Abstract
We recently have shown that mice deficient for the 86-kDa component (Ku80) of the DNA-dependent protein kinase exhibit growth retardation and a profound deficiency in V(D)J (variable, diversity, and joining) recombination. These defects may be related to abnormalities in DNA metabolism that arise from the inability of Ku80 mutant cells to process DNA double-strand breaks. To further characterize the role of Ku80 in DNA double-strand break repair, we have generated embryonic stem cells and pre-B cells and examined their response to ionizing radiation. Ku80−/− embryonic stem cells are more sensitive than controls to γ-irradiation, and pre-B cells derived from Ku80 mutant mice display enhanced spontaneous and γ-ray-induced apoptosis. We then determined the effects of ionizing radiation on the survival, growth, and lymphocyte development in Ku80-deficient mice. Ku80−/− mice display a hypersensitivity to γ-irradiation, characterized by loss of hair pigmentation, severe injury to the gastrointestinal tract, and enhanced mortality. Exposure of newborn Ku80−/− mice to sublethal doses of ionizing radiation enhances their growth retardation and results in the induction of T cell-specific differentiation. However, unlike severe combined immunodeficient mice, radiation-induced T cell development in Ku80−/− mice is not accompanied by extensive thymocyte proliferation. The response of Ku80-deficient cell lines and mice to DNA-damaging agents provides important insights into the role of Ku80 in growth regulation, lymphocyte development, and DNA repair.
The energy absorbed from γ-ray irradiation can induce DNA base modifications and DNA strand breaks in a cell. Among the various forms of DNA damage produced by γ-irradiation, DNA double-strand breaks (DSBs) are potentially the most lethal. Four complementation groups (groups 4, 5, 6, and 7) of mammalian cell mutants have been described that are specifically defective in the repair of DSBs (for review see refs. 1 and 2). Three of these groups have been shown to correspond to components of the DNA-dependent protein kinase (DNA-PK), whereas the fourth mutant is deficient in the gene encoding XRCC4. Although the biochemical properties of the XRCC4 gene are still unclear (3), much more is known about the function of the DNA-PK. DNA-PK is a serine/threonine kinase that consists of a catalytic subunit, DNA-PKcs (complementation group XRCC7), and a DNA-binding regulatory subunit, the Ku heterodimer [Ku70 (XRCC6) and Ku80 (XRCC5)]. Ku binds to DSBs and recruits the catalytic subunit to the lesion, thereby activating its enzymatic function (1, 2). Putative downstream effectors of DNA-PK include transcription factors Sp1, Oct1, c-fos, c-Jun; the tumor suppressor p53 (4), and the 34-kDa subunit of replication protein A (5).
In addition to their hypersensitivity to γ-irradiation, mutant cells from complementation groups 4–7 have been found to be defective in V(D)J recombination, the process by which genomic segments encoding the variable (V), diversity (D), and joining (J) elements of Ig and T cell receptor genes are assembled (3, 6–12). A direct link between DSB repair and V(D)J recombination was discovered through analysis of severe combined immunodeficient (SCID) mice, which are both radiosensitive and immunodeficient (for review see refs. 13 and 14). Recent evidence indicates that SCID cells are defective in the DNA-PK catalytic subunit (9, 10, 15). The kinase activity of DNA-PK is absent, and the radiation sensitivity and V(D)J defect in SCID is complemented by the gene encoding DNA-PKcs. In addition, a nonsense mutation in the C-terminal region of DNA-PKcs has been discovered in SCID cells that is likely to impair its function (16, 17).
Except for the immune deficiency and radiosensitivity, SCID mice are phenotypically normal. However, a null mutation in Ku80 subunit of the DNA-PK results in a more severe phenotype (8). Ku80−/− mice are approximately half the size of their control littermates, and primary embyronic fibroblasts derived from Ku80−/− mice exhibit premature senescence, suggesting a link between Ku80 and growth control. Although both SCID and Ku80 mutant mice are deficient in V(D)J recombination, the SCID recombination defect is coding junction-specific, whereas Ku80−/− mice are deficient in processing both coding and signal joints (8, 18).
To better understand the nature of the DNA repair defect associated with Ku80 deficiency, we have established Ku80−/− embryonic stem (ES) cells and pre-B cell lines, and investigated the response of mutant cell lines and mice to DNA-damaging agents. Ku80−/− cells are hypersensitive to γ-irradiation, as evidenced by their low survival as assayed by colony formation efficiencies and their susceptibility to apoptosis. Ku80−/− ES cells also show a cross-sensitivity to methyl methanesulfonate (MMS), a DNA alkylating agent that generates single-strand breaks. Consistent with the radiation-hypersensitive phenotype of the cell lines, Ku80 mutant mice also display an extreme radiosensitivity, characterized by loss of hair pigmentation, severe injury to the gastrointestinal tract, and enhanced mortality. The growth of Ku80−/− mice and the proliferation of thymocytes after γ-irradiation also is impaired. These studies extend the analysis of the Ku80−/− defect both in vitro and in vivo, and provide important insights into the role that Ku80 plays in growth regulation and DSB repair.
MATERIALS AND METHODS
Cell Lines.
The Ku80−/− ES cell line was generated from Ku80+/− ES cells by selection in 1 mgml−1 G418. Of 200 colonies screened, one was found to be Ku80−/−. For radiation survival experiments, ES cells were grown without feeder layer in DMEM + 10% fetal calf serum supplemented with 10,000 units/ml of lymphocyte inhibitory factor (GIBCO/BRL). The Abelson murine leukemia retrovirus was used to transform bone marrow cells from a 6-week-old Ku80 mutant mouse and from a wild-type littermate as previously described (19). All B cell lines expressed B220 and CD43 on the cell surface (data not shown), indicating that these cell lines originated from immature pre-B cells. Pre-B cells were maintained in RPMI supplemented with 10% fetal calf serum, 50 μM β-mercaptoethanol, and antibiotics.
Generation and Screening of Mice.
Ku80 mutant mice (C57BL/6–129/Sv) were generated from Ku80+/− intercrosses and were screened by Southern blotting or PCR as described (8). SCID mice were obtained from the mouse facility at Memorial Sloan-Kettering Cancer Center.
Irradiation.
Exponentially growing ES cells were exposed to graded doses of γ-rays, MMS, or UV light, then trypsinized and plated onto pre-gelatinized Petri dishes. Eight days later cells were fixed and stained, and colonies containing greater than 50 cells were counted for clonogenic survival. The response of pre-B cell lines to ionizing radiation was analyzed by flow cytometry. Cells were removed from control and γ-irradiated cultures at different times, fixed at 4°C in 70% ethanol, and then stained with 5 μg/ml of propidium iodide. Apoptotic cells were identified based on reduced DNA stainability of propidium iodide (20).
To determine the effect of radiation on mouse growth and lymphocyte development, newborn mice from Ku80+/− × Ku80+/− crosses were irradiated within 72 hr after birth, and their body weights were measured at 2-day intervals. Twenty-five percent of the offspring from these crosses were Ku80−/−, which initially were identified by their reduced body size and subsequently confirmed by PCR (8). At least one unirradiated sex-matched Ku80−/− littermate control was used for comparing body weights. The effects of γ-irradiation on Ku80 heterozygtes vs. wild-type littermates were indistinguishable, and both Ku80+/− and Ku80+/+ were used as controls. For in vivo survival experiments, 2- to 4-month-old animals (n = 6) of each genotype were irradiated at graded doses between 200 and 800 cGy. To examine the radiation toxicity to various tissues, mice of each genotype were given 8 Gy of whole body irradiation and were euthanized for histopathological analysis after 2, 3, and 4 days.
Flow Cytometric Analysis of Lymphocyte Development.
Thymi were harvested from wild-type and Ku80 mutant mice, and single-cell suspensions were prepared. Cells were stained with combinations of phycoerythrin (PE)-conjugated anti-CD8, fluorescein isothiocyanate (FITC)-conjugated anti-CD4, Red 613-conjugated anti-CD8, PE-conjugated anti-αβ T cell receptor, and PE-conjugated anti-CD3ɛ. Cells from bone marrow and spleen were depleted of red blood cells. Bone marrow cells were stained with FITC-conjugated anti-CD43 and PE-conjugated anti-B220. Splenocytes were stained with combinations of FITC-conjugated anti-IgM, PE-conjugated anti-B220, PE-conjugated anti-CD8, and FITC-conjugated anti-CD4. All antibodies were purchased from PharMingen with the exception of anti-CD8 (GIBCO/BRL).
Histopathological Analysis.
Tissues were fixed in 10% buffered formalin, embedded in paraffin blocks, sectioned at 4–5 microns, and stained with hematoxylin and eosin. Sections were photographed at 20× and 100× magnification as indicated.
RESULTS
Effect of Ionizing Radiation on Growth.
From the fetal period until the first few weeks of postnatal development, mice body weights increase exponentially with time (21). During this period Ku80-deficient mice exhibit a defect in cellular proliferation that results in a 40–60% reduction in body weights (8). To determine the effect of ionizing radiation on early postnatal development, we monitored the growth of Ku80 mutant mice and controls after exposing newborn animals to graded doses of γ-irradiation. A dose of 100 cGy had no detectable effect on wild-type mice (not shown). However, the growth of Ku80−/− mice was even further retarded relative to unirradiated Ku80−/− littermates (Fig. 1A), and these mice invariably died within 2 to 4 weeks (Fig. 1B). A dose of 50 cGy at birth also resulted in a significant growth retardation in Ku80−/− homozygotes (Fig. 1C), but did not lead to mortality in four of six mice. Below 25 cGy, there was no detectable effect of ionizing radiation on growth (not shown). When similar experiments were performed on SCID mice, we did not detect significant growth retardation at 100 cGy, and their survival was barely affected at 200 cGy (seven of nine mice survived at least 2 months).
Figure 1.
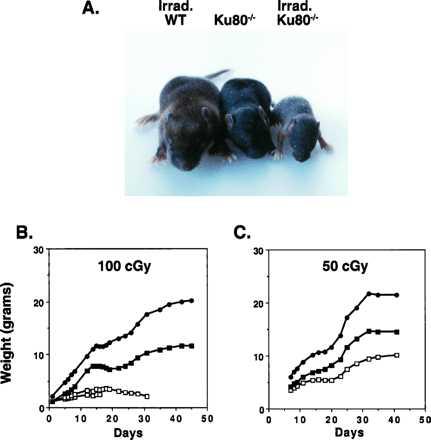
The effect of low-dose ionizing radiation on postnatal growth. (A) Photograph of 10-day-old wild-type and Ku80−/− littermates. Wild-type and Ku80−/− mice were irradiated within 24 hr of birth by using a 137Cs source with a rate of 83 cGy. Unirradiated Ku80−/− mouse is shown for comparison. There is no difference in size between irradiated and unirradiated wild-type mice (not shown). (B) Postnatal growth of one wild-type and three Ku80−/− male littermates, two of which were treated with 100 cGy irradiation. Weights of individual animals (wild-type mice, •; irradiated Ku80−/− mice, □; and untreated Ku80−/− mice, ▪) are plotted against time. Irradiated Ku80−/− mice subsequently died 16 days and 30 days postirradiation as indicated. (C) The effect of 50-cGy irradiation on the growth of Ku80−/− mice. Growth of female wild-type, irradiated, and untreated Ku80−/− mice are shown. Data are representative of five independent experiments.
Effect of Sublethal Doses of Ionizing Radiation on T Cell and B Cell Development.
Over 80% of thymocytes from Ku80−/− mice are developmentally arrested at the double negative stage (DN), characterized by the lack of expression of CD4 and CD8 markers. In some Ku80−/− animals, CD4+CD8+ double positive (DP) cells are also detectable and range from 0 to 20% of the total number of Ku80−/− thymocytes (Fig. 2A, Center). Recent studies have shown that agents that induce double-strand breaks, such as γ-irradiation, can effect the differentiation of DN thymocytes to DP thymocytes and lead to a significant increase in thymus cellularity in both SCID and Rag−/− mice (22–25). Fig. 2A (Right) shows that radiation also rescued development of DP thymocytes in Ku80−/− mice. Three weeks after 50-cGy irradiation the majority (90%) of Ku80−/− thymocytes were CD4+CD8+, with no single positive CD4+ or CD8+ cells observed. Similar CD4 vs. CD8 profiles were detected 2–5 weeks after irradiation and with doses as low as 25 cGy (data not shown). By contrast, these low doses of irradiation did not affect differentiation of SCID thymocytes, which required doses between 100–200 cGy to overcome the developmental arrest and promote the appearance of DP thymocytes (data not shown). Irradiated Ku80−/− thymocytes were unable to proceed beyond the CD4+CD8+ stage as there was no surface expression of either T cell antigen receptor β (Fig. 2B) or CD3ɛ (not shown) in the thymus, nor were there CD4+ or CD8+ single positive cells in the periphery (Fig. 2C). Thymocyte proliferation was not induced by γ-irradiation in Ku80−/− mice or controls. Thymi from irradiated Ku80−/− mice did not increase significantly in size and remained approximately 100 times smaller than irradiated controls [1.5 × 106 cells in the former vs. 2 × 108 in the latter (n = 4)]. Neither have we observed any thymic lymphomas to date (7 months after 50-cGy irradiation, n = 3). These results contrast with those in SCID mice in which irradiation increased thymus cellularity from 4- to 20-fold 3 weeks after treatment and eventually led to thymic lymphomas in 100% of the animals (23).
Figure 2.
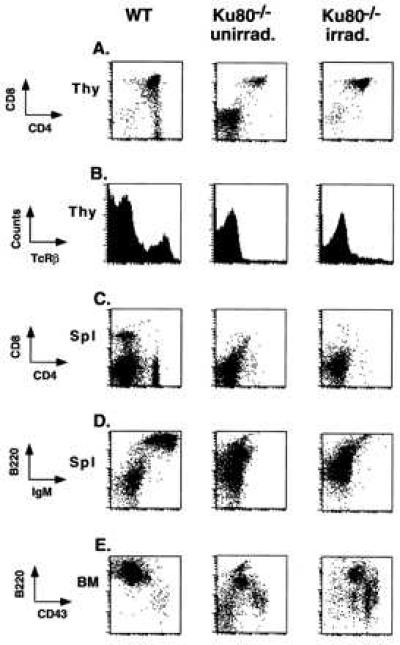
Ionizing radiation induces T cell specific differentiation in Ku80−/− mice. Cells from different lymphoid organs from wild-type mice (Left), untreated Ku80−/− mice (Center), and day 21 Ku80−/− mice subjected to 50 cGy at birth (Right) were stained for the expression of various surface markers and analyzed by multiparameter flow cytometry. Thymocytes were analyzed for the expression of (A) CD4/CD8 and (B) T cell antigen receptor β(TCRβ) expression; splenocytes were stained for (C) CD4/CD8 and (E) CD43/B220; and bone marrow cells were analyzed for the expression of (D) IgM/B220. For data analysis, viable lymphoid cells were electronically gated according to forward and side scatter by using Cell-Quest software (Becton Dickinson). Data are plotted on a logarithmic scale except for B, which displays the cell counts on a linear scale.
In contrast to the rescued development of DP thymocytes, γ-irradiation failed to simultaneously restore B cell development. B cells from Ku80−/− bone marrow remained at the immature CD43+ pro-B cell stage (Fig. 2E), and splenocytes lacked surface expression of IgM (Fig. 2D). Together, these data show that T cell but not B cell differentiation can be induced by low doses of γ-irradiation in Ku80−/− mice. However, T cell differentiation occurred without extensive cell proliferation.
In Vitro Radiation Sensitivity.
To quantify the radiation sensitivity of Ku80 mutant ES cells, we carried out clonogenic survival assays as described (26). Fig. 3A clearly shows that Ku80−/− ES cells were more sensitive to ionizing radiation than wild-type cells. Ku80−/− ES cells showed a biphasic survival curve with a sharp decrease in survival at low doses, followed by a shallower linear response at higher doses. The basis for this dose–response is not understood, but has been observed in several x-ray-sensitive cell lines, including those from XRCC5 and XRCC4 complementation groups (27, 28). Whereas Ku80−/− ES cells showed a similar sensitivity to UV light as controls (not shown), Ku80−/− ES cells were more sensitive to the effects of MMS, a DNA alkylating agent that results in single-strand breaks (Fig. 3B). By contrast, cells from SCID mice do not exhibit cross-sensitivity to MMS and are only slightly more sensitive to UV light (29).
Figure 3.
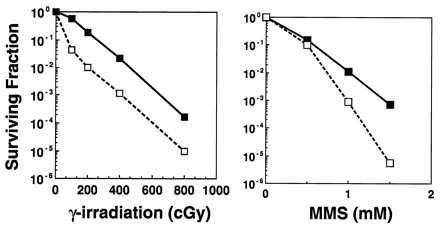
The effect of DNA damaging agents on Ku80-deficient ES cells. Clonogenic survival assays were performed on Ku80−/− (□) and wild-type (▪) ES cells exposed to graded doses of (A) ionizing radiation and (B) MMS. Ku80-deficient cells show significantly decreased ability to form colonies after exposure to ionizing radiation and MMS compared with wild-type controls.
As a model for apoptosis, we established pre-B cell lines from Ku80-deficient and wild-type bone marrow and examined the response of these cells to γ-irradiation. Apoptotic cells from both Ku80−/− and wild-type lymphoid lines could be easily identified by their diminished stainability with propidium iodide, which resulted in the accumulation of a sub G0/G1 peak in the DNA content histogram (Fig. 4). In the absence of irradiation, Ku80−/− cultures consistently contained a 10–20% greater fraction of apoptotic cells than wild-type cell cultures (Fig. 4 A and B, compare controls). After treating Ku80−/− cells with 200 cGy, we observed a massive increase in the fraction of cells with sub G0/G1 content and a concomitant decrease in the cycling fraction (Fig. 4B). By 2 hr postirradiation 69% of the Ku80−/− cells had sub G0/G1 content, which increased to >90% by 4 hr postirradiation. On the other hand, by 24 hr postirradiation only 66% of the wild-type cells appeared to be apoptotic (Fig. 4A). Our data demonstrate that both in terms of clonogenic survival (Fig. 3) and apoptosis (Fig. 4), Ku80-deficient cells are much more sensitive to γ-irradiation.
Figure 4.
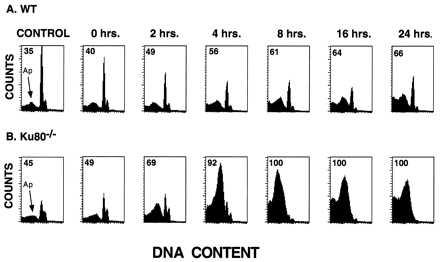
Radiation response of Ku80−/− pre-B cells. Representative DNA content histograms of (A) wild-type and (B) Ku80−/− pre-B cell lines before and after treatment with 200 cGy of γ-irradiation. Samples were processed for DNA content analysis by flow cytometry at different times postirradiation. γ-irradiation increased the appearance of cells with fractional DNA content, which coincided with a loss of cells in the cycling population. The sub-G0 peak corresponding to apoptotic cells is indicated by an arrow, and the proportion of apoptotic cells is quantified.
In Vivo Radiation Sensitivity.
The radiation sensitivity of adult (2 to 4 months old) wild-type and Ku80−/− mice was compared. At 200 cGy, all wild-type and Ku80 homozygotes survived for at least 12 weeks. However, Ku80-deficient animals lost their hair within a month after irradiation. After an additional 1–2 months, their hair grew back but it lacked the original agouti-color pigmentation and appeared gray (Fig. 5A). By contrast, we did not notice any change in the hair pigmentation of irradiated wild-type mice during the same period (Fig. 5A). Even at 400 cGy, all wild-type and heterozygous mice survived at least 2 months. However, all six of the irradiated Ku80−/− mice died within 2 weeks (Fig. 5B). At an intermediate dose of 300 cGy, two of four Ku80−/− mice died but only after 8 weeks postirradiation. We conclude that the dose that causes 50% mortality in 2 weeks is between 300 and 400 cGy, which is similar to the value of <370 cGy previously reported for SCID mice (30).
Figure 5.
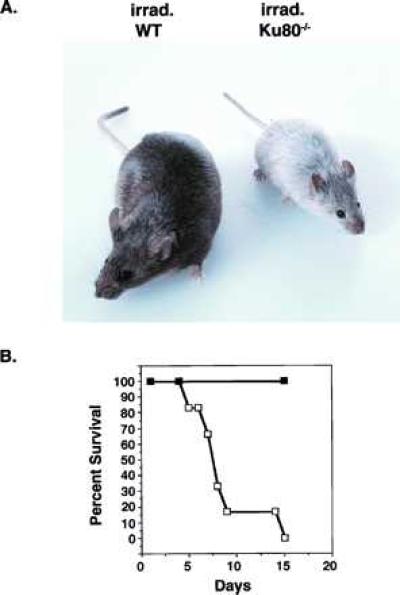
Radiation sensitivity of Ku80−/− mice. (A) Adult (3 months old) agouti wild-type and Ku80−/− mice were subjected to 300 cGy γ-irradiation, and mice were photographed 2 months after the treatment. Irradiation resulted in the appearance of gray hair in Ku80−/− mice but did not affect the color coat of wild-type mice. (B) Survival of Ku80−/− (□) and wild-type (▪) mice subjected to 400 cGy. Six 3-month-old mice from each group were treated simultaneously and then monitored for 2 weeks. Whereas all of the wild-type mice survived, 100% of the Ku80−/− mice died within this time period.
To determine the radiation sensitivity of various tissues in Ku80 mutant mice, animals were treated with 8 Gy, and tissues were examined histologically at 2, 3, and 4 days postirradiation. Most tissues, such as brain, lung, heart, and pancreas, appeared morphologically within normal limits. The lymphoid organs, including thymus, spleen, lymph nodes, and bone marrow, which already were markedly atrophic in the mutant mouse, showed even more profound cellular depletion after the radiation exposure. In addition, a severe radiation-induced injury to the gastrointestinal tract was observed in the Ku80 mutant mice at much lower doses than for the wild-type controls. Whereas the villi from wild-type mice intestines remained intact 4 days after irradiation, villi from Ku80 mutant mice were blunted and lined by degenerative epithelial cells (Fig. 6 A and B). Whereas wild-type mice infrequently had single cell degeneration and necrosis of the proliferative crypt cell population, the crypts of Ku80−/− mice were almost completely obliterated. (Fig. 6 C and D). At 2 days postirradiation, there were moderate degrees of crypt depopulation in Ku80 mutant mice, whereas the effects at 3 days postirradiation were intermediate (not shown). A similar pattern of injury was observed in the large intestine of mutant mice, which showed extensive mucosal collapse by 4 days from the loss of surface epithelial and crypt cells (Fig. 6 E and F). In the absence of radiation, histological analysis did not reveal any intestinal abnormalities in either Ku80−/− or control mice (data not shown).
Figure 6.
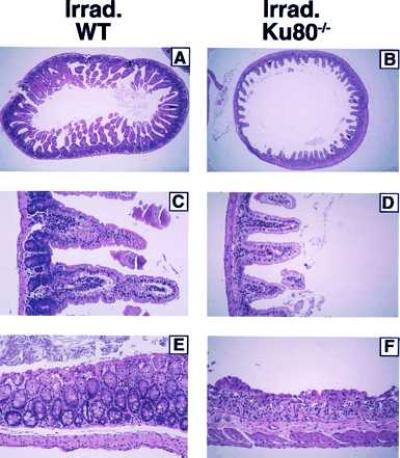
Histological appearance of intestinal lesions induced by γ-irradiation. Three-month-old wild-type and Ku80−/− mice were subjected to 800 cGy and then euthanized at 2, 3, and 4 days postirradiation. Tissue specimens were stained with hematoxylin and eosin, and photographs from postirradiation day 4 experiments are shown. Comparison of the small intestines in irradiated (A) wild-type and (B) Ku80−/− at ×20 magnification shows the marked blunting and loss of villi in Ku80−/− mice. At higher ×100 magnification (C) and (D) the striking absence of crypt cells is demonstrated. Sections of the large intestines (E) and (F) also show denudement of epithelia and collapse of mucosa because of loss of crypts in the Ku80−/− mice.
DISCUSSION
To investigate the role of Ku80 in mammalian DSB repair, we have determined the effects of ionizing radiation on Ku80-deficient cells, tissues, and whole animals. We found that Ku80−/− mice and cells are severely affected by doses of radiation that have no noticeable effect on wild-type controls. Ku80−/− mice are particularly susceptible to whole-body irradiation early in development, when doses of >50 cGy exacerbate their growth deficiency and result in a high frequency of mortality. Interestingly, these low doses of radiation have no discernable affect on the growth or survival of SCID mice, indicating that early in development Ku80−/− mice are more radiosensitive than SCID mice. On the other hand, the radiation response of adult Ku80−/− mice appears to be similar to that of SCID mice (30). Doses >400 cGy produce severe toxic effects to the gastrointestinal tract, and 100% of the animals die within 2 weeks postirradiation. Lower doses (<300 cGy) of ionizing radiation result in abnormalities in the hair pigmentation of Ku80−/− mice, possibly because of the hypersensitivity of Ku80−/− melanocytes to ionizing radiation.
In newborn mice, sublethal doses of ionizing irradiation were found to promote Ku80−/− thymocyte maturation to the CD4+CD8+ DP stage. However, T cell-specific differentiation did not result in an increase in thymus cellularity, nor have we observed any thymic lymphomas to date as previously reported for irradiated newborn SCID mice (23). Although the signaling pathway by which irradiation induces development and expansion of DP thymocytes is not well understood, it is possible that in the absence of Ku80, irradiation does not supply all the necessary signals to fully effect this developmental transition. Alternatively, the lack of significant expansion of Ku80−/− DP thymocytes may be because of the intrinsic sensitivity of DP cells to apoptosis (31), which may be further enhanced in the absence of Ku80. Consistent with this idea, we found that rapidly dividing Ku80−/− pre-B cells also were much more susceptible to spontaneous and γ-ray-induced apoptosis than wild-type controls. Given these observations, it will be interesting to test if the Ku80−/− pre-T cell or pre-B cell populations can be restored to normal lymphocyte numbers in Ku80−/− mice that express T cell receptor β or B cell receptor μ transgenes, respectively.
Ku80−/− ES cells were also hypersensitive to radiation, particularly at low doses.
At doses of irradiation >50 cGy the differential sensitivity between Ku80−/− and controls did not increase, suggesting the possibility of a radiation-resistant population within the Ku80−/− ES cell culture. These observations are consistent with earlier studies on cells from complementation groups 4, 5, and 7, which showed residual DSB rejoining ability and a reduced sensitivity to radiation at the S/G2 phase of the cell cycle. It has been postulated that this residual capacity for DSB repair represents a separate (DNA-PK independent) repair pathway that operates during S/G2, whereas the DNA-PK dependent machinery is active during the G1/S phase of the cell cycle. Recently, cell synchronization experiments on SCID cells have provided additional support for this model (32). Interestingly, unlike cells from SCID mice, Ku80−/− ES cells also exhibit a cross-sensitivity to MMS, which suggests that the Ku80 mutation also may affect the way DNA alkylated bases are processed.
We previously have shown that mice lacking Ku80 exhibit an unexpected growth defect in addition to their immune deficiency. In the current study we have demonstrated that low levels of ionizing radiation exacerbate the growth retardation, increase apoptosis in pre-B cells, and induce the maturation, but not the proliferation, of CD4+CD8+ DP thymocytes. These data suggest that the levels of DNA damage to which Ku80−/− mice are exposed during physiological conditions also would have severe phenotypic consequences. Abnormalities in growth and lymphocyte development both may be manifestations of the inability of Ku80−/− cells to conduct DNA repair during normal cellular metabolism.
The effects of ionizing radiation on Ku80−/− mice are particularly pronounced in rapidly proliferating cells, such as intestinal crypt cells, lymphocytes, and in tissues from newborn animals. Previous analyses of Ku80 gene expression indicates that levels of Ku80 are especially abundant in proliferative tissues and are enhanced when cells are stimulated to proliferate (33, 34). In light of this finding, it is intriguing that Ku80−/− mice are more radiosensitive than SCID mice specifically during the rapid burst in growth in early development. This result suggests that Ku80−/− cells are either less proficient in repairing damaged DNA during this period or that Ku80 has functions in proliferating cells independent of its association with DNA-PKcs. Further investigations should reveal why mutations in different components of the DNA-PK complex result in discrete phenotypes.
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA-31397, CA-56909, and CA-61801.
ABBREVIATIONS
- DNA-PK
DNA-dependent protein kinase
- DSB
DNA double-strand break
- ES
embryonic stem
- SCID
severe combined immunodeficient
- MMS
methyl methanesulfonate
- PE
phycoerythrin
- DN
double negative
- DP
double positive
References
- 1.Jeggo P A, Taccioli G A, Jackson S P. BioEssays. 1995;17:949–956. doi: 10.1002/bies.950171108. [DOI] [PubMed] [Google Scholar]
- 2.Weaver D T. Trends Genet. 1995;11:388–392. doi: 10.1016/s0168-9525(00)89121-0. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Otevrel T, Gao Y, Cheng H-L, Seed B, Stamato T, Taccioli G, Alt F W. Cell. 1995;83:1079–1089. doi: 10.1016/0092-8674(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 4.Anderson C W. Trends Biochem Sci. 1993;18:433–437. doi: 10.1016/0968-0004(93)90144-c. [DOI] [PubMed] [Google Scholar]
- 5.Pan Z Q, Amin A A, Gibbs E, Niu H, Hurwitz J. Proc Natl Acad Sci USA. 1994;91:8343–8347. doi: 10.1073/pnas.91.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taccioli G E, Rathbun G, Oltz E, Stamato T, Jeggo P A, Alt F W. Science. 1993;260:207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- 7.Smider V, Rathmell W K, Lieber M R, Chu G. Science. 1994;266:288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- 8.Nussenzweig A, Chen C, Soares V C, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Nature (London) 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 9.Kirchgessner C U, Patil C K, Evans J W, Cuomo C A, Fried L M, Carter T, Oettinger M A, Brown J M. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 10.Blunt T, Finnie N J, Taccioli G E, Smith G C M, Demengeot J, Gottlieb T M, Mizuta R, Varghese A J, Alt F W, Jeggo P A, Jackson S P. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Jin S, Gao Y, Weaver D T, Alt F W. Proc Natl Acad Sci USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang H, Nussenzweig A, Kurimasa A, Soares V, Li X, Cordon-Cardo C, Li W, Cheong N, Nussenzweig M C, Illiakis G, Chen D, Li G. J Exp Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taccioli G E, Alt F W. Curr Opin Immunol. 1995;7:436–440. doi: 10.1016/0952-7915(95)80085-9. [DOI] [PubMed] [Google Scholar]
- 14.Bosma M J, Carroll A M. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 15.Peterson S R, Kurimasa A, Oshimura M, Dynan W S, Bradbury E M, Chen D J. Proc Natl Acad Sci USA. 1995;92:3171–3174. doi: 10.1073/pnas.92.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danska J S, Holland D P, Mariathasan S, Williams K M, Guidos C J. Mol Cell Biol. 1996;16:5507–5517. doi: 10.1128/mcb.16.10.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blunt T, Gell D, Fox M, Taccioli G E, Lehmann A R, Jackson S P, Jeggo P A. Proc Natl Acad Sci USA. 1996;93:10285–10290. doi: 10.1073/pnas.93.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu C, Bogue M A, Lim D-S, Hasty P, Roth D B. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg N, Baltimore D. J Exp Med. 1976;143:1453–1460. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz M A, Lassota P, Traganos F. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 21.Baker J, Liu J-P, Robertson E J, Efstratiadis A. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 22.Murphy W J, Durum S K, Anver M R, Ferris D K, McVicar D W, O’Shea J J, Ruscetti S K, Smith M R, Young H, Longo D L. J Immunol. 1994;153:1004–1014. [PubMed] [Google Scholar]
- 23.Danska J S, Pflumio F, Williams C J, Huner O, Dick J E, Guidos C J. Science. 1994;266:450–455. doi: 10.1126/science.7524150. [DOI] [PubMed] [Google Scholar]
- 24.Zuniga-Pflucker J C, Jiang D, Schwartzberg P L, Lenardo M J. J Exp Med. 1994;180:1517–1521. doi: 10.1084/jem.180.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidos C J, Williams C J, Wu G E, Paige C J, Danska J S. J Exp Med. 1995;181:1187–1195. doi: 10.1084/jem.181.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G C, Nussenzweig A. In: Stress-Inducible Cellular Responses. Feige U, Morimoto R I, Yahara I, Polla B S, editors. Vol. 77. Basel: Birkhauser; 1996. pp. 425–450. [Google Scholar]
- 27.Lee S E, Pulaski C R, He D M, Benjamin D M, Voss M, Um J, Hendrickson E A. Mutat Res. 1995;366:279–291. doi: 10.1016/0921-8777(95)00002-2. [DOI] [PubMed] [Google Scholar]
- 28.Stamato T D, Dipatri A, Giaccia A. Radiat Res. 1988;115:325–333. [PubMed] [Google Scholar]
- 29.Hendrickson E A, Qin X-Q, Bump E A, Schatz D G, Oettinger M, Weaver D T. Proc Natl Acad Sci USA. 1991;88:4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biedermann K A, Sun J, Giaccia A J, Tosto L M, Brown J M. Proc Natl Acad Sci USA. 1991;88:1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith C A, Williams G T, Kingston R, Jenkinson E J, Owen J J T. Nature (London) 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 32.Lee S E, Mitchell R A, Cheng A, Hendrickson E A. Mol Cell Biol. 1997;17:1425–1433. doi: 10.1128/mcb.17.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Q-Q, Plet A, Imbert J, Lafage-Pochitaloff M, Cerdan C, Blanchard J-M. Cytogenet Cell Genet. 1994;65:221–227. doi: 10.1159/000133635. [DOI] [PubMed] [Google Scholar]
- 34.Yaneva M, Jhiang S. Biochim Biophys Acta. 1991;1090:181–187. doi: 10.1016/0167-4781(91)90099-8. [DOI] [PubMed] [Google Scholar]


