Abstract
Venous leg ulcers are a prevalent nonhealing wound of the lower extremity. Although topically applied growth factors successfully improve wound repair in animal studies, similar studies on humans with venous leg ulcers have not been successful. This study was designed to evaluate the acute safety and biologic feasibility of peri-ulcer injection of a replication-incompetent adenoviral construct expressing platelet-derived growth factor-β (PDGF-β). In this phase I study, we demonstrate the initial safety, feasibility, and biologic plausibility of using H5.020CMV.PDGF-β to treat venous leg ulcer disease.
Introduction
Chronic wounds have become a major health concern for the United States and abroad. In the United States alone, chronic wounds cost the health-care system ~$9.7 billion annually.1,2 A large portion of these wounds are localized to the lower extremities, where their prevalence has been estimated at 0.2–2% of the adult population.3,4 Multiple etiologies have been implicated as a cause for lower extremity wounds, including venous disease, arterial insufficiency, and insensate neuropathy.4,5 Venous disease, being the most common cause, is estimated to be responsible for 40–70% of chronic lower extremity wounds. Furthermore, it is believed that between 500,000 and 2 million Americans suffer from venous leg ulcer disease.3,5
Successful treatment of venous leg ulcer disease has been difficult to achieve. Lower limb compression has been the standard of care for these patients, but unfortunately, is too often unsuccessful. Additionally, to achieve optimal results, this technique requires a prolonged 24-week treatment period, and even then only achieves success rates between 30 and 70% (refs. 6,7,8).
Due to the suboptimal results obtained from leg compression therapy alone, alternative methods to treat nonhealing venous leg ulcer disease have been explored. One promising alternative is the administration of growth factors to the wound site, which are known to be important for wound healing. One particular growth factor of interest in treating chronic nonhealing wounds is platelet-derived growth factor (PDGF). This protein is normally deposited into tissue in large quantities by degranulating platelets shortly after injury, where it is known to assist in wound repair.9,10 PDGF is also normally secreted by several other cell lines active in wound repair, such as macrophages, endothelial cells, fibroblasts, and keratinocytes.11 When supplemented onto wounds in various animal models, PDGF has been shown to increase the rate at which wounds heal.10,12,13,14 PDGF administration has also been used in humans and approved by the US Food and Drug Administration (FDA) to treat diabetic foot ulcers.15 Additionally, trials using PDGF to treat venous leg ulcers have resulted in FDA approval.
Previous studies have attempted to treat venous leg ulcer disease by using various topically administered growth factors including PDGF, acidic fibroblast growth factor, basic fibroblast growth factor, insulin-like growth factor, epidermal growth factor, keratinocyte growth factor, transforming-α, and transforming growth factor-β. Many of these studies, however, have gone unpublished due to lack of efficacy.16 The reasons for failed efficacy of these novel pharmaceuticals are not clear but may be due to problems in growth factor penetration into the wound. Without adequate penetration, these growth factors may not reach their target cells and consequently fail to initiate the wound healing process. In addition, the application of growth factors is often daily, which interrupts the use of standard limb compression therapy.
Recently, the use of gene transfer to introduce PDGF into animal wounds has been shown to be superior to topical application.17,18,19,20 It is our contention that the use of cytokine growth factors should augment the healing of chronic wounds and that topical application is an ineffective method of application. This study was designed to evaluate the acute safety and biologic feasibility of peri-ulcer injection of a replication-incompetent adenoviral construct expressing PDGF-β under the control of the cytomegalovirus (CMV) promoter and early enhancer.
Results
General
We discussed the study with 247 subjects to determine their interest and obtained consent from 26 subjects, who were formally screened. During this process, 11 were found to be ineligible for the following reasons: four had wounds smaller than 5 cm2, two were seropositive for hepatitis C, one did not complete all screening tests, one subject had an erythrocyte sedimentation rate > 60; one subject had hemoglobin <10, one subject had a hemoglobin <10 and an erythrocyte sedimentation rate >60, and 1 individual had a positive antinuclear antibody. The average age of the participants was 46.4 years (SD 6.9) with a median age of 48 years. Of the 15 subjects, 11 (73 %) were male and 9 (60%) were Caucasian. The average weight was 126.2 kg (SD 59.8) and the median weight was 115 kg. Twelve subjects (80%) had more than one wound and 10 (67%) had a history of previous wounds. At screening, the average wound was 24.4 cm2 (SD 21.5) and the median wound size was 19.5 cm2. Mean wound size for each dose-group is described in Table 1 and for each participant in Table 2. The average wound duration was 24.9 months (SD 31.5) and the median wound duration was 12 months. Twelve subjects had additional wounds that were usually smaller and of shorter duration.
Table 1.
Averaged by dose group
Table 2.
Data for each subject
Wound response
In general, the injection was well tolerated and the subjects showed improvement in their wounds over the 28 days of the primary study. No subjects reported pain from the injection. No signs of local wound infection, wound erythema, or cellulitis were observed as a consequence of the injection. Wound improvement was noted by several parameters during the first 28 days of the study. Wound pain was noted by 13 of 15 subjects at baseline. By day 28 no one reported an increase in pain; by week 28, only 3 of 15 still reported pain and two of these subjects noticed much less pain. For all subjects, granulation tissue increased during the study period. Granulation tissue was often exuberant, it often filled the wound (i.e., was not just present in the site of injection), and, between days 7 and 14, for those in dose-group 1–3, the wounds often superficially bled during dressing changes. All biopsy sites healed without complication.
A decrease in wound size between baseline and day 28 was noted in 14 of 15 subjects (93%). The overall mean and median percentage changes between baseline and day 28 were 45.2% (SD 36.2) and 41.3% (25%, 75%:20.0%, 83.7%), respectively, and the mean and median log healing rates were 0.048 (SD 0.065) and 0.019 (25%, 75%:0.008, 0.065), respectively (Tables 1 and 2). No statistically significant dose by “wound size change” effect was noted (percentage change, P = 0.1047 and log healing rate, P = 0.1235, respectively). By day 28 two subjects healed and at the end of 24 weeks of follow-up seven more subjects (47%) were found to have healed. Many subjects had more than one wound (12 of 15, 80%). Only the target wound was treated (largest and oldest eligible wound). For four of these multiwound subjects, a total of seven nontarget wounds healed.
Routine blood testing
Based on our reference laboratory's criteria there were several laboratory abnormalities, which in general were expected. Abnormalities were noted before the experimental injection (screening and day 1) as well as days 3, 7, 14, and 28. The laboratory results most frequently reported as abnormal included: values obtained as part of complete blood count or differential, lactate dehydrogenase, glucose (in a subject with a history of diabetes), erythrocyte sedimentation rate, chloride, or CO2. One subject, in group 4, had detectable rheumatoid factor (RF) titers. This subject had trace RF titers at baseline (37 IU/ml), a low level positive at day 28 (105 IU/ml), and negative titers at month 3 (<30 IU/ml). No signs or symptoms consistent with a rheumatologic illness were noted. This finding could have represented dose-limiting toxicity (DLT).
H5 adenovirus detection
By design, H5.020CMV.PDGF-B is an adenovirus (H5) that cannot replicate. Subjects were tested at day 3, 7, 14, 21, and 28 with samples from blood, wound swabs, and bandage swabs for the presence of this virus and its ability to replicate. As expected, the adenovirus could not be cultured from any of these specimens (i.e., H5 adenovirus could not replicate and did not gain this ability after exposure to the study subjects). In total, using PCR probes, 7 of 15 subjects in 10 out of 75 specimens revealed viral DNA on the wound or on the wound-exposed bandage. All positive tests occurred at days 3 or 7. No subjects at dose 1 had a positive test. One of three subjects from dose-level 2, three of three subjects from dose-level 3, and three of six subjects from dose-level 4 had positive tests. Adenovirus DNA was noted on 5 day three wounds, 3 day three bandages, and 2 day seven wounds.
Antibodies to H5 were noted in all 14 subjects. Eight of 15 had antibodies at screening. All had antibodies at month 3. The largest increases, some greater than 100-fold, were noted from the subjects that had the highest H5 antibodies at baseline and these subjects were distributed across all dose groups. It is important to realize that while this test was developed specifically to measure adenovirus H5 antibodies, the assay does crossreact and measure antibody responses to wild-type adenovirus, too.
PDGF antibody
Three of 14 subjects had measurable antibodies to PDGF-BB (optical density of >0.1 at a dilution of 1:100). These were single subjects in dose groups 1, 3, and 4. The antibodies persisted to a dilution of 1:400. Their titers were undetectable at week 6. By their sixth month of care, two of these subjects healed. One of these subjects also had detectable RF titers.
Detection of adenoviral DNA within wound bed
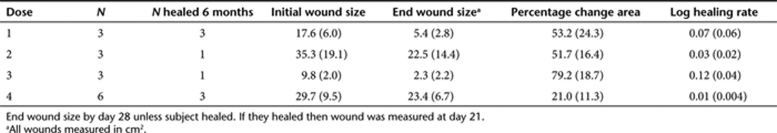
To assess the presence of adenoviral DNA in the wound bed, we used DNA in situ hybridization to detect the CMV promoter within the adenoviral genome. Tissue biopsies before adenoviral administration at day 1 and following adenoviral administration at days 3 and 28 were analyzed. As shown in Figure 1, adenoviral DNA was not detected before adenoviral administration at day 1. However, at day 3 adenoviral DNA was detected in both the ulcer bed and keratinocyte layer. Biopsies taken at day 28 postadenoviral injection were also found to be negative for adenoviral DNA.
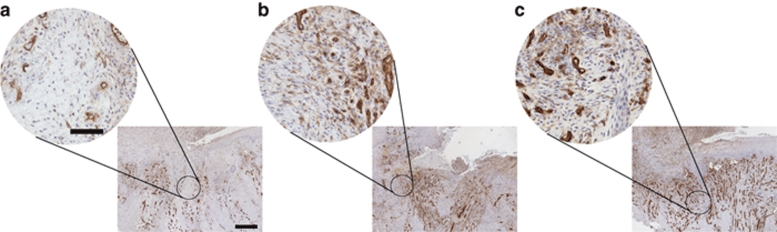
Figure 1.
DNA in situ hybridization for the cytomegalovirus (CMV) promoter. (a) Day 1 venous leg ulcer biopsy 5-µm sections showing no CMV-expressing cells in the wound bed (left panel) or in the keratinocyte layer (right panel). (b) Day 3 venous leg ulcer 5-µm sections showing CMV-expressing cells (arrows) in both the wound bed and keratinocyte layer. (c) Day 28 venous leg ulcer 5-µm section showing no CMV-expressing cells, suggesting that the infection has been cleared. (d) Day 3 no probe control venous leg ulcer 5-µm sections showing no positive staining. Bar = 50 µm. Samples were counterstained with Nuclear Fast Red (Vector Laboratories).
Assessment of wound neovascularization
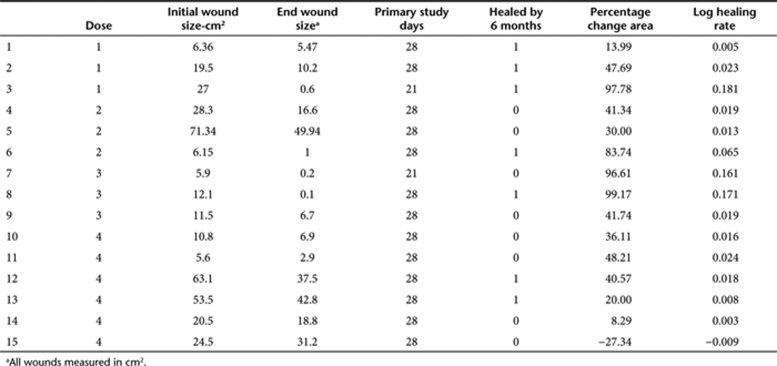
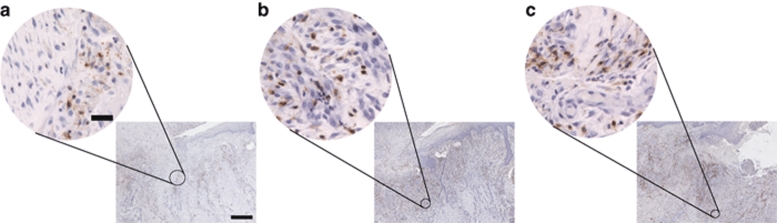
An important sign of wound healing is the creation of new blood vessels, or neovascularization, within the wound. To assess the degree of neovascularization, we used an endothelial cell–specific antibody to CD31 for labeling capillaries within the ulcer. These labeled cells were then quantified per high power field (HPF, ×400) in each of four quandrants within the wound biopsy as shown in Figure 2. A representative patient biopsy at days 1, 3, and 28 using CD31 immunohistochemistry can be seen in Figure 3. The average number of CD31+ staining vessels were found to be 3.8 (SD 0.9), 5.2 (SD 2.3), and 4.6 (SD 1.7) on days 1, 3, and 28, respectively (P = 0.41). Although a trend was noted comparing day 1 to days 2 or 28 (P = 0.06 and 0.09, respectively). The number of vessels noted did vary by region in that more vessels were counted in the subepidermal superficial quadrant than the others (P < 0.0001, all other comparisons P > 0.30). There was no apparent effect of increasing H5 adenovirus dose on CD31+ staining vessels.
Figure 2.
Representative image of a wound biopsy after hematoxylin and eosin staining showing the regions used for cell quantitation and vessel density analysis. (a) Superficial subepithelial region, (b) superficial ulcer region, (c) subepithelial dermal (or deep) region, and (d) ulcer deep region. Bar = 1 mm.
Figure 3.
To assess wound neovascularization after administration of H5.020CMV.PDGF-β, we immunostained tissue biopsies for an endothelial specific antigen CD31 and quantified the number of positive staining vessels per HPF at (a) day 1, (b) day 3, and (c) day 28. Bar = low power: 500 µm, high power: 150 µm. CMV, cytomegalovirus; HPF, high power field; PDGF-β, platelet-derived growth factor-β.
Assessment of tissue inflammation
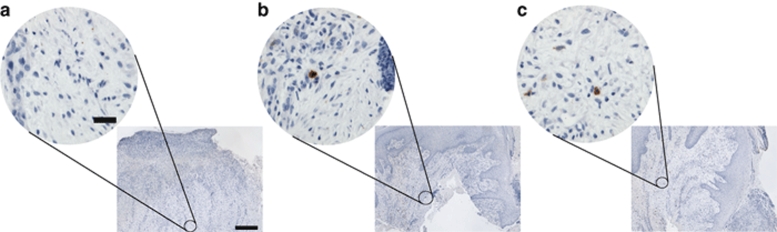
To determine whether the administration of the adenoviral construct caused an inflammatory response within the injected tissue, the number of cells expressing the common leukocyte antigen (CD45) was quantified per HPF using immunohistochemistry. The average number of CD45+ cells noted per HPF on days 1, 3, and 28, respectively, was 12.6 (SD 7.0), 11.7 (SD 7.5), and 13.3 (SD 7.4). This can be seen in Figure 4, from a representative patient biopsy at days 1, 3, and 28. These values were found not to vary significantly by day or location (P > 0.30), suggesting no increased inflammatory response after injection of H5.020CMV.PDGF-β.
Figure 4.
To assess wound inflammation from H5.020CMV.PDGF-β, we immunostained tissue biopsies for the common leukocyte antigen (CD45) and quantified the number of cells staining positive at (a) day 1, (b) day 3, and (c) day 28. No significant increase in CD45+ cells were noted in tissue biopsies taken at day 1, day 3 and day 28 (P > 0.30). Bar = low power: 500 µm, high power: 50 µm. CMV, cytomegalovirus; PDGF-β, platelet-derived growth factor-β.
Assessment of progenitor cell recruitment
Recruitment of bone marrow–derived stem cells to sites of ischemia is known to be important for normal wound healing. To determine whether the delivery of Ad-PDGF-B caused an increase in the recruitment of CD133+ cells, an antigen known to be expressed on endothelial progenitor cells, we quantified the number of these cells within a section of wound biopsy per HPF. The average number of CD133+ cells on days 1, 3, and 28 were 3.8 (SD 0.6), 5.2 (SD 0.8), and 4.6 (SD 1.4), respectively. This can be seen in Figure 5, from a representative patient biopsy at days 1, 3, and 28. The number of cells did not vary by region (P = 0.78) but did vary by day (P = 0.0023). This finding was primarily due to differences between day 1 and day 2 (Sidak adjusted P = 0.0015).
Figure 5.
To determine the effect of recruitment on progenitor cells within the wound after administration of H5.020CMV.PDGF-β, we immunostained tissue biopsies for CD133 an antigen found on undifferentiated cell types such as endothelial progenitor cells. High and low power views of representative wound biopsies at (a) day 1, (b) day 3, and (c) day 28. Note more cells staining positive for CD133 at day 3 as compared to day 1 (P = 0.0015). Bar = low power: 500 µm, high power: 50 µm. CMV, cytomegalovirus; PDGF-β, platelet-derived growth factor-β.
Adverse events and serious adverse events
Seventeen adverse events (AEs) were reported during the primary portion of the study. The most frequently reported AEs were related to pre-existing illness such as hypertension or diabetes (four reports each) as well as single reports for others. None of the AEs were thought to be related to the study. No serious AEs were noted during the initial 28-day postinjection follow-up. One serious AE was noted ~5 months postinjection. At this time a study subject was hospitalized for endocarditis. He initially appeared to recover and his wound healed. However, he died of a cardiovascular cause 11 months after injection.
Discussion
The goals of this study was to evaluate the acute safety of a wound-edge injection of H5.020CMV.PDGF-B and the biologic feasibility of using H5.020CMV.PDGF-B to treat patients with chronic venous leg ulcers while they receive limb compression therapy. In general, the injections were well tolerated and subjects were able to use a compression bandage during the 28 days of the initial study period. Only one individual in the study may have exhibited DLT based on the conversion of a trace positive to truly positive RF serology, which then resolved without clinical significance. Shortly after this subject was enrolled, the inclusion and exclusion criteria of the study were modified to prevent the enrollment of a subject with a slightly positive RF serology. In our early phase gene transfer study of H5.020CMV.PDGF-B, because of a lack of measured DLT, by default our maximally tolerated dose was dose-level 4.
We were also able to demonstrate that H5.020CMV.PDGF-B was able to transfect cells in the wound. Transfection with H5.020CMV.PDGF-B resulted in the migration of endothelial precursor cells to the wound and the formation of granulation tissue. The injection of H5.020CMV.PDGF-β also resulted in a decrease in the overall size of the target wound by day 28 in 14 of 15 subjects. Although we found very few signs of systemic toxicity, it is possible that the adenoviral injection had a systemic effect based on the observation that “uninjected” parts of the target wound improved as did nontarget not injected wounds on the few subjects with additional wounds. Our clinical observations of improved wound healing and the presence of granulation tissue have also been reported with topical recombinant human PDGF.15 In addition, although this may have been related to a lack of sensitivity of our assay, we found an antibody response to H5 (our adenoviral vector) in most subjects. Finally, antibodies were found to develop against PDGF in a few subjects that later resolved, but again our test was relatively nonspecific. Finally, based on all of our findings, the greatest effect from a single injection of H5.020CMV.PDGF-β may have been in dose groups 2 or 3.
Several investigators have previously demonstrated that improvements in wound size by the fourth week of care can be used to predict that a venous leg ulcer will heal by the 16th to 24th week of care, which is the usual length of a trial to study venous leg ulcers.21,22 For this study, we used previously validated historical comparisons based on surrogate markers for a healed wound by the 24th week of care in order to predict who might have healed.23 Seventy-three percent of our subjects had wound changes consistent with healing.23 After week 4, it is important to note that the subjects returned to their previous wound care providers and they no longer consistently used limb compression. By report, only three were actively engaged in limb bandage compression therapy. It is very unlikely that an individual with a venous leg ulcer will heal without limb compression. Because of noncompliance with limb compression a natural historic comparison population does not exist. However, the likelihood of similar subjects who used limb compression healing, based on wound size and duration, is about 31% by the 24th week of care.24 This healing rate is similar to the control arms in randomized clinical trials.22 However, the difference between our results (47% at week 24) and the historical expectation was a risk difference of 16%, which is a number needed to treat ~6 or a relative risk of 1.52 favoring H5.020CMV.PDGF-β. This relative risk is similar to that noted as part of FDA studies where recombinant human PDGF was applied daily for more >12 weeks to treat diabetic foot ulcers.8,25,26,27
Our study may provide evidence that supports the notion that successful vasculogenesis, or de novo formation of new blood vessels from the differentiation of stem cells, requires the interplay of many different cell types including multiple cells that are now classified as endothelial progenitor cells.28,29,30 In our study, clinically exuberant granulation tissue throughout the wound was noted within the first 2 weeks of injection. The histopathology of biopsy specimens from day 3 wounds transfected by H5.020CMV.PDGF-β demonstrated a statistically significant increase in CD133 cells, a marker of an immature bone marrow–derived endothelial progenitor cell.31 While the number of these cells in the wound-edge biopsy increased at day 3, they then decreased at day 28. Interestingly, they also predominated in the sections of the biopsies that represent the site of injections. To the best of our knowledge, this is the first report of PDGF-β application resulting in CD133 migration to wounded tissue. In contrast, the number of blood vessels assessed by positive staining for CD31, which is believed to be a more mature marker for a cell destined to become a mature vascular endothelial cell, did increase at day 28 and the vessels were most prevalent in the subepidermal region of the biopsy. This is probably related to the formation of vessels in healing tissue.
Our study was first designed in 1999, at which time we received funding from the National Institute of Arthritis Musculoskeletal and Skin disease to conduct this study. Our investigational new drug application was approved in late 2003 and we published our clinical trial protocol in 2004 (ref. 32). Because of regulatory concerns about the production of our vector and the safety of conducting gene transfer studies, we were not able to enroll our first study subject until January 2005 and we enrolled our final study subject in summer 2008. This study took nearly 10 years to complete. Our delays were often related to what are now historical events related to the field of gene transfer studies.33 Many of these events resulted in improved oversight and government regulation concerning the conduct of such studies. In fact, our vector production and preclinical animal studies were conducted three separate times by different organizations, because of the need of these organizations to provide services at Good Manufacturing Practice and Good Laboratory Practice. The pace of our study was further slowed because of the logistic difficulties associated with identifying subjects to screen, screening only one subject at a time, and a safety requirement such that we could enroll only one subject in the primary phase of our study at a time. In many ways the regulatory infrastructure needed to conduct a gene transfer study on a subject who does not have lethal or genetic illnesses was created while this study was underway. Finally, in order to fill our study, which ultimately required 15 subjects, we had to discuss the study with 247 subjects and screen 26. Many potential subjects decided not to participate because of the need to stay in a research unit over two nights for observation and because of the potential risks of the study described in the consent form.
In conclusion, we have demonstrated within the reliability of a Phase I study that the doses of H5.020CMV.PDGF-β used in this study should be considered for further human testing. We believe that a single administration of H5.020CMV.PDGF-β has the potential to improve the likelihood that a subject with a hard-to-heal venous leg ulcer will heal. We believe that the mechanism of action may involve an effect on angiogenesis. Based on our study observations it is not apparent that H5.020CMV.PDGF-β needs to be injected into the edge of the wound insofar as it appears to have an effect over the full wound and perhaps full subject. Further studies need to be conducted in order to establish the efficacy of this compound.
Materials and Methods
Production of H5.020CMV.PDGF-β. The human PDGF-β complementary DNA clone was obtained by Meenhard Herlyn (at the Wistar Institute) from B. Westermark, University Hospital, Uppsala, Sweden. A 1.2-kb fragment was cut out of the complementary DNA clone using restriction enzymes PstI and EcoRI, which encode the entire open reading frame. This gene was then subcloned into the pSL301 transfer plasmid provided by the Vector Core in the Division of Medical Genetics at the University of Pennsylvania. The pSL301 plasmid provides a CMV immediate early promoter and SV40 polyA region. Final production of the adenoviral vector was produced at the Center for Cell and Gene Therapy at Baylor University. The process was conducted under the Good Manufacturing Practice and Good Laboratory Practice regulations required by the FDA for vector production.34 Preclinical animal toxicity studies were performed by Southern Research Institute.
Study design. The study protocol has been previously published.32 Briefly, this was a Phase I study dose-escalation study conducted at a single site, the University of Pennsylvania. As per study protocol all subjects received only one dose of H5.020CMV.PDGF-β along with limb compression therapy. The protocol was approved by the University's institutional review board for human subjects and the General Clinical Research Centers (now Clinical and Translational Science Award) advisory committee of the University of Pennsylvania. All subjects gave written informed consent and the study conformed to the Declaration of Helsinki protocols. The trial was registered on clintrial.gov. All subjects were screened within 4 weeks of starting the study. The inclusion criteria included: an examination by the study investigator consistent with a venous leg ulcer (i.e., varicose veins, venous blush, wound in the gaiter area of the leg, dermatitis, lipodermatosclerosis); the prior use of and ability to tolerate limb compression therapy, which had been used for at least 6 weeks without improvement in wound size; a wound size >5 cm2 but <60 cm2; wound duration of >6 months; ankle brachial index ≥0.85; white blood cells ≥3,500/mm3, platelets <1,000,000/mm3 but >100,000/mm3 and hemoglobin > 10.0g%; age ≥18 years; females of childbearing potential and all males must agree to use a medically approved method of contraception; duplex ultrasound not consistent with an acute deep venous thrombosis; and the target wound must be free of all necrotic debris. Subjects were excluded from the study if they had: any active cancer other than a keratinocyte skin cancer; a life expectancy of <6 months; liver function tests (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin) >1.5× upper limit of normal for the reference lab; erythrocyte sedimentation rate of >60; a positive RF titer; an antinuclear antibody test of dilutional titer of >1:160; intercurrent organ damage, abnormal laboratory tests, or medical problems that in the opinion of the investigator would jeopardize their ability to participate in this study or to heal their wound; a recognized rheumatic disease (e.g., lupus, scleroderma, dermatomyositis, rheumatoid arthritis, polymyalgia, etc.) or any concurrent medical illness that could be exacerbated by H5.020CMV.PDGF-β administration; any requirement for systemic corticosteroids or immunosuppressives, or history of corticosteroid or immunosuppressive use in the 4 weeks before study entry; seropositivity for hepatitis B or C surface antigen; or refused to use a limb compression bandage.
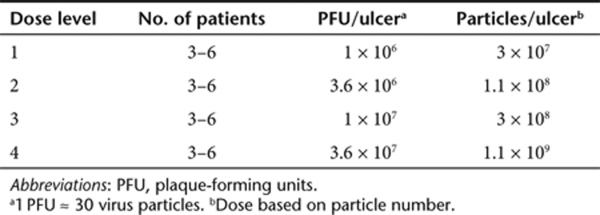
Dose administration. Using a 3–6 design (Table 3), a single dose of H5.020CMV.PDGF-β reconstituted in 0.1 ml of saline was injected using a 27-gauge tuberculin syringe. The dose was administered subcutaneously around ~3 cm of wound edge (perimeter) and occurred during day 1 of an in-patient stay at the Clinical and Translational Research Center of the University of Pennsylvania that lasted for about 48 hours. A limb compression bandage was applied after the administration of H5.020CMV.PDGF-β (day 1) and then reapplied at the day 3, 7, 14, 21, and 28 visits. Days 1–28 were the primary critical active phase days of the study. Per the 3–6 dose escalation scheme used in the study, dose escalation occurred if 0 of 3 subjects experienced DLT. However, if one subject experienced DLT, then three more subjects were treated at that dose, meaning that six individuals received the dose. If a total of two or more of the six experienced a DLT, then the dose was deemed too toxic and the next lower dose would be evaluated again in up to six subjects. If, in total, zero of three or fewer than two of six subjects experienced DLT at a dose level, then the next higher dose was evaluated until up to six subjects had been enrolled in dose-level 4. This pattern of administration was repeated for all subsequent dose levels until a maximally tolerated dose was declared or dose-level 4 was completed (assuming that all subjects had been dosed successfully). The maximally tolerated dose was defined as the highest dose for which fewer than two of six subjects experience DLT. The decision to progress to the next level was made with the guidance of the Data Safety Monitoring Board after all had been dosed and salient safety information reviewed up to day 28 in a dose-group.
Table 3.
Dose-escalation schedule
Observations. Each patient was monitored closely for adverse reactions resulting from H5.020CMV.PDGF-β treatment. All tests were conducted as described in Supplementary Table S1. These observations included history, physical examination, and tests (i.e., routine blood chemistries as well as experimental tests such as viral shedding assays, viral cultures, antibody testing for PDGF, etc.). All abnormal routine laboratory values were based on abnormal values as determined by the University of Pennsylvania Pathology Laboratories. Abnormalities of specific interest defined a priori as DLT are listed in Supplementary Table S2 (however, some were modified during the study with FDA approval). In addition, any clinical evidence of autoimmune disease was defined as DLT. Local toxicity was considered DLT and graded as follows: erythema and induration >2 cm from the edge of the wound and/or ulcer enlargement of >20% on day 3; more than a 33% increase in pain score on day 3; any permanent dysfunction related to local toxicity; and any fever >39.0 °C not successfully controlled with nonsteroidal anti-inflammatory medications. In addition, any vector-related serious AE, as determined by the Data Safety Monitoring Board, was considered a DLT. All AEs were additionally reviewed periodically by the Data Safety Monitoring Board, which was allowed to suggest changes to the DLT policy, and the investigator.
At all visits the wound was evaluated, traced, and photographed. An excisional biopsy was performed after the technique of Herrick35 just before the administration of H5.020CMV.PDGF-β and was repeated at days 3 and 28. Briefly, three full-thickness rectangular excisional biopsies measuring ~12 × 3 mm were obtained from within the tissue injected with H5.020CMV.PDGF-β and were positioned to include the ulcer tissue, wound edge, and portion of intact skin. The tissue biopsy was immediately placed into 10% neutral buffered formalin at room temperature and processed as indicated in immunohistochemistry section. In order to assure that transfected tissue was biopsied, the periwound edge was marked after injection using gentian violet and the area injected was also marked on the acetate trace. It should, however, be noted that the day 3 biopsy potentially removed transfected tissue from the study subject.
Postprimary study surveillance of the subjects continued at 6 weeks and at 3, 6, and 12 months following day 28 of the primary phase of the study (Supplementary Table S1). Excepting the follow-up visits, study subjects after day 28 returned to their previous health-care providers for wound care.
Experimental testing
HAdV5-specific neutralizing antibody assay: Anti-HAdV5 neutralizing antibody titers in serum samples were measured by assessing the ability of serum to inhibit transduction of the corresponding reporter vector, HAdV5LacZ into HEK 293 cells. The reporter vector was incubated with twofold serial dilutions of heat-inactivated sera for 1 hour at 37 °C. Serum samples were diluted with naive mouse serum (Sigma-Aldrich, St Louis, MO) so that the final serum concentration at all dilutions was 5%. Subsequently, the serum–vector mixture was added onto HEK 293 cells in 96-well flat-bottomed plates (at a multiplicity of infection of 10 virus particles per cell) and incubated for 18–22 hours. Cells were washed in phosphate-buffered saline (PBS) × 2 and developed with a Galacto-Star kit (Applied Biosystems, Foster City, CA). The resulting luminescence was measured with a luminometer (Clarity; BioTek Instruments, Winooski, VT). The neutralizing antibody titer was reported as the highest serum dilution that inhibited AAV-CMV-LacZ transduction (β-galactosidase expression) by ≥50%, compared with the naive serum control. These assays were conducted on serum samples obtained at days 1 and 28, week 6 and month 3 after adenoviral injection. Samples were stored before assaying at −80 °C. Samples were not available for one subject.
PDGF-β-specific enzyme-linked immunosorbent assay: Polypropylene plates (96-well, cat. no. 3369; Costar, Corning, NY) were coated with 100 µl of recombinant human PDGF-BB (500 ng/ml, cat. no. P4306; Sigma, St Louis, MO) overnight at 4 °C. The plates were blocked with PBS containing 3% bovine serum albumin (cat. no. A3803; Sigma) for 2 hours at room temperature. Serum samples were twofold serially diluted (starting at 1/100) with PBS containing 1% fetal bovine serum and added in triplicate to the plates for incubation for 2 hours at room temperature. Then 100 µl of rabbit antihuman-PDGF-β antibody (cat. no. P6101; Sigma) was diluted 1:500 in PBS containing 1% fetal bovine serum, which was added to the plate as a positive control. Next, the plates were washed three times with PBS containing 0.1% Tween-20, and then incubated for 1 hour with peroxidase-conjugated antihuman or anti-rabbit IgG (diluted 1:10,000 in PBS; Sigma-Aldrich). After three washes, the plates were incubated with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate (100 µl/well, cat. no. 50-66-00; KPL, Gaithersburg, MD) for 30 minutes at room temperature to allow for color development. The optical density was then measured at a wavelength of 405 nm. These assays were conducted on serum samples obtained at days 1 and 28, week 6 and month 3 after adenoviral injection. Samples were stored before assaying at −80 °C. Samples were not available for one subject.
Adenoviral viral shedding assays and culturing: This testing was conducted by the Clinical Virology Laboratory of the Children's Hospital of Philadelphia. Two tests were conducted on two specimens obtained at five different time points (once just before the administration of the vector and then on days 3, 7, 14, 21, and 28) from each study subject. The tests were performed during dressing changes on swabs of the wound and swabs from the surface of bandages in contact with the wound. Whole blood was also assayed for viral DNA using qualitative PCR. The cytopathic effect assay for adenovirus was performed on A549 cells to detect replication-competent adenovirus produced by homologous recombination of the replication-deficient vector and wild-type adenovirus and/or latent E1a in trans. Replication-competent adenovirus occurs as an impurity of manufacture at an extremely low level (<1 replication-competent adenovirus in 3 × 1010 particles). A cytopathic effect assay test was performed on 293 cells used to detect the presence of replication-deficient adenovirus vector. The presence of 51 serotypes of adenovirus was tested by incubating patient samples on highly sensitive A549 cells. Adenovirus growth suggested by cytopathic effect assay was confirmed by immunofluorescence using monoclonal antibodies to adenovirus.
Immunohistochemistry: Postfixed biopsy samples were dehydrated in ethanol, cleared in xylene, and embedded in paraffin using the Leica ASP 300 Tissue processor (Leica, Wetzlar, Germany). Five micrometer sections were used for immunohistochemical analysis. All staining was performed using the Dako Autostainer Plus (Golstrup, Denmark). Before quenching endogenous peroxidase activity, antigen retrieval was performed: 20 minutes in Proteinase K (S3020; Dako) for CD31 staining, 20 minutes in Target Retrieval Solution (S1699; Dako) at 95 °C followed by a 20-minute cool down at room temperature for PDGF-β and CD133. The following primary antibodies were used: CD45, Leucocyte Common Antigen (N1514, Ready to Use; Dako), CD31, Endothelial Cell (N1596, Ready to Use, Dako), CD133/1 (AC133) (#130-090-422, 1:100; Miltenyi Biotec, Bergisch Gladbach, Germany), and PDGF BB antibody, prediluted (ab15500, 1:2; Abcam, Cambridge, MA). Detection of the primary antibodies CD45 and CD31 was achieved through the Dako Universal LSAB 2 Kit/HRP, Rabbit/Mouse (K0675) kit; CD133 was detected using Catalyzed Signal Amplification System (K1500; Dako), and PDGF-BB was detected with Biotinylated Anti-Rat IgG (heavy + light chains), mouse adsorbed secondary (BA-4001, 1:200; Vector Laboratories, Burlingame, CA) and ABC kit (6100; Vector Laboratories). All staining was visualized with diaminobenzidine (K3466; Dako) and counterstained with hematoxylin (S3301; Dako).
Adenoviral DNA in situ hybridization: Adenoviral DNA was detected in paraffin-embedded biopsy sections using digoxigenin-labeled DNA hybridized to the CMV promoter. The CMV promoter was PCR amplified from pAd-Track-CMV using the forward primer CTG ACG GTT CAC TAA ACC AG and the reverse primer TAG TAA TCA ATT ACG GGG TCA TTA G. This DNA segment was then used as a template for digoxigenin-labeling using the Random Primed Dig DNA Labeling Kit (Roche, Indianapolis, IN).
Tissue DNA in situ hybridization was performed as described in the Roche Dig application manual.36 Briefly, 5-µm tissue sections were dewaxed and rehydrated in a graded ethanol series. Sections were permeabilized using Proteinase K (25 µg/ml) for 25 minutes at 37 °C. The sections were then hybridized with probe cocktail containing the CMV digoxigenin-labeled DNA and heated to 95 °C on a slide warmer for 6 minutes. The no probe control contained no digoxigenin-labeled DNA in the probe cocktail. After cooling on ice, the sections were incubated overnight at 42 °C in the probe hybridization cocktail. The following day sections were washed in sodium salt citrate and blocked in blocking reagent (Roche). Digoxigenin was detected using anti-dig Fab fragments conjugated to alkaline phosphatase (Roche). Color was developed using BM purple (Roche), counterstained with nuclear fast red (Vector Laboratories), dehydrated through ethanol series and xylenes, and mounted in xylene-containing mounting media (Fisher Scientific, Pittsburgh, PA).
Tissue analysis: Tissue sections were divided into four regions based on location within the biopsy specimen (see Figure 1). Within each region, using five or more HPFs ×400, the number of CD31+ vessels, CD45+ cells, and CD133+ cells was quantified and averaged.
Statistics. Statistical analysis included descriptive evaluation based on means with standard deviations and medians with 25 and 75% quartiles, repeated measures analysis of variance, and an evaluation of differences based on the day the tissue was obtained or the location using a Sidak test for multiple comparisons. All analyses were conducted using Stata 9.2 (StataCorp, College Station, TX).
SUPPLEMENTARY MATERIALTable S1. Subject evaluations.Table S2. Dose-limiting toxicity (DLT)
Supplementary Material
Subject evaluations.
Dose-limiting toxicity (DLT)
Acknowledgments
The project described was supported by contract number N01-AR092238 and grant number K24AR002212 from the National Institute of Arthritis, Musculoskeletal and Skin Disease and UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We acknowledge Meenhard Herlyn (Wistar Institute, Philadelphia PA) for his guidance, encouragement, and contributions to this study. We also acknowledge and thank the Data Safety Monitoring Board for their service and commitment to this study. The members were Vincent Falanga (Chair), Gail Woodbury, Eric Bernstein, Yuko Palesch and Karl Sylvester. The work was conducted in Philadelphia, Pennsylvania, USA.
REFERENCES
- Ruckley CV. Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology. 1997;48:67–69. doi: 10.1177/000331979704800111. [DOI] [PubMed] [Google Scholar]
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Coon WW, Willis PW., and , Keller JB. Venous thromboembolism and other venous disease in the Tecumseh community health study. Circulation. 1973;48:839–846. doi: 10.1161/01.cir.48.4.839. [DOI] [PubMed] [Google Scholar]
- Hallbook T. Leg ulcer epidemiology. Acta Chir Scand. 1988;544:17–20. [PubMed] [Google Scholar]
- Phillips TJ. Chronic cutaneous ulcers: etiology and epidemiology. J Invest Dermatol. 1994;102:38S–41S. doi: 10.1111/1523-1747.ep12388556. [DOI] [PubMed] [Google Scholar]
- O'Meara S, Tierney J, Cullum N, Bland JM, Franks PJ, Mole T, et al. A systematic review of compression treatment for venous leg ulcers. BMJ. 2009;3385:1047–1063. [Google Scholar]
- Skene AI, Smith JM, Doré CJ, Charlett A., and , Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ. 1992;305:1119–1121. doi: 10.1136/bmj.305.6862.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human Skin Equivalent Investigators Group. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- Clark RAF (1996). Wound repair: overview and general considerations In: Clark RAF (ed). The Molecular and Cellular Biology of Wound Repair, 2 edn. Plenum: New York. pp. 3–50
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Danilenko DM, Ring BD, Tarpley JE, Morris B, Van GY, Morawiecki A, et al. Growth factors in porcine full and partial thickness burn repair. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. Am J Pathol. 1995;147:1261–1277. [PMC free article] [PubMed] [Google Scholar]
- Pierce GF., and , Mustoe TA. Pharmacologic enhancement of wound healing. Annu Rev Med. 1995;46:467–481. doi: 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Tarpley JE, Tseng J, Bready J, Chang D, Kenney WC, et al. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995;96:1336–1350. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer HD, Longaker MT., and , Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol. 1997;109:132–138. doi: 10.1111/1523-1747.ep12319188. [DOI] [PubMed] [Google Scholar]
- Wieman TJ, Smiell JM., and , Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- Falanga V, Eaglstein WH, Bucalo B, Katz MH, Harris B., and , Carson P. Topical use of human recombinant epidermal growth factor (h-EGF) in venous ulcers. J Dermatol Surg Oncol. 1992;18:604–606. doi: 10.1111/j.1524-4725.1992.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Crombleholme TM. Adenoviral-mediated gene transfer in wound healing. Wound Repair Regen. 2000;8:460–472. doi: 10.1046/j.1524-475x.2000.00460.x. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Sablich TJ, Adzick NS., and , Crombleholme TM. Recombinant adenoviral mediated gene transfer in ischemic impaired wound healing. Wound Repair Regen. 1999;7:148–153. doi: 10.1046/j.1524-475x.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- Sylvester KG, Nesbit M, Radu A, Herlyn M, Adzick NS., and , Crombleholme TM. Adenoviral-mediated gene transfer in wound healing: acute inflammatory response in human skin in the SCID mouse model. Wound Repair Regen. 2000;8:36–44. doi: 10.1046/j.1524-475x.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- Gruss CJ, Satyamoorthy K, Berking C, Lininger J, Nesbit M, Schaider H, et al. Stroma formation and angiogenesis by overexpression of growth factors, cytokines, and proteolytic enzymes in human skin grafted to SCID mice. J Invest Dermatol. 2003;120:683–692. doi: 10.1046/j.1523-1747.2003.12112.x. [DOI] [PubMed] [Google Scholar]
- Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds-developing Products for Treatment (2006). Food and Drug Administration. pp. 1–18 [DOI] [PubMed]
- Kantor J., and , Margolis DJ. Expected healing rates for chronic wounds. Wounds. 2000;12:155–158. [Google Scholar]
- Gelfand JM, Hoffstad O., and , Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol. 2002;119:1420–1425. doi: 10.1046/j.1523-1747.2002.19629.x. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Allen-Taylor L, Hoffstad O., and , Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12:163–168. doi: 10.1111/j.1067-1927.2004.012207.x. [DOI] [PubMed] [Google Scholar]
- Bartus CL., and , Margolis DJ. Reducing the incidence of foot ulceration and amputation in diabetes. Curr Diab Rep. 2004;4:413–418. doi: 10.1007/s11892-004-0049-x. [DOI] [PubMed] [Google Scholar]
- Falanga V, Fujitani RM, Diaz C, Hunter G, Jorizzo J, Lawrence PF, et al. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo-controlled trial. Wound Repair Regen. 1997;7:208–213. doi: 10.1046/j.1524-475x.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg. 1995;21:71–78; discussion 79. doi: 10.1016/s0741-5214(95)70245-8. [DOI] [PubMed] [Google Scholar]
- Humpert PM, Bärtsch U, Konrade I, Hammes HP, Morcos M, Kasper M, et al. Locally applied mononuclear bone marrow cells restore angiogenesis and promote wound healing in a type 2 diabetic patient. Exp Clin Endocrinol Diabetes. 2005;113:538–540. doi: 10.1055/s-2005-872886. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A., and , Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D, Brittan M., and , Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Cromblehome T, Herlyn M, Cross P, Weinberg L, Filip J, et al. Clinical protocol. Phase I trial to evaluate the safety of H5.020CMV.PDGF-b and limb compression bandage for the treatment of venous leg ulcer: trial A. Hum Gene Ther. 2004;15:1003–1019. doi: 10.1089/hum.2004.15.1003. [DOI] [PubMed] [Google Scholar]
- Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- FDA ICH E9 (1998) E9 Statistical Principles for Clinical Trials . < www.FDA.gov >. September
- Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN., and , Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol. 1992;141:1085–1095. [PMC free article] [PubMed] [Google Scholar]
- Nonradioactive In Situ Hybridization Application Manual . < http://www.roche-applied-science.com/PROD_INF/MANUALS/InSitu/InSi_toc.htm >. Accessed 5 January 2009
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subject evaluations.
Dose-limiting toxicity (DLT)