Abstract
We have used Syrian hamsters to examine the role of pre-existing immunity to adenovirus (Ad) 5 in the toxicity of the oncolytic Ad vector INGN 007. Groups of hamsters were or were not immunized with Ad5. Half the hamsters were immunosuppressed using cyclophosphamide (CP), then injected intravenously (i.v.) with 3× the maximum tolerated dose (MTD) of INGN 007 (in immunocompetent hamsters), and toxicity and vector replication in the liver were quantitated. In nonimmunized immunocompetent hamsters, toxicity was observed early but the hamsters recovered by day 6 after vector injection. In nonimmunized immunosuppressed hamsters, the vector was lethal by 3 days. Pre-existing neutralizing antibody (NAb) prevented liver infection and hepatotoxicity in both immunocompetent and immunosuppressed hamsters. In another study, passive immunization of immunosuppressed hamsters 1 day before a lethal dose (1× MTD) of INGN 007 prevented liver infection and replication, but immunization 1 day after vector administration was barely effective. When immunosuppressed hamsters were passively immunized 1 day after injection of 1/3rd the MTD of INGN 007, then significant protection was observed against liver infection and toxicity. Therefore, serum NAb are sufficient to prevent oncolytic Ad vector liver infection and toxicity. We saw no evidence that pre-existing immunity was associated with increased vector toxicity.
Introduction
A number of viruses including adenoviruses (Ads) have been employed as replication-defective vectors that deliver therapeutic genes into target tissues.1,2,3 Ads have also been designed as conditionally replicative oncolytic vectors for cancer gene therapy in which the viruses themselves act as anticancer therapeutic agents.4 Oncolytic Ad vectors rely on infection and lysis of cancer cells and spread throughout the tumor. Phase I and II clinical trials have been conducted with various oncolytic Ad vectors; so far, these vectors have caused minimal toxicity and have displayed antitumor efficacy in some studies in combination with chemotherapy and/or radiation therapy.5 An oncolytic Ad vector named H101 was recently approved for treatment of head and neck cancer by direct intratumoral (i.t.) injection of the vector along with chemotherapy.6,7
Most animal model studies and clinical trials with oncolytic Ad vectors have used direct i.t. administration of the vector into accessible tumors. However, systemic delivery of oncolytic Ad vectors may be required to treat inaccessible tumors or metastatic lesions, and this poses concerns about antitumor efficacy and toxicity. First, >90% of the Ad vector delivered systemically is absorbed by the liver, thereby reducing/preventing the vector from reaching the tumor.8,9 Second, Ad vectors are highly immunogenic, leading to cellular and humoral immune responses.10,11,12,13,14,15 Further, much of the human population has pre-existing antibody (Ab) to Ad serotype 5 (Ad5) (refs. 16,17), which might neutralize Ad5-based vectors as soon as the vector is injected. Also, pre-existing immunity to the vector will be boosted following administration of the vector. These observations could explain why clinical trials with systemic oncolytic Ad therapy did not show more anticancer efficacy.18,19,20
Regarding vector-induced toxicity, pre-existing immunity might reduce toxicity,10 as most of the vector might be neutralized before the vector reaches any organ. Pre-existing immunity is likely to reduce vector efficacy if the vector is delivered systemically. However, pre-existing immunity has been reported to not affect vector efficacy after i.t. injection of vector.21,22 On the other hand, activation of the immune system in response to the vector in addition to the direct toxicity of the vector might lead to increased vector-associated toxicity.14,23,24 For example, activation of the complement pathway by recombinant Ad vectors might induce toxicity in patients having pre-existing immunity.25 In another study with mice, pre-existing immunity caused increased mortality, even though there was less tissue toxicity in preimmunized animals compared to naive animals.26 Another study on preimmunization showed enhanced toxicity caused by a replication-defective Ad5 vector in a subcutaneous mouse cancer model.27 In another study, pre-existing immunity did not seem to prevent toxicity after a systemic injection of a replication-defective Ad vector into Rhesus monkeys.13 Clearly, it is important to understand the role of pre-existing immunity in vector-associated toxicity.
Studies on the relationship between oncolytic Ad vectors and antivector immunity have been limited because of a lack of appropriate animal models inasmuch as Ads replicate poorly in tissues of most nonhuman species. There have been efforts to model pre-existing immunity in nude or severe combined immunodeficient mice to test the efficacy and toxicity of oncolytic Ad vectors; pre-existing immunity was generated by passive transfer of immune serum/purified Ab from human or rabbit.10,28 However, there are several limitations to this model: although the passively introduced Ab can neutralize the virus, this model cannot address the immune responses mediated by the Fc region of the Ab, as the Ab is from a different species. Also, the role of T-cells is not addressed.
We have shown that the Syrian hamster is a good model for studying oncolytic Ad5-based vectors, as the hamsters are both immunocompetent and permissive for human Ad5 (refs. 17,29,30,31,32,33). We have also modeled pre-existing immunity in Syrian hamsters using Ad5 for generating the pre-existing immunity and evaluated the effect of pre-existing immunity on oncolytic Ad5-based vector INGN 007 (also named VRX-007) antitumor efficacy.17 INGN 007 is a fully replication-competent vector that lacks most of the Ad E3 genes and overexpresses Adenovirus Death Protein.34 We showed that i.t. administration of the oncolytic Ad vector, INGN 007, into subcutaneous tumors significantly suppressed tumor growth, and that pre-existing antibody did not affect vector antitumor efficacy in immunocompetent animals (in immunosuppressed conditions pre-existing immunity did reduce the vector efficacy).17,33 Further, pre-existing immunity reduced vector spread from the site of the i.t. injection to normal tissues.17
This study addresses the role of antivector immunity including pre-existing immunity in oncolytic Ad vector toxicity. Most of the previous studies on this topic employing systemic Ad vector administration were conducted with replication-defective vectors, and the few studies that employed replication-competent vectors used animal models that were either immunodeficient or nonpermissive for human Ads.10,13,26,35,36 We have now used Syrian hamsters to study the effect of pre-existing immunity, particularly neutralizing antibody (NAb), after a lethal intravenous (i.v.) dose of INGN 007. The maximum tolerated dose (MTD) of INGN 007 in immunocompetent hamsters is 1.9 × 1012 virus particles (vp)/kg [~1.9 × 1010 plaque forming units (pfu) per hamster] (M. Thomas and W. Wold, unpublished results). Intravenous injection of INGN 007 or Ad5 at this dose leads to toxicity and productive infection (i.e., replication) of the liver, lungs, kidney, adrenals, and pancreas.31 Replication is especially high in the liver.31,37 Replication involves infection of cells, expression of Ad “early” genes in the nucleus, viral DNA replication, expression of “late” genes, assembly of virus in the nucleus, then lysis of cells and release of virus. Both INGN 007 and Ad5 injected i.v. at the MTD causes transient liver toxicity that resolves by 28 days (ref. 29). In this study, we administered 3× the MTD. In naive hamsters that are immunosuppressed by cyclophosphamide (CP), this dose is 100% lethal at 3 days after the 1st dose or 1 day after the 3rd dose. As mentioned above, pre-existing immunity might reduce vector toxicity, might increase the toxicity associated with the vector, or might not affect vector toxicity. Further, we have investigated whether passive immunization can be used to reduce toxicity associated with systemic vector administration both preventively and curatively (i.e., passive immunization before or after vector administration, respectively).
Results
Circulating antibodies play an important role in preventing liver damage and toxicity
We examined the role of pre-existing immunity to Ad5 in vector replication and toxicity in the liver following i.v. injection of INGN 007. Two groups of hamsters were or were not immunized with Ad5 (replication-competent). Fourteen days postimmunization, half of the hamsters from these two groups were immunosuppressed with CP. Hamsters treated with CP under the conditions we used have only ~1/10th the normal level of leukocytes when the vector is injected, and the level of leukocytes declines further upon repeated CP injection.17,33,37 Immunosuppression with CP prevented an immune response against the vector17,33,37 and allowed us to evaluate the effect of anti-Ad NAbs present in the circulation before vector injection in preimmunized hamsters. We have shown previously that the half-life of circulating anti-Ad5 NAb is about 10 days under these conditions of CP immunosuppression.17 The hamsters were then injected i.v. with buffer or INGN 007. As a very rigorous test of the importance of NAb, we used three consecutive daily injections of the MTD (1.9 × 1010 pfu) of INGN 007 as determined elsewhere in immunocompetent hamsters (M. Thomas and W. Wold, unpublished results).
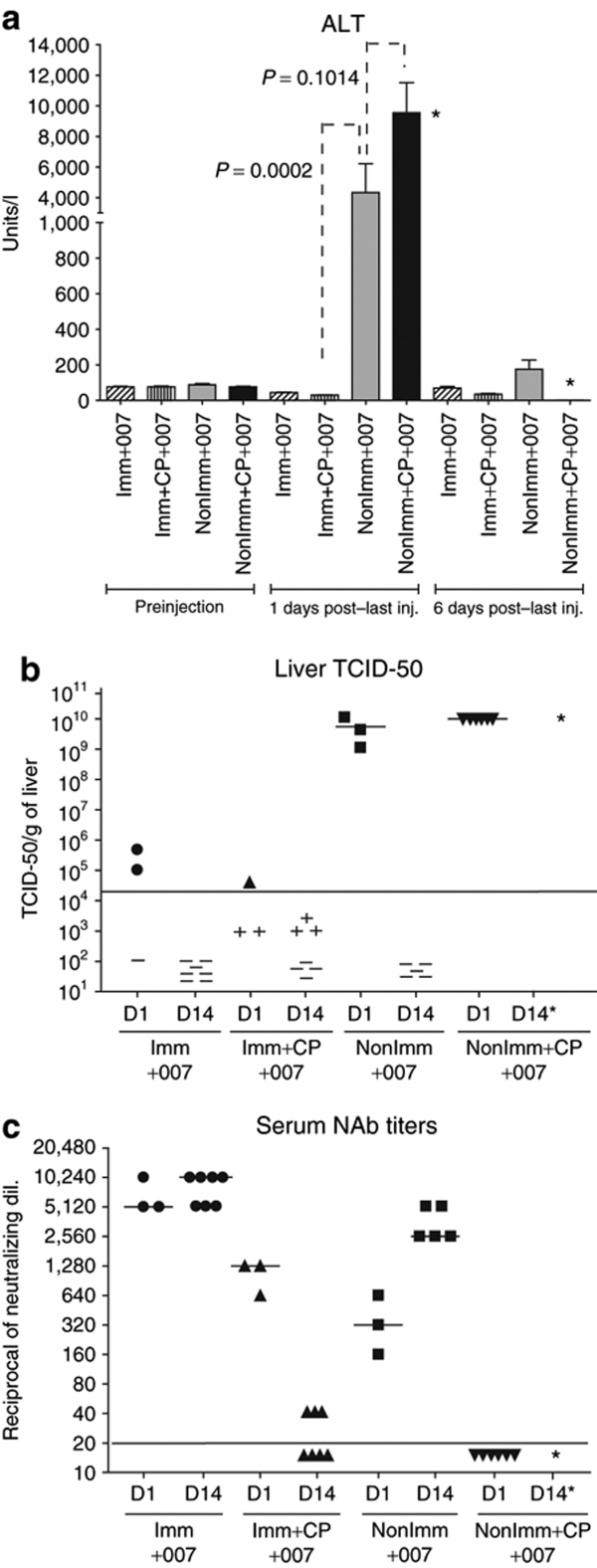
We observed that all the hamsters that were not immunized with Ad5, were immunosuppressed with CP, and were injected with INGN 007 (NonImm+CP+007) were moribund by day 1 (the day after the 3rd injection of INGN 007) and had to be euthanized. We showed previously that progeny vector is present at day 1 under these experimental conditions.31 The serum alanine aminotransferase (ALT) levels were extremely high in this group, indicating severe liver damage (Figure 1a). Similar results were obtained for aspartate aminotransferase (AST) levels (data not shown). The liver damage in the NonImm+CP+007 group corresponded to a large number of infectious vps in the liver (≥1 × 1010 tissue culture infectious dose-50 (TCID-50)/g of liver) at day 1 after the last injection (day 3 after the 1st injection) (Figure 1b). In contrast, the hamsters that were immunized with Ad5, immunosuppressed, and injected with INGN 007 (Imm+CP+007) had normal serum levels of liver enzymes (Figure 1a), which corresponded with very low levels of infectious vector in the liver (Figure 1b). These results indicate that the presumptive pre-existing circulating NAb alone were effective in preventing liver infection and toxicity caused by an extremely high dose of INGN 007.
Figure 1.
Pre-existing immunity prevents vector toxicity. Preimmunized or nonimmunized hamsters, with or without CP immunosuppression, were injected i.v. with INGN 007 (three consecutive injections of 1.9 × 1010 pfu/injection). (a) Serum was collected preinjection and at 1 and 6 days after last injection of INGN 007 and analyzed for the liver enzyme ALT. (b) TCID-50 assay detecting infectious virus particles present in the liver at days 1 and 14. (c) A neutralization assay was performed with the serum. The NAb titers are plotted as the highest dilution of serum that resulted in at least 50% inhibition of CPE when incubated with 100 pfu of INGN 007. *Hamsters from this group (NonImm+CP+007) were moribund on day 1 and had to be killed. ALT, alanine aminotransferase; CP, cyclophosphamide; CPE, cytopathic effect; D, day; Imm+CP+007, immunized with Ad5, immunosuppressed, and injected with INGN 007; i.v., intravenous; NAb, neutralizing antibody; NonImm+CP+007, not immunized with Ad5, were immunosuppressed with CP, and were injected with INGN 007; pfu, particle forming units; TCID-50, tissue culture infectious dose-50.
With respect to the immunocompetent hamsters, the hamsters that were not immunized (NonImm+007), which also received 3× the MTD of INGN 007 (for immunocompetent hamsters), had serum liver enzymes that were elevated on day 1 (after the last injection), but went down to near normal levels by day 6 (after the last injection) (Figure 1a). These hamsters had high amounts of infectious virus in the liver at day 1 (after the last injection), but the infection was cleared by day 14 (after the last injection) (Figure 1b). The hamsters mounted a NAb response that could be detected by day 1 (after the last injection) (~1:320) and that increased to high levels (~1:2,560) at day 14 (after the last injection) (Figure 1c). Apparently, the normal host immune response to the virus effectively cleared the virus infection by day 14. With the immunocompetent hamsters that were immunized (Imm+007), there was no elevation in liver enzymes at 1 or 6 days (Figure 1a). Some INGN 007 was detected in the liver at 1 day (~105 TCID-50/g liver in two of three animals), but none at 14 days (Figure 1b). These hamsters had high levels of NAb at day 1 (~1:5,120) that were boosted to ~1:10,240 at day 14 (Figure 1c). So, as was the case with the immunosuppressed groups, pre-existing immunity prevented liver toxicity in the immunocompetent hamsters. This immunity also greatly reduced the amount of infectious vector in the liver.
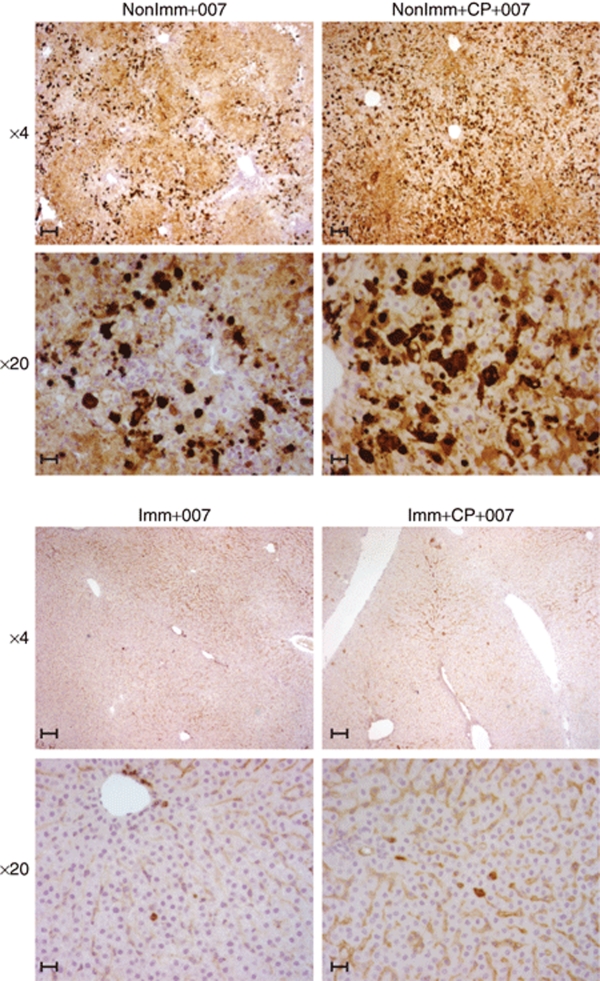
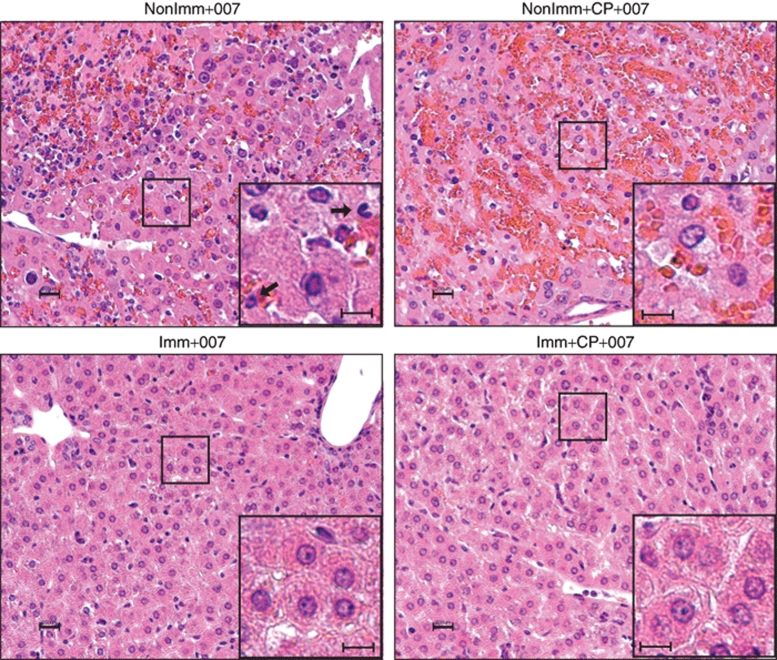
Immunohistochemistry (IHC) analyses further indicated that the livers from nonimmunized hamsters were infected to a much greater extent than their immunized counterparts (Figure 2). Also, histopathology analysis of hematoxylin–eosin stained liver sections indicated infiltration of neutrophils in nonimmunized immunocompetent animals injected with INGN 007 (NonImm+007) (Figure 3). In the NonImm+CP+007 group, no infiltrating cells were seen, presumably because the CP treatment had depleted the immune effector cells. The interstitial aggregation of erythrocytes (the orange staining) indicates that blood leaked out of the vasculature due to extensive damage. With the immunized hamsters, in accord with the observation that pre-existing immunity prevented infection of hepatocytes (Figures 1b and 2), there were no visible infiltrating immune cells and there was little liver damage (Imm+007 and Imm+CP+007 groups) (Figure 3).
Figure 2.
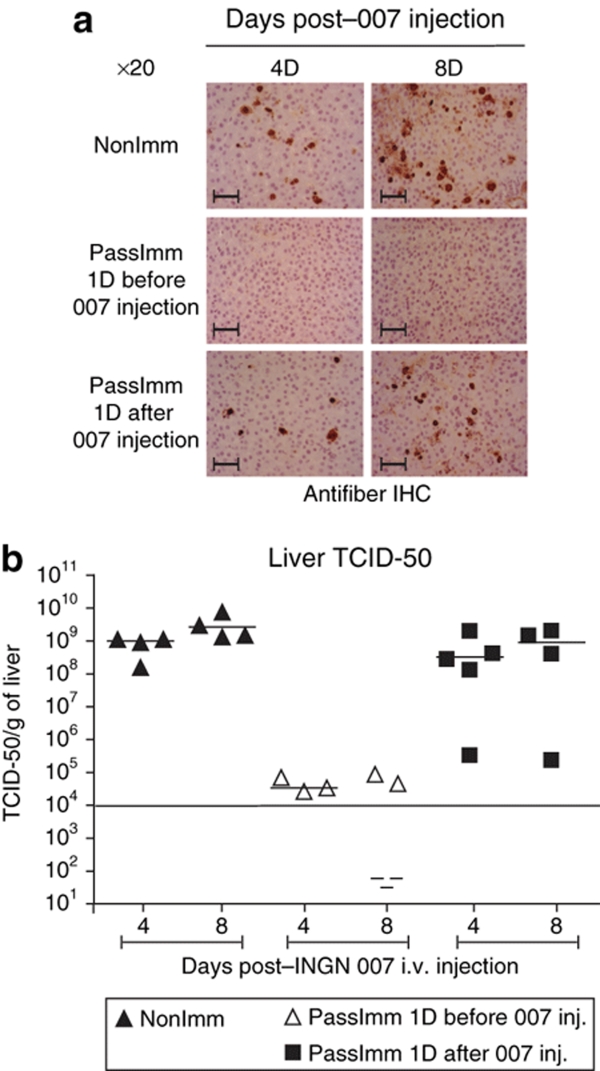
Pre-existing immunity, specifically NAb to Ad5, largely prevents INGN 007 infection of the liver. Hamsters were or were not immunosuppressed, then administered three consecutive i.v. injections of INGN 007 (1.9 × 1010 pfu/injection). Livers were collected at 1 day following the last injection, and liver sections were processed for immunohistochemistry with antifiber antibody. Bar = 200 µm. Ad5, adenovirus 5; CP, cyclophosphamide; Imm+CP+007, immunized with Ad5, immunosuppressed, and injected with INGN 007; i.v., intravenous; NAb, neutralizing antibody; NonImm+CP+007, not immunized with Ad5, were immunosuppressed with CP, and were injected with INGN 007; pfu, particle forming units.
Figure 3.
Pre-existing immunity prevents damage to the liver. H&E staining of liver sections collected 1 day after three consecutive i.v. injections of INGN 007 (1.9 × 1010 pfu/injection). This is the same experiment as in Figure 2. Inset: arrows indicate neutrophils. Bar = 200 µm. CP, cyclophosphamide; H&E, hematoxylin and eosin; Imm+CP+007, immunized with Ad5, immunosuppressed, and injected with INGN 007; i.v., intravenous; NonImm+CP+007, not immunized with Ad5, were immunosuppressed with CP, and were injected with INGN 007; pfu, particle forming units.
The results in Figures 1–3 indicate that pre-existing immunity to Ad5 largely prevents INGN 007 infection and damage to the liver. Further, nearly complete prevention of liver infection can be afforded by NAb, as indicated by the results with the immunosuppressed hamsters. We found no indication that pre-existing or induced immunity caused liver toxicity or any toxicity as suggested by in-life observations, gross necropsy observations, or histopathology. However, in the NonImm+007 group, infiltration of neutrophils into the liver at day 1 (Figure 3, top left) may contribute to toxicity.
Passive immunization is an effective method to prevent toxicity of the vector in immunosuppressed conditions
The majority of cancer patients undergo radiotherapy and/or chemotherapy. These treatments can depress the immune system. The use of an oncolytic Ad vector in such patients with a compromised immune system might lead to increased toxicity due to disseminated infection. One approach to this potential problem is to passively immunize the patients by adoptive transfer of anti-Ad antibody before injecting the vector. Also, patients treated with vector could potentially be passively immunized should a disseminated infection develop. We tested these ideas in our Syrian hamster model.
We pooled serum from five different hamsters that were immunized with Ad5. The neutralization titer was determined to be 1:10,240. Then, 0.4 ml of the pooled immune serum were injected intraperitoneally (i.p.) to passively immunize the hamsters. The neutralization titer was 1:320 at 1 day after the passive immunization (Supplementary Figure S1). Four groups of hamsters were immunosuppressed with CP. Group 1 was passively immunized by injecting the immune serum one day before INGN 007 injection. Group 2 was passively immunized one day after INGN 007 injection. Group 3 was passively immunized but injected with buffer. Group 4 was not immunized and was injected with INGN 007. INGN 007 was injected i.v. with a single dose of 1.9 × 1012 vp/kg (ca. 2 × 1010 pfu), which is the MTD for immunocompetent hamsters and is a lethal dose for immunosuppressed hamsters.
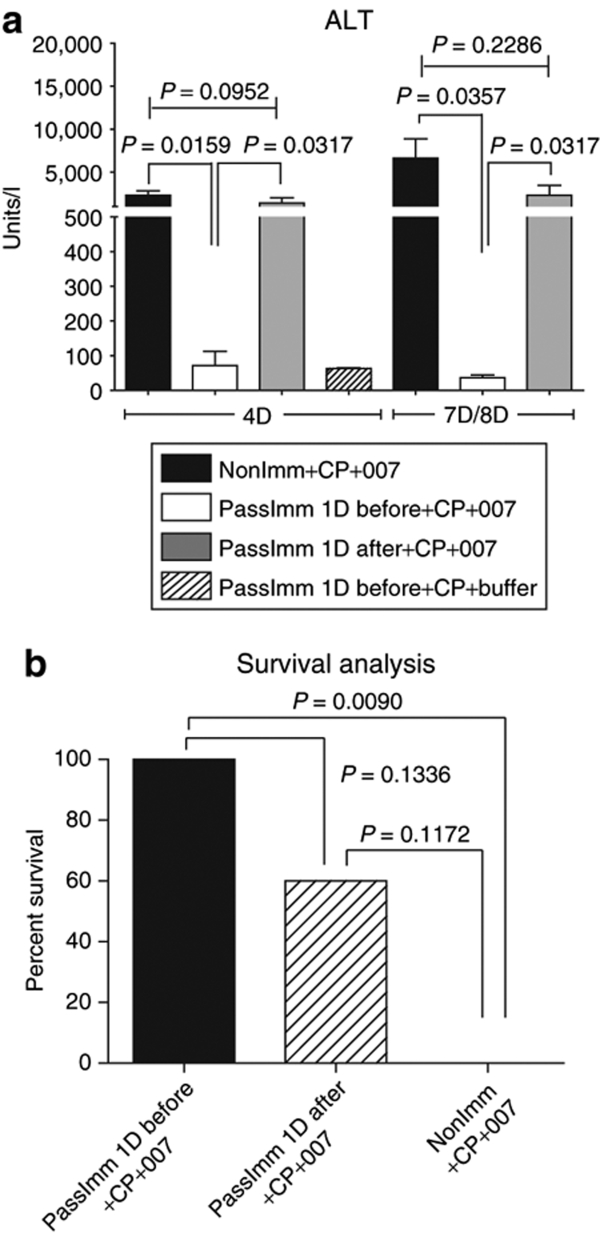
Analysis of serum ALT (Figure 4a) and AST (data not shown) levels at 4 and 8 days after INGN 007 injection indicated that passive immunization is very effective in preventing liver toxicity when the hamsters were immunized 1 day before vector injection (PassImm 1D before versus PassImm 1D after: P < 0.05 both at day 4 and day 8 after vector injection; PassImm 1D before versus NonImm+CP: P < 0.05 both at day 4 and day 8). Normal ALT levels in passively immunized and buffer-injected animals (PassImm 1D before+CP+buffer group) indicates that the serum transfer itself was not toxic (Figure 4a). Some of the hamsters died between day 7 and day 8, and some were moribund at day 8; serum was collected only from the moribund hamsters. Survival analysis revealed that passive immunization 1 day before INGN 007 injection resulted in 100% survival of the hamsters whereas 100% of the nonimmunized hamsters were dead by day 8 (P = 0.009) (Figure 4b). Passive immunization 1 day after vector injection seemed to provide some protection (60% survival), but the result was not statistically significant (P = 0.1172) (Figure 4b). IHC analysis of liver sections with antifiber Ab showed that passive immunization 1 day before vector administration almost completely prevented infection of the liver (Figure 5a, middle panels) whereas passive immunization 1 day after vector injection was only barely effective, if at all (Figure 5a, lower panels). TCID-50 analysis of liver extract confirmed the above observations (Figure 5b).
Figure 4.
Passive immunization before vector injection prevents liver toxicity and increases survival. Hamsters were immunosuppressed using CP. Then, hamsters were not immunized or were passively immunized (i.p. injection of immune serum) 1 day before or 1 day after an i.v. dose of INGN 007 (1.9 × 1012 vp/kg). The dose of virus used is the MTD for immunocompetent Syrian hamsters. (a) Serum was collected at day 4 and day 8 after virus injection and serum ALT levels were assayed. Serum was also collected at day 7 from moribund animals in the NonImm+CP+007 group. (b) Survival of hamsters at day 8 after vector injection. ALT, alanine aminotransferase; CP, cyclophosphamide; D, day; i.p., intraperitoneal; i.v., intravenous; MTD, maximum tolerated dose; vp, virus particles.
Figure 5.
Passive immunization before vector injection reduces liver infection. The experiment was conducted as outlined in Figure 4. (a) The livers were collected 4 and 8 days after virus injection and processed for immunohistochemistry analysis with antifiber antibody to detect adenoviral infection of hepatocytes. (b) TCID-50 assay detecting infectious virus in liver extracts. Bar = 600 µm. CP, cyclophosphamide; D, day; IHC, immunohistochemistry; i.v., intravenous; TCID-50, tissue culture infectious dose-50.
In this experiment, it is likely that passive immunization before vector injection was so effective because the NAb neutralized the vector before the liver could be infected. When the hamsters were immunized after vector injection, then the liver became highly infected; the slight protective effect seen could be because the NAb prevented the spread of vector from the initially infected hepatocytes to the hepatocytes in subsequent rounds of replication.
Evaluating the curative effect of passive immunization with a sublethal dose of virus
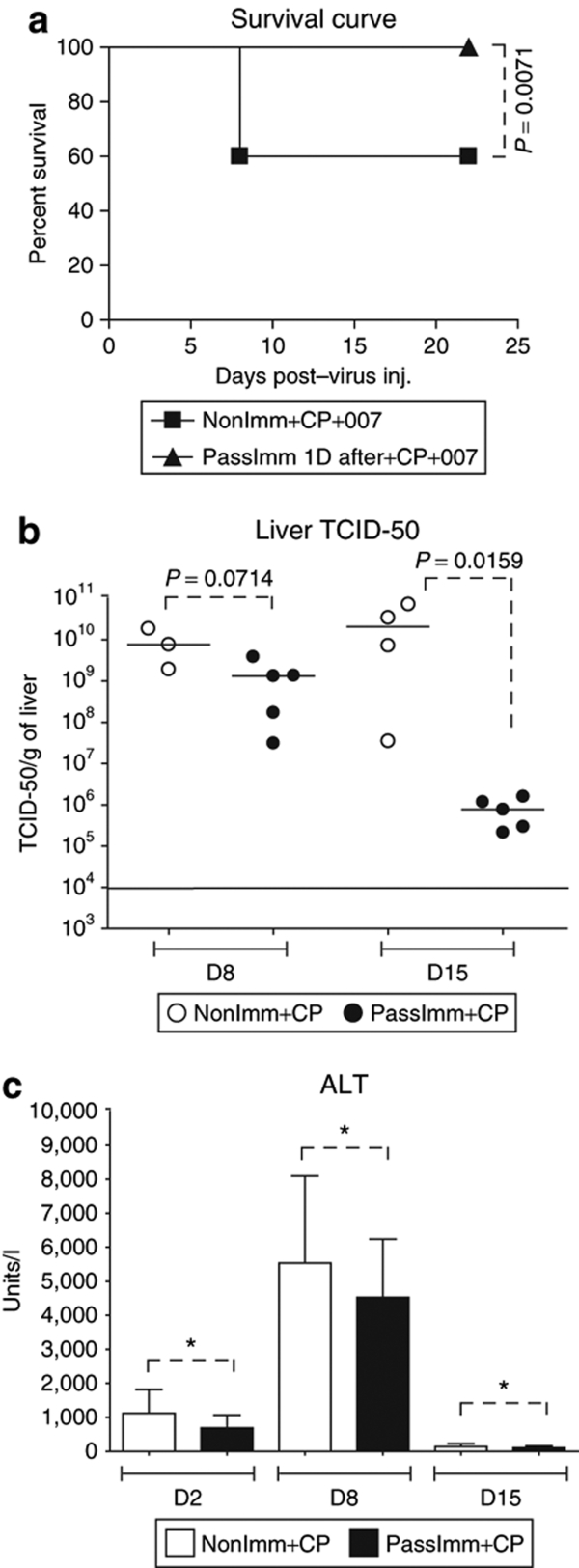
In the experiment described above (Figures 4 and 5), a lethal dose of vector was used, which kills the immunosuppressed animals by 7–8 days postinjection. We next evaluated the effect of passive immunization 1 day after injection with a sublethal dose of vector (1/3rd the MTD, i.e., 0.63 × 1012 vp/kg). We also looked at day 8 and day 15 after vector injection, a late time when there might be multiple rounds of replication, cell lysis, and cell reinfection. As seen in Figure 6a, at this dose of INGN 007, only 40% of the animals in the nonimmunized immunosuppressed group (NonImm+CP+007) died (as opposed to 100% death in the nonimmunized immunosuppressed group at day 8 using the MTD for immunocompetent hamsters, Figure 4b). Importantly, none of the passively immunized animals died (Figure 6a), indicating that the passive immunization was effective (P = 0.0071). When liver extracts were assayed for INGN 007 at day 8 after vector injection, the PassImm+CP group had lower TCID-50 levels than did the NonImm+CP group, with a trend toward significance (P = 0.0714) (Figure 6b). At day 15, the TCID-50 titers were significantly less (P = 0.0159) in the PassImm+CP group compared to the NonImm+CP group (Figure 6b). These results indicate that when the hamsters are passively immunized after an i.v. challenge with a dose of vector that does not overwhelm the animals, then the passive immunization is effective in preventing morbidity and subsequent proliferation of the vector, probably because the NAb prevented reinfection of hepatocytes following the replication and release of the vector from those cells initially infected after i.v. injection of the vector.
Figure 6.
Passive immunization after vector injection prevents mortality and reduces vector load in the liver. Immunosuppressed hamsters were injected i.v. with INGN 007 at 1/3rd MTD (0.63 × 1012 vp/kg). At 1 day postinjection, one group of hamsters was passively immunized with immune serum while another group remained nonimmunized. (a) Survival curve as analyzed by Kaplan–Meier analysis. (b) Livers were collected 8 and 15 days after virus injection and subjected to TCID-50 assay to determine infectious virus titers. (c) Serum ALT levels were assayed 2, 8, and 15 days after virus injection. *P > 0.05 (nonsignificant). ALT, alanine aminotransferase; CP, cyclophosphamide; i.v., intravenous; MTD, maximum tolerated dose; NonImm+CP+007, not immunized with Ad5, were immunosuppressed with CP, and were injected with INGN 007; TCID-50, tissue culture infectious dose-50; vp, virus particles.
In this same experiment, the serum ALT levels were analyzed as an indicator of liver damage (Figure 6c). The ALT (Figure 6c) and AST (data not shown) levels appeared to be lower in the passively immunized group than in the nonimmunized group, but the differences were not statistically significant.
Comparing Figure 6b and Figure 6c, it seems that more virus in the liver corresponds to higher serum ALT levels, but these data were not statistically significant. It is interesting to note that the serum ALT levels were normal in the NonImm+CP group at day 15, even though there were large amounts of vector present at day 15 in the liver of these hamsters (Figure 6b). We have observed a similar scenario before in which there is a great deal of infectious vector in the liver but no apparent increase in the serum ALT/AST levels.37
Discussion
Options for administration of oncolytic Ad vectors to cancer patients include direct i.t. injection or systemic delivery. Systemic delivery, if feasible, would be desirable because then metastasized tumors could be treated. However, concerns about the safety of systemic Ad administration were raised following the death of a gene therapy trial patient, who was being treated with a replication-deficient recombinant Ad vector for ornithine transcarbamylase deficiency.38 In subsequent clinical trials with conditionally replicating oncolytic Ad vectors, however, it was demonstrated that systemic administration of such vectors is a feasible approach.18,20 Nevertheless, systemic administration of Ad vectors to cancer patients is associated with liver toxicity of various grades.39
Pre-existing immunity to the vector and immunity induced following vector injection pose a major problem for systemic Ad vector delivery and also i.t. injection of vector. Also, pre-existing immunity might be a significant barrier for successful transduction of target tissues.10,40 Although anti-Ad immunity is prevalent in the human population,16,17 there are few studies that have evaluated the role of pre-existing immunity on vector-associated toxicity.10,13,26,41 Previous reports on this issue have been conflicting.10,13,27 In this study, we have used Syrian hamsters to address the role of pre-existing immunity to Ad5 in the toxicity of the Ad5-based oncolytic Ad vector INGN 007 after a lethal i.v. challenge with INGN 007. We found that pre-existing immunity completely prevented vector-associated liver toxicity in immunocompetent hamsters after a lethal i.v. challenge of three consecutive injections of the MTD of INGN 007. Liver toxicity was also prevented in hamsters that were first immunized, then immunosuppressed before the i.v. challenge. TCID-50 assays and IHC analysis of liver sections showed that very little vector was able to reach the liver and infect the hepatocytes in the preimmunized groups. The amount of vector used in these immunosuppressed hamsters (3× the MTD in immunocompetent hamsters) was extremely high, and indeed, all the naive immunosuppressed hamsters were moribund 1 day after the last i.v. injection. None of the hamsters in the immunized group showed signs of morbidity at the 8 day harvest time. Because these preimmunized animals were immunosuppressed at the time of vector injection, and they had very few circulating leukocytes that were also prevented from expansion as a result of this CP administration protocol,17 our results indicate that pre-existing circulating NAb are sufficient to prevent liver infection, liver toxicity, and morbidity. Presumably, most of the vector was neutralized by the circulating NAb as soon as it was injected into the blood stream.
As another approach to address the role of anti-Ad5 serum antibodies in vector infection and vector toxicity, we passively immunized the hamsters 1 day before or 1 day after vector injection. These hamsters were immunosuppressed by CP so that we could study the effect of antibodies in the absence of an immune response to the injected vector. We found that passive immunization before administration of a lethal dose (for immunosuppressed hamsters) of vector (1.9 × 1012 vp/kg) was nearly completely protective. There was little infection of the liver as indicted by TCID-50 and IHC assays, almost no elevation in serum ALT and AST, and no morbidity after 8 days. Most likely the passively administered NAb neutralized most of the injected vector before it could infect the liver. The NAb would also likely neutralize any vector released from productively infected cells of the liver and other tissues.
Passive immunization 1 day after injection of this same high dose of vector did not prevent liver infection and liver toxicity, although the toxicity seemed to be slightly less than in nonimmunized immunosuppressed hamsters, which is also reflected in the slightly reduced morbidity compared to the nonimmunized immunosuppressed hamsters (P = 0.1172). The slight reduction in toxicity and morbidity may be due to neutralization of vector released from the hepatocytes that were initially infected. The dose of vector (the MTD in immunocompetent hamsters) used in the immunodeficient hamsters in this experiment was so high that we could not discern a statistically significant effect of the NAb added after the liver (and other tissues) had already been infected. In practice, such a high dose of replication-competent vector would never be used to treat a patient systemically. Also, naturally occurring infections do not reach such high serum titers.42 When we injected 1/3rd of the MTD, then added immune serum at 1 day postinfection, a clear benefit was seen from the passive immunization (Figure 6). Infection of the liver was reduced at 8 days (P = 0.0714) and especially at 15 days (P = 0.0159) postinjection. All the animals survived versus 60% survival in the untreated group (P = 0.0071). Most likely these passively administered NAb neutralized the vector resulting from initial infection of hepatocytes and release after cell lysis; i.e., the NAb prevent the spread of the vector. This protective effect of passive immunization of preinfected hamsters is remarkable because the dose of vector used, 6.3 × 1011 vp/kg, is very high considering that the hamsters were immunosuppressed with CP.
Our results suggest that passive immunization can be used to control the spread of oncolytic Ad vectors. Passive immunization would be of particular interest in the case of direct i.t. vector injection as the circulating NAb will prevent vector spread from the site of injection to normal tissues thereby reducing toxicity associated with vector spillover with minimal or no effect on the antitumor efficacy.17,33 As no antiviral drug is currently approved specifically for Ad infection,37 passive immunization with anti-Ad NAb might be used as a preventive measure against Ad infections in cases such as solid organ transplant patients and pediatric hematopoietic stem cell transplant patients, where the patients are immunosuppressed and therefore are prone to Ad infection.
What levels of NAb are required to prevent Ad vector infection of tissues and the resulting toxicity? Studies by other groups have shown that very low levels of NAb in passively immunized nude mice, which are nonpermissive for Ad5, effectively prevent systemic Ad vector toxicity.10 In our studies, we also found that passive immunization of immunosuppressed hamsters giving rise to a NAb level in the serum of 1:320 (as compared to 1:5,120–1:10,240 NAb titers in immunized immunocompetent animals) before vector injection effectively prevented vector-induced toxicity. Therefore, in Syrian hamsters, which are permissive for Ad5, small amounts of NAb are protective.
Regarding the general issue of toxicity in the hamster model, the immunity data strongly suggest that toxicity is not caused by adaptive immunity, especially serum antibodies. Most likely toxicity is due to vector replication in the liver and other organs, and possibly to an innate response to the injected bolus of vector. Early toxicity is in accord with previous reports in mice and Rhesus monkeys that showed hepatic injury soon after systemic Ad vector administration.12,13 However, in our studies, toxicity is also very likely caused by vector replication. In naive immunocompetent animals, a great deal of vector was found in the liver at day 1 after the last i.v. injection, and this was associated with significant liver toxicity (Figures 1–3). This vector seen at day 1 after the 3rd consecutive daily injection may represent some of the input vector (although we did not detect any replication-defective Ad vector by TCID-50 assay of the liver at 1 day postinjection of the vector,31 so it likely represents new vector replication). As an adaptive immune response develops, the vector is cleared from the liver and other organs and toxicity is minimized (Figure 1).29,31 Further support for the idea that vector replication causes toxicity comes from our observation that an E1-minus replication-defective Ad vector showed less toxicity as compared to the replication-competent viruses in immunocompetent Syrian hamsters.29 In immunosuppressed conditions, the hepatotoxicity is very likely caused by replication of the input vector and subsequent rounds of vector replication and cell lysis. Lysis of hepatocytes results in increased toxicity at later days in immunosuppressed animals (compare ALT levels at days 2, 4, and 8 in Figures 4 and 6).
As a point of interest, high levels (>1010 TCID-50/g liver) of infectious INGN 007 in the liver of immunosuppressed hamsters at 15 days postinjection was not associated with elevated serum ALT levels (Figure 6b,c). This contrasts with the high serum ALT levels and high TCID-50 levels in the liver at earlier times after vector injection (Figures 1 and 6). We have observed this phenomenon before with wild-type Ad5 (ref. 37). We do not know whether the vector at day 15 is actually replicating in the liver, we only know that it is capable of replicating following extraction from the liver and TCID-50 assay. But, if the vector is replicating in the liver, then somehow the hamsters adjust such that there is little toxicity.
In summary, we report that pre-existing NAb blocks liver infection thereby preventing hepatotoxicity associated with the vector. Pre-existing immunity was not associated with elevated hepatotoxicity in either immunocompetent or immunosuppressed hamsters (as opposed to a study reporting enhanced toxicity in preimmune animals27). Therefore, pre-existing immunity is beneficial from the toxicity point of view. However, pre-existing immunity might be a major concern for both cancer therapy and gene therapy in general where systemic administration of the vector is required. On the other hand, pre-existing immunity is beneficial when the vector is injected i.t., as pre-existing immunity prevents vector spillover from the tumor and replication in normal tissues but does not affect vector efficacy.17 Further, we found that passive immunization of immunosuppressed hamsters with hyper immune serum before injection of a very large dose of vector effectively prevented liver infection and hepatotoxicity. Passive immunization of immunosuppressed hamsters after vector administration was effective in reducing vector loads in the liver at later time points and preventing death when infecting dose was <100% lethal. Therefore passive immunization might be considered as an option for curative and preventive measures against disseminated Ad infections.
Materials and Methods
Vectors and viruses. INGN 007 (also named VRX-007) is an oncolytic Ad vector based on Ad serotype 5 (Ad5). INGN 007 is identical to wild-type Ad5, except that INGN 007 lacks most of the E3 genes and overexpresses the E3-11.6K Adenovirus Death Protein.34,43,44 INGN 007 and Ad5 virus stocks were obtained from Introgen Therapeutics (Houston, TX). Virus stocks were grown in HEK 293 cells, purified by column chromatography, and vp titers were determined by high-performance liquid chromatography. Infectious titers were determined in our laboratory by plaque assays and TCID-50 assays on A549 cells.45 The vp:pfu ratio was 12 for INGN 007.
Animals. Four- to five-week-old female Syrian (Golden) hamsters (Mesocricetus auratus) were obtained from Harlan Sprague Dawley (Indianapolis, IN). The Institutional Animal Care and Use Committee of Saint Louis University approved the studies, and they were conducted in accordance with institutional and federal regulations.
Immunosuppression. The animals where immunosuppressed by dosing CP (Sigma-Aldrich, St Louis, MO).17,33,37 CP was administered beginning 7 days before INGN 007 injection and subsequently twice every week by i.p. injection for the duration of the study. The initial dose of CP was 140 mg/kg body weight and the subsequent doses were 100 mg/kg. The dose and schedule were based on our previous studies.17,33,37 Immunosuppressed hamsters were housed in sterile caging and fed irradiated chow and antibiotic (Baytril-Bayer HealthCare, Shawnee Mission, KS) treated water.
Intravenous injection of virus. The hamsters were anesthetized with ketamine–xylazine mix (i.p. injection), and injected i.v. (into the jugular vein) with 1.9 × 1012 vp/kg or 1.9 × 1010 pfu/hamster of INGN 007 diluted in 200 µl of 10 mmol/l Tris pH 8.2, 10% glycerol. This dose was established previously as the i.v. MTD for INGN 007 in immunocompetent hamsters.
Immunization. Pre-existing immunity was generated by a single intramuscular injection of Ad5 (2 × 1011 vp/hamster).17
Passive immunization. Five hamsters were immunized with Ad5 (intramuscular injection of 2 × 1011 vp) and were boosted with same amount of virus after 14 days. Serum was collected 14 days postboost, pooled, and NAb titers were determined (see below). To passively immunize the immunosuppressed hamsters, 0.4 ml of this pooled serum was injected i.p. The neutralizing titer of this immune serum was 1:10,240. The serum neutralizing titer in the hamsters following passive immunization was 1:320 at 1 day postinjection of the hyperimmune serum (Supplementary Figure S1).
IHC and histopathology. The liver of each animal was fixed in 10% neutral-buffered formalin. Following fixation, the liver was trimmed, embedded in paraffin, sectioned (5 µm), and stained with hematoxylin–eosin. For IHC, unstained slides were prepared from formalin-fixed, paraffin-embedded tissues. Antigen retrieval using DIVA Decloaker (Biocare Medical Concord, CA) was conducted before incubation with an anti-Ad-fiber mouse monoclonal antibody (4D2; NeoMarkers, Fremont, CA). Secondary antibody incubation was performed with horseradish peroxidase–conjugated goat anti-mouse IgG from Dako (Envision + system, Carpinteria, CA). Slides were incubated with diaminobenzidene substrate and counterstained with hematoxylin.
Blood/serum collection and analysis. Blood was collected in anticoagulant tubes (BD microtainer with EDTA; BD, Franklin Lakes, NJ) from anesthetized animals either via the jugular vein or via retro orbital bleeds for hematology. For serum chemistry analysis, blood was collected in serum separator tubes (BD microtainer with serum separator; BD). Hematology and serum chemistry analyses (the liver enzymes ALT and AST) were performed by the Clinical Pathology laboratory in the Department of Comparative Medicine at Saint Louis University.
Neutralization assay. A549 cells were plated in 96-well plates at 8 × 105 cells per plate in a volume of 100 µl per well 1 day before the assay. Serum samples were incubated at 56 °C for 30 minutes to inactivate complement. Serum samples (in four replicate wells) were diluted twofold across a round-bottom 96-well plate, in media containing 20% fetal bovine serum (to normalize the total serum concentration across the plate).17 One row contained no serum sample to observe the effect of virus only. A volume of 100 pfu of INGN 007 was added to each well and the serum–virus mix was incubated for 1 hour at 37 °C. After incubation, the serum–virus mixtures (100 µl total volume) were transferred to the 96-well plate containing A549 cells and incubated for additional 1 hour at 37 °C. The media was then removed and replaced with fresh DME containing 5% fetal bovine serum. At 7 days postinfection, the wells were individually scored (+/−) for cytopathic effect. Neutralizing titers were determined by the highest dilution of serum that resulted in at least 50% inhibition of cytopathic effect (≤2 of 4 wells positive for cytopathic effect).17
Virus quantitation in tissues. The right lateral lobe of the liver was collected in sterile tubes, and blood was collected in anticoagulant tubes. All the tissues were snap-frozen in liquid nitrogen and stored at −80 °C. The solid tissues were weighed and then homogenized in phosphate-buffered saline with a single tungsten carbide bead using the TissueLyser (Qiagen, Valencia, CA). For determining infectious virus titers, tissue homogenates were freeze–thawed three times, sonicated for 7 minutes, centrifuged, and titered by TCID-50 assays on A549 cells.17,33,37,46
Statistical analysis. The Kruskal–Wallis test was used to detect the overall treatment effect, and the Mann–Whitney U test was performed for pairwise comparisons. For survival curve analysis, the log-rank test was used to determine the statistical significance. P ≤ 0.05 was considered to be significant.
SUPPLEMENTARY MATERIALFigure S1. Serum NAb titers after passive immunization. Immunosuppressed hamsters were passively immunized with 0.4 ml of hyperimmune serum (NAb titer of 1:10240) by i.p. injection. One day after passive immunization, serum was collected and NAb titers were determined by neutralization assay. An average NAb titer of 1:320 was achieved in immunosuppressed hamsters which were not treated with the vector before passive immunization. Serum NAb recovered from the 'PassImm 1D after+CP+007' group were lower because this group was passively immunized 1 day after INGN 007 injection, and, presumably, binding of the NAb to the vector reduced the amount of NAb that could be detected in the assay.
Supplementary Material
Serum NAb titers after passive immunization. Immunosuppressed hamsters were passively immunized with 0.4 ml of hyperimmune serum (NAb titer of 1:10240) by i.p. injection. One day after passive immunization, serum was collected and NAb titers were determined by neutralization assay. An average NAb titer of 1:320 was achieved in immunosuppressed hamsters which were not treated with the vector before passive immunization. Serum NAb recovered from the 'PassImm 1D after+CP+007' group were lower because this group was passively immunized 1 day after INGN 007 injection, and, presumably, binding of the NAb to the vector reduced the amount of NAb that could be detected in the assay.
Acknowledgments
This research was supported by the grant CA118022 to W.S.M.W. from the National Institutes of Health. We thank Introgen Therapeutics, Inc. for kindly providing the purified INGN 007 and Ad5 stocks. W.S.M.W. is the founder of VirRx, Inc. VirRx has a financial interest in INGN 007.
REFERENCES
- Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- Wilson DR. Viral-mediated gene transfer for cancer treatment. Curr Pharm Biotechnol. 2002;3:151–164. doi: 10.2174/1389201023378445. [DOI] [PubMed] [Google Scholar]
- Hunt KK., and , Vorburger SA. Tech.Sight. Gene therapy. Hurdles and hopes for cancer treatment. Science. 2002;297:415–416. doi: 10.1126/science.297.5580.415. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV., and , Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TC, Galanis E., and , Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Yu W., and , Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- Shashkova EV, Doronin K, Senac JS., and , Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, et al. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol Ther. 2008;16:1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu DC, Charlton D., and , Henderson DR. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A., and , Guillet JG. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–3473. doi: 10.1002/eji.1830251239. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE., and , Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, Yu QC, et al. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC., and , Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari DS., and , Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R., and , Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Dhar D, Spencer JF, Toth K., and , Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Varterasian ML, Wadler S, Hecht JR, Benson A, Galanis E, et al. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:1498–1504. doi: 10.1200/JCO.2003.09.114. [DOI] [PubMed] [Google Scholar]
- Liu TC., and , Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–432. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- Bramson JL, Hitt M, Gauldie J., and , Graham FL. Pre-existing immunity to adenovirus does not prevent tumor regression following intratumoral administration of a vector expressing IL-12 but inhibits virus dissemination. Gene Ther. 1997;4:1069–1076. doi: 10.1038/sj.gt.3300508. [DOI] [PubMed] [Google Scholar]
- Molnar-Kimber KL, Sterman DH, Chang M, Kang EH, ElBash M, Lanuti M, et al. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9:2121–2133. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gönczöl E., and , Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ertl HC., and , Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Cichon G, Boeckh-Herwig S, Schmidt HH, Wehnes E, Müller T, Pring-Akerblom P, et al. Complement activation by recombinant adenoviruses. Gene Ther. 2001;8:1794–1800. doi: 10.1038/sj.gt.3301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnavski AN, Calcedo R, Bove M, Gao G., and , Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- Vlachaki MT, Hernandez-Garcia A, Ittmann M, Chhikara M, Aguilar LK, Zhu X, et al. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol Ther. 2002;6:342–348. doi: 10.1006/mthe.2002.0669. [DOI] [PubMed] [Google Scholar]
- Tsai V, Johnson DE, Rahman A, Wen SF, LaFace D, Philopena J, et al. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin Cancer Res. 2004;10:7199–7206. doi: 10.1158/1078-0432.CCR-04-0765. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer JM, Shashkova EV, et al. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector Cancer Gene Ther 2009(epub ahead of print) [DOI] [PMC free article] [PubMed]
- Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, et al. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies Cancer Gene Ther 2009(epub ahead of print) [DOI] [PMC free article] [PubMed]
- Spencer JF, Sagartz JE, Wold WS., and , Toth K.New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster Cancer Gene Ther 2009(epub ahead of print) [DOI] [PMC free article] [PubMed]
- Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ., and , Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE., and , Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M., and , Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Córdova E, et al. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR, et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci USA. 2008;105:7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Schulick AH, Vassalli G, Dunn PF, Dong G, Rade JJ, Zamarron C, et al. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J Clin Invest. 1997;99:209–219. doi: 10.1172/JCI119149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81:6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold WSM., and , Horwitz MS. Fields Virology. Wolters Kluwer Lippincott Williams & Wilkins: Philadelphia, PA; 2007. Adenoviruses. In: Knipe, DM and Howley, PM (eds; pp. 2395–2436. [Google Scholar]
- Lichtenstein DL, Toth K, Doronin K, Tollefson AE., and , Wold WS. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ., and , Wold WS. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Kuppuswamy M, Shashkova EV, Doronin K., and , Wold WS. Preparation and titration of CsCl-banded adenovirus stocks. Methods Mol Med. 2007;130:223–235. doi: 10.1385/1-59745-166-5:223. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF., and , Wold WS. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med. 2007;130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum NAb titers after passive immunization. Immunosuppressed hamsters were passively immunized with 0.4 ml of hyperimmune serum (NAb titer of 1:10240) by i.p. injection. One day after passive immunization, serum was collected and NAb titers were determined by neutralization assay. An average NAb titer of 1:320 was achieved in immunosuppressed hamsters which were not treated with the vector before passive immunization. Serum NAb recovered from the 'PassImm 1D after+CP+007' group were lower because this group was passively immunized 1 day after INGN 007 injection, and, presumably, binding of the NAb to the vector reduced the amount of NAb that could be detected in the assay.