arrest, and extravasation should be as closely matched as possible. In a proof-of-concept study, Power et al. chased down lung metastases using highly virus permissive carcinoma cells loaded with VSV and injected intravenously.21 However, further studies suggested that the tracking of tumor metastases in that model was indeed purely regional targeting. That is, the lung location of the metastases was simply compatible with the intravenous route of administration of the virus-loaded carriers. Lymphocytic leukemia cells proved more effective at allowing the VSV-loaded cell carriers to navigate the circulation beyond the first pass effect of the lungs.21 These cells were also more able to pass through the small capillary beds where larger solid tumor cell carriers are easily arrested. However, divergence of the histological type of the transformed cell used to carry virus from the tumor type being treated somewhat dilutes the purity of the approach (using “like” to find, and treat, “like”). Conversely, use of transformed lymphocytic cell lines move closer to the T cell–mediated strategies discussed above, with their possible advantages for tumor-specific, or lymphoid organ–specific, targeting.
A major attraction of a transformed cell carrier would be the ease of preparation of an “off-the-shelf” product, which could be preapproved for patient treatment. In contrast, administering tumor cells to patients itself presents multiple regulatory headaches. However, a multitude of clinical trials using irradiated allogeneic, or autologous, tumor cell vaccinations indicates that this is not as difficult as it might seem. Studies will have to prove that tumor cell carriers can still be effective even when irradiated, or otherwise doomed to die (perhaps by transduction with a suicide gene), following transfer into the patient.
Where to Next for Cell Carriers?
Selecting tumor tropic cell types, characterization, and cell engineering
It is probably fair to say that there are now plenty of potential oncolytic, or other, virus types, which could be matched to the “ideal” cell carrier for cancer therapy. The rate-limiting step remains the lack of a clearly characterized cell population that can (i) accumulate to high levels in tumor and (ii) avoid other tissues and organs.
A literature survey suggests that very few of the candidate cells (Table 1) accumulate in tumor models at levels exceeding 10% of the adoptively transferred dose—and this is usually at the high end of most estimates. Therefore, a challenge for the short term is the identification of improved cellular phenotypes, which accumulate reproducibly in tumors following adoptive transfer. This has three components: (i) identification of improved tumor tropic cell types from preclinical models; (ii) confirmation that these cell types are also found at high levels in the corresponding human tumors in patients; and (iii) improved cell engineering so that the appropriate cell type can be manufactured in vitro at high enough levels for adoptive transfer.
We would speculate that the responses observed in studies of intravenous oncolytic virus administration2 are due to the ability of viral particles to bind to a subpopulation of circulating cells, which subsequently chaperoned virions to the tumor, in the face of high levels of NAb.68 There is, therefore, much to be learned from the scientific study of clinical trials of systemic viral delivery, in order to identify which cell populations in vivo lead to viral delivery as opposed to elimination.
In a complementary approach, we recently approached this issue by conducting a systematic screen of tumors growing in mice. Our rationale was to replace best guess models (in which known cell types were used for tumor trafficking) with unbiased screening models (in which no assumptions, or predeterminations, were made about which cell types may be optimal for tumor trafficking). We identified a population of cells recovered from B16ova melanoma tumors growing in immune-competent mice that had undergone adoptive transfer with unfractionated, labeled bone marrow cells.69 These cells, which reproducibly localize to tumors at levels in excess of those associated with our gold standard cell type (antigen-specific OT-I T cells), express both Sca1 and NK1.1 markers. This implied that they represent a stem cell population, which might recognize specific properties associated with the tumor environment (Figure 3). The next step, however, has highlighted a major problem associated with these studies. Although we screened these cells for expression of different markers, it is unclear as to which, if any, of these arbitrary markers are functionally associated with the phenotype of tumor trafficking. In fact, the likelihood is that many unknown molecules contribute to the phenotype. A very clear understanding of which markers are associated with the therapeutic cell carrier population is absolutely required. Without it, it will not be possible to develop the in vitro cell engineering required for to generate high numbers of purified cell carriers for clinical use.
Testing new carrier cells
There is still likely to be considerable benefit to be gained from proceeding with a “best (educated) guess” approach to introduce new cell carriers of oncolytic viral vectors. For example, it may be possible to exploit the chaotic and highly irregular vascular network of tumors.70 In particular, if carrier cells are particularly inflexible and poorly deformable, such cells may be selectively retained in tumor vasculature as opposed to other capillary beds. This rationale has led us to explore the possibility of using sickle red blood cells as carriers of oncolytic vectors.71 We have shown that both normal red blood cells, as well as sickle red cells from human patients, can be loaded with either VSV or reovirus. These cells can also be found in tumors at high levels within 30 minutes of adoptive transfer. Therapeutic studies with this approach are underway.
Going forward, it will be important to be both brave and imaginative. As discussed above, several cell types may present themselves as potential carriers not because they are effective antitumor agents, but precisely because they are associated with tumor growth and development. Such cell types may initially be difficult to accept as therapeutic agents. Thus, mesenchymal stem cells can have tumor-promoting properties by themselves.64,65,66 However, in the context of carriage of oncolytic vectors, net destructive power may be greater than overall tumor enhancement. Similarly, other apparently dubious candidates may be worth exploring. Tumor immunologists, for example, take an understandably rather dim view of regulatory T cells. This is because their accumulation within tumors is strongly associated with evasion of antitumor T-cell responses.72,73 However, it is just such a phenotype that should encourage the oncovirologist to develop regulatory T cell–mediated delivery of viruses to tumors.
Engineering cell trafficking
Even if better cellular vehicles become available, either from unbiased selection or best guess approaches, it may still be possible to improve trafficking efficiencies still further through physical intervention. The ability to concentrate very powerful magnetic fields upon localized areas of the body may offer new opportunities to focus adoptively transferred cells into tumors at artificially high levels. In this respect, Muthana et al. demonstrated that macrophages, which have a basal level of tumor accumulation following adoptive transfer, can be preloaded with magnetic iron particles without detectable loss of function.74 These magnetic macrophages could then be recruited to human tumor xenografts by placing a powerful magnet over the tumor. Such an approach offers maximal therapeutic potential in the context of enhancing the “regional” targeting approach—for example, by applying magnetic fields to organs in which tumor metastases are known to reside.75 If a magnetic field could be used to increase regional accumulation of the cell carriers, the cells may then become much more effective in the context of homing to tumor cells over short ranges. Indeed, effective development of this approach may even remove the need to use a cell carrier with any tumor tropic properties at all. If the magnetic field can be strong and focused enough to recruit any magnetized cells to the sites of metastatic disease, sufficient virus might then be released in the vicinity of tumor cells to allow for productive infection and therapy.
In addition, a combination of cell carriers with other conventional modalities—such as radiotherapy—may enhance tumor delivery. Irradiation upregulates adhesion molecule expression on endothelial cells,76,77 and there is evidence of a differential effect upon the expression of adhesion molecules by tumor and normal endothelium.78 Also, a radiotherapy-induced enhancement of the release of inflammatory cytokines has been described in association with increased leukocyte infiltration.76,77 In this respect, preclinical studies have shown that local radiotherapy can increase the delivery of adoptively transferred tumor-infiltrating lymphoctyes.79 Thus, based upon the hypothesis that conventional radiotherapy could be followed by the cellular delivery of oncolytic viruses, we have recently opened a trial to determine the ability radiotherapy to enhance tumor delivery of 111Indium-labeled lymphocytes.
The Future of Cell Carriers
The emergence of cell carriers to deliver oncolytic viruses is an inevitable result of the technical problems associated with exposing potentially therapeutic oncolytic virus vectors to the hazards of the systemic circulation and to an immune system, which is incapable of distinguishing friendly, therapeutic viruses from malevolent, pathogenic viruses. Exploitation of cells as carriers is based on mimicry of natural mechanisms by which invading pathogens associate with normal host cells to avoid instant, or delayed, neutralization by serum components, neutralizing antibodies, and host scavenger cells. Cell carriers can be used with a tropism for tumor cells themselves, the environment created by tumors, or for anatomic locations where tumors are known to be resident. Cell carriers offer tumor-selective distribution and delivery components to the protection that cells can provide to viruses, which are packaged either onto, or into, them. The current range of cell carriers already includes varied cell types (including T cells, macrophages, MSC, endothelial progenitor cells, tumor cells) and multiple viruses (including retroviruses, VSV, reovirus, vaccinia virus, Newcastle disease virus, adenovirus, measles virus). However, further developments will depend on innovation, technology development, and interdiscipline collaboration. Innovative strategies will discover new tumor tropic cell types using both unprejudiced and best guess selection protocols. The technology required to improve cell-engineering technologies is critical for generation of clinical grade batches of cells with the most relevant biological phenotypes for tumor trafficking. And fruitful collaborations with virologists (identifying new oncolytic viral payloads), biomedical engineers (developing physical targeting strategies such as magnetism) and cell biologists, immunologists, and hematologists (identifying new cell carriers) will all be required to take the science of cell carriers for cancer therapy into the next phase of efficacy and clinical translation.
Several years ago, our cynical, but realistic, Clinical Fellows referred us to a classic of Greek Literature to inspire our future development of protocols for systemic delivery of viral vectors into patients with metastatic tumors. In Aristophanes' play, Pisthetairos finally persuades the birds to create Nepheloccygia (Cloud Cuckoo Land) and the new city in the sky becomes essential for the transmission of messages between Earth and the Gods. Maybe it will be cell-based carriers that eventually provide the elusive, but critical, link allowing direct communication between the oncolytic viruses of the laboratory and the tumors of the patients.
Acknowledgments
R.G.V. is supported by NIH grants CA107082, CA130878, and CA132734, Mayo Foundation, and The Richard M. Schulze Family Foundation. We thank Toni L. Higgins for expert secretarial assistance.
REFERENCES
- Henderson J. Aristophanes: The Birds. Focus Publishing: Newburyport, MA; 1999. [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- Liu TC., and , Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8:301–313. [PubMed] [Google Scholar]
- Kirn DH., and , Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum Gene Ther. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- Pizzato M, Marlow SA, Blair ED., and , Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C, Qiao J, Kottke T, Diaz RM, Ahmed A, Sanchez-Perez L, et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med. 2005;11:1073–1081. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- Kottke T, Qiao J, Diaz RM, Ahmed A, Vroman B, Thompson J, et al. The perforin-dependent immunological synapse allows T-cell activation-dependent tumor targeting by MLV vector particles. Gene Ther. 2006;13:1166–1177. doi: 10.1038/sj.gt.3302722. [DOI] [PubMed] [Google Scholar]
- Thanarajasingam U, Sanz L, Diaz R, Qiao J, Sanchez-Perez L, Kottke T, et al. Delivery of CCL21 to metastatic disease improves the efficacy of adoptive T-cell therapy. Cancer Res. 2007;67:300–308. doi: 10.1158/0008-5472.CAN-06-1017. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Lekkerkerker AN, van Kooyk Y., and , Geijtenbeek TB. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res. 2006;4:169–176. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, et al. Persistence of infectious HIV on follicular dendritic cells. J Immunol. 2001;166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- Smith-Franklin BA, Keele BF, Tew JG, Gartner S, Szakal AK, Estes JD, et al. Follicular dendritic cells and the persistence of HIV infectivity: the role of antibodies and Fcγ receptors. J Immunol. 2002;168:2408–2414. doi: 10.4049/jimmunol.168.5.2408. [DOI] [PubMed] [Google Scholar]
- Harrington K, Alvarez-Vallina L, Crittenden M, Gough M, Chong H, Diaz RM, et al. Cells as vehicles for cancer gene therapy: the missing link between targeted vectors and systemic delivery. Hum Gene Ther. 2002;13:1263–1280. doi: 10.1089/104303402760128504. [DOI] [PubMed] [Google Scholar]
- Ilett EJ, Prestwich RJ, Kottke T, Errington F, Thompson JM, Harrington KJ, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumor killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–699. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Diaz RM, Kaluza K, Pulido J, Galivo F, Wongthida P, et al. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol Ther. 2008;16:1910–1918. doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HT, Hasegawa K, Dietz AB, Russell SJ., and , Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Hwang TH., and , Kirn DH. Vaccinia virus and oncolytic virotherapy of cancer. Curr Opin Mol Ther. 2005;7:359–365. [PubMed] [Google Scholar]
- Pizzato M, Blair ED, Fling M, Kopf J, Tomassetti A, Weiss RA, et al. Evidence for nonspecific adsorption of targeted retrovirus vector particles to cells. Gene Ther. 2001;8:1088–1096. doi: 10.1038/sj.gt.3301494. [DOI] [PubMed] [Google Scholar]
- Chester J, Ruchatz A, Gough M, Crittenden M, Chong H, Cosset FL, et al. Tumor antigen-specific induction of transcriptionally targeted retroviral vectors from chimeric immune receptor-modified T cells. Nat Biotechnol. 2002;20:256–263. doi: 10.1038/nbt0302-256. [DOI] [PubMed] [Google Scholar]
- Groot F, Welsch S., and , Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- Hübner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, et al. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Kashefi K, Hollinshead M., and , Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- Piguet V., and , Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh L, Leung K, Loré K, Levin R, Panet A, Schwartz O, et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Köhler K, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Pan YW, Scarlett JM, Luoh TT., and , Kurre P. Prolonged adherence of human immunodeficiency virus-derived vector particles to hematopoietic target cells leads to secondary transduction in vitro and in vivo. J Virol. 2007;81:639–649. doi: 10.1128/JVI.01089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colombatto P, Brunetto M, et al. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- Pfirschke C., and , Schirrmacher V. Cross-infection of tumor cells by contact with T lymphocytes loaded with Newcastle disease virus. Int J Oncol. 2009;34:951–962. doi: 10.3892/ijo_00000221. [DOI] [PubMed] [Google Scholar]
- Power AT., and , Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- Thorne SH., and , Contag CH. Integrating the biological characteristics of oncolytic viruses and immune cells can optimize therapeutic benefits of cell-based delivery. Gene Ther. 2008;15:753–758. doi: 10.1038/gt.2008.42. [DOI] [PubMed] [Google Scholar]
- Thorne SH, Negrin RS., and , Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Thorne SH, Tam BY, Kirn DH, Contag CH., and , Kuo CJ. Selective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacy. Mol Ther. 2006;13:938–946. doi: 10.1016/j.ymthe.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- Dudley ME., and , Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Powell DJ, Rosenberg SA., and , Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer Clin Cancer Res 2006126106–6115.20 Pt 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden M, Gough M, Chester J, Kottke T, Thompson J, Ruchatz A, et al. Pharmacologically regulated production of targeted retrovirus from T cells for systemic antitumor gene therapy. Cancer Res. 2003;63:3173–3180. [PubMed] [Google Scholar]
- Yotnda P, Savoldo B, Charlet-Berguerand N, Rooney C., and , Brenner M. Targeted delivery of adenoviral vectors by cytotoxic T cells. Blood. 2004;104:2272–2280. doi: 10.1182/blood-2003-11-3803. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA., and , Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C. Adoptive T cell therapy: addressing challenges in cancer immunotherapy. J Transl Med. 2005;3:17. doi: 10.1186/1479-5876-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Dudley ME, Hogan KA, Wunderlich JR., and , Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–6539. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Karne NK, Kerkar SP, Heller CK, Palmer DC, Johnson LA, et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology. 2009;116:981–989.e1. doi: 10.1016/j.ophtha.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81:1009–1016. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH. Strategies to achieve systemic delivery of therapeutic cells and microbes to tumors. Expert Opin Biol Ther. 2007;7:41–51. doi: 10.1517/14712598.7.1.41. [DOI] [PubMed] [Google Scholar]
- Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH., and , Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- Zumsteg A., and , Christofori G. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Opin Oncol. 2009;21:60–70. doi: 10.1097/CCO.0b013e32831bed7e. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P., and , Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Sica A, Schioppa T, Mantovani A., and , Allavena P. Tumor-associated macrophages are a distinct M2 polarised population promoting tumor progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Jevremovic D, Gulati R, Hennig I, Diaz RM, Cole C, Kleppe L, et al. Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumors. Am J Physiol Heart Circ Physiol. 2004;287:H494–H500. doi: 10.1152/ajpheart.00064.2004. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M., and , Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ., and , Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B., and , Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10:657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AT., and , Bell JC. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther. 2008;15:772–779. doi: 10.1038/gt.2008.40. [DOI] [PubMed] [Google Scholar]
- White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- Lang SI, Kottke T, Thompson J., and , Vile RG. Unbiased selection of bone marrow derived cells as carriers for cancer gene therapy. J Gene Med. 2007;9:927–937. doi: 10.1002/jgm.1089. [DOI] [PubMed] [Google Scholar]
- Brown SL, Ewing JR, Nagaraja TN, Swerdlow PS, Cao Y, Fenstermacher JD, et al. Sickle red blood cells accumulate in tumor. Magn Reson Med. 2003;50:1209–1214. doi: 10.1002/mrm.10646. [DOI] [PubMed] [Google Scholar]
- Willmon CL, Thompson J, Foley R, Aguinaga MDP, Terman D., and , VIle RV.Sickle cells as carriers of oncolytic viruses Mol Ther: J Amer Soc Gene Ther 200917S206#538 [Google Scholar]
- Barnett BG, Rüter J, Kryczek I, Brumlik MJ, Cheng PJ, Daniel BJ, et al. Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol. 2008;622:255–260. doi: 10.1007/978-0-387-68969-2_20. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthana M, Scott SD, Farrow N, Morrow F, Murdoch C, Grubb S, et al. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 2008;15:902–910. doi: 10.1038/gt.2008.57. [DOI] [PubMed] [Google Scholar]
- Kaluza K., and , Vile RG. Magnetic cells for cancer therapy: adopting magnets for cell-based cancer therapies. Gene Ther. 2008;15:1511–1512. doi: 10.1038/gt.2008.139. [DOI] [PubMed] [Google Scholar]
- Hallahan D, Kuchibhotla J., and , Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56:5150–5155. [PubMed] [Google Scholar]
- Mollà M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, et al. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2003;57:264–273. doi: 10.1016/s0360-3016(03)00523-6. [DOI] [PubMed] [Google Scholar]
- Hallahan DE., and , Weichselbaum R. Role of gene therapy in radiation oncology. Cancer Treat Res. 1998;93:153–167. doi: 10.1007/978-1-4615-5769-2_7. [DOI] [PubMed] [Google Scholar]
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG., and , Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]