Abstract
Recent studies in a variety of leukemias and solid tumors indicate that there is significant heterogeneity with respect to tumor-forming ability within a given population of tumor cells, suggesting that only a subpopulation of cells is responsible for tumorigenesis. These cells have been commonly referred to as cancer stem cells (CSCs) or cancer-initiating cells (CICs). CICs have been shown to be relatively resistant to conventional anticancer therapies and are thus thought to be responsible for disease relapse. As such, they represent a potentially critical therapeutic target. Oncolytic viruses are in clinical trials for cancer and kill cells through mechanisms different from conventional therapeutics. Because these viruses are not susceptible to the same pathways of drug or radiation resistance, it is important to learn whether CICs are susceptible to oncolytic virus infection. Here we review the available data regarding the ability of several different oncolytic virus types to target CICs for destruction.
Introduction
Over the past 40 years, anticancer agents have entered clinical practice and improved survival for many patients with cancer. Unfortunately, even for patients who achieve remission following treatment with surgery, chemotherapy, radiation therapy, and some of the newer targeted inhibitors and antibodies, relapse often occurs months or years later. Hypotheses to explain this phenomenon include ideas such as tumor cell acquisition of resistance, suboptimal surgical debulking and inability of chemotherapy and radiotherapy to target all cancer cells within a given patient. The cancer stem cell (CSC) theory postulates that there are subpopulations of cells, also known as cancer-initiating cells (CICs), responsible for relapse.
The term “CSCs” was originally coined to describe features of these cells that are similar to bona fide normal stem cells.1 Whether CSCs arise from normal stem cells is still under debate; their origin is likely to vary among different cancers. By definition, CSCs possess the shared properties with normal stem cells of self-renewal and pluripotency. Normal stem cells also express drug resistance genes, such as the ATP-binding cassette protein efflux pump ABCG2, which presumably provide these cells with a measure of protection from environmental toxins.2 Similarly, many identified CSCs express such genes and are relatively resistant to chemotherapy and irradiation, making them prime candidates as the source of relapse.3 Because CSCs may not share other features with normal stem cells, and due to their uncertain origin, there is a growing preference to refer to them by the less controversial and more precise terms of CICs. Nevertheless, their role in producing daughter cells that constitute the bulk of a tumor is similar to the role of normal stem cells in generating the bulk of an organ (or blood, in the case of bone marrow stem cells). Although considerably more study is required to faithfully classify, isolate, and characterize the biology and role of CICs, therapeutic targeting of these cells may reduce relapse rates and improve long-term outcome for patients with many types of cancers.4
The past decade has seen an explosion of research into the field of gene therapy and therapeutic, or so-called “oncolytic,” viruses.5 Such viruses fall into broad categories of (i) wild-type animal viruses that do not typically infect human cells but are cytotoxic to human cancer cells, (ii) attenuated mutants of human viruses in which critical genes for virus replication that are dispensable in cancer cells have been deleted or mutated, and (iii) viruses that have been attenuated by serial passage in culture, such as most live virus vaccines. These agents hold much promise as they have been shown to be efficacious against malignant tissues, yet minimally toxic to their normal cell and tissue counterparts. Oncolytic viruses are effective against a wide variety of human cancers in preclinical models and encouraging results from clinical trials are beginning to accumulate. Novel methods of delivery, including cell-based schemes, appear to increase their ability to reach distant metastatic sites of disease, counteracting a major criticism that they will be useful only for localized disease.6 Oncolytic viruses may also be engineered to deliver therapeutic transgenes, thereby increasing their antitumor effects.
The question of whether oncolytic viruses are well suited to eliminate CICs has begun to be addressed. Oncolytic viruses seem like ideal candidates to target CICs because they are cytotoxic and are not subject to the typical mechanisms of drug resistance such as drug efflux pumps and defective apoptotic signaling.7 In addition, viruses may be engineered to express therapeutic transgenes that specifically target properties that CICs rely upon for self-renewal and cell division. Indeed, initial studies suggest that oncolytic viruses may be effective against and may be directed toward CICs.8 The focus of this review is to highlight recent studies using oncolytic viruses against CICs to determine whether oncolytic viruses are able to eradicate CICs and prevent tumor formation or relapse. We have separated our discussion of DNA viruses from RNA viruses because they exhibit fundamental differences in their life cycles that might impact their ability to kill CICs (DNA viruses replicate in the nucleus whereas RNA viruses replicate in the cytoplasm).
DNA Viruses
Herpes simplex virus
The majority of studies with herpes simplex virus (HSV)–derived mutants have been conducted in models of malignant brain tumors, partly because of HSV's neurotropism as well as the fact that these were among the first solid tumors in which CICs were identified. Initial studies of brain tumors mainly used serum-free basal medium supplemented with epidermal growth factor and fibroblast growth factor (EF medium) to culture surgical specimens.9,10 This medium, also referred to as neural stem cell medium, was originally designed for culture of neuronal stem cells to maintain their “stemness” properties in vitro.11 When cultured in EF medium, primary brain tumor specimens form three-dimensional spheres and show many features resembling normal stem cells, including self-renewal, expression of stem cell markers such as CD133 and nestin, and multilineage differentiation. Most importantly, cells derived from these “tumorspheres” are able to form xenografts in mice that recapitulate their original morphological phenotype.10,12
The HSV-1 RL1 gene encodes ICP34.5, the “neurovirulence factor,” which reverses the host cell's protein kinase R mediated shutoff of protein synthesis, thereby enabling efficient virus replication even in the presence of a robust interferon (IFN) response.13,14 Virus mutants deleted for RL1 alone (e.g., HSV1716) or RL1 combined with deletion of the UL31 gene that encodes the large subunit of ribonucleotide reductase, ICP6 (e.g., G207, OncoVexGM-CSF), have proven safe in clinical trials by direct intratumoral injections in brain, melanoma and head/neck squamous cell carcinoma.15,16,17,18,19,20,21 While severely attenuating virus replication in normal postmitotic cells, lack of ICP34.5 expression also impairs their replication to some degree in cancer cells. Taking advantage of differential expression of nestin in glioma cells versus normal astrocytes, Kambara et al. restored ICP34.5 expression in an ICP34.5-deleted virus under the control of the nestin enhancer. This virus, rQnestin34.5, showed selective targeting to glioma cells.22 Nestin is also expressed in many brain tumor stem cells and its expression is decreased in differentiated cells. Otsuki et al. showed that primary glioma tumorspheres were effectively targeted by rQnestin34.5 compared to the ICP34.5-null control virus, rHSVQ1 (ref. 23). Moreover, pretreatment with the histone deacetylase inhibitor, valproic acid, augmented viral propagation and antitumor efficacy in glioma cells including primary sphere cells. The authors found that valproic acid inhibited the IFN response and significantly downregulated IFN-responsive antiviral genes following HSV-1 infection. Valproic acid pretreatment also counteracted exogenously applied IFNβ-mediated inhibition of viral propagation. Interestingly, IFNβ treatment inhibited rQnestin34.5 replication by >99.99% in primary sphere cells while showing far less effects in other tested glioma cultured lines. Similarly, Zhang has found that breast CICs isolated from cell lines are somewhat resistant to infection with an oncolytic HSV-2 mutant, but could be made sensitive with addition of the histone deacetylase inhibitor, trichostatin A (X. Zhang, Baylor College of Medicine, personal communication, 6 Jun 2009). Taken together, these findings suggest that an intact IFN response may be an unique feature of CICs compared with bulk cancer cells.
Using the same rQnestin34.5 virus, Mahller et al. showed that CICs from a neuroblastoma cell line, LA-N-5, were susceptible to infection by oncolyic HSV-1 (ref. 8). Neuroblastoma is a childhood cancer thought to arise from the embryonic neural crest.24 LA-N-5 cells cultured in EF medium as tumorspheres were enriched for expression of CD133 and ABCG2 and more resistant to doxorubicin compared to the serum-grown bulk cultures. Spheres derived from LA-N-5 and two other neuroblastoma cell lines, IMR-32 and CHP-134, showed multilineage differentiation indicating pluripotency of neuroblastoma tumorspheres. In vitro cytotoxicity and viral production assays confirmed that both LA-N-5 bulk cells and tumorspheres were sensitive to nestin-targeted HSV-1 infection. In addition, mice inoculated with LA-N-5 bulk cells preinfected with rQnestin34.5 showed no flank tumor formation for >2 months whereas mice injected with saline or control virus–treated cells showed rapid tumorigenesis.
Because these studies used established cell lines, it is important to determine whether similar results are found in primary human CICs. One source of such CICs is from the bone marrow of patients with neuroblastoma, as reported by Hansford et al., obtained by culturing bone marrow aspirates in EF medium.25 The resulting sphere-forming cells were highly tumorigenic, requiring as few as 10 cells to initiate tumor growth in immunocompromised mice. In collaboration with Hansford et al., we have found two of three primary CICs to be as susceptible as LA-N-5 cells to oncolytic HSV infection (P.-Y. Wang and T.P. Cripe, unpublished results). Reasons for resistance of the third culture are under investigation.
In invasive glioma models, Wakimoto et al. showed that the HSV-1 mutant, G47Δ (ICP6−, γ234.5−, α47−), effectively targeted glioblastoma (GBM) CICs.26 G47Δ is derived from G207 (ICP6−, ICP34.5−) with the additional deletion of the α47 coding sequence and the US11 promoter, which places expression of the US11 gene under the control of the α47 promoter. Expression of the normally late-expressed US11 gene under the immediate-early α47 promoter partially compensates for the lack of ICP34.5 and enhances viral growth in infected cells.27 Four GBM surgical specimens were cultured in vitro as tumorspheres and confirmed to exhibit CIC characteristics (CD133 and nestin expression, differentiation potential, and tumorigenicity in an orthotopic xenograft model).26 Tumorigenicity between the different GBM cells appeared to correlate with CD133 expression. Only 50 GBM8 sphere-derived cells (92.8% CD133+) were required to consistently form tumors in mice, whereas nearly 5 × 104 GBM8 cells cultured in FCS condition (<20% CD133+) and 5 × 103 GBM4 sphere cells (38.1% CD133+) were required to create tumors in vivo. In comparison with wild-type and different HSV-1 F strain mutants, G47Δ showed less antitumor efficacy in GBM spheres than wild-type and ICP6-deficient FΔ6 but significant more than ICP34.5 mutants (G207 & R3616), which had a minimal effect on cell survival. G47Δ targets both CD133+ and CD133− GBM cells, and infected GBM cells were unable to form secondary spheres in vitro, suggesting GBM CICs are susceptible to virus infection. A single intratumoral injection of G47Δ prolonged survival of mice bearing CIC-derived tumors. Similarly, recent studies by Friedman et al. suggest glioma progenitor cells are sensitive to killing by HSV ICP34.5-defective mutants, but only if the cells express sufficient amounts of the major HSV receptor, nectin-1 (ref. 28). It is of interest to note that some CICs have been shown to have relatively intact and even hyperactivated DNA repair mechanisms,29 a property thought to be responsible for their relative resistance to chemotherapy and irradiation, and activated DNA repair pathways enhance replication of HSV mutants.30
Adenovirus
In order to replicate, adenovirus (Ad) promotes entry of cells into the G1 phase of the cell cycle by binding Rb via the immediate-early protein E1A and releasing the transcriptional factor E2F.31 Thus, Ad is capable of infecting both dividing and nondividing cells. Because many tumor cells harbor defects in the Rb/p16 pathway, mutant Ads with a 24 base pair deletion of the E1A Rb binding site (Δ24) showed tumor selectivity as viral replication was abrogated in normal cells with intact Rb/p16 (refs. 32,33). Most Ad serotypes, including commonly used Ad5, enter into cells through their viral fiber knob binding to the host cell surface coxsackie-Ad receptor,34 which is highly expressed on normal epithelial cells but lacking in many tumor cells.35 Modification of the viral capsid to change the Ad tropism has been a common strategy to overcome the lack of coxsackie-Ad receptor in tumor cells.
Eriksson et al. showed capsid-modified E1A mutated Ads, Ad5/3-Δ24 (ref. 36), which uses the Ad serotype 3 receptor that is highly expressed in tumor cells, and Ad5.pk7-Δ24 (ref. 37), which enters through heparan sulfate proteoglycans, were able to kill breast CICs.38 Breast CICs have been identified as a CD44+/CD24−/low population in breast carcinoma.39,40 These cells represent a small subpopulation and exclusively retain tumorgenicity in the xenograft model. Like brain tumor CICs, breast CICs can be propagated in vitro as spheres (“mammospheres”) and retain stem cell–like characteristics. CD44+/CD24−/low cells freshly sorted from pleural effusions were enriched in a Hoeschst 33342 “side population” (from 1% to 7%), a known characteristic of bone marrow stem cells, and expressed the stem cell markers oct4 and sox2 (ref. 38). In vitro, both Ad5/3-Δ24 and Ad5.pk7-Δ24 effectively killed unsorted and CD44+/CD24−/low cells compared to an Ad5 wild-type or a replication-deficient control, suggesting tumor tropism and selectivity of these capsid-modified viruses. In vivo, no tumor grew in mice injected with Ad5/3-Δ24-infected CD44+/CD24−/low cells. In addition, intratumoral injection of Ad5/3-Δ24 or Ad5.pk7-Δ24 into tumors derived from CD44+/CD24−/low CICs stopped tumor growth and prolonged animal survival. Bauerschmitz et al. from the same group later demonstrated that tissue-specific promoter (TSP)–controlled Ad5/3 variants also target CD44+/CD24− breast CICs.41 Cyclo-oxygense-2 (Cox-2), telomerase (hTERT), and multidrug resistance (mdr) protein promoters were used to control E1A expression and showed activated promoter activity in CD44+/CD24− cells. In vitro, Ad5/3-mdr-Δ24 was the most oncolytic for CD44+/CD24−/low cells freshly collected from two out of three pleural effusions and Ad5/3-hTERT-Δ35 and Ad5/3-Cox2L-Δ24 closely followed. In vivo, these viruses all showed significant antitumor efficacy compared with a mock control against CD44+/CD24−/low-derived tumors. Interestingly, in both studies, the proportion of CD44+/CD24−/low cells in the recipient tumor returned to the same level as presorted cells [~3% for pleural effusion41 and ~10% for short-term cultured cell line, JIMT-1 (ref. 38)], even when injected with 100% CD44+/CD24−/low cells, consistent with asymmetric cell division. After virus treatment, CD44+/CD24−/low from JIMT-1 tumors decreased to 1.1% (Ad5.pk7-Δ24) and ~5% (Ad5/3-Δ24), and the Hoechst side population in CD44+/CD24−/low cells decreased from 14% (untreated) to 3%, suggesting the capsid-modified Ads indeed target CICs in the tumors.38 In contrast, the proportion of CD44+/CD24−/low cells from fresh pleural-derived tumors remained similar or slightly lower (3.1% vs. 2.6%) after treatment with Ad5/3-mdr-Δ24 (ref. 41), suggesting perhaps a less effective targeting of the CICs in the primary tumor setting. Although promising results were shown in both studies, complete tumor eradication was not seen in either case. The fact that the viruses efficiently infected CD44+/CD24−/low cells in vitro indicates a barrier for viruses to reach all tumor cells in vivo.
Studies of brain tumor CICs with Ad have also been pursued. Jiang et al. showed that tumorspheres derived from surgical GBM specimens express high levels of coxsackie-Ad receptor and the Ad internalization receptors, αvβ3 and αvβ5 integrins, which enabled the Ad mutant Delta-24-RGD to effectively target these cells in vitro and prolong survival in tumor-bearing mice.42 They further demonstrated that Delta-24-RGD-mediated cell death is via an autophagy process, as indicated by accumulation of autophagic vacuoles and Atg5 and LC3II proteins in the infected cells.
Skog et al. first reported using Ad vectors other than the most commonly used serotypes 3 or 5 to target brain CICs.43 Their results showed Ad16p and chimpanzee Ad CV23 effectively target GBM cell lines and both CD133+ and CD133− cells freshly isolated from primary brain tumors. In contrast, Ad5 was mainly effective in higher passage established cell lines but very poor in primary specimens, indicating differences between primary and culture-adapted cells relative to Ad5 treatment.
Myxoma virus
Myxoma virus is a member of the poxviridae family, contains a double-stranded DNA genome and causes disease (myxomatosis) in rabbits. Although the virus does not infect normal human cells, it is highly replicative in and lytic for many different human cancer cells.44 The primary reason for the permissiveness of these cells is their altered signaling pathways, particularly Akt.45 In preliminary studies in Paul Beaudry's group at the University of Calgary, Alberta, Canada, Redding et al. reported that myxoma virus effectively infected neuroblastoma CIC cultures isolated by Hansford et al.46 This finding may indicate that neuroblastoma CICs have altered Akt signaling, which is known to be an important survival signal in neuroblastoma cells,47 and suggest this may be an effective virus-tumor combination.
RNA Viruses
Reovirus
Reovirus is a double-stranded RNA virus generally considered to be benign in humans, typically causing at most mild respiratory or gastrointestinal ailments. Interestingly, the virus is highly cytopathic for a variety of cultured cancer cell lines and induces rapid tumor regression in mouse xenograft models of a variety of human cancers including those from colon, ovarian, breast, lymphoma, brain and spinal cancer derived cells.48,49,50,51,52,53 Reovirus is oncolytic in its naturally isolated state and over the past decade has been in development as a potential cancer therapeutic (Reolysin).54 After promising results from phase I/II clinical trials on various cancers, Reolysin is scheduled to begin phase III clinical trials for head and neck cancer in the 4th quarter of 2009.
Recently, we (P.M. and P.W.K.L.) used biopsy cores taken from the primary tumor of a breast cancer patient to grow tumors in the mammary fat pad of immunocompromised mice.55 Subsequent reovirus injection induced regression of these patient-derived breast tumors. In this study, breast CICs were identified by the two published methods, tumor cells with CD44+/CD24−/low cell surface expression and cells positive for aldehyde dehydrogenase 1 expression (Aldefluor+).39,56 CIC and non-CIC populations in the reovirus-treated tumors were found to be equally infected and eradicated. As predicted, it appears that characteristics of CICs that make them resistant to common chemotherapeutics and radiation treatments are not hindering factors in reovirus cancer therapy.29,57,58,59,60,61
Reovirus permissiveness has been shown to correlate with the activation status of the Ras signaling pathway(s) in the host cell.62 Although Ras mutations are present in only about 30% of all human cancers, the fact that mutations in upstream activators and/or downstream effectors of Ras can also result in enhanced Ras signaling suggests that most cancers are theoretically treatable by reovirus.63 Ras proteins function as molecular switches in many cellular processes including apoptosis, cell cycle transitions, protein translation, cytoskeletal rearrangement, and intracellular vesicle transport.64 Although we did not assess Ras activation status, we found that isolated breast CICs and non-CICs had similar levels of total Ras expression.55 This finding is congruent with the observation that the two cancer cell subpopulations were equally sensitive to reovirus infection.
The cellular changes undergone by a cancer cell or induced by Ras transformation have favorable effects on multiple steps of reovirus replication.65 First, once the virus enters a cell it must undergo an uncoating step in order to initiate the infectious process. This step requires sufficient levels of cathepsin lysosomal proteases that are upregulated in cancer cells and by Ras signaling.65,66,67 Second, Ras-mediated transformation lends a favorable cellular environment that enhances infectivity of the progeny virus particles, possibly by facilitating post-translational modification of viral proteins, and their subsequent assembly into virions. Finally, cancer cells are significantly more sensitive to reovirus-induced apoptosis at late stages of the infection, resulting in enhanced release, and therefore spread of the virus through subsequent rounds of infection.51,65,68 In this regard, it is interesting to note that JNK activation and NF-κB have been implicated in reovirus-induced apoptosis and both are altered by Ras-induced transformation and oncogenesis.64,69,70,71,72 The cumulative effect of these cellular changes results in the heightened sensitivity of cancer cells to reovirus infection, which thus far appears to also manifest in breast CICs. It will be important to determine whether reovirus can also target and kill CICs from other tumor types.
Vesicular stomatitis virus
A member of the rhabdoviridae family, vesicular stomatitis virus (VSV) is a negative sense single-stranded RNA virus that is highly sensitive to the antiviral IFN response. This acute sensitivity to IFN-induced changes effectively blocks infection of the virus in normal cells. It also makes the virus an ideal naturally oncolytic agent as cancer cells often have a deregulated IFN response, allowing for uninhibited VSV replication.73 In addition, tumor cells with defects in Ras, p53 or Myc signaling pathways have been shown to be permissive to VSV.74
VSV is an animal pathogen, which can also infect humans. In humans, VSV infections are usually asymptomatic, but it can cause flu-like symptoms, raising some concern about using the virus as a cancer therapeutic in its naturally isolated state. Because the original observations that a laboratory VSV isolate (strain Indiana) efficiently infects and kills tumor cells in vitro and in vivo,73 natural mutants and engineered versions have been isolated with improved oncolytic cancer-killing capabilities and tumor specificity.75 For example, the HSV thymidine kinase gene has been incorporated into the VSV genome.76 This suicide cassette is designed to enhance killing of VSV-infected tumor cells and also uninfected neighboring tumor cells through the “bystander effect.” In another example, interleukin-4 cDNA was incorporated into the VSV genome in order to enhance anti-tumor immunity.76 Using an antibody binding domain, Bergman et al. engineered a VSV variant that could target Her2/neu overexpressing cells, which is most typical of breast cancers77 and plays an important biologic role in breast CICs.78 Naturally isolated VSV variants AV1 and AV2 have mutated matrix (M) proteins (responsible for blocking IFN induction in infected cells), and are further attenuated for replication in normal cells but still retain full cancer-killing ability.79 This finding led to engineered versions such as recombinant VSV ΔM51, which has reduced toxicity but is highly effective at targeting various primary and metastasized tumors in vivo.79,80
Numerous studies have convincingly shown the ability of VSV and the various recombinant strains to induce tumor regression in multiple cancers (e.g., melanoma, lung, colon, brain).81 In view of increasing evidence of the role of CICs in cancer, the question whether this RNA virus is also able to target and kill CICs remains. Using the VSV recombinant strain, ΔM51, Redding et al. are testing the efficacy of VSV to infect and kill the primary human neuroblastoma CICs isolated by Hansford et al.25 mentioned previously. Preliminary results suggest these cells may be resistant to VSV infection (N. Redding and P. Beaudry, University of Calgary, personal communication, 5 June 2009). If confirmed, resistance of neuroblastoma CICs to VSV may lead to interesting studies on their biology, including their IFN response.
Concluding Remarks
For many different cancer types there is now considerable evidence supporting the stem cell theory of cancer, which suggests that a relatively small subpopulation of cells (CICs) within a tumor are actually tumorigenic and responsible for generating the bulk of nontumorigenic cancer cells. Although not yet proven, the identification, isolation, and characterization of such cells is likely to be paramount to discover effective new cancer therapies that prevent relapse and improve long-term overall survival. With the recent resurgence of interest in the use of oncolytic viruses as cancer therapeutics, their effects on CICs may ultimately determine whether they play a significant role in improving survival rates. Because they are not subject to the same mechanisms mediating resistance to cytotoxic chemotherapy and irradiation, there is ample reason to postulate oncolytic viruses will be effective at eradicating CICs.
Although this topic is only beginning to be addressed as the identification of CICs in various cancers are revealed, the data so far present a mixed picture. Some CICs appear susceptible to virus infection and some do not, depending on the virus mutation and mechanism of attenuation (Table 1). The disrupted IFN response found in many cancer cells creates an oncolytic virus therapeutic window; virus replication is permitted in cancer cells but is thwarted by normal cells. Some data indicate CICs may have a more robust, intact IFN response than the bulk cancer cells, suggesting this may be a mechanism of relative resistance to virus infection. Thus the selection of genetic mutants that can counteract that response may be critical. Fortunately, histone deacetylase inhibitors also appear to counteract the IFN effects and restore virus permissiveness, suggesting effective treatment with oncolytic viruses in some cases may require such concomitant “virus-enabling” therapies. Although there do not appear to be universal themes yet emerging, of this we are certain: with the myriad different virus types and genetic mutations under investigation as oncolytic agents and the rapidly expanding list of CICs being discovered, the interaction of oncolytic viruses with CICs will be a fruitful area of investigation for years to come.
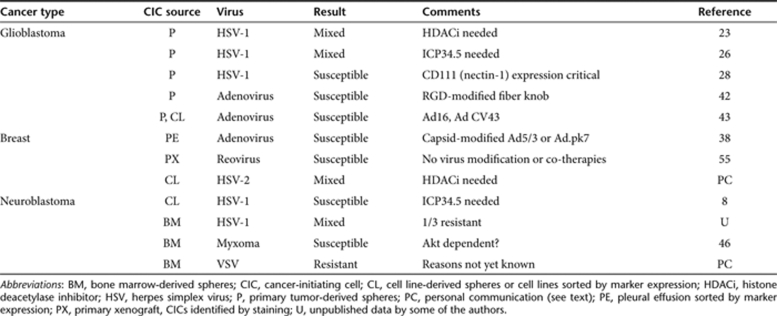
Table 1.
Summary of virotherapy studies using CICs
Acknowledgments
This work was funded in part by TeeOffAgainstCancer.org and NIH grants R01-CA114004 and R21-CA133663 to TPC as well as by operating grants to P. Lee from the Canadian Institutes for Health Research, Canadian Breast Cancer Foundation and Cancer Care Nova Scotia.
REFERENCES
- Jordan CT. Cancer stem cells: controversial or just misunderstood. Cell Stem Cell. 2009;4:203–205. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MO, Kanemura Y, Tajria J, Mori H, Kobayashi S, Hara M, et al. Functional expression of ABCG2 transporter in human neural stem/progenitor cells. Neurosci Res. 2005;52:75–82. doi: 10.1016/j.neures.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Donnenberg VS., and , Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45:872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- Dingli D., and , Michor F. Successful therapy must eradicate cancer stem cells. Stem Cells. 2006;24:2603–2610. doi: 10.1634/stemcells.2006-0136. [DOI] [PubMed] [Google Scholar]
- Liu TC., and , Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- Power AT., and , Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- Coukos G, Makrigiannakis A, Kang EH, Rubin SC, Albelda SM., and , Molnar-Kimber KL. Oncolytic herpes simplex virus-1 lacking ICP34.5 induces p53-independent death and is efficacious against chemotherapy-resistant ovarian cancer. Clin Cancer Res. 2000;6:3342–3353. [PubMed] [Google Scholar]
- Mahller YY, Williams JP, Baird WH, Mitton B, Grossheim J, Saeki Y, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS ONE. 2009;4:e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Reynolds BA., and , Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Bolovan CA, Sawtell NM., and , Thompson RL. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol. 1994;68:48–55. doi: 10.1128/jvi.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M., and , Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKie RM, Stewart B., and , Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357:525–526. doi: 10.1016/S0140-6736(00)04048-4. [DOI] [PubMed] [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- Mace AT, Ganly I, Soutar DS., and , Brown SM. Potential for efficacy of the oncolytic Herpes simplex virus 1716 in patients with oral squamous cell carcinoma. Head Neck. 2008;30:1045–1051. doi: 10.1002/hed.20840. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre- and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Okano H, Chiocca EA., and , Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- Maris JM, Hogarty MD, Bagatell R., and , Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- Hansford LM, McKee AE, Zhang L, George RE, Gerstle JT, Thorner PS, et al. Neuroblastoma cells isolated from bone marrow metastases contain a naturally enriched tumor-initiating cell. Cancer Res. 2007;67:11234–11243. doi: 10.1158/0008-5472.CAN-07-0718. [DOI] [PubMed] [Google Scholar]
- Wakimoto H, Kesari S, Farrell CJ, Curry WT, Zaupa C, Aghi M, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–3481. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD., and , Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GK, Langford CP, Coleman JM, Cassady KA, Parker JN, Markert JM, et al. Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111 J Neurooncol 2009. epub ahead of print [DOI] [PMC free article] [PubMed]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Aghi M, Rabkin S., and , Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, et al. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- Tomko RP, Xu R., and , Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribacka C., and , Hemminki A. Virotherapy as an approach against cancer stem cells. Curr Gene Ther. 2008;8:88–96. doi: 10.2174/156652308784049372. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Ranki T, Kanerva A, Ristimäki A, Hakkarainen T, Särkioja M, Kangasniemi L, et al. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5.pk7-Delta24, for the treatment of advanced breast cancer. Gene Ther. 2007;14:58–67. doi: 10.1038/sj.gt.3302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Guse K, Bauerschmitz G, Virkkunen P, Tarkkanen M, Tanner M, et al. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24−/low cells. Mol Ther. 2007;15:2088–2093. doi: 10.1038/sj.mt.6300300. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ., and , Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- Bauerschmitz GJ, Ranki T, Kangasniemi L, Ribacka C, Eriksson M, Porten M, et al. Tissue-specific promoters active in CD44+CD24−/low breast cancer cells. Cancer Res. 2008;68:5533–5539. doi: 10.1158/0008-5472.CAN-07-5288. [DOI] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- Skog J, Edlund K, Bergenheim AT., and , Wadell G. Adenoviruses 16 and CV23 efficiently transduce human low-passage brain tumor and cancer stem cells. Mol Ther. 2007;15:2140–2145. doi: 10.1038/sj.mt.6300315. [DOI] [PubMed] [Google Scholar]
- Stanford MM., and , McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding N, Zhou H-Y, Lun X, Senger D, Forsyth P, Robbins S, et al. The utility of oncolytic viruses against neuroblastoma 2009. In The 5th International Meeting on Replicating Oncolytic Virus Therapeutics, Banff, Canada
- Sartelet H, Oligny LL., and , Vassal G. AKT pathway in neuroblastoma and its therapeutic implication. Expert Rev Anticancer Ther. 2008;8:757–769. doi: 10.1586/14737140.8.5.757. [DOI] [PubMed] [Google Scholar]
- Alain T, Hirasawa K, Pon KJ, Nishikawa SG, Urbanski SJ, Auer Y, et al. Reovirus therapy of lymphoid malignancies. Blood. 2002;100:4146–4153. doi: 10.1182/blood-2002-02-0503. [DOI] [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA., and , Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Norman KL, Coffey MC, Hirasawa K, Demetrick DJ, Nishikawa SG, DiFrancesco LM, et al. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- Smakman N, van den Wollenberg DJ, Borel Rinkes IH, Hoeben RC., and , Kranenburg O. Sensitization to apoptosis underlies KrasD12-dependent oncolysis of murine C26 colorectal carcinoma cells by reovirus T3D. J Virol. 2005;79:14981–14985. doi: 10.1128/JVI.79.23.14981-14985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- Yang WQ, Senger DL, Lun XQ, Muzik H, Shi ZQ, Dyck RH, et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Ther. 2004;11:1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- Yap TA, Brunetto A, Pandha H, Harrington K., and , Debono JS. Reovirus therapy in cancer: has the orphan virus found a home. Expert Opin Investig Drugs. 2008;17:1925–1935. doi: 10.1517/13543780802533401. [DOI] [PubMed] [Google Scholar]
- Marcato P, Dean CA, Giacomantonio CA., and , Lee PW. Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther. 2009;17:972–979. doi: 10.1038/mt.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Lee TK, Zheng BJ, Chan KW., and , Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Strong JE, Coffey MC, Tang D, Sabinin P., and , Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Malumbres M., and , Pellicer A. RAS pathways to cell cycle control and cell transformation. Front Biosci. 1998;3:d887–d912. doi: 10.2741/a331. [DOI] [PubMed] [Google Scholar]
- Marcato P, Shmulevitz M, Pan D, Stoltz D., and , Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–1530. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- Golden JW, Linke J, Schmechel S, Thoemke K., and , Schiff LA. Addition of exogenous protease facilitates reovirus infection in many restrictive cells. J Virol. 2002;76:7430–7443. doi: 10.1128/JVI.76.15.7430-7443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain T, Kim TS, Lun X, Liacini A, Schiff LA, Senger DL, et al. Proteolytic disassembly is a critical determinant for reovirus oncolysis. Mol Ther. 2007;15:1512–1521. doi: 10.1038/sj.mt.6300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smakman N, van den Wollenberg DJ, Elias SG, Sasazuki T, Shirasawa S, Hoeben RC, et al. KRAS(D13) Promotes apoptosis of human colorectal tumor cells by ReovirusT3D and oxaliplatin but not by tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2006;66:5403–5408. doi: 10.1158/0008-5472.CAN-05-4108. [DOI] [PubMed] [Google Scholar]
- Clarke P, Meintzer SM, Wang Y, Moffitt LA, Richardson-Burns SM, Johnson GL, et al. JNK regulates the release of proapoptotic mitochondrial factors in reovirus-infected cells. J Virol. 2004;78:13132–13138. doi: 10.1128/JVI.78.23.13132-13138.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JL, Rodgers SE, Clarke P, Ballard DW, Kerr LD, Tyler KL, et al. Reovirus-induced apoptosis requires activation of transcription factor NF-kappaB. J Virol. 2000;74:2981–2989. doi: 10.1128/jvi.74.7.2981-2989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ., and , Baldwin AS. Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- Pruitt K, Pruitt WM, Bilter GK, Westwick JK., and , Der CJ. Raf-independent deregulation of p38 and JNK mitogen-activated protein kinases are critical for Ras transformation. J Biol Chem. 2002;277:31808–31817. doi: 10.1074/jbc.M203964200. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Porosnicu M., and , Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol. 2001;75:3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF., and , Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Porosnicu M, Markovic D., and , Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman I, Whitaker-Dowling P, Gao Y, Griffin JA., and , Watkins SC. Vesicular stomatitis virus expressing a chimeric Sindbis glycoprotein containing an Fc antibody binding domain targets to Her2/neu overexpressing breast cancer cells. Virology. 2003;316:337–347. doi: 10.1016/j.virol.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Iovino F., and , Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- Sinkovics JG., and , Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz) 2008;56 Suppl 1:3s–59s. doi: 10.1007/s00005-008-0047-9. [DOI] [PubMed] [Google Scholar]